Abstract
Background:
Clinical results of percutaneous needle fasciotomy (PNF) in Japanese patients with Dupuytren’s disease are reported.
Methods:
In this prospective study, 51 patients (103 fingers: 1 index, 9 middle, 47 ring, and 46 small) underwent PNF at 99 metacarpophalangeal (MCP) and 68 proximal interphalangeal (PIP) joints. Patients were assessed postoperatively after 1 day, at 1, 2, 4, 6, and 8 weeks, and at 3, 6, 9, and 12 months. Correction of contracture was measured in degrees, and an improvement index (% improvement) was described previously by Tonkin et al. A correction of the contracture to 5° or less at each joint and at each digital ray represented a successful correction. The recurrence rates in MCP and PIP joints were also evaluated. Correlations between the Tubiana classification stage and successful correction, % improvement, and recurrence rate were evaluated. The relationships between recurrence rate and the diathesis score (more/less than 5 points) and between recurrence rate and age at surgery (<50/≥50 years) were also examined.
Results:
In MCP and PIP joints, the improvement maintained at final follow-up was 89% and 57%, respectively, with successful corrections in 89% and 76%, respectively. PNF corrected digital rays at various Tubiana stages: stage 1 = 100%, stage 2 = 82%, stage 3 = 46%, and stage 4 = 0%. Improvements were preserved in stage 1 = 83%; stage 2 = 62%; stage 3 = 58%, and stage 4 = 60%. Recurrence of Dupuytren’s disease was significant for the PIP joint, severe Tubiana stage, and younger patients.
Conclusions:
Clinical results of PNF in Japanese patients with Dupuytren’s contractures were similar to those of whites.
Percutaneous needle fasciotomy (PNF) is an alternative treatment for Dupuytren’s contracture compared with open partial fasciectomy. However, only a few reports exist in the English literature, documenting the clinical results following PNF treatment for Dupuytren’s disease in an Asian population.1 Cheng et al1 have reported on a case series of 7 patients; it was concluded that PNF is a safe and effective procedure for Chinese patients. Dupuytren’s disease is less common in an Asian population than in a white population. Because of the small number of subjects, no study has been performed on a relatively large case series that would allow statistical analysis. In the present study, we report on the clinical results of PNF treatment for a series of 51 Japanese patients with Dupuytren’s disease.
The purpose of this study was to report and generally compare the clinical results of PNF for Dupuytren’s disease in Japanese patients with previously published results in white populations.
MATERIALS AND METHODS
This prospective study was designed and approved by the local Medical Ethics Committee in September 2011. From October 2011 to April 2013, every patient with Dupuytren’s contracture who presented at our department was assessed for enrollment in the study. Seventy-seven patients were consecutively enrolled in this study. PNF was offered to all those patients with Dupuytren’s disease who had a clearly defined cord, who had a contracture of at least 30° or more as measured by the total passive extension deficit (TPED), and who were willing to participate in the study.
Recurrent cases of Dupuytren’s contracture following a clinical trial of injectable collagenase Clostridium histolyticum (CCH) were also included in this study (2 patients). Excluded were 26 patients with previous trauma distal to their wrist, those with arthritis in their finger joint (4 patients), those with a contracture of less than 30° measured by TPED (20 patients), and those who did not want to be treated with PNF and preferred limited fasciectomy (LF) instead (2 patients). Therefore, this case series consisted of 51 patients with 103 fingers and involved 99 metacarpophalangeal (MCP) joints and 68 proximal interphalangeal (PIP) joints. Written consent was obtained from all patients at study entry. The average age at surgery was 65 years (range, 39–90 years). Patients included 43 men and 8 women.
At initial presentation, the flexion contractures of the MCP joints and the PIP joints of the involved finger rays were measured. These figures were added to the TPED and classified according to Tubiana’s staging system.2 Abe’s diathesis score3 was also calculated.
Various criteria were used to determine efficacy. A successful correction was a correction of the contracture to 5° or less at each joint and digital ray. Recurrence was considered as an increase in the TPED of at least 20° compared with the value documented at 6 weeks in each joint and digital ray. Correction of the contracture was measured in degrees and by an improvement index (% improvement), successful correction, and a determination of the recurrence rate in the MCP joint and in the PIP joint. The correlation between Tubiana stage and contracture correction measures that included successful correction, % improvement as defined by Tonkin et al,4 and recurrence rate was examined, as were the relationships between the diathesis score (more/less than 5 points) and recurrence rate and between age at surgery (<50/≥50 years old) and recurrence rate. Surgical complications following PNF were also assessed.
Patients were assessed postoperatively after 1 day, after 1, 2, 4, 6, and 8 weeks, and after 3, 6, 9, and 12 months. During these visits, measurements were performed by the first author. In addition, complications, including recurrence, were documented at each visit.
We conformed to the Declaration of Helsinki, and this research protocol was approved by the appropriate ethical committee.
Operative Technique
All the fingers on a hand were treated during a single session. Portal sites between skin creases in areas of definite cords were carefully chosen and marked with a surgical marker. Portals were spaced 5 mm apart and were not made in skin creases. After marking portals, 0.1 mL or less of a lidocaine 1% wt/vol solution and epinephrine (1:100,000) were injected into each site to be treated.
Working in a distal-to-proximal direction, a 25-gauge needle was used as a scalpel to release the cord at multiple levels. As previously described by Eaton,5 3 basic moves—clear, perforate, and sweep—were performed to transect diseased cords. The needle was changed frequently to maintain sharpness.
After division of the cord, affected fingers were passively extended to obtain maximal release. Portals and nodules were subsequently injected with a 10 mL lidocaine 1% wt/vol solution and 20 mg triamcinolone acetate mixture because its efficacy had been proved by McMillan and Binhammer6 in their randomized controlled trial study, and it is also known that steroids downregulate cell proliferation and induce apoptosis by affecting collagen ratios and fibroblast activity at the molecular level.7,8
Postoperatively, a light gauze bandage dressing was applied and removed the next day if the skin had not ruptured. Immediately after surgery, a fiberglass splint was applied, and its use at night was recommended for up to 3 months. No restrictions were applied to curb usual daily activities. Patients were encouraged to start increasing the motion of their hand immediately after the procedure, although they did not receive formal hand therapy.
Statistical Analysis
Statistical evaluation was performed using statistical software (StatMate 5, Atoms, Tokyo). The characteristics of both patient groups and the characteristics of the hands and digits were analyzed with cross tables. Categorical data were analyzed with Fisher’s exact test. All other data were analyzed using the Student’s t test. Statistical significance was set at P < 0.05.
RESULTS
According to Tubiana’s staging system, the number of digits per classification was as follows: stage 1 = 28, stage 2 = 42, stage 3 = 28, and stage 4 = 2. Forty patients had less than 5 points in Abe’s diathesis score and 11 patients had more than 5 points. There were 36 left hands and 31 right hands that included 1 index, 9 middle, 47 ring, and 46 small finger rays that underwent PNF.
Mean MCP joint contracture was corrected from 43° (range, 30–65°) to 2° (range, 0–10°) by 6 weeks after surgery and was maintained at 11° (range, 0–35°). The immediate % improvement (1 day after surgery) was 98% (range, 85–100%), and by 12 months after surgery, it was being maintained at 89% (range, 60–100%). Mean PIP joint contracture was corrected from 42° (range, 15–65°) to 8° (range, 0–35°) by 6 weeks after surgery, and by 12 months postoperatively, it was being maintained at 17° (range, 0–45°). Immediate % improvement (1 day after surgery) was 89% (range, 75–100%), and 12 months postoperatively, it was being maintained at 57% (range, 0–100%). PNF resulted in a successful correction in 82% of joints, including 89% of the MCP joints and 76% of the PIP joints. There was a statistically significant difference between the MCP joints and the PIP joints (P = 0.007).
The success of PNF in the correction of Dupuytren’s contractures declined progressively from stage 1 to stage 4 (Fig. 1). At final follow-up, 83% of the improvement was preserved in stage 1 (range, 33–100%), 62% in stage 2 (range, 0–100%), 58% in stage 3 (range, 25–100%), and 60% in stage 4 (range, 50–70%). However, no significant difference was observed between different stages (Fig. 2).
Fig. 1.
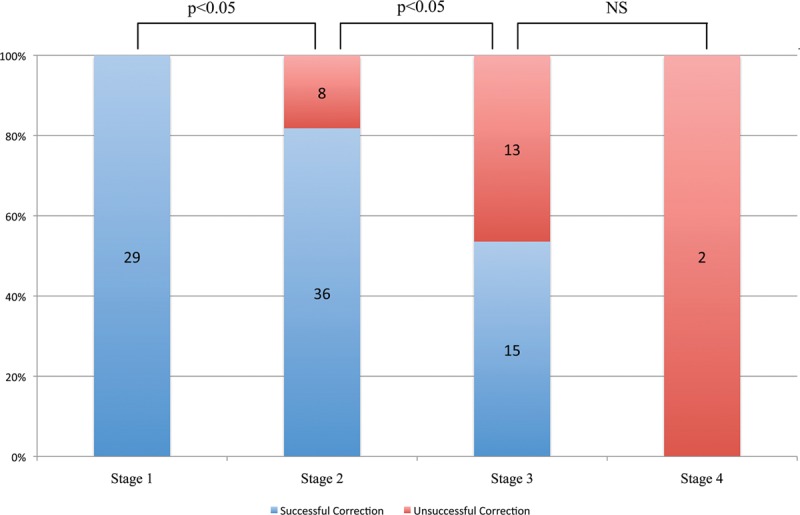
Tubiana stage vs successful correction. Immediate correction was significantly better in the lower Tubiana stages. P values were computed using Fisher’s exact test. NS indicates not significant.
Fig. 2.
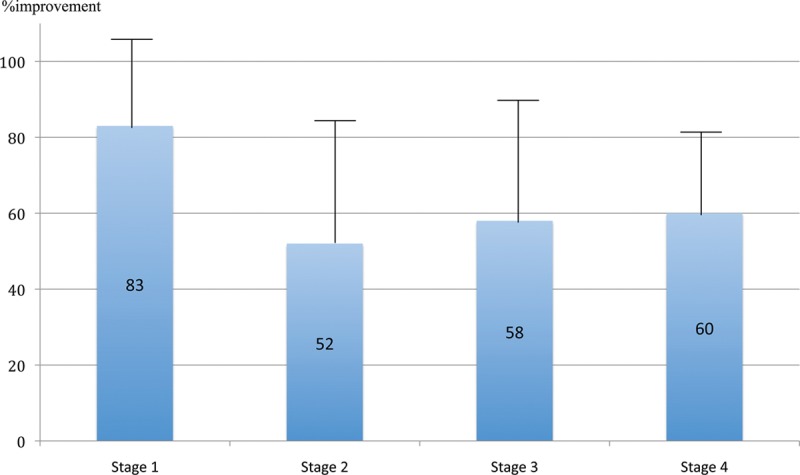
Tubiana stage vs % improvement at final follow-up. PNF was equally effective at each Tubiana stage of disease. There was no significant difference between the results for different stages (Student’s t test).
Recurrence
In terms of the overall recurrence rate, P values and the odds ratio for each parameter are summarized in Table 1. Of note, the odds ratio was significant for the type of joint (MCP vs PIP), Tubiana stage (1/2 vs 3/4), and age (<50 vs ≥50 years). The data for Tubiana stage versus recurrence are shown in Figure 3.
Table 1.
Recurrence Rates of Dupuytren’s Disease by Each Category Assessed
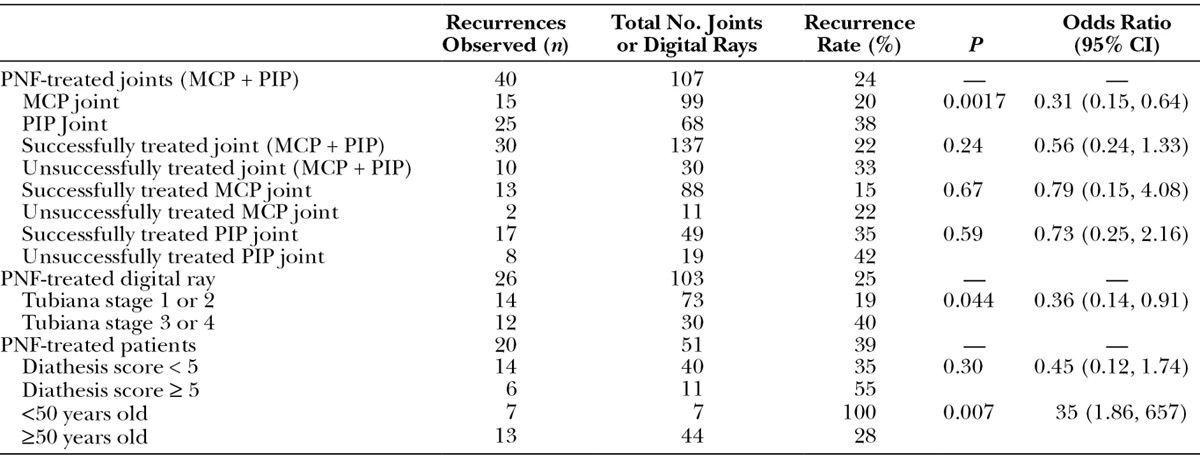
Fig. 3.
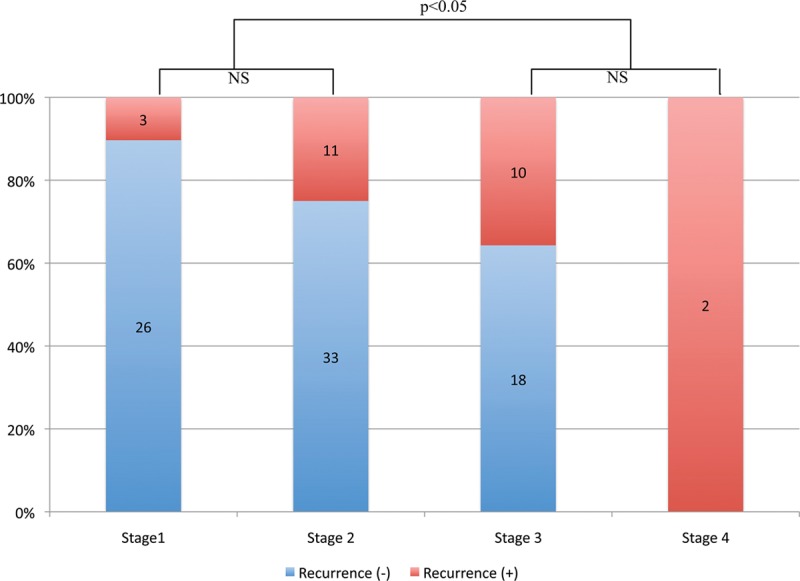
Tubiana stage vs recurrence. There was a statistically significant difference between the mild form (Tubiana stages 1 and 2) of Dupuytren’s disease and the severe form (Tubiana stages 3 and 4). P values were computed using Fisher’s exact test. NS indicates not significant.
Complications
Skin tears occurred in 18 digits. All tears healed with no intervention other than local wound care. Six patients had temporary neuropraxia that lasted for 3 months.
One patient sustained persistent paresthesia, but when Semmes-Weinstein needles were used, the sensibility had not diminished, suggesting that this was caused by partial laceration of the digital nerve.
DISCUSSION
Our study revealed 6 clinical results of PNF that were of interest in terms of Japanese patients: surgical correction was better maintained in MCP joints than in PIP joints; PNF was equally effective for each Tubiana stage of disease; PIP joint contracture, severe forms of Dupuytren’s disease, and an age at surgery < 50 years old were risk factors for recurrence; Abe’s diathesis score did not predict recurrence following PNF after a 1-year follow-up; the recurrence rate can be represented in various ways using different denominators ranging from 22% to 39%; and except for skin tears, complications were rare.
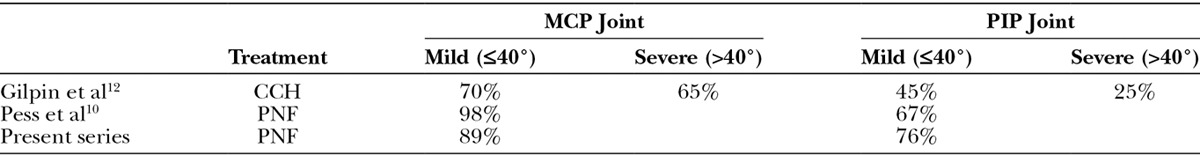
Several authors have already reported that corrections are better maintained in MCP joints than in PIP joints following PNF.1,9–11 Therefore, the results of our study are in line with their results (Table 2). In terms of successful corrections, short-term efficacy was better for MCP joints than for PIP joints according to our results. Similarly, short-term efficacy was better for mild forms of the disease than for moderately severe and severe forms of Dupuytren’s disease classified by Tubiana stage. Our findings concur with other case series published in the literature for PNF and CCH12–14 (Table 3).
Table 2.
% Improvement at Final Follow-up in PNF Studies
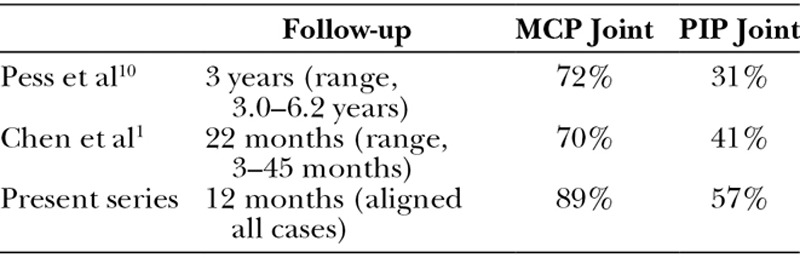
Table 3.
Relationship Between Successful Correction (5° or Less Contracture Immediately by PED) and Preoperative Contracture
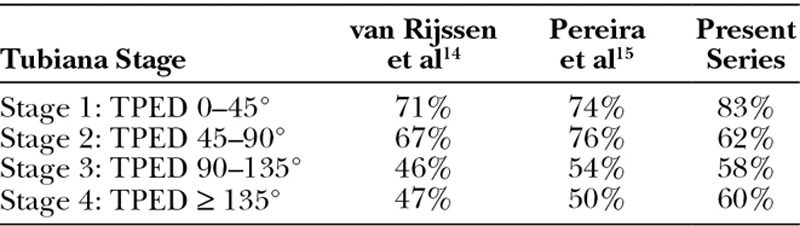
For % improvement, our results were in agreement with published reports of whites (Table 4) that documented a trend toward better results for lower Tubiana stages, but differences that were not significant between different stages.15,16 In the present study, there was no significant difference between the results for different stages of the disease, but a difference was evident between the MCP joints and the PIP joints. When assessing the stage of disease according to the classification of Tubiana, it becomes important to establish whether the deformity occurs at the MCP joint or at the PIP joint because the prognosis is different. The surgical outcome of Dupuytren’s disease depends on the degree of contracture of the PIP joint, especially for preoperative contractures greater than 60°, leading to significantly worse outcomes.17–20 Measurements that include both the MCP joints and PIP joints may misrepresent the state of disease.
Table 4.
Tubiana Stage vs % Improvement Following PNF
Pess et al11 reported that recurrences were frequent in younger patients (<55 years old) and for PIP contractures following PNF. van Rijssen et al12 claimed that the diathesis score, or each factor, did not correlate with recurrence, and only age at surgery was a statistically significant factor. Our results are consistent with those 2 reports; Abe’s diathesis score did not predict recurrence following PNF, and we suspect that the score is based on the result of the LF study that used different criteria for recurrence.
A number of definitions can be found in the literature for recurrence.11–25 In LF studies, the definition commonly used is “the reappearance of Dupuytren’s tissue in a zone previously operated on.”22 On the other hand, PNF studies have defined recurrence differently or as “an increase of TPED 20–30°.”11,26 This difference is because the pathological cords are divided but not removed by PNF. In CCH studies, recurrence has been described as a “return to contracture of at least 20° in those with successfully treated joints.”24
Reported recurrence rates vary in range from 12% to 73% for LF,12,18,25,27,28 from 0% to 100% for CCH,13,24,29–31 and from 9% to 85% for PNF.10,12,26,32 Chen et al33 assumed that recurrence rates following PNF were 4 times higher than CCH and 2 times higher than LF based on published data. However, it is our opinion that a direct comparison seems to be impossible because of the lack of standardized definitions for recurrence. Moreover, the disparity of the recurrence rate between PNF and CCH may be explained by the fact that unsuccessfully treated joints were excluded in CCH studies.
Recurrence rates are highly dependent on the time between treatment and the follow-up examination. It is commonly accepted that recurrence rates increase in proportion to follow-up duration.12,34–36 Different follow-up durations make it impossible to meaningfully compare recurrence results, even within the same intervention. We think that recurrence should be evaluated annually. Therefore, in this study, we decided on a follow-up duration of 12 months and plan to align future comparisons accordingly and beyond this duration. Werker et al37 concluded that clearly defined objective definitions for the correction of contracture and for recurrence are needed for more meaningful comparisons of results achieved with different surgical interventions.
Similar to the published PNF studies, complications were rare in our case series. Badois et al31 reported that complications were lower in PNF treatment compared with LF treatment. In their series, complications of PNF included skin breaks (16%), digital dysesthesia due to nerve damage (2%), and local infection (2%). In comparison, the frequency of complications following LF was not negligible; Bulstrode et al37 reported that complications occurred in 253 patients (18.2%) who underwent open fasciectomy and included nerve division (2.0%), arterial injury (0.8%), infection (9.6%), hematoma (2.0%), sympathetic dystrophy (2.4%), and skin slough (2.4%).
Although major complications of tendon rupture, infection, or digital dysesthesia have been reported as being rare, CCH has been associated with adverse effects such as injection-site pain, swelling, pruritus, tenderness, ecchymosis, skin laceration, palmar—and to a lesser extent dorsal—edema, and lymph node enlargement at the elbow and/or in the axillary region.24,29,30,38,39 According to Warwick et al,39 88% of patients who underwent CCH experienced one or more treatment-emergent adverse effects.
There were several limitations to this study. The number of patients was relatively small. Also, the 1-year follow-up may be too short to accurately determine the actual recurrence rate that may change over an extended period of time. Recurrence rates are highly dependent on the time between treatment and the follow-up examination. Based on this, it is important to standardize follow-up periods because discrepant durations can bias results for recurrence.
Our study is the first series to include more than 50 subjects. Importantly, this provided a reasonable sample size for a more robust statistical analysis than has been possible previously in Japanese patients. Also, the meaning of correction and recurrence was clearly defined for patients in this study, and a 1-year follow-up was provided. Standardizing the reporting of results will allow for more meaningful comparisons of different treatments for Dupuytren’s disease in the future. A longer follow-up study is necessary to better understand the treatment of Dupuytren’s disease in the Japanese population.
CONCLUSIONS
The clinical results of PNF in Japanese patients were very similar to the results reported in whites, at least in relation to a 1-year follow-up. The recurrence rate can be represented in various ways even with same sample and aligned follow-up period. It is important to standardize definition for recurrence and follow-up periods because discrepant definition and durations can bias results for recurrence for future meaningful comparison.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Cheng HS, Hung LK, Tse WL, et al. Needle aponeurotomy for Dupuytren’s contracture. J Orthop Surg (Hong Kong) 2008;16:88–90. doi: 10.1177/230949900801600120. [DOI] [PubMed] [Google Scholar]
- 2.Tubiana R. Surgical management. In: Tubiana R, editor. The Hand. Paris: WB Saunders Company; 1999. p. 480. [Google Scholar]
- 3.Abe Y, Rokkaku T, Ofuchi S, et al. An objective method to evaluate the risk of recurrence and extension of Dupuytren’s disease. J Hand Surg Br. 2004;29:427–430. doi: 10.1016/j.jhsb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Tonkin MA, Burke FD, Varian JP. Dupuytren’s contracture: a comparative study of fasciectomy and dermofasciectomy in one hundred patients. J Hand Surg Br. 1984;9:156–162. [PubMed] [Google Scholar]
- 5.Eaton C. Percutaneous fasciotomy for Dupuytren contracture. J Hand Surg Am. 2011;36:910–915. doi: 10.1016/j.jhsa.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 6.McMillan C, Binhammer P. Steroid injection and needle aponeurotomy for Dupuytren contracture: a randomized, controlled study. J Hand Surg Am. 2012;37:1307–1312. doi: 10.1016/j.jhsa.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Meek RM, McLellan S, Reilly J, et al. The effect of steroids on Dupuytren’s disease: role of programmed cell death. J Hand Surg Br. 2002;27:270–273. doi: 10.1054/jhsb.2001.0742. [DOI] [PubMed] [Google Scholar]
- 8.Meek RM, McLellan S, Crossan JF. Dupuytren’s disease. A model for the mechanism of fibrosis and its modulation by steroids. J Bone Joint Surg Br. 1999;81:732–738. doi: 10.1302/0301-620x.81b4.9163. [DOI] [PubMed] [Google Scholar]
- 9.Beaudreuil J, Lermusiaux JL, Teyssedou JP, et al. Multi-needle aponeurotomy for advanced Dupuytren’s disease: preliminary results of safety and efficacy (MNA 1 study). Joint Bone Spine. 2011;78:625–628. doi: 10.1016/j.jbspin.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Foucher G, Medina J, Navarro R. Percutaneous needle aponeurotomy: complications and results. J Hand Surg Br. 2003;28:427–431. doi: 10.1016/s0266-7681(03)00013-5. [DOI] [PubMed] [Google Scholar]
- 11.Pess GM, Pess RM, Pess RA. Results of needle aponeurotomy for Dupuytren contracture in over 1,000 fingers. J Hand Surg Am. 2012;37:651–656. doi: 10.1016/j.jhsa.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 12.van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129:469–477. doi: 10.1097/PRS.0b013e31823aea95. [DOI] [PubMed] [Google Scholar]
- 13.Gilpin D, Coleman S, Hall S, et al. Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am. 2010;35:2027.e1–2038.e1. doi: 10.1016/j.jhsa.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Witthaut J, Jones G, Skrepnik N, et al. Efficacy and safety of collagenase Clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg Am. 2013;38:2–11. doi: 10.1016/j.jhsa.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 15.van Rijssen AL, Gerbrandy FS, Ter Linden H, et al. A comparison of the direct outcomes of percutaneous needle fasciotomy and limited fasciectomy for Dupuytren’s disease: a 6-week follow-up study. J Hand Surg Am. 2006;31:717–725. doi: 10.1016/j.jhsa.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Pereira A, Massada M, Sousa R, et al. Percutaneous needle fasciotomy in Dupuytren’s contracture: is it a viable technique? Acta Orthop Belg. 2012;78:30–34. [PubMed] [Google Scholar]
- 17.Legge JW, McFarlane RM. Prediction of results of treatment of Dupuytren’s disease. J Hand Surg Am. 1980;5:608–616. doi: 10.1016/s0363-5023(80)80119-5. [DOI] [PubMed] [Google Scholar]
- 18.Abe Y, Rokkaku T, Ofuchi S, et al. Surgery for Dupuytren’s disease in Japanese patients and a new preoperative classification. J Hand Surg Br. 2004;29:235–239. doi: 10.1016/j.jhsb.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Donaldson OW, Pearson D, Reynolds R, et al. The association between intraoperative correction of Dupuytren’s disease and residual postoperative contracture. J Hand Surg Eur Vol. 2010;35:220–223. doi: 10.1177/1753193409353849. [DOI] [PubMed] [Google Scholar]
- 20.Misra A, Jain A, Ghazanfar R, et al. Predicting the outcome of surgery for the proximal interphalangeal joint in Dupuytren’s disease. J Hand Surg Am. 2007;32:240–245. doi: 10.1016/j.jhsa.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Hueston JT. Current state of treatment of Dupuytren’s disease. Ann Chir Main. 1984;3:81–92. doi: 10.1016/s0753-9053(84)80066-6. [DOI] [PubMed] [Google Scholar]
- 22.McFarlane RM, McGrouther DA. Complications and their management. In: McFarlane RM, McGrouther DA, Flint M, editors. Dupuytren’s Disease: Biology and Treatment. Edinburgh: Churchill Livingstone; 1990. pp. 377–382. [Google Scholar]
- 23.Leclercq C. Results of surgical treatment. In: Tubiana R, Leclercq C, Hurst LC, editors. Dupuytren’s Disease. London: Martin Dunitz; 2000. pp. 239–249. [Google Scholar]
- 24.Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg Am. 2007;32:767–774. doi: 10.1016/j.jhsa.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Jurisić D, Ković I, Lulic I, et al. Dupuytren’s disease characteristics in Primorsko-goranska County, Croatia. Coll Antropol. 2008;32:1209–1213. [PubMed] [Google Scholar]
- 26.van Rijssen AL, Werker PM. Percutaneous needle fasciotomy in Dupuytren’s disease. J Hand Surg Br. 2006;31:498–501. doi: 10.1016/j.jhsb.2006.03.174. [DOI] [PubMed] [Google Scholar]
- 27.Dias JJ, Braybrooke J. Dupuytren’s contracture: an audit of the outcomes of surgery. J Hand Surg Br. 2006;31:514–521. doi: 10.1016/j.jhsb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Ullah AS, Dias JJ, Bhowal B. Does a ‘firebreak’ full-thickness skin graft prevent recurrence after surgery for Dupuytren’s contracture?: a prospective, randomised trial. J Bone Joint Surg Br. 2009;91:374–378. doi: 10.1302/0301-620X.91B3.21054. [DOI] [PubMed] [Google Scholar]
- 29.Hurst LC, Badalamente MA, Hentz VR, et al. CORD I Study Group. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968–979. doi: 10.1056/NEJMoa0810866. [DOI] [PubMed] [Google Scholar]
- 30.Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase Clostridium histolyticum (CORDLESS study): 3-year data. J Hand Surg Am. 2013;38:12–22. doi: 10.1016/j.jhsa.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Watt AJ, Curtin CM, Hentz VR. Collagenase injection as nonsurgical treatment of Dupuytren’s disease: 8-year follow-up. J Hand Surg Am. 2010;35:534–539, 539.e1. doi: 10.1016/j.jhsa.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Badois FJ, Lermusiaux C, Masse C, et al. Nonsurgical treatment of Dupuytren disease using needle fasciotomy. Rev Rhum (Engl Ed) 1993;60:692–697. [PubMed] [Google Scholar]
- 33.Chen NC, Shauver MJ, Chung KC. Cost-effectiveness of open partial fasciectomy, needle aponeurotomy, and collagenase injection for Dupuytren contracture. J Hand Surg Am. 2011;36:1826.e32–1834.e32. doi: 10.1016/j.jhsa.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Dickie WR, Hughes NC. Dupuytren’s contracture: a review of the late results of radical fasciectomy. Br J Plast Surg. 1967;20:311–314. doi: 10.1016/s0007-1226(67)80055-9. [DOI] [PubMed] [Google Scholar]
- 35.Norrote G, Apoil A, Travers V. A ten-year follow-up of the results of surgery for Dupuytren’s disease. A study of 58 cases. Ann Chir Main. 1988;7:277–281. doi: 10.1016/s0753-9053(88)80024-3. [DOI] [PubMed] [Google Scholar]
- 36.Leclercq C, Tubiana R. Recurrence in Dupuytren’s contracture. In: Tubiana R, editor. The Hand. Philadelphia: WB Saunders; 1999. pp. 484–492. [Google Scholar]
- 37.Werker PM, Pess GM, van Rijssen AL, et al. Correction of contracture and recurrence rates of Dupuytren contracture following invasive treatment: the importance of clear definitions. J Hand Surg Am. 2012;37:2095.e7–2105.e7. doi: 10.1016/j.jhsa.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 38.Bulstrode NW, Jemec B, Smith PJ. The complications of Dupuytren’s contracture surgery. J Hand Surg Am. 2005;30:1021–1025. doi: 10.1016/j.jhsa.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Warwick D, Arner M, Pajardi G, et al. POINT X Investigators. Collagenase Clostridium histolyticum in patients with Dupuytren’s contracture: results from POINT X, an open-label study of clinical and patient-reported outcomes. J Hand Surg Eur Vol. 2015;40:124–132. doi: 10.1177/1753193413519926. [DOI] [PMC free article] [PubMed] [Google Scholar]


