Abstract
Background:
Diagnostic and therapeutic strategies for lower-limb lymphedema have not yet been established. The purpose of this study was to estimate the lymphodynamic condition and therapeutic efficacy of lymphovenous anastomosis (LVA) in lower-limb lymphedema patients using 2-phase 99mTc-phytate lymphoscintigraphy with single-photon emission computed tomography-computed tomography (SPECT-CT).
Methods:
In this study, consecutive patients with lower-limb lymphedema who underwent 2-phase lymphoscintigraphy using 99mTc-phytate were enrolled between June 2013 and June 2014. SPECT-CT was also performed to clarify the relationships between functional and morphological information. In both the early and delayed images, inguinal lymph node accumulation, dermal backflow, and their sequential alternations were evaluated, and liver-to-blood ratio and inguinal lymph node-to-blood ratio were calculated. All participants were classified into 6 types of lymphodynamic conditions based on the image findings. Patients with both dermal backflow and associated normal lymphatic vessel accumulation proceeded to LVA and underwent a second lymphoscintigraphy after the operation.
Results:
Of all 30 participants, the largest population was categorized as type 4, which had consistent inguinal lymph node accumulation defect with dermal backflow. In 12 operated cases, dermal backflow was degraded in 10 cases by LVA. Liver-to-blood ratio in both early and delayed images and inguinal lymph node-to-blood ratio in delayed image significantly increased after LVA.
Conclusions:
Lymphoscintigraphy with SPECT-CT can provide both functional and morphological information simultaneously in patients with lower-limb lymphedema. Using these procedures, a type categorization for the patients was devised, which reflects their lymphodynamic conditions. The therapeutic efficacy of LVA could also be estimated quantitatively by the derived findings.
Lymphedema is usually caused by lymphatic disorder due to various causative conditions, including surgery, radiation, trauma, and infectious diseases. In the clinical setting, although investigators have struggled to reveal the actual mechanism of lymphodynamic disorders, the diagnostic and therapeutic strategies against lymphedema remain unestablished.
Lymphoscintigraphy using 99mTc-human serum albumin diethylenetriamine pentaacetic acid (99mTc-HSAD)1–3 is commonly adopted to evaluate the lymphodynamic condition in lymphedema patients, and its type categorization using this modality has already been established.4 However, as 99mTc-HSAD promptly distributes to entire vascular systems due to its small particle size (2–3 nm), discriminating lymphatic vessels from veins may prove to be difficult. Compared to this, 99mTc-phytate has a larger particle size (200–1000 nm) and is usually trapped in inguinal lymph nodes.5 Such characteristics of this tracer might facilitate further comprehension of lymphodynamic conditions, including main lymphatic vessels’ flow and lymphovenous shunt. Thus, this tracer was chosen for lymphoscintigraphy in this study. Recently, single-photon emission computed tomography-computed tomography (SPECT-CT) combined systems have been introduced for clinical use. We believe that 99mTc-phytate lymphoscintigraphy, integrated with SPECT-CT fusion image, allows us to interpret the detailed lymphodynamic conditions by combining functional and morphological information6,7 and helps to establish a new clinical staging system for lower-limb lymphedema. Common surgical treatment for patients with lower-limb lymphedema is lymphovenous anastomosis (LVA).8–10 This modality may also promote the comprehension of LVA’s therapeutic efficacy.
The purpose of this study was to estimate the lymphodynamic condition and therapeutic efficacy of LVA in lower-limb lymphedema patients using 2-phase 99mTc-phytate lymphoscintigraphy with SPECT-CT.
MATERIALS AND METHODS
Patient Enrollment and Physical Examinations
Consecutive patients with lower-limb lymphedema who underwent an initial lymphoscintigraphy between June 2013 and June 2014 were enrolled in this study. Patients with deep vein thrombosis and generalized edema were excluded. At their initial visit, all participants were interviewed about their history of lower-limb lymphedema and its causative conditions. International Society of Lymphology (ISL) stage11 and lower extremity lymphedema (LEL) stage,12 derived by calculating a lower-limb swelling index, LEL index, were confirmed according to their symptoms and/or physical examination findings. All patients provided written informed consent, and this study was approved by the Ethical Committee of Nippon Medical School Hospital.
Lymphoscintigraphic Imaging with SPECT-CT
Tc-99m-phytate 185 MBq in a total volume of 1 mL was subcutaneously injected into the first interdigital spaces on both sides of the feet by using a 27-gauge needle. The interval between the 2 injections was less than 1 minute. Injection sites were massaged for 3 minutes to facilitate tracer transport into lymphatic systems. Acquisitions were performed using a SPECT-CT combined system equipped with a dual-headed gamma camera, Symbia T2 (Siemens Healthcare Japan, Tokyo, Japan). Anterior and posterior planar images from chest to foot were acquired, starting at 2 time points, 12 and 90 minutes after the injection. The acquisition from each time point proceeded over 10 minutes. SPECT-CT images from foot to inguinal area were acquired over 30 minutes, starting at 25 minutes after the injection, and a total of 30 projection images were obtained over an orbit of 360 degrees in 6- degree increments at a rate of 30 seconds per projection. The image matrix size was 128, the pixel size was 4.8 mm, and a low energy high-resolution collimator was used. Images were acquired on the 140 keV photopeak, with a 20% symmetrical energy window.
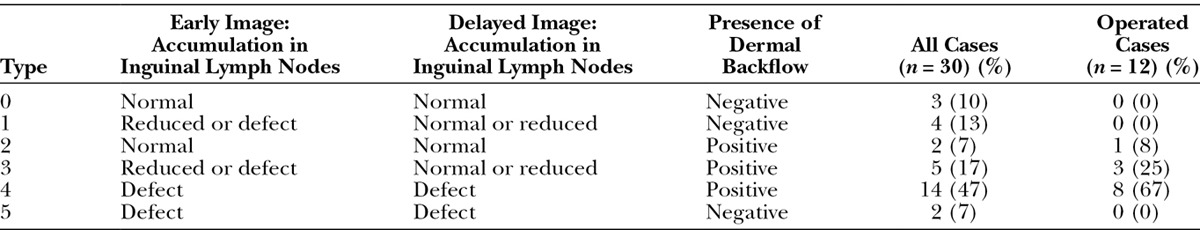
In both early and delayed lymphoscintigraphic images with SPECT-CT, the lymphodynamic findings, including lymphatic vessel accumulation, inguinal lymph node accumulation, and dermal backflow, were interpreted with the consensus of 2 experienced nuclear radiologists. Furthermore, inguinal lymph node accumulation on the affected side was graded into normal, reduced, and defect in both early and delayed planar images, compared with those on the unaffected side. Dermal backflow was assessed in the SPECT-CT images to comprehend its 3D distribution. After these findings, all participants were categorized into 6 types (type 0–5) as shown in Table 3. Patients without abnormal findings were categorized as type 0; patients with abnormal inguinal lymph node accumulation and negative dermal backflow were categorized as type 1; those with normal inguinal lymph node accumulation and positive dermal backflow were categorized as type 2; those with abnormal inguinal lymph node accumulation and negative dermal backflow were categorized as type 3; those with inguinal lymph node accumulation defect and positive dermal backflow were categorized as type 4; and patients with inguinal lymph node accumulation defect and negative dermal backflow were categorized as type 5.
Conducting LVA and Evaluation of Its Efficacy with Postoperative Lymphoscintigraphy
Patients with both dermal backflow and associated normal lymphatic vessel accumulation proceeded to LVA to reduce lymphostasis. Indocyanine green lymphography13–15 was also performed immediately before LVA to detect superficial lymphatic vessels and ensure the indication of LVA. The patients who experienced LVA underwent a second lymphoscintigraphy 1 month after the operation. Its imaging procedure was identical to that in the preoperative test.
In the operated cases, postoperative lymphoscintigraphy was implemented to evaluate the efficacy of LVA, and the images were visually interpreted. Furthermore, liver and inguinal lymph node accumulations in initial and second lymphoscintigraphy were quantitatively evaluated. In both early and delayed planar images, regions of interest were drawn on the entire liver and left ventricle. Liver-to-blood ratio (LBR), which indicates the shunt volume from lymphatic vessels to veins, was then calculated by dividing the mean liver count by the mean blood count. Inguinal lymph node-to-blood ratio (ILBR), which indicates the transport capacity of main lymphatic vessels in lower limb, was also calculated by dividing maximal inguinal lymph node count by the mean blood count. These quantitative parameters, in both early and delayed images, were respectively compared between pre- and postoperative tests.
Statistical Analysis
Quantitative variables were expressed as mean ± SD for normally distributed data. For data not distributed normally, such as LEL index, LBR, and ILBR, data were presented as median with 25th and 75th percentiles. Categorical variables were presented as number (%).
In comparison of operated and nonoperated patients, age was compared using a Welch test as it was a parametric variable with unequal variance. ISL and LEL stages were compared using a G test with William’s correction. Categorical variables, such as gender, previous cellulitis, and causative diseases, were compared using a Fisher’s exact probability test. In the cases undergoing LVA, inguinal lymph node accumulation between pre- and postoperative images was tested for significance using a G test with Williams’ correction. The perioperative variations in LEL index, LBR, and ILBR were tested for significance using a Wilcoxon signed-rank test.
A P value < 0.05 was considered statistically significant. All statistical analyses were performed using StatMate IV software version 4.01 (Advanced Technology for Medicine and Science, Tokyo, Japan).
RESULTS
Patient Characteristics
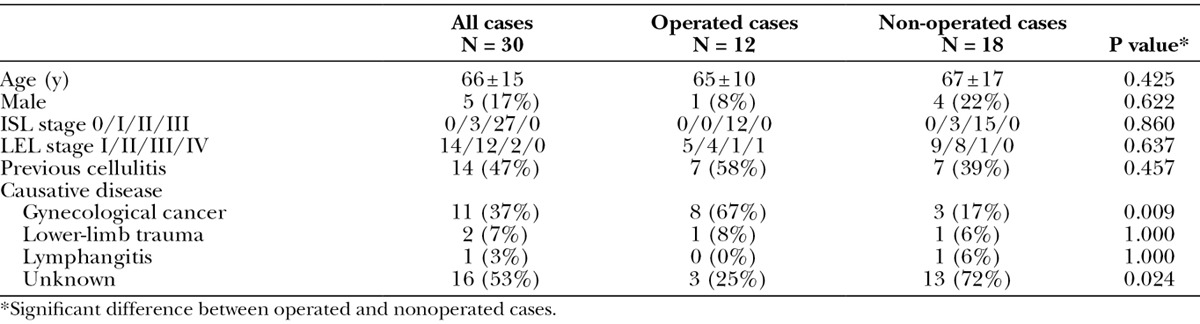
In total, 30 participants were included in this study, and Table 1 shows their characteristics. The study population included 5 men and 25 women, with a mean age of 66 years. In the ISL staging system, 27 (90%) were categorized as stage II, and 3 (10%) were categorized as stage III. In the LEL staging system, 14 (47%) were categorized as stage I, 12 (40%) were categorized as stage II, and 2 were categorized as stage III. Their known causative diseases were gynecological cancer (37%), trauma of lower limb (7%), and lymphangitis (3%).
Table 1.
Patient Characteristics
Initial Lymphoscintigraphic Findings and Type Categorization
In the inguinal lymph node accumulation on the affected side, 21 (70%) showed accumulation defect and 4 (13%) showed reduced accumulation in the early image, and 17 (57%) showed accumulation defect and 8 (27%) showed reduced accumulation in the delayed image (Table 2). Of all participants, 21 (70%) demonstrated dermal backflow (Table 3). The results of categorization into 6 types were shown in Table 3, and type 4 had the largest population of participants (14 of 30; 47%).
Table 2.
Inguinal Lymph Node Accumulation in Early and Delayed Lymphoscintigraphic Images
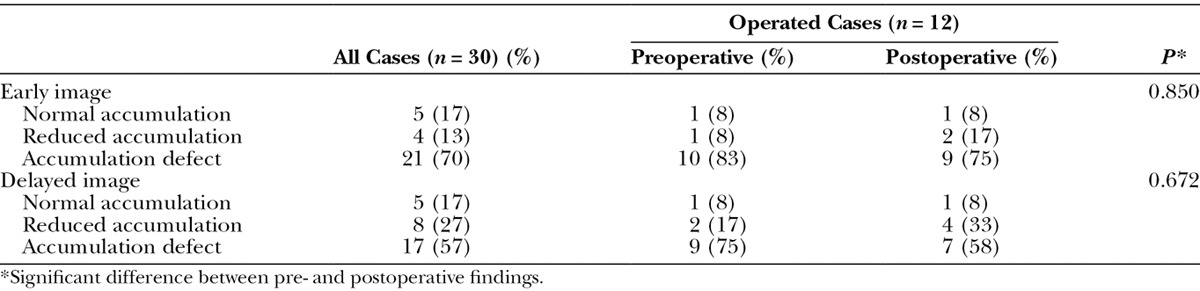
Table 3.
Categorization of Lower-limb Lymphedema Patients in Accordance with Lymphoscintigraphic Findings
Conducting LVA and Its Efficacy
Twenty-one patients were confirmed to have an indication for LVA, according to the criteria in this study. Of these, 9 did not undergo LVA, as they chose to reject the treatment. The remaining 12 patients (1 man and 11 women; 65 ± 10 years) underwent LVA.
In the patients undergoing LVA, their ISL stage remained unchanged, and their LEL index significantly decreased in the postoperative test (P = 0.003) (Fig. 1). Furthermore, cellulitis did not occur in each participant after the operation. In the postoperative lymphoscintigraphy, dermal backflow disappeared in 2 cases (17%) and decreased in 8 cases (67%) compared with the initial test. As shown in Figures 2 and 3, LVA increased LBR in both early and delayed images [33.99 (23.05–45.31) to 41.59 (35.36–51.41), P = 0.011; 61.93 (54.45–77.73) to 87.87 (67.20–104.96), P = 0.013], and additionally, increased ILBR in the delayed image [1.86 (1.67–3.29) to 3.74 (2.62–7.13), P = 0.003]. ILBR in early image did not increase [1.79 (1.67–3.17) to 2.08 (1.48–4.27), P = 0.610] after LVA (Fig. 3).
Fig. 1.
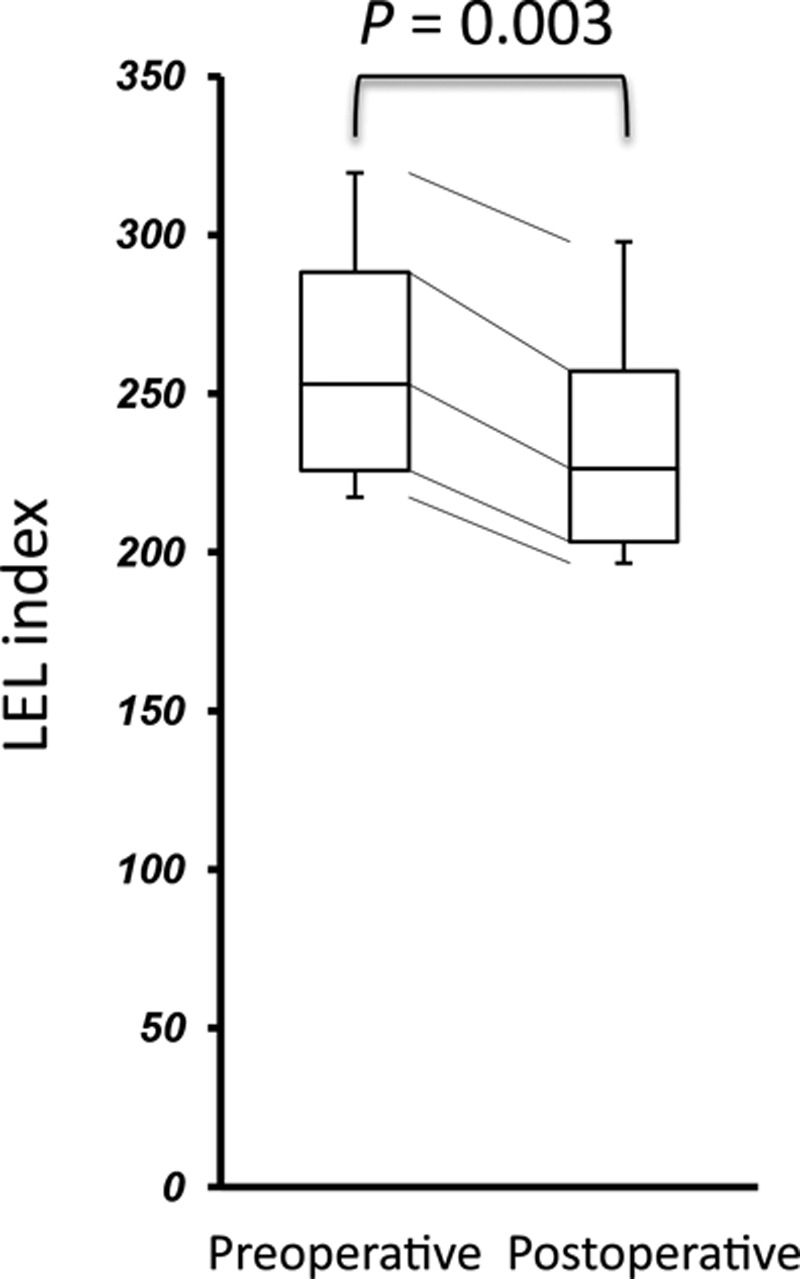
Comparison of LEL index between pre- and postoperative conditions.
Fig. 2.
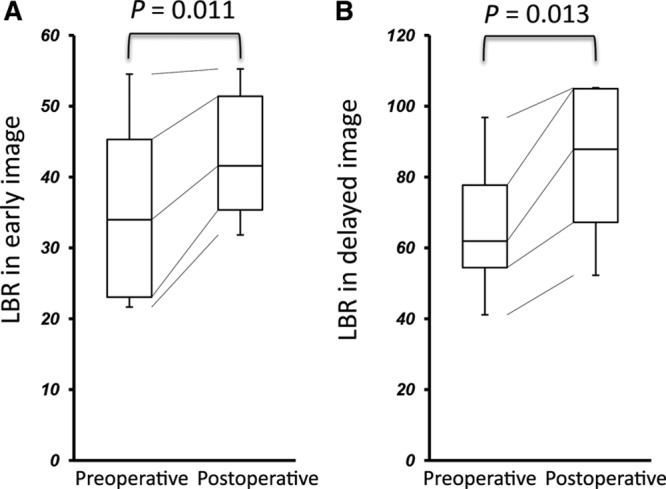
Comparison of LBR between pre- and postoperative conditions. A, Early. B, Delayed images.
Fig. 3.
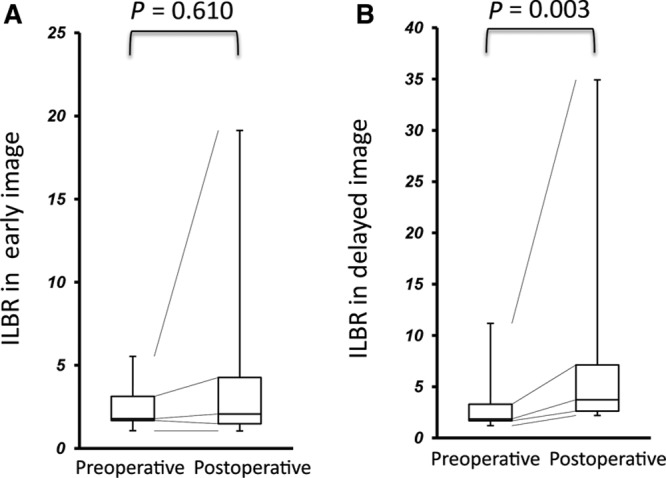
Comparison of ILBR between pre- and postoperative conditions. A, Early. B, Delayed images.
DISCUSSION
The present study was performed to assess the lymphodynamic conditions in patients with lymphedema using 2-phase 99mTc-phytate lymphoscintigraphy integrated with SPECT-CT. Of the 30 participants, 25 (83%) showed abnormal inguinal lymph node accumulation in lymphoscintigraphy, and 21 (70%) demonstrated dermal backflow. In 10 of 12 operated cases, dermal backflow was degraded after LVA, and this treatment significantly improved their LEL index, LBR, and ILBR.
Two-phase 99mTc-phytate Lymphoscintigraphy with SPECT-CT
Lymphoscintigraphy has been widely adopted to evaluate the lymphodynamic condition in patients with lower-limb lymphedema.1–3 Maegawa et al4 categorized secondary lymphedema cases as 5 types using 99mTc-HSAD lymphoscintigraphy, applying the categorization system to confirming LVA indication. However, as 99mTc-HSAD promptly distributes to the entire vascular systems, due to its small particle size (2–3 nm), discriminating lymphatic vessels from veins may be difficult. Compared with this, because 99mTc-phytate has a larger particle size (200–1000 nm), this tracer migrates along lower-limb main lymphatic vessels more slowly than 99mTc-HSAD and is largely trapped in inguinal lymph nodes.5 Such characteristics of this tracer might facilitate a more detailed observation of lymphodynamic abnormality, by comparing 2-phase images at 12 and 90 minutes, and clarify lymphatic transport capacity in relation to inguinal lymph node accumulation (Fig. 4A). For these reasons, we believe that 99mTc-phytate is a more suitable tracer than 99mTc-HSAD for comprehending lymphodynamic conditions in lower-limb lymphedema patients.
Fig. 4.
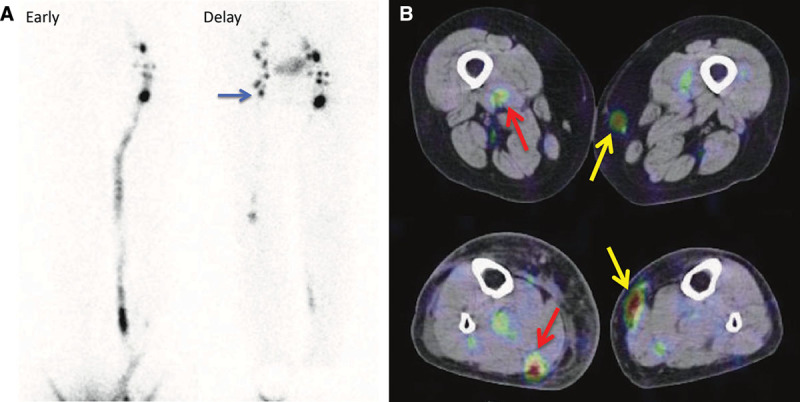
An 85-year-old woman with type 2 of right lower leg lymphedema (ISL stage: II and LEL index: 299) caused by a leg trauma. The early images show inguinal lymph node accumulation defect on the affected side, and the inguinal lymph node accumulation improved (blue arrow) in the delayed images. Collecting lymphatic vessel accumulation along great saphenous vein (yellow arrow) and that along small saphenous vein (red arrow) were clarified by SPECT-CT images. A, Lymphoscintigraphy early and delayed images. B, SPECT-CT image.
Recently, SPECT-CT combined systems have been introduced for clinical use. Conventionally, as lymphoscintigraphy usually obtains anterior and posterior planar images alone, lacking morphological information, it is not possible to comprehend the detailed location and distribution of tracer accumulation. Conversely, because SPECT-CT simultaneously provides both functional and morphological information, it can discriminate lymphatic vessels from veins (Figs. 4B, 5B, and 5C) and can also discriminate dermal backflow from lymphatic vessel accumulation (Fig. 5D). Additionally, it also can locate the suitable lymphatic vessels for LVA (Fig. 6C).
Fig. 5.
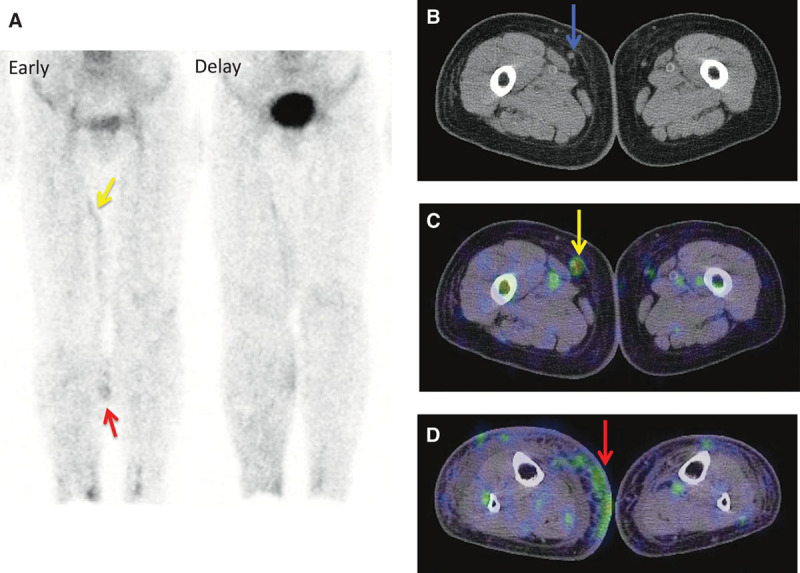
An 81-year-old woman with bilateral lower-limb lymphedema caused by a surgery for uterine cervical cancer. The right lower limb, with more severe lymphedema than the opposite side, was regarded as affected side (ISL stage: II and LEL index: 338). Both the early and delayed images show inguinal lymph node accumulation defect. Although the planar images cannot discriminate the lymphatic vessel in the medial lower thigh from dermal backflow (A, red arrow), SPECT-CT images provide the correct information as dermal backflow (D, red arrow). Furthermore, whereas the planar images cannot determine whether the tubular accumulation in the medial thigh is lymphatic vessel or vein, SPECT-CT showed vein to be the definitive answer, according to its morphological information. Therefore, this case was categorized as type 5. A, Lymphoscintigraphy early and delayed images. B, CT image. C, SPECT-CT image of thigh. D, SPECT-CT image of lower thigh.
Fig. 6.
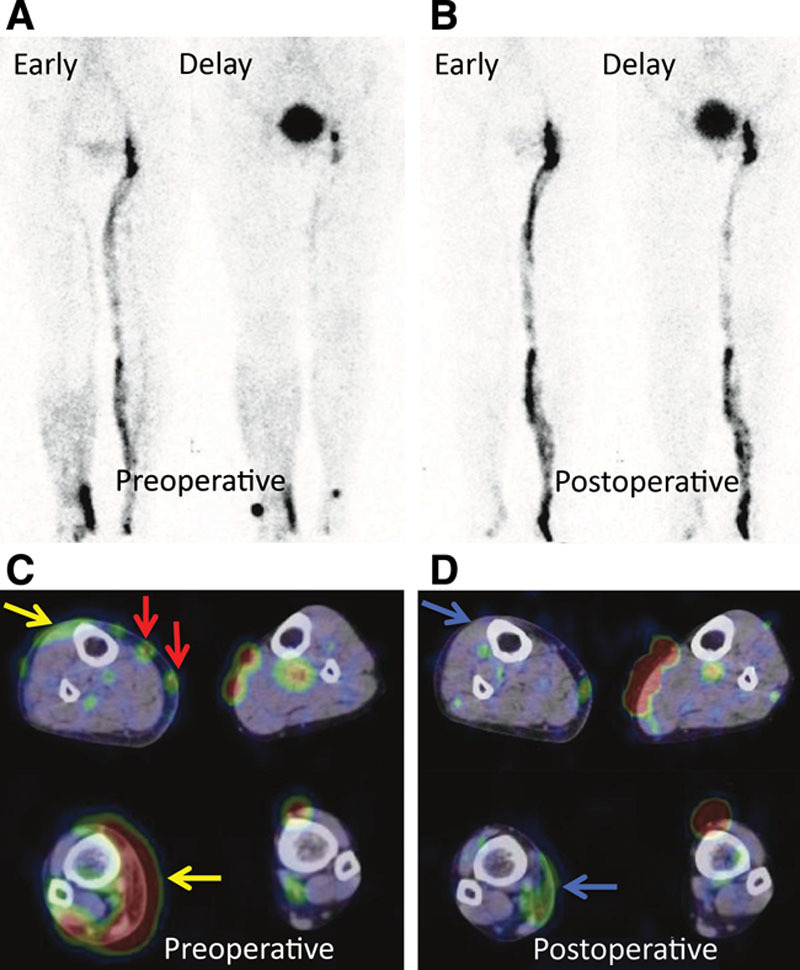
A 69-year-old woman with type 5 of right lower-limb lymphedema (ISL stage: II and LEL index: 245) caused by a surgery for uterine cervical cancer. Preoperative images show inguinal lymph node accumulation defect and dermal backflow (yellow arrow) on the affected side and demonstrate the normal lymphatic vessel accumulation in the medial lower thigh (red arrow). After this, LVA was performed and the dermal backflow was relieved after surgery (blue arrow). LEL index also reduced from 245 to 234. Preoperative (A) and postoperative (B) lymphoscintigraphy early and delayed images. Preoperative (C) and postoperative (D) lymphoscintigraphy SPECT-CT images.
Type Categorization of Lower-limb Lymphedema
In this study, 2-phase 99mTc-phytate lymphoscintigraphy with SPECT-CT provided the information about inguinal lymph node accumulation via lower-limb main lymphatic vessel, dermal backflow, and hepatic accumulation. Inguinal lymph node accumulation may reflect lower-limb lymphatic transport capacity, dermal backflow may reflect the degree of lymphostasis, and hepatic accumulation may reflect the degree of lymphovenous shunt. According to this information, all patients could be categorized as 6 types (type 0–5) (Table 3). Several cases showed no dermal backflow and lymphatic vessel accumulation. In such cases, it is presumed that lymphatic fluid in lymphatic capillaries does not flow into lymphatic vessels but directly flows into blood capillaries via already existing lymphovenous shunt.16–18 Whereas we currently do not have enough data for indicating the relationship between this categorization and severity of lower-limb lymphedema, we believe that this functional information will facilitate establishing a new clinical staging system.
Conducting LVA and Evaluating Its Efficacy
In this study, patients with both dermal backflow and associated normal lymphatic vessel accumulation proceeded to LVA to relieve lymphostasis, and its efficacy was evaluated. Previously, although several investigators attempted to institute a quantification method for evaluating LVA efficacy, the standard method has not been established.
In the visual analysis of this study, pre- and postoperative lymphoscintigraphy with SPECT-CT could demonstrate that LVA relieved lower-limb edema and dermal backflow. Figure 6 is a representative case that shows improved dermal backflow in the postoperative SPECT-CT images. Furthermore, in 2 operated cases, their inguinal lymph node accumulation was improved from defect to positive by LVA (Table 3).
In the present study, 2 quantitative variables, LBR and ILBR, were calculated in pre- and postoperative images. LBR was obtained by dividing the mean liver count by the mean blood count, signifying the degree of hepatic accumulation. As 99mTc-phytate is commonly ingested by Kupffer cells located in hepatic sinusoid, the majority of the tracer, which entered into blood vessels via lymphovenous shunt, accumulates in the liver.19 Therefore, this parameter indicates the degree of lymphovenous shunt. As shown in Figure 2, because LBR significantly increased after LVA in both early and delayed images (P = 0.011 and 0.013, respectively), LVA would have increased their lymphovenous shunt. The other variable was ILBR, which indicates lymphatic transport capacity. In the operated cases, although ILBR in early image showed little change due to instability of blood pool accumulation, ILBR in delayed image significantly increased after LVA (P = 0.003) (Fig. 3). These results indicate that ILBR is an accurate measure of LVA-induced improvement in lymphatic transport capacity. Whereas LVA aims to alleviate lymphedema by delivering lymphatic fluid into venous system, hence reducing lymphostasis, these data indicate that relieving lymphedema may also improve lymphatic transport capacity. This can be associated with the previous clinical and basic science studies, indicating that the pathological and physiological abnormalities in lymphatic systems caused by obesity may recover through weight loss.20–22
Study Limitations
The main limitation of this study was the small sample size, which limits the statistical reliability of the analyses. Another limitation was the lack of a control group or other treatment group. Moreover, future studies that examine the correlation between the test results and clinical symptoms may be required. Increasing the number of cases and long-term follow-up will be necessary in establishing a new clinical staging system for lower-limb lymphedema. In the comparison of pre- and postoperative tests, LBR and ILBR proved to be good indicators for the efficacy of LVA. However, we have not yet compared these parameters between the lymphoscintigraphy images using 99mTc-phytate and 99mTc-HSAD. This would also be a subject for future analysis.
Nevertheless, this is the first report on the usefulness of 99mTc-phytate lymphoscintigraphy with SPECT-CT for patients with lower-limb lymphedema. This method could prove to be useful for estimating lymphodynamic condition and the efficacy of LVA quantitatively in patients with lower-limb lymphedema.
CONCLUSIONS
99mTc-phytate lymphoscintigraphy with SPECT-CT can provide both functional and morphological information simultaneously in patients with lower-limb lymphedema. Using this modality, 6-type categorization for the patients was devised, and it accurately reflects their lymphodynamic conditions. Furthermore, the therapeutic efficacy of LVA could also be estimated quantitatively by comparing the pre- and postoperative findings.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Nawaz K, Hamad M, Sadek S, et al. Lymphscintigraphy in peripheral lymphedema using technetium-labelled human serum albumin: normal and abnormal patterns. Lymphology. 1985;18:181–186. [PubMed] [Google Scholar]
- 2.Suehiro K, Morikage N, Murakami M, et al. Re-evaluation of qualitative lymphangioscintigraphic findings in secondary lower extremity lymphedema. Surg Today. 2014;44:1048–1055. doi: 10.1007/s00595-013-0819-7. [DOI] [PubMed] [Google Scholar]
- 3.Szuba A, Shin WS, Strauss HW, et al. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med. 2003;44:43–57. [PubMed] [Google Scholar]
- 4.Maegawa J, Mikami T, Yamamoto Y, et al. Types of lymphoscintigraphy and indications for lymphaticovenous anastomosis. Microsurgery. 2010;30:437–442. doi: 10.1002/micr.20772. [DOI] [PubMed] [Google Scholar]
- 5.Seok JW, Choi YS, Chong S, et al. Sentinel lymph node identification with radiopharmaceuticals in patients with breast cancer: a comparison of 99mTc-tin colloid and 99mTc-phytate efficiency. Breast Cancer Res Treat. 2010;122:453–457. doi: 10.1007/s10549-010-0973-1. [DOI] [PubMed] [Google Scholar]
- 6.Blum KS, Radtke C, Knapp WH, et al. SPECT-CT: a valuable method to document the regeneration of lymphatics and autotransplanted lymph node fragments. Eur J Nucl Med Mol Imaging. 2007;34:1861–1867. doi: 10.1007/s00259-007-0458-6. [DOI] [PubMed] [Google Scholar]
- 7.Pecking AP, Wartski M, Cluzan RV, et al. SPECT-CT fusion imaging radionuclide lymphoscintigraphy: potential for limb lymphedema assessment and sentinel node detection in breast cancer. Cancer Treat Res. 2007;135:79–84. doi: 10.1007/978-0-387-69219-7_6. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien BM, Sykes P, Threlfall GN, et al. Microlymphaticovenous anastomoses for obstructive lymphedema. Plast Reconstr Surg. 1977;60:197–211. doi: 10.1097/00006534-197708000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Campisi C, Davini D, Bellini C, et al. Lymphatic microsurgery for the treatment of lymphedema. Microsurgery. 2006;26:65–69. doi: 10.1002/micr.20214. [DOI] [PubMed] [Google Scholar]
- 10.Campisi C, Eretta C, Pertile D, et al. Microsurgery for treatment of peripheral lymphedema: long-term outcome and future perspectives. Microsurgery. 2007;27:333–338. doi: 10.1002/micr.20346. [DOI] [PubMed] [Google Scholar]
- 11.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology. 2013;46:1–11. [PubMed] [Google Scholar]
- 12.Yamamoto T, Matsuda N, Todokoro T, et al. Lower extremity lymphedema index: a simple method for severity evaluation of lower extremity lymphedema. Ann Plast Surg. 2011;67:637–640. doi: 10.1097/SAP.0b013e318208fd75. [DOI] [PubMed] [Google Scholar]
- 13.Unno N, Inuzuka K, Suzuki M, et al. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J Vasc Surg. 2007;45:1016–1021. doi: 10.1016/j.jvs.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T, Narushima M, Doi K, et al. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg. 2011;127:1979–1986. doi: 10.1097/PRS.0b013e31820cf5df. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T, Matsuda N, Doi K, et al. The earliest finding of indocyanine green lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: the modified dermal backflow stage and concept of subclinical lymphedema. Plast Reconstr Surg. 2011;128:314e–321e. doi: 10.1097/PRS.0b013e3182268da8. [DOI] [PubMed] [Google Scholar]
- 16.O’Mahony S, Britton TB, Ballinger JR, et al. Delivery of radiolabelled blood cells to lymphatic vessels by intradermal injection: a means of investigating lymphovenous communications in the upper limb. Nucl Med Commun. 2010;31:121–127. doi: 10.1097/MNM.0b013e328330dd14. [DOI] [PubMed] [Google Scholar]
- 17.Edwards JM, Kinmonth JB. Lymphovenous shunts in man. Br Med J. 1969;4:579–581. doi: 10.1136/bmj.4.5683.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamp GF, Peters AM. Peripheral lymphovenous communication in lymphoedema. Nucl Med Commun. 2012;33:701–707. doi: 10.1097/MNM.0b013e32835313d4. [DOI] [PubMed] [Google Scholar]
- 19.Alavi A, Staum MM, Shesol BF, et al. Technetium-99m stannous phytate as an imaging agent for lymph nodes. J Nucl Med. 1978;19:422–426. [PubMed] [Google Scholar]
- 20.Mehrara BJ, Greene AK. Lymphedema and obesity: is there a link? Plast Reconstr Surg. 2014;134:154e–160e. doi: 10.1097/PRS.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martel C, Li W, Fulp B, et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest. 2013;123:1571–1579. doi: 10.1172/JCI63685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim HY, Rutkowski JM, Helft J, et al. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am J Pathol. 2009;175:1328–1337. doi: 10.2353/ajpath.2009.080963. [DOI] [PMC free article] [PubMed] [Google Scholar]


