Abstract
Background:
In cases of bilateral breast reconstruction when the deep inferior epigastric perforator (DIEP) free flap alone does not provide sufficient volume for body-specific reconstruction, stacking each DIEP flap with a second free flap will deliver added volume and maintain a purely autologous reconstruction. Stacking the profunda artery perforator (PAP) flap with the DIEP flap offers favorable aesthetics and ideal operative efficiency. We present the indications, technique, and outcomes of our experience with 4-flap breast reconstruction using stacked DIEP/PAP flaps.
Methods:
The authors performed 4-flap DIEP/PAP breast reconstruction in 20 patients who required bilateral reconstruction without adequate single donor flap volume. The timing of reconstruction, average mastectomy/flap weights, and operative time are reported. Complications reviewed include fat necrosis, dehiscence, hematoma, seroma, mastectomy flap necrosis, and flap loss.
Results:
Twenty patients underwent 4-flap DIEP/PAP breast reconstruction. Surgical time averaged 7 hours and 20 minutes. The primary recipient vessels were the antegrade and retrograde internal mammary vessels. No flap losses occurred. Complications included 1 hematoma, 1 incidence of arterial and venous thrombosis successfully treated with anastomotic revision, 1 incidence of thigh donor site dehiscence, and 3 episodes of minor mastectomy skin flap necrosis.
Conclusions:
Four-flap breast reconstruction is a favorable autologous reconstructive option for patients requiring bilateral reconstruction without adequate single donor flap volume. Stacking DIEP/PAP flaps as described is both safe and efficient. Furthermore, this combination provides superior aesthetics mirroring the natural geometry of the breast. Bilateral stacked DIEP/PAP flaps represent our first choice for breast reconstruction in this patient population.
Perforator-based autologous breast reconstruction provides a natural, long-lasting, and aesthetically pleasing result. Rivaling other forms of breast reconstruction, this reconstructive modality remains our practice’s preferred method for breast reconstruction, with the deep inferior epigastric perforator (DIEP) flap representing the gold standard. In patients who do not have enough abdominal tissue to reconstruct body-appropriate breasts, stacking flaps has proven to be an efficacious technique for providing adequate volume.1–9 Bilateral DIEP flaps are typically stacked for unilateral reconstruction.1,9 When bilateral reconstruction is required and the abdominal tissue does not provide enough volume, the addition of a second flap in a stacked fashion with a DIEP can provide favorable autologous reconstruction.
The task of performing 4 free flaps in one surgery is feasible by choosing an additional flap to pair with the DIEP that allows for an efficient operation and provides an aesthetically pleasing outcome. In an effort to provide perforator-based autologous breast reconstruction to all patients, further options are not infrequently warranted, commonly due to a need for additional volume or an inability to use the abdomen as a donor site. These secondary options have included the superior gluteal, inferior gluteal, transverse upper gracilis, and profunda artery perforator (PAP) flaps.10–13 Our practice now most commonly uses the PAP flap as our second-line flap because of its long pedicle, ease of harvest, and favorable donor site.13 We have concluded that the PAP flap is also the ideal flap to stack with the DIEP in 4-flap bilateral breast reconstruction. This combination of stacked flaps is safe and efficient and offers superior aesthetic results for bilateral perforator-based autologous breast reconstruction when the abdomen does not provide sufficient volume (Fig. 1).
Fig. 1.
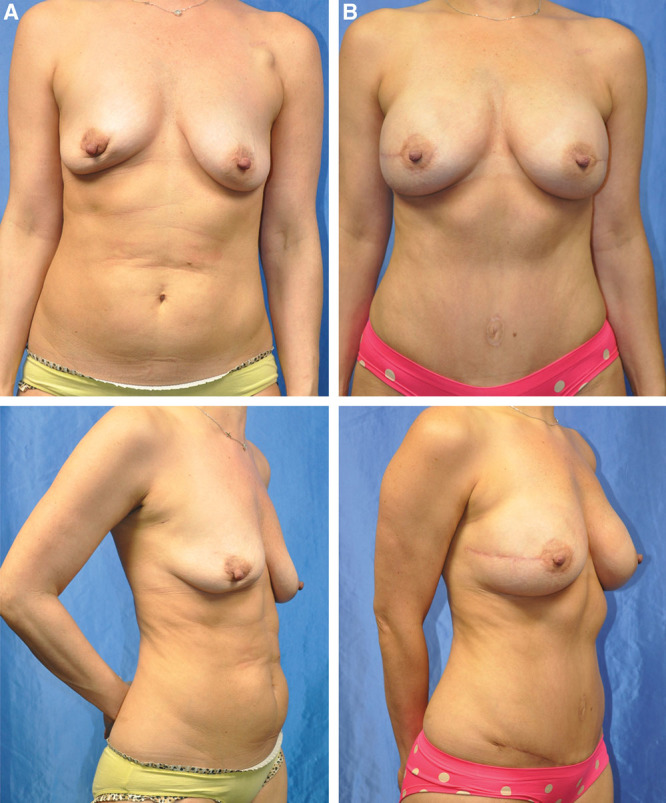
Preoperative (A) and postoperative (B) view of a 42-year-old woman with stage II right breast cancer with positive margins after a lumpectomy who elected to undergo bilateral nipple-sparing mastectomies through a radial incision with immediate bilateral stacked DIEP and PAP flap breast reconstruction. Results at 3 months follow-up.
At first encounter, performing 4 free flaps in one setting can seem overwhelming, but with appropriate flap selection and optimal operative planning, successful 4-flap breast reconstruction can be achieved. Our practice has stacked DIEP and superior gluteal artery perforator (SGAP) flaps in the past with favorable results but felt that the DIEP and SGAP combination for 4-flap breast reconstruction could be improved upon. The SGAP requires patient repositioning, which is an unnecessary inefficiency when performing 4-flap breast reconstruction.
Ultimately, the combination of stacking the PAP and DIEP flaps was born less due to the inadequacy of the SGAP but more due to the superiority of the PAP. A series of 3 patients in our practice presented after bilateral autologous reconstruction with volume deficiency (Fig. 2). Our treatment plan involved the addition of bilateral free flaps, either adding bilateral PAP flaps to existing DIEP reconstruction or vice versa. A favorable cosmesis in breast shape was recognized as the DIEP flap provided upper-pole fullness while the PAP flap offered an attractive, rounded lower breast pole (Fig. 3). Moving forward, those patients requiring bilateral breast reconstruction without adequate single site donor site volume were reconstructed with bilateral PAP and DIEP free flap breast reconstruction. This 4-flap DIEP/PAP stacked breast reconstruction has been recognized by our group to be the ideal option for perforator-based autologous bilateral breast reconstruction when DIEP flaps will not offer enough volume.
Fig. 2.
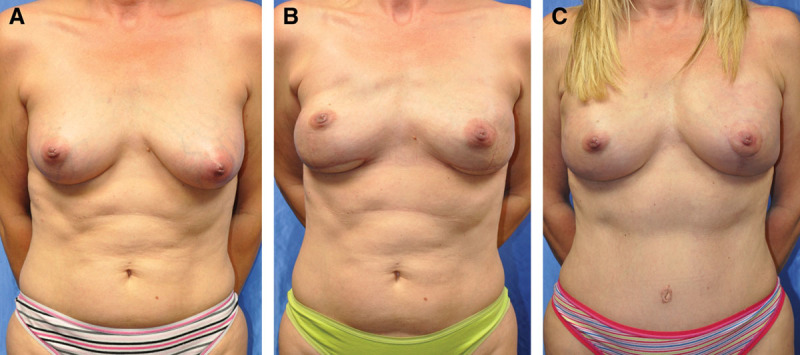
A 44-year-old woman with stage I left breast cancer underwent a bilateral nipple-sparing mastectomy with PAP flap breast reconstruction, leaving the patient with a superior tissue deficiency. A second delayed bilateral DIEP breast reconstruction was performed to correct the deficiency. A, Preoperative view. B, Three-month postoperative view after PAP flap. C, Three months status post-bilateral delayed DIEP staged reconstruction with left mastopexy for symmetry.
Fig. 3.
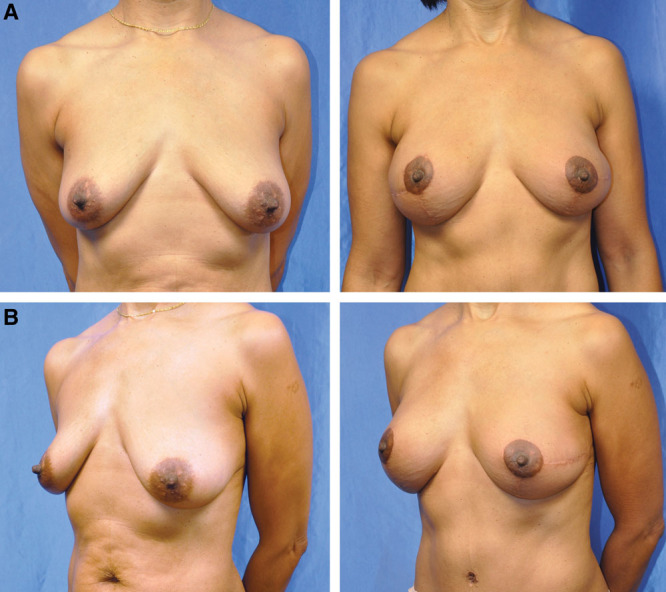
Preoperative (A) and postoperative (B) view of a 43-year-old woman with BRCA 2 gene mutation after bilateral nipple-sparing mastectomy with immediate 4-flap operation. Results at 3 months status post-second stage.
We describe our technique for 4-flap breast reconstruction in 20 patients using DIEP/PAP stacked flaps.
Methods
Candidates for 4-flap breast reconstruction are those patients in need of bilateral reconstruction who are deemed to not possess adequate volume for single free flap breast reconstruction based on physical examination. Decision making is based on evaluation of current breast size and shape, including the presence of ptosis, body size, and donor site volume. Those patients with donor site–specific contraindications for either the abdomen or thigh are excluded from consideration. Each patient participates in extensive counseling regarding all primary options for breast reconstruction and additional options that would add volume to a single free flap reconstruction.
The patient undergoes preoperative computed tomography angiogram evaluation of her abdomen and thighs, identifying ideal DIEP flap and PAP flap, respectively. The standard preoperative markings are placed with the aid of a handheld Doppler in the office the day before surgery (Fig. 4). In surgery, the patient is placed supine with bilateral arms and legs prepared into the field (Fig. 5). The arms are secured at the patient’s side, and the legs are positioned in frog-legged style. The DIEP and PAP flaps are harvested simultaneously. In the senior authors’ early experience, the PAP was harvested in the prone position, but the supine frog-legged position is more than adequate to successfully harvest the flap and close the defect.13 The surgeon that finishes harvesting this flap first then exposes the internal mammary vessels. Both unilateral anastomoses are performed.
Fig. 4.
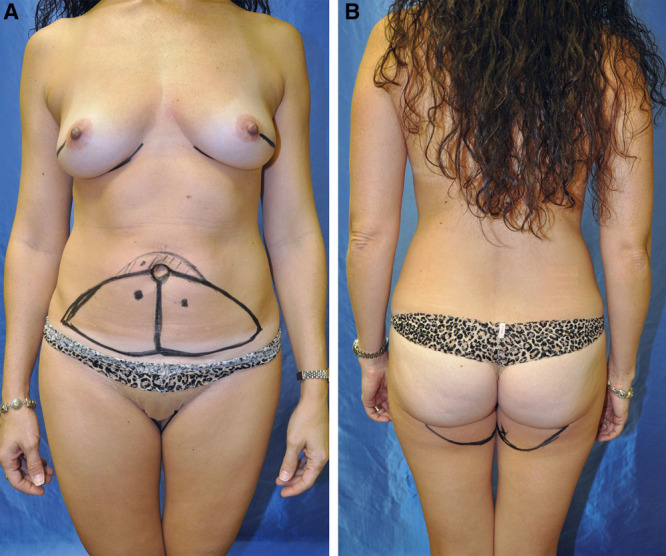
Preoperative markings for DIEP (A) and PAP (B) flaps. Computed tomography angiogram imaging is obtained for perforator localization. The long arc of the PAP crescent is marked at the length of the inframammary fold.
Fig. 5.
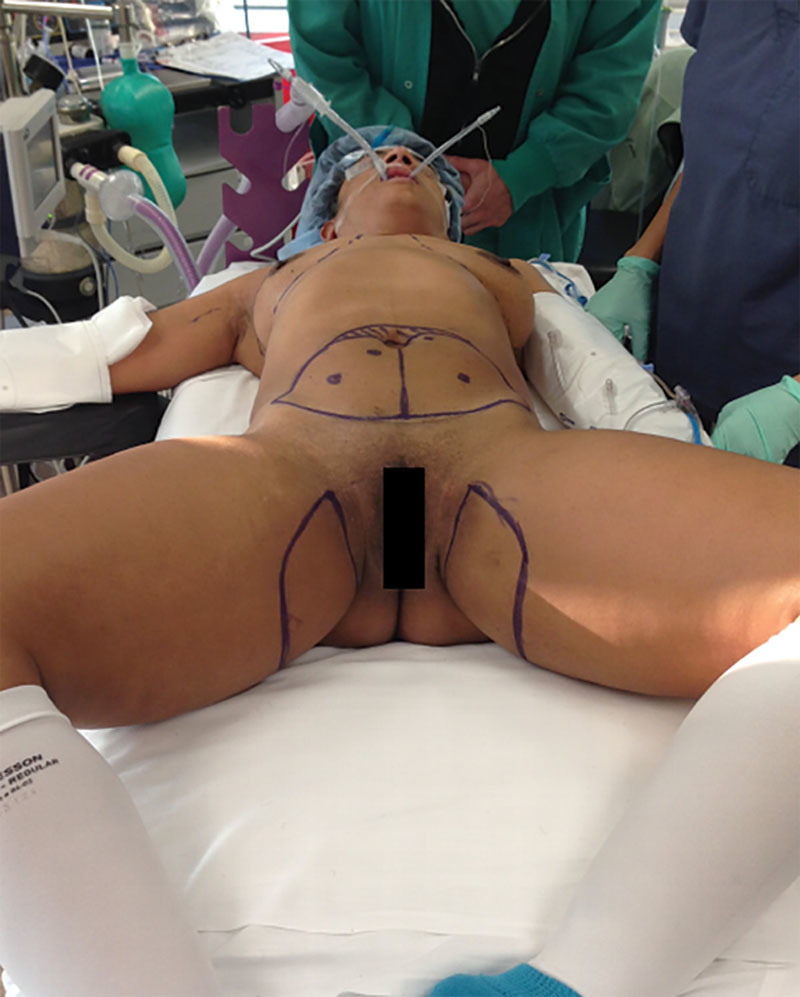
Intraoperative markings with patients in supine position for the entire operation.
The DIEP flap is placed in a superior position, and its pedicle is anastomosed to the retrograde internal mammary vessels. The use of the retrograde internal mammary vessels is a key component of the surgical plan because harvesting is efficient with minimal further dissection and the thoracodorsal system is spared in the event these vessels are needed at a later time. The triangular shape of the DIEP fills the upper pole with acceptable volume as it tapers into a pseudo-axillary tail. The PAP is then used to reconstruct the lower pole, and its pedicle is anastomosed to the antegrade internal mammary vessels. The crescent shape and favorable fat quality of the PAP flap provide a pleasing curvature and fullness to the lower portion of the breast (Fig. 6). The crisscross pattern of the anastomoses allows the pedicles to lie in an optimal orientation without unfavorable kinking or tension (Fig. 7). Finally, the flaps are inset and secured to the chest wall and then de-epithelialized as appropriate. A skin paddle for each flap is left for monitoring. In our practice, flaps are seldom buried, at which point internal venous Dopplers are placed. All donor sites are closed in layers with the patient in the supine position.
Fig. 6.
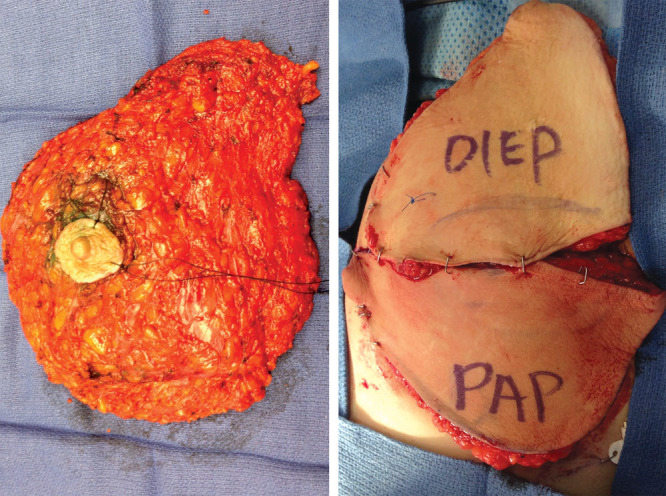
The positioning of the DIEP and PAP flaps resembles the shape of the mastectomy specimen after mastectomy.
Fig. 7.
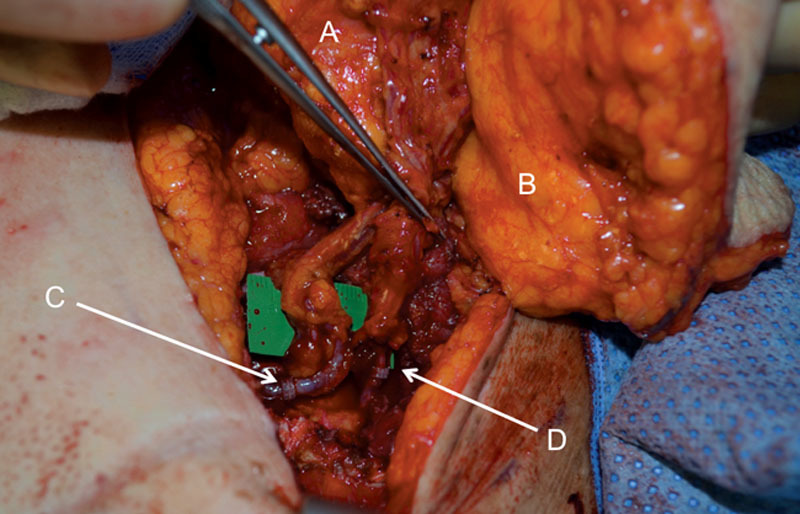
The vessels of the DIEP, which sits superiorly, will be anastomosed to the retrograde internal mammary vessels. The vessels of the PAP flap, which sits inferiorly, will be anastomosed to the antegrade vessels. PAP flap (A), DIEP flap (B), retrograde IM vessel anastomosis with DIEP vessels (C), and antegrade IM vessel anastomosis with the PAP vessels (D). IM indicates internal mammary.
EXPERIENCE REVIEW
Bilateral stacked DIEP/PAP flap breast reconstruction was performed on a total of 20 patients from December 2012 to August 2014, with a mean follow-up period of 14 ± 6 months. Three patients were staged with either bilateral PAP and staged DIEP flaps or vice versa using the thoracodorsal system for the staged recipient vessels. The other 17 patients underwent bilateral 4-flap breast reconstruction as described above. Of the 17 nonstaged reconstructions, the antegrade and retrograde internal mammary vessels were used as recipient vessels in 15 cases. Only the antegrade vessels were used in the 3 patients by stacking the flaps in a chimeric fashion anastomosing the second flap to the primary flap pedicle. Of the total 40 breast reconstructions, 13 (33%) were delayed and 27 (67%) immediate. The patient population had an average body mass index of 23.7 ± 2.4 and was relatively healthy with 1 obese patient and 2 patients with hypertension. No patients smoked or suffered from diabetes.
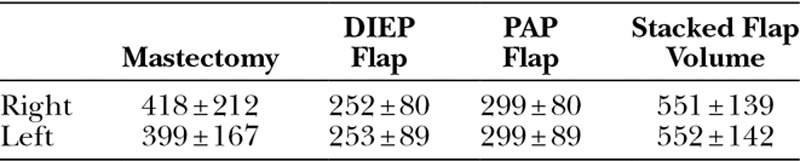
The average operative time was 7 hours 20 minutes ± 42 minutes (SD). Length of hospital stay for all patients was 4 days. The average mastectomy weight was 409 g (112–866 g). Average flap weights were 252 g (138–508 g) for DIEP flaps and 299 g (193–488 g) for PAP flaps. Total average reconstructed breast volume was 551 g (404–836 g) (Table 1).
Table 1.
Average Mastectomy and Flap Weights for 4-flap DIEP/PAP Stacked Breast Reconstructions Reported in Grams
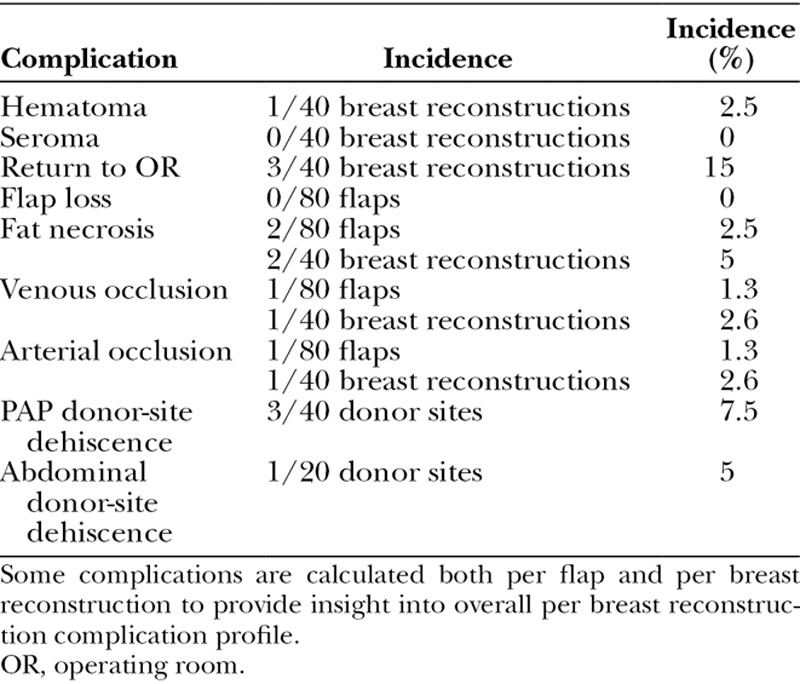
Complications were at or below levels reported in the literature for 1- and 2-flap reconstructions.14 No flaps were lost. One episode of arterial thrombosis and 1 episode of venous thrombosis were each treated with anastomosis revision resulting in flap salvage. One hematoma was evacuated. One abdominal donor site and 3 PAP donor sites developed minor wound dehiscence. All of these groups healed with local wound care. Operable fat necrosis was seen in 2 patients (Table 2). The incidence of complications was calculated per flap and per breast reconstruction to evaluate for possible increased risk of complications seen with increased number of free flaps.
Table 2.
Complication Profile for 4-Flap DIEP/PAP Stacked Breast Reconstruction
DISCUSSION
The incidence of bilateral breast reconstruction has risen with increased detection of genetic mutations and general patient desire for prophylactic mastectomies.15 The reconstruction of bilateral breasts in patients with inadequate abdominal tissue has posed a further reconstructive challenge and pushed the reconstructive surgeon to pursue more varied options. Fat grafting offers a secondary volume enhancement. However, in filling a large volume deficit, multiple procedures may be warranted as 50–60% retention is seen even at 150-cm3 grafting volumes.16 The addition of an implant to a DIEP changes the paradigm of the operation to a hybrid autologous reconstruction, introducing the risks of implant reconstruction, such as asymmetry, contracture, and infection necessitating implant removal. The flap may help reduce the increased detrimental effects seen with the combination of an implant and radiation, but the risks of nonautologous reconstruction remain.17 Pedicle-based flaps, such as the thoracodorsal artery perforator flap and intercostal artery perforator flap, represent local tissue options to augment DIEP reconstruction. Both can require patient repositioning, use a donor site often seen as a viable “back-up” reconstructive option, and predominantly offer additional volume more laterally.18,19
As microsurgical technique and operative room efficiency improve, the applications of perforator-based free flaps diversify. Multiple free flaps for a single operation are becoming a more comfortable endeavor and, as a result, more widely applied. The patient in need of more volume than the abdomen is able to offer has numerous options for additional volume, but our practice believes additional free flaps offer the known benefits of autologous reconstruction with little added risk. Specifically, 4-flap breast reconstruction with DIEP/PAP stacked flaps for bilateral breast reconstruction is our premier choice in this situation.
Stacking free flaps, the use of 2 separate flaps for a single reconstructive unit, is not a novel concept in breast reconstruction. In 1992, Arnez and Scamp2 described performing a bipedicled free transverse rectus abdominis myocutaneous flap by anastomosing each pedicle to a different recipient vessel, and in 1993, Pennington et al3 first described the use of stacked flaps in a chimeric fashion in reconstructing a breast with one free transverse rectus abdominis myocutaneous flap connected to a periumbilical perforator of the second flap. With the introduction and evolution of the DIEP flap, using stacked DIEP flaps for unilateral large volume reconstruction was reported as safe and effective.1,9 Further techniques have been published describing unilateral breast reconstruction by stacking various abdominal flaps.4–7 Alternatively, stacked flaps, such as the PAP flap, for unilateral reconstruction have also been successfully performed.7
Four-flap DIEP/PAP breast reconstruction offers the benefits of completely autologous reconstruction and an efficiency and aesthetic outcome not seen with other options for stacked autologous reconstruction. The largest series of bilateral stacked flaps to date details the stacking of DIEP and SGAP flaps in 25 cases.8 Time of surgery for this series averaged over 10 hours, which is longer compared with our series (7 hours 20 minutes), likely due to the necessary change in patient positioning and more complex dissection of the SGAP flap compared with the PAP. With a focus on safety and efficiency, an emphasis is placed on not increasing morbidity with the addition of extra flaps. The complication profile of the DIEP/SGAP series is limited, making a comparison difficult.8 More applicable is a comparison to single flap reconstruction. In comparison to Allen’s review14 of 758 DIEP free flap breast reconstructions, our series of stacked DIEP/PAP reconstructions shows no increase in morbidity with additional free flaps.
The use of the retrograde internal mammary vessels as a recipient vessel for free flap reconstruction has been a consistently viable option. Plus, its use as first-choice recipient vessels for the second flap eliminates the need for further dissection and saves the thoracodorsal vessels should they be needed at a later time. The long pedicle of the PAP flap makes the second anastomosis more practical compared with the SGAP.
The aesthetics created by 4-flap DIEP and PAP autologous reconstruction is quite favorable (Fig. 8). The DIEP flap offers sought-after upper-pole fullness often deficient from single-flap DIEP breast reconstruction. This may negate the need for revision fat grafting. The use of the PAP flap for the lower pole provides lower-pole fullness with a natural contour. Two additional scars are created, but the PAP donor site is well hidden and has a low wound complication profile (Fig. 9). We have also recognized that by using 2 flaps for each breast, the weight of each individual flap is slightly lower than usual, which may provide decreased drainage burden on the pedicle and, thus, decreased fat necrosis. This theoretical benefit can be further elucidated via further future investigation.
Fig. 8.
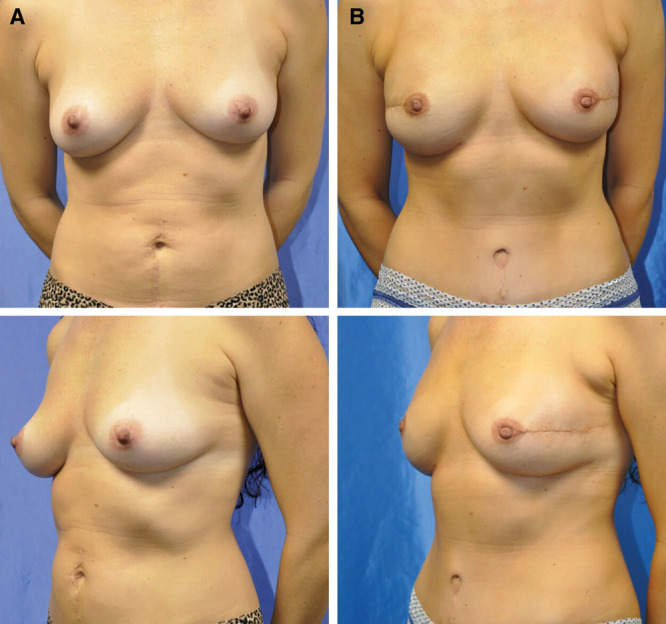
A 37-year-old woman with BRCA 2 gene mutation after BL NSM through radial incisions and BL immediate stacked DIEP and PAP flaps. A, Preoperative view. B, Three-month postoperative view. BL indicates bilateral; NSM, nipple-sparing mastectomy.
Fig. 9.
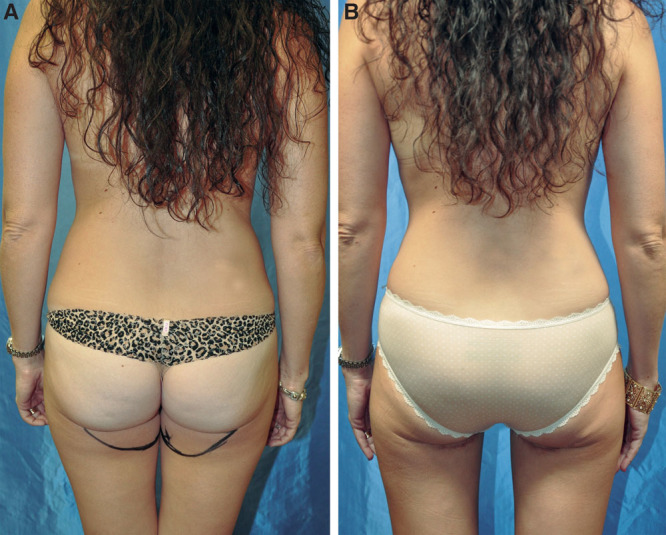
PAP donor-site scar hidden in the buttock crease. Preoperative (A) and postoperative (B) views.
Limitations to application of this technique are predominantly experience related. Not only does a learning curve dictate operative efficiency and outcomes but so too does a well-trained surgical staff and facility familiar with the intricacies of microsurgical breast reconstruction. However, these goals can be realized in centers committed to providing autologous breast reconstruction.
The patient with limited abdominal tissue in need of bilateral breast reconstruction can pose a challenge when seeking a truly autologous breast reconstruction. However, our practice has produced favorable results in an efficient manner with low morbidity by performing 4-flap breast reconstruction with stacked DIEP and PAP flaps using the antegrade and retrograde internal mammary vessels as recipient vessels. This represents our primary option for breast reconstruction in this patient population.
CONCLUSION
Four-flap breast reconstruction with stacked DIEP/PAP flaps should be considered when the abdomen alone fails to provide the necessary volume. Working with a two-person microsurgeon team, utilizing both the antegrade and retrograde internal mammary vessels as recipient vessels, and maintaining a supine position throughout the surgery, provide necessary efficiency without added complications. Additionally, the aesthetics of the DIEP/PAP combination are quite favorable.
ACKNOWLEDGMENT
This retrospective review article conforms to the Helsinki Declaration.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Ali RS, Garrido A, Ramakrishnan V. Stacked free hemi-DIEP flaps: a method of autologous breast reconstruction in a patient with midline abdominal scarring. Br J Plast Surg. 2002;55:351–353. doi: 10.1054/bjps.2002.3834. [DOI] [PubMed] [Google Scholar]
- 2.Arnez ZM, Scamp T. The bipedicled free TRAM flap. Br J Plast Surg. 1992;45:214–218. doi: 10.1016/0007-1226(92)90080-h. [DOI] [PubMed] [Google Scholar]
- 3.Pennington DG, Nettle WJ, Lam P. Microvascular augmentation of the blood supply of the contralateral side of the free transverse rectus abdominis musculocutaneous flap. Ann Plast Surg. 1993;31:123–126; discussion 126–127. doi: 10.1097/00000637-199308000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal JP, Gottlieb LJ. Double pedicle deep inferior epigastric perforator/muscle-sparing TRAM flaps for unilateral breast reconstruction. Ann Plast Surg. 2007;58:359–363. doi: 10.1097/01.sap.0000239818.28900.81. [DOI] [PubMed] [Google Scholar]
- 5.Figus A, Fioramonti P, Ramakrishnan V. Stacked free SIEA/DIEP flap for unilateral breast reconstruction in a thin patient with an abdominal vertical midline scar. J Resconstr Microsurg. 2007;23:523–525. doi: 10.1055/s-2007-1022692. [DOI] [PubMed] [Google Scholar]
- 6.Hamdi M, Khuthaila DK, Van Landuyt K, et al. Double-pedicle abdominal perforator free flaps for unilateral breast reconstruction: new horizons in microsurgical tissue transfer to the breast. J Plast Reconstr Aesthet Surg. 2007;60:904–912; discussion 913–914. doi: 10.1016/j.bjps.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Blechman KM, Broer PN, Tanna N, et al. Stacked profunda artery perforator flaps for unilateral breast reconstruction: a case report. J Reconstr Microsurg. 2013;29:631–634. doi: 10.1055/s-0033-1348065. [DOI] [PubMed] [Google Scholar]
- 8.DellaCroce FJ, Sullivan SK, Trahan C, et al. Body lift perforator flap breast reconstruction: a review of 100 flaps in 25 cases. Plast Reconstr Surg. 2012;129:551–561. doi: 10.1097/PRS.0b013e31824127fc. [DOI] [PubMed] [Google Scholar]
- 9.DellaCroce FJ, Sullivan SK, Trahan C. Stacked deep inferior epigastric perforator flap breast reconstruction: a review of 110 flaps in 55 cases over 3 years. Plast Reconstr Surg. 2011;127:1093–1099. doi: 10.1097/PRS.0b013e318205f223. [DOI] [PubMed] [Google Scholar]
- 10.Allen RJ, Tucker C. Superior gluteal artery perforator free flap for breast reconstruction. Plast Reconstr Surg. 1995;95:1207–1212. doi: 10.1097/00006534-199506000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Allen RJ, Levine JL, Granzow JW. The in-the-crease inferior gluteal artery perforator flap for breast reconstruction. Plast Reconstr Surg. 2006;118:333–339. doi: 10.1097/01.prs.0000227665.56703.a8. [DOI] [PubMed] [Google Scholar]
- 12.Wechselberger G, Schoeller T. The transverse myocutaneous gracilis free flap: a valuable tissue source in autologous breast reconstruction. Plast Reconstr Surg. 2004;114:69–73. doi: 10.1097/01.prs.0000127797.62020.d4. [DOI] [PubMed] [Google Scholar]
- 13.Allen RJ, Haddock NT, Ahn CY, et al. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg. 2012;129:16e–23e. doi: 10.1097/PRS.0b013e3182363d9f. [DOI] [PubMed] [Google Scholar]
- 14.Gill PS, Hunt JP, Guerra AB, et al. A 10-year retrospective review of 758 DIEP flaps for breast reconstruction. Plast Reconstr Surg. 2004;113:1153–1160. doi: 10.1097/01.prs.0000110328.47206.50. [DOI] [PubMed] [Google Scholar]
- 15.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27:1362–1367. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 16.Choi M, Small K, Levovitz C, et al. The volumetric analysis of fat graft survival in breast reconstruction. Plast Reconstr Surg. 2013;131:185–191. doi: 10.1097/PRS.0b013e3182789b13. [DOI] [PubMed] [Google Scholar]
- 17.Chang DW, Barnea Y, Robb GL. Effects of an autologous flap combined with an implant for breast reconstruction: an evaluation of 1000 consecutive reconstructions of previously irradiated breasts. Plast Reconstr Surg. 2008;122:356–362. doi: 10.1097/PRS.0b013e31817d6303. [DOI] [PubMed] [Google Scholar]
- 18.Levine JL, Soueid NE, Allen RJ. Algorithm for autologous breast reconstruction for partial mastectomy defects. Plast Reconstr Surg. 2005;116:762–767. doi: 10.1097/01.prs.0000176892.38430.8a. [DOI] [PubMed] [Google Scholar]
- 19.Losken A, Hamdi M. Partial breast reconstruction: current perspectives. 124:722–736. doi: 10.1097/PRS.0b013e3181b179d2. [DOI] [PubMed] [Google Scholar]


