Abstract
Using the energy of ATP hydrolysis, the Na+/K+-ATPase is able to transport across the cell membrane Na+ and K+ against their electrochemical gradients. The enzyme is strongly inhibited by ouabain and its derivatives, some that are therapeutically used for patients with heart failure (cardiotonic steroids). Using lanthanide resonance energy transfer (LRET), we trace here the conformational changes occurring on the external side of functional Na+/K+-ATPases induced by the binding of ouabain. Changes in donor/acceptor pair distances are mainly observed within the α subunit of the enzyme. To derive a structural model matching the experimental LRET distances measured with bound ouabain, molecular dynamic simulations were carried out with energy restraints applied simultaneously using a novel methodology with multiple noninteracting fragments. The restrained simulation, initiated from the X-ray structure of the E2(2K+) state, became strikingly similar to the X-ray structure of the sodium-bound state. The final model shows that ouabain is trapped within the external ion permeation pathway of the pump.
Graphical abstract
Introduction
Since its discovery [1], the Na+/K+-ATPase has been extensively studied because of its importance in restoring the Na+ and K+ gradients in cells [2] and its clinical relevance as the site of action for cardiotonic steroids used by heart failure patients.
The Na+/K+ pump is a P-type ATPase that hydrolyzes one molecule of ATP to drive the transport of 3Na+ by 2K+ against their electrochemical gradients. To function, the Na+/K+ pump requires two subunits: α and β. The α subunit is about 1,000 amino acids long containing ten transmembrane α helices (αM1–M10) with intracellular N- and C-termini. The entire transport machinery resides within the α subunit [3,4]. The β subunit (~300 amino acids) has only one transmembrane segment with a large external glycosylated C-terminus domain. It functions as a chaperone subunit with some regulatory transport properties [5–8]. A third tissue-specific player in mammals is a FXYD type subunit that spans once across the membrane with an extracellular N-terminus and it is important for enzymatic modulation [9–11].
Due to its physiological and clinical relevance [12–21], interactions of cardiotonic steroids with the Na+/K+ pump has been extensively studied [22–37]. Ouabain (a type of cardiotonic steroid) is frequently used as the inhibitor of the Na+/K+ pump in ligand-receptor studies. Ouabain accesses the Na+/K+ pump from the extracellular side. Functional and structural studies show that ouabain binds to E2P states containing no ions, or loaded with Na+, K+ or even Mg2+ [31,35–37]. Ouabain’s site of action is in the transmembrane region of the α subunit [31,34,36,37]. In particular, it appears to sit deep within the ion permeation pathway in a cleft between transmembrane segments αM1–M6. Crystallographic data show that ouabain binding produces substantial rearrangements within the α subunit’s transmembrane segments αM1–M4 [31,36,37].
Here we use a spectroscopic approach to determine donor/acceptor pair distances upon binding of ouabain to fully functional Na+/K+-ATPases. Distances were determined by assessing lanthanide resonance energy transfer (LRET) between 11 donor/acceptor pairs on the extracellular side of the α and β subunits. In agreement with structural data [31,36,37], ouabain binding produced significant movements of the α subunit, while the β remained unperturbed. We have used molecular dynamics simulations to model the ouabain-bound state of the Na+/K+-ATPase that corresponds to the LRET distances obtained experimentally. The initial model of the Na+/K+-ATPase was built based on the crystal structure of the pump in the E2P state [4], and was simulated in the presence of additional restraints corresponding to the donor/acceptor pair distances estimated in the presence of ouabain. To impose the experimentally obtained distances, we have developed a computational strategy, in which the donor/acceptor pairs are invisible to each other. Six donor/acceptor pairs, including three LBT (lanthanide binding tag, see Material and Methods) segments and three Cys-BODIPY FL elements are inserted into the atomic model of the pump. This atomic model is then subject to molecular dynamics simulation in which experimentally measured LRET distances are imposed as harmonic restraints between donor/acceptor elements. To mimic the experimental setup, where each donor-acceptor distance is measured independently, the interactions between each donor/acceptor pair are turned off in the simulation. In this setup, the donor and acceptor elements from a single distance measurement see each other and interact with the rest of the protein, but the other donor/acceptor pairs are invisible to them. This setup allows us to simultaneously impose the six experimentally measured distances without interference of the long LBT loops.
Our results indicate that αM1 and αM2 helices opened up to accommodate the bound ouabain, as the αM3 and αM4 helices moved toward the ouabain binding site. Interestingly, the transmembrane region of the final model is closer to the crystal structure of the Na+ bound E1P-ADP state [38] rather than the E2P-ouabain bound structure [37].
Results
The objective of these experiments is the direct estimation of inter and intra subunit conformational changes that occur when a functional Na+/K+ pump binds the inhibitor ouabain. To this end, experiments were conducted in oocytes expressing squid Na+/K+ pump constructs containing one donor (LBT(Tb3+)) and one acceptor (BODIPY FL dye). All constructs were tested to be functional and sensitive to ouabain (Figure S1). Molecular distances were estimated from donor and sensitized emission decays in the absence and in the presence of ouabain.
Inter-subunit distance determinations upon ouabain binding
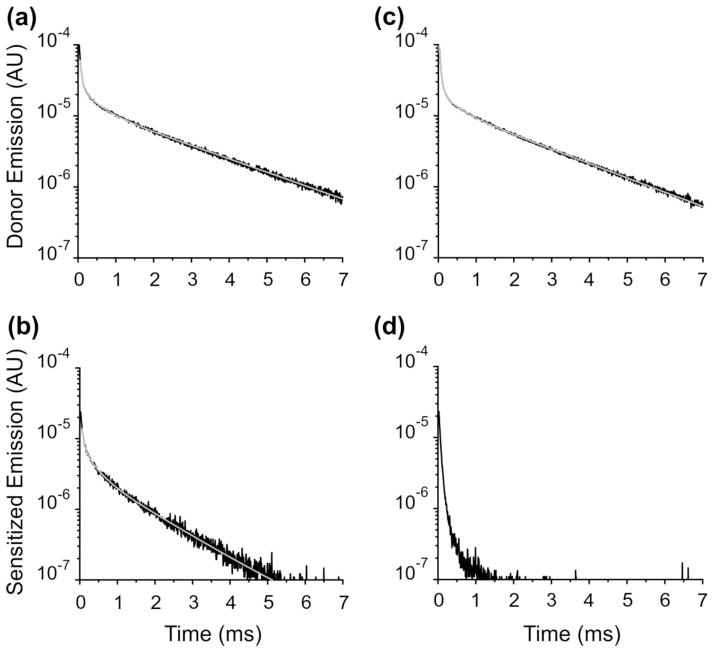
We estimated α-β distances in three donor-acceptor pairs, first in the absence and then in the presence of ouabain bound. Figure 1 (A & B) shows an example in the absence of ouabain, when the donor is in position 324LBT(αM3–M4) and the acceptor is a BODIPY FL dye attached to a cysteine located at the external end of the β subunit’s transmembrane segment (T71C(β)). The donor emission (with no acceptor) shows the characteristic slow decay of ~2.4 ms when a Tb3+ is tightly bound to the LBT moiety (Figure 1A). The sensitized emission decay detected in the first dark region of the Tb3+ emission spectrum is much faster (τ=1.36 ms; Figure 1B), indicating an energy transfer between the Tb3+ in the LBT and the fluorophore. Is the observed energy transfer specific to this donor-acceptor pair? To address this question we performed experiments with pumps formed by the 324LBT(αM3–M4) and the wild-type β subunit subjected to the same experimental protocol including BODIPY FL labelling (Figure 1C & 1D). In this case, the donor emission decay is similar (Figure 1C) confirming the presence of 324LBT(αM3–M4) subunits in the membrane. However, the sensitized emission is virtually absent (Figure 1D), demonstrating that the decay observed previously is specific to the donor/acceptor pair assessed.
Fig. 1.
LRET-based measurements of energy transfer in the Na+/K+-ATPase in the absence of ouabain. (a) Donor emission of the pump construct 324LBT(αM3–M4)/T71C(β). Solid line represents an exponential fit with three time constants. The fastest two components are associated with unspecific Tb3+ binding. The slowest component (τD = 2.39 ms) corresponds to the emission decay from Tb3+ bound to LBT. (b) Sensitized emission of the pump construct 324LBT(αM3–M4)/T71C(β). Solid line denotes an exponential fit with two components. The slowest component (τSE =1.36 ms) represents the sensitized emission of the acceptor reflecting the energy transfer between the donor and the acceptor. (c) Donor emission of the pump construct 324LBT(αM3–M4)/βWT. The slow luminescence decay (τD = 2.36 ms) reflects the presence of protein in the membrane. (d) Sensitized emission of the pump construct 324LBT(αM3–M4)/βWT. In this case, only unspecific signal is observed due to the lack of acceptor in the protein.
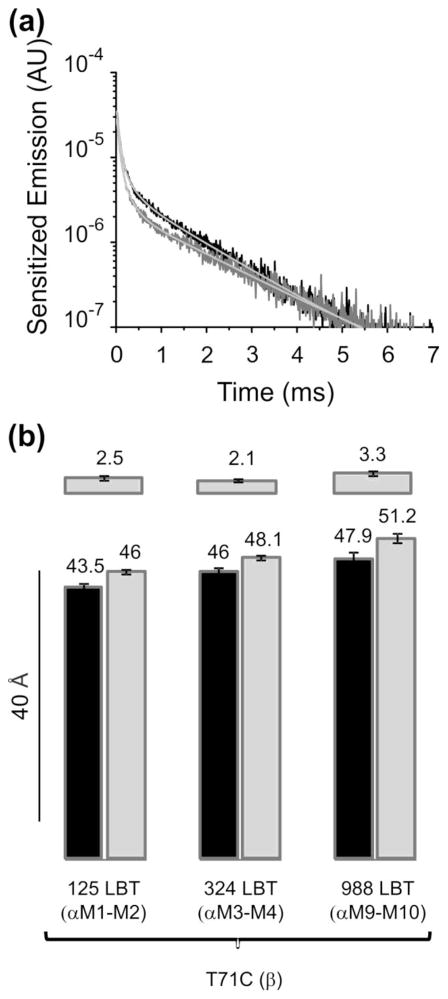
Next we asked whether the sensitized emission of the 324LBT(αM3–M4)/T71C(β) pair changes in the presence of ouabain in its binding site. Figure 2A shows the sensitized emission decays in the same oocyte before (black) and after incubating the oocyte with 100 μM ouabain (gray). The presence of the inhibitor slowed down the decay from 1.34 to 1.59 ms, suggesting that the donor and the acceptor increased their distance after ouabain binding. The donor emission decay is not changed by the presence of ouabain (Figure S2), showing that the inhibitor molecule does not distort the integrity of the LBT-Tb3+ complex. Therefore the changes in sensitized emission with ouabain reflect unambiguous movements between the donor and the acceptor. Similar results were obtained with donor-acceptor pairs 125LBT(αM1–M2)/T71C(β) and 988LBT(αM9–M10)/T71C(β) (Figure S3). Figure 2B displays the estimated distances before and after ouabain incubation of the three inter-subunit donor-acceptor pairs. In all cases, the presence of ouabain in the pump increased the distances between α and β subunits.
Fig. 2.
Changes in energy transfer in the Na+/K+-ATPase with ouabain bound. (a) Sensitized emissions of the pump construct 324LBT(αM3–M4)/T71C(β). Sensitized emission in the absence of ouabain decayed with a τSE of 1.34 ms (black trace). In the same oocyte, preincubation with ouabain slowed down the decay to 1.59 ms (gray trace). (b) Distance estimations. Distance estimated for constructs 125LBT(αM1–M2)/T71C(β) (n=13), 324LBT(αM3–M4)/T71C(β) (n=10) and 988LBT(αM9–M10)/T71C(β) (n=5) in the absence of ouabain (black) and in the presence of ouabain bound (gray). Upper bars show the Δ distances caused by ouabain bound to the pump.
Intra-subunit distance determinations upon ouabain binding
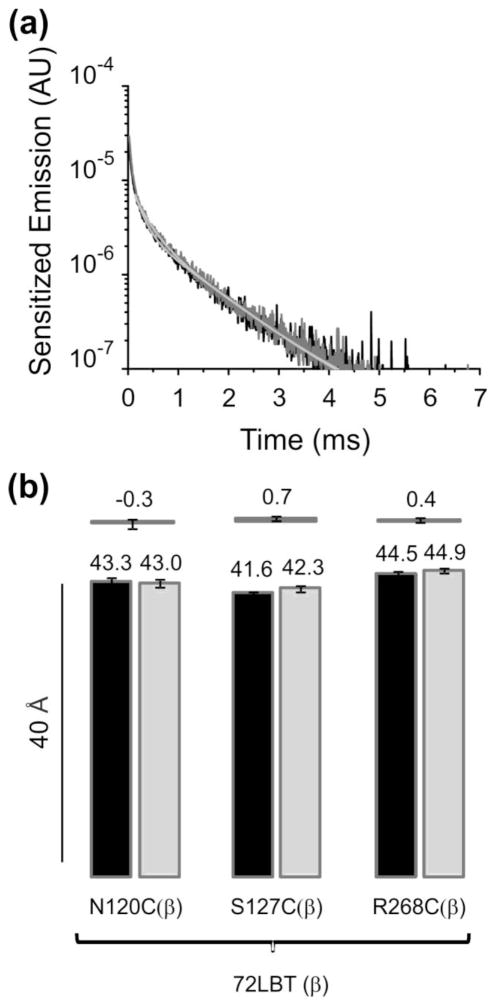
The inter-subunit distance changes observed could originate from movements of the subunits α or β, or a combination of both. To estimate intra β subunit distances we introduced a LBT moiety at position 72, close to the external end of the transmembrane segment (72LBT(β)) and mutated to cysteine several residues in different regions of the subunit. Figure 3A shows the sensitized emission decays with the pump construct αWT/72LBT(β)-N120C(β) without (black) and with ouabain bound (gray). The time constants are indistinguishable (1.13 and 1.07 ms), suggesting that the β subunit does not change upon ouabain binding. Distance estimations for this pump construct and, constructs αWT/72LBTβ-S127C(β) and αWT/72LBT(β)-R268C(β) do not show conformational changes of the β subunit in presence of ouabain (Figure 3B). It is then likely that inter-subunit distance changes detected previously come from the α subunit.
Fig. 3.
Intra β subunit distance changes upon ouabain binding to the Na+/K+-ATPase. (a) Sensitized emissions of the pump construct αWT/72LBT(β)-N120C(β). Sensitized emission in the absence of ouabain decayed with a τSE of 1.13 ms (black trace) while in the presence of ouabain bound (same oocyte) it decays with a τSE of 1.07 ms (gray trace). (b) Distance estimations. Distance estimated for constructs αWT/72LBT(β)-N120C(β) (n=8), αWT/72LBT(β)-S127C(β) (n=10) and αWT/72LBT(β)-R268C(β) (n=5) without ouabain (black) and in the presence of ouabain bound (gray). Upper bars show the Δ distances caused by the presence of ouabain in the pump.
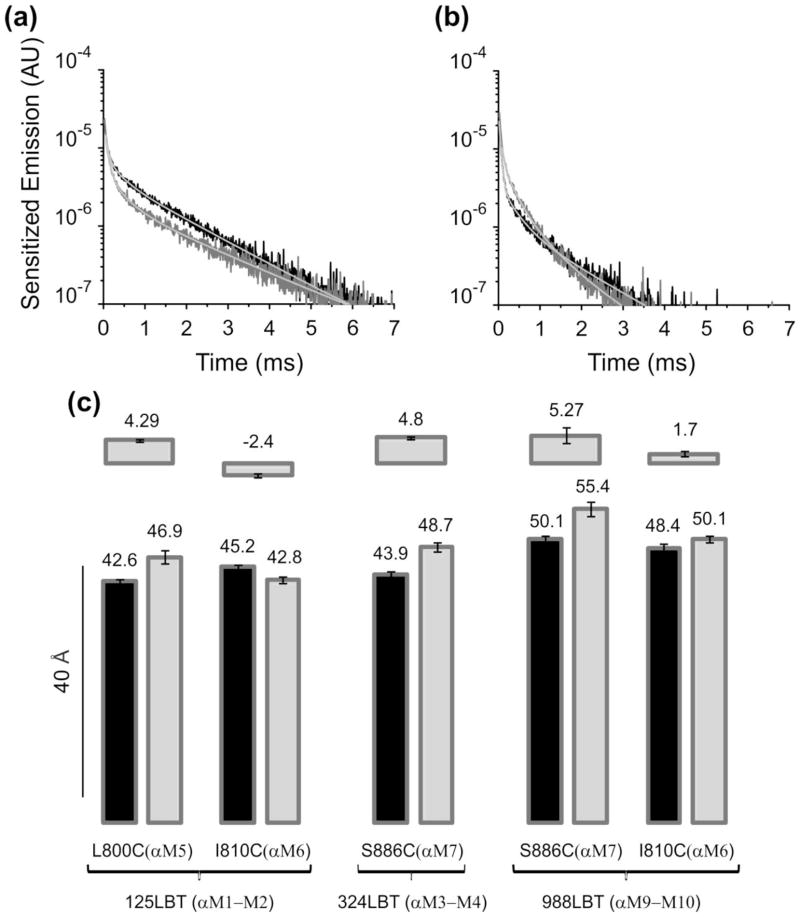
Intra α subunit distances were estimated with 125LBT(αM1–M2), 324LBT(αM3–M4) and 988LBT(αM9–M10) as donors, and cysteines at the external end of transmembrane segments 5 through 7. Figures 4A & 4B show two examples with pump constructs 324LBT(αM3–M4)-S886C(αM7)/βWT and 125LBT(αM1–M2)-I810C(αM6)/βWT. In both cases, ouabain in the pump altered the sensitized emission decay. In the first case it was slowed down while in the second it was sped up. Distance estimations from these results and those obtained with three other intra α subunit pump constructs are shown in Figure 4C. Remarkably, in all cases the distance between the donor and the acceptor was changed by the presence of the ouabain molecule in the pump.
Fig. 4.
Intra α subunit distance changes upon ouabain binding to the Na+/K+-ATPase. (a) Sensitized emissions of the pump construct 324LBT(αM3–M4)-S886C(αM7)/βWT. In the absence of ouabain, sensitized emission decayed with a τSE of 1.45 ms (black trace). Ouabain bound to these pumps slowed down the sensitized emission decay to 1.69 ms (gray trace). (b) Sensitized emissions of the pump construct 125LBT(αM1–M2)-I810C(αM6)/βWT. In this example, ouabain binding accelerated τSE from 1.30 (black trace; absence) to 1.12 ms (gray trace; presence). (c) Distance estimations. Distance estimated for all intra α subunit constructs tested: 125LBT(αM1–M2)-L800C(αM5)/βWT (n=7), 125LBT(αM1–M2)-I810C(αM6)/βWT (15), 324LBT(αM3–M4)-S886C(αM7)/βWT (n=7), 988LBT(αM9–M10)-S886C(αM7)/βWT (n=4) and 988LBT(αM9–M10)-I810C(αM6)/βWT (n=5). Black bars correspond to distances without ouabain while gray bars represent distances upon ouabain binding. Upper bars show the Δ distances produced by ouabain binding.
Discussion
We used a LRET-based method to study the conformational changes induced by ouabain binding to the Na+/K+-ATPase. We designed donor and acceptors sites in all external loops of the α subunit, as well as in different sites within the β subunit. We were unable to detect changes in donor-acceptor distances within the β subunit, which is compatible with crystal structures of Na+/K+-ATPase with ouabain bound [31,36,37]. In these structures, binding of ouabain produced rearrangements at the extracellular end of the transmembrane segments αM1–αM4. Similarly, our LRET determination show that ouabain binding produce distance changes between the linkers αM1–M2, αM3–M4 and αM9–M10 and regions of the β and α subunits. We have used molecular dynamics simulations to model the ouabain-bound state of the Na+/K+-ATPase that corresponds to the LRET distances obtained. The initial model of the Na+/K+-ATPase was built based on the crystal structure of the pump in the E2P state [4]. This model was then simulated in the presence of additional restraints corresponding to the experimentally estimated distances between donor-acceptor pairs in the presence of ouabain, to give the final model that represents the ouabain bound state (see Methods). Figure 5A and 5B shows side views of the superposition of the initial and final models, with an ouabain molecule bound. The αM5–M10 regions (shown in green) remained very similar between the two models, and for clarity only that from the final model is shown. The transmembrane segment of the β subunit is represented in red. In our models, the relative position of the β subunit’s transmembrane segment remained similar as observed in different ouabain bound crystal structures [31,36,37]. The moving parts upon ouabain binding were the αM1–M4 helices. Their positions in the initial model are shown in light gray and in a different color for the final model. During the simulation, αM1 and αM2 helices opened up to accommodate the bound ouabain (Figure 5C; top view). These two helices moved together and bulged in the middle; their displacement at the extracellular end of the helices is approximately 6Å. In addition, αM3 and αM4 helices moved towards the ouabain binding site. Interestingly, the αM4 helix bends in the middle of the membrane in a hinge-like motion resulting in a displacement of approximately 7Å at near the extracellular side. This movement of the αM4 segment is followed by the αM3 helix, which moves as a rigid body. Even though the details of the local movements predicted by modeling cannot be directly compared with known ouabain-bound crystal structures since likely represent different conformations, general principles about these motions can be drawn. First, αM1 and αM2 helices are opened up to accommodate the ouabain molecule, suggesting that initial access of ouabain originate from a state in which the external access channel is opened like in the SERCA1a E2:BeF3− structure [39]. A further movement towards the cytoplasmic end of αM1 and αM2 helices has also been observed in ouabain bound crystal structures [36,37] and functionally suggested by ouabain induced changes of trypsin accessibility in the αM2–αM3 linker [40]. Second, αM4 helix appears to be rather flexible and prompt to adopt changes in conformation upon ouabain binding as observed in our simulations and ouabain bound crystal structures [36,37]. Third, once ouabain is bound, the molecule is trapped within the ion permeation pathway unable to freely escape to the external solutions, as seen in our simulations and ouabain bound crystal structures [36,37].
Fig. 5.
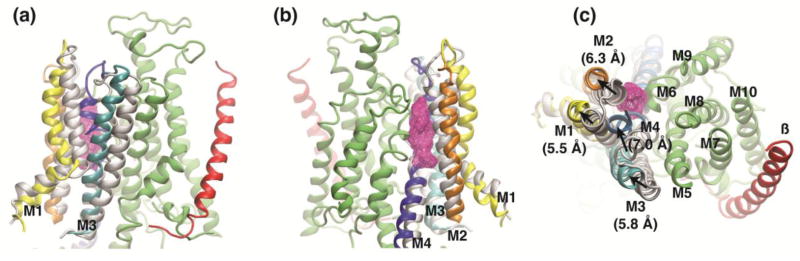
Modeling the ouabain-bound state of the Na+/K+-ATPase. (a) & (b), Snapshot (side views rotated ~ 180°) of the Na+/K+ ATPase after a short simulation in which the LRET distances corresponding to the ouabain-bound conformation are imposed as harmonic restraints between LBT(Tb3+) and the BODIPY FL. The final conformation is shown in green (αM5–M10), yellow (αM1), orange (αM2), cyan (αM3), and blue (αM4). The β subunit is shown in red. The initial conformation of αM1–M4 segments is shown in light gray. Ouabain is shown as a wired mesh in magenta. (c) Movement of the extracellular half of the transmembrane helices αM1–M4 is shown in top view. The numbers in the parenthesis correspond to the movement of most extracellular residue of each transmembrane helix after simulating the system while enforcing the LRET distances.
Comparison of the final model with the crystal structure of the E2 ouabain [37] (Figure 6B) and the Na+ bound states [38] (Figure 6C) shows that while our initial structure was very similar to the E2 ouabain structure (Figure 6A), the final model is remarkably closer to the Na+ bound state (Figure 6C). Distinct differences still exist between the positions of αM1–M2 helices in the two models, which can be due to the fact that the cytoplasmic domains of the pump and in particular the A domain were constrained to their initial positions (as in E2P) during the simulations. However, αM3 and αM4 helices have moved to the same position as in the Na+ bound state. The bending movement of the αM4 segment, which partially coordinates the ions in the middle of membrane, is similar to the movement observed between the Na+/K+-ATPase’s crystal structures with K+ bound [4] and Na+ bound [38] states, suggesting that the LRET distances as well as the final model obtained here correspond to the Na+ bound high affinity binding state of ouabain [35]. These discrepancies between the crystal structures of Na+/K+-ATPase with ouabain and our model are readily explained by the fact that all models derived from crystal structures have been obtained in the absence of Na+, reflecting distinct conformations of ouabain bound Na+/K+-ATPases.
Fig. 6.
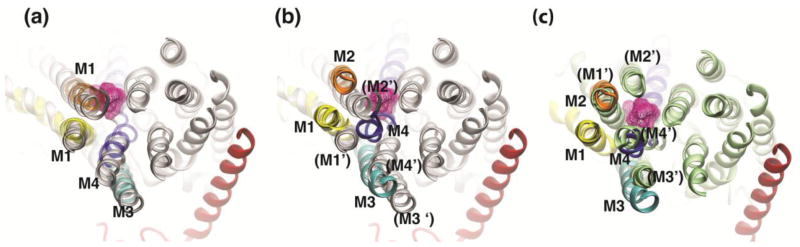
Comparison of the ouabain bound model with the Na+-bound state and the E2P Ouabain bound state. (a) Superposition of the Na+/K+-ATPase in the E2P ouabain bound state (white [37]) with the initial conformation of the squid model. For clarity only M1–M4 of the squid model are shown in yellow, orange, cyan, and blue, respectively. (b) Superposition of the Na+/K+ ATPase in the E2P ouabain bound state shown in white with the ouabain-bound conformation (αM1–αM4) obtained in the simulations after imposing the LRET distances. (c) Superposition of the Na+/K+-ATPase in the Na+-bound state E1 shpown in green [38] with the final model of the ouabain-bound state, for which only αM1–M4 helices are shown.
Materials and Methods
LRET measurements and Na+/K+ ATPase LBT constructs
We use a variant of the LRET energy transfer technique in which the donor is a Tb3+ bound with high affinity to a 17 amino acid long tag (YIDTNNDGWYEGDELLA) that can be inserted at any desired location within a protein[41]. The Lanthanide Binding Tag (LBT) contains a Trp residue that acts like an antenna to excite the Tb3+. In this study we used four LBT constructs in the squid (Loligo opalescens) Na+/K+-ATPase [42]. In three of them, LBT was inserted in the α subunit and in one construct the LBT was inserted in the β subunit. 125LBT(αM1–M2) denotes an α subunit LBT construct in which the LBT was inserted after position 125 located between transmembrane segments 1 and 2. Following the same nomenclature, the other two α subunit LBT constructs are 324LBT(αM3–M4) and 988LBT(αM9–M10). 72LBT(β) represents the construct in which the LBT was inserted in the β subunit at the external end of the transmembrane segment. The acceptor was a BODIPY FL fluorophore molecule attached to a cysteine mutation in the α or β subunit. In total, we studied 11 donor-acceptor pairs: 3 α-β, 5 α-α and 3 β-β. Measurements using LRET technique and its advantages have been lengthily discussed [43]. The specific details of our system and measurements have been described previously [34,44]. For each oocyte expressing a donor-acceptor pair we performed distance determinations in the absence or in the presence of ouabain bound to the Na+/K+ATPase. Oocytes were preincubated with 100 μM ouabain for 5 min before measurements were made. In all constructs ouabain inhibition is irreversible in the time frame of our experiments rendering an unperturbed high affinity binding site. The donor’s emission decay was acquired in a solution containing 5 μM Tb3+ (TbCl3, Sigma Aldrich) in oocytes that were not labeled with BODIPY FL dye (Invitrogen). The sensitized emissions were acquired in oocytes that were pre-incubated with BODIPY FL dye for 20 minutes. The sensitized emission decays were detected within the first dark region of the Tb3+ emission spectrum (500–540 nM). Distances between the donor and acceptor were estimated from the Förster relation using the time constants of the donor emission (τD) and the sensitized emission (τSE) as: R=R0 (τSE/(τD-τSE))1/6 where R0 is 42 Å. Pump’s rearrangements induced by ouabain binding were estimated in terms of the displacement Δ (=Rouab-R), where R and Rouab correspond to LRET-based distances first in the absence of ouabain and subsequently in the presence of ouabain bound, respectively. Means ± SEM are given or plotted and n the number of experiments is indicated by n.
Structural modeling
The homology model of the squid (Loligo opalescens) Na+/K+ ATPase was generated based on the crystal structure of the Na+/K+-ATPase from shark rectal gland (PDB code: 2ZXE) using the program Modeller [45]. Missing residues in the α and β subunits of the crystallographic structure were also omitted in the squid homology model.
Figure S4 shows an overview of the modeling system. Three LBT loops were inserted at positions corresponding to 125LBT(αM1–M2), 324LBT(αM3–M4), and 988LBT(αM9–M10). The LBT coordinates were taken from the PDB code 1TJB[41]. Using the program Modeller, the coordinates of the squid homology model was combined with the LBT coordinates to produce 10 different models containing the three insertions simultaneously, from which the most favorable model was used for further simulations. Three cysteine mutations were introduced at positions T71C(β), L800C(αM5) and S886C(αM7). These cysteines were covalently attached to BODIPY FL. The Cys-BODIPY FL moiety was parameterized using the General Automated Atomic Model Parameterization (GAAMP) (http://gaamp.lcrc.anl.gov/xsede.html) [46].
The final model was subject to molecular dynamics simulations using NAMD [47]. During the simulations, the extracellular domain of the β subunit was removed to avoid further clashes with the inserted loops. Harmonic restraints were used to impose distance restraints between each LBT(Tb3+)/BODIPY FL pair, constraining them to the distances estimated experimentally after ouabain is bound. All the LBT(Tb3+)/BODIPY FL pairs were introduced simultaneously in the structure to make full use of all the available information from the experiments. To avoid unphysical steric clashes and to mimic the experimental conditions in which each donor/acceptor distance was determined separately, these LBT loops as well as Cys-BODIPY FL side chains were treated as non-interacting fragments with respect to each other in the simulations. According to this protocol, selected nonbonded interactions (i.e. Lennard-Jones and electrostatic interactions) between atom pairs belonging to individual LBT loops or individual BODIPY FL attachments were skipped (omitted) from the energy and force calculations during the simulations. In practice, this protocol was implemented by manipulating the non-bonded exclusion list in the protein structure file (PSF) of the system for selected pairs of atoms
The protocol allows simultaneous simulations of the LBT-inserted structure with the wild-type squid model (as shown in Figure Supplementary 4). In each simulation, the LBTs as well as its ten neighboring residues, i.e. five residues before and five residues after the insertion are replicated and superimposed onto the wild-type structure. To confine the structure of the squid to adopt only one conformation, the backbone atoms of the five residues before and after the insertion are harmonically restrained to follow the coordinates of the wild-type squid structure.
To avoid structural deformation of the LBT loops, the atomic distance between each Tb3+ atom and its surrounding acidic residues in the LBT structure (2 Glu and 2 Asp residues) were constrained to their crystallographic distances. In addition, the backbone of the cytoplasmic domain of the pump, as well as the TM regions within 5Å of the cytoplasmic solution was restrained.
The parameters of ouabain were obtained using the General Automated Atomic Model Parameterization (GAAMP) (http://gaamp.lcrc.anl.gov/xsede.html) [46]. The Cys-BODIPY FL parameters were also obtained similarly by first parameterizing the iodacetamide-cystine compounds and them manually adding the BODIPY FL.
The simulation systems include the α subunit of the Na+/K+ ATPase as well as the transmembrane region of the β subunit (residues 39–74). The lipid bilayer and solvent were not included in the system to facilitate conformational changes of the protein. The simulations are carried out using the CHARMM36 forcefield for the proteins [48] and the newly determined parameters for ouabain and BODIPY FL attachments. Electrostatic calculations were cut off at 12Å. The simulations were carried out at a constant temperature of 300K with a timestep of 1fs. Each simulation was run for 120ps, during which the position of LBT insertions and their adjacent transmembrane helices have stabilized toward a stable conformation. LRET distances were imposed on the system as harmonic restraints between each BODIPY-LBT(Tb3+) pair with a force constant of 1.0 kcal/mol.Å. Additionally, residues 900–949 and 960–990 of the transmembrane domain were constrained to their initial position to prevent tumbling and lateral movement of the protein when additional restraints are applied. The αM9 and αM10 TM helices containing residues 900–949 and 960–990 do not vary among the Na+-bound, ouabain-bound and the K+-bound structures of the pump and thus, restraining their backbone to the crystallographic positions is not expected to affect the results. The simulations included ouabain at the high affinity position that was identified previously [34]. During the simulation, ouabain fluctuates around its initial position. The root mean square deviation of ouabain between its initial position and its final position is 5.2Å, in which ouabain has moved 4Å down toward the cytoplasmic region.
Supplementary Material
Highlights.
Proposed model of the ouabain bound conformation of a functional Na+/K+-ATPase
Ouabain binding changes distances at the external end of the α subunit helices
A molecular model shows ouabain trapped within the ion permeation pathway
The ouabain bound model corresponds to a Na+-occluded state of the Na+/K+-ATPase
Acknowledgments
This work was supported by NIH grants R01-GM062342 (BR), R01-GM030376 (FB) and U54-GM087519 (FB, BR and MH), and the Intramural Research Program of the National Institutes of Health, NINDS (MH and PM). JESR was a recipient of a postdoctoral fellowship from Consejo Nacional de Ciencia y Tecnología (Mexico).
Footnotes
Author Contributions
FB, BR and MH conceived and supervised the project. JESR performed the LRET experiments. PM designed and obtained pump constructs and participated in some experiments. FKA and BR designed and performed molecular dynamic simulations. All authors contributed to writing the manuscript.
COMPETING FINANCIAL INTERESTS
The authors report no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957;23:394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- 2.Hodgkin AL, Keynes RD. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955;128:28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morth JP, Pedersen BP, Toustrup-Jensen MS, Sorensen TL, Petersen J, Andersen JP, Vilsen B, Nissen P. Crystal structure of the sodium-potassium pump. Nature. 2007;450:1043–9. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- 4.Shinoda T, Ogawa H, Cornelius F, Toyoshima C. Crystal structure of the sodium-potassium pump at 2.4 A resolution. Nature. 2009;459:446–50. doi: 10.1038/nature07939. [DOI] [PubMed] [Google Scholar]
- 5.Jaisser F, Canessa CM, Horisberger JD, Rossier BC. Primary sequence and functional expression of a novel ouabain-resistant Na,K-ATPase. The beta subunit modulates potassium activation of the Na,K-pump. J Biol Chem. 1992;267:16895–903. [PubMed] [Google Scholar]
- 6.Lutsenko S, Kaplan JH. An essential role for the extracellular domain of the Na,K-ATPase beta-subunit in cation occlusion. Biochemistry. 1993;32:6737–43. doi: 10.1021/bi00077a029. [DOI] [PubMed] [Google Scholar]
- 7.Geering K. The functional role of beta subunits in oligomeric P-type ATPases. J Bioenerg Biomembr. 2001;33:425–38. doi: 10.1023/a:1010623724749. [DOI] [PubMed] [Google Scholar]
- 8.Hasler U, Crambert G, Horisberger JD, Geering K. Structural and functional features of the transmembrane domain of the Na,K-ATPase beta subunit revealed by tryptophan scanning. J Biol Chem. 2001;276:16356–64. doi: 10.1074/jbc.M008778200. [DOI] [PubMed] [Google Scholar]
- 9.Garty H, Karlish SJ. Role of FXYD proteins in ion transport. Annu Rev Physiol. 2006;68:431–59. doi: 10.1146/annurev.physiol.68.040104.131852. [DOI] [PubMed] [Google Scholar]
- 10.Mahmmoud YA, Vorum H, Cornelius F. Identification of a phospholemman-like protein from shark rectal glands. Evidence for indirect regulation of Na,K-ATPase by protein kinase c via a novel member of the FXYDY family. J Biol Chem. 2000;275:35969–77. doi: 10.1074/jbc.M005168200. [DOI] [PubMed] [Google Scholar]
- 11.Therien AG, Goldshleger R, Karlish SJ, Blostein R. Tissue-specific distribution and modulatory role of the gamma subunit of the Na,K-ATPase. J Biol Chem. 1997;272:32628–34. doi: 10.1074/jbc.272.51.32628. [DOI] [PubMed] [Google Scholar]
- 12.Lingrel JB. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu Rev Physiol. 2010;72:395–412. doi: 10.1146/annurev-physiol-021909-135725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mijatovic T, Van Quaquebeke E, Delest B, Debeir O, Darro F, Kiss R. Cardiotonic steroids on the road to anti-cancer therapy. Biochim Biophys Acta. 2007;1776:32–57. doi: 10.1016/j.bbcan.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol. 2007;293:C509–36. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 15.Larre I, Castillo A, Flores-Maldonado C, Contreras RG, Galvan I, Munoz-Estrada J, Cereijido M. Ouabain modulates ciliogenesis in epithelial cells. Proc Natl Acad Sci USA. 2011;108:20591–6. doi: 10.1073/pnas.1102617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cereijido M, Contreras RG, Shoshani L, Larre I. The Na+-K+-ATPase as self-adhesion molecule and hormone receptor. Am J Physiol Cell Physiol. 2012;302:C473–81. doi: 10.1152/ajpcell.00083.2011. [DOI] [PubMed] [Google Scholar]
- 17.Larre I, Lazaro A, Contreras RG, Balda MS, Matter K, Flores-Maldonado C, Ponce A, Flores-Benitez D, Rincon-Heredia R, Padilla-Benavides T, Castillo A, Shoshani L, Cereijido M. Ouabain modulates epithelial cell tight junction. Proc Natl Acad Sci U S A. 2010;107:11387–92. doi: 10.1073/pnas.1000500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaustein MP, Hamlyn JM. Signaling mechanisms that link salt retention to hypertension: endogenous ouabain, the Na+ pump, the Na+/Ca2+ exchanger and TRPC proteins. Biochim Biophys Acta. 2010;1802:1219–29. doi: 10.1016/j.bbadis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamlyn JM, Blaustein MP. Salt sensitivity, endogenous ouabain and hypertension. Curr Opin Nephrol Hypertens. 2013;22:51–8. doi: 10.1097/MNH.0b013e32835b36ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierre SV, Xie Z. The Na,K-ATPase receptor complex: its organization and membership. Cell Biochem Biophys. 2006;46:303–16. doi: 10.1385/cbb:46:3:303. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem. 2004;279:17250–9. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- 22.Antolovic R, Schoner W, Geering K, Canessa C, Rossier BC, Horisberger JD. Labeling of a cysteine in the cardiotonic glycoside binding site by the steroid derivative HDMA. FEBS Lett. 1995;368:169–72. doi: 10.1016/0014-5793(95)00637-o. [DOI] [PubMed] [Google Scholar]
- 23.Artigas P, Gadsby DC. Ouabain affinity determining residues lie close to the Na/K pump ion pathway. Proc Natl Acad Sci USA. 2006;103:12613–8. doi: 10.1073/pnas.0602720103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brinkmann K, Linnertz H, Amler E, Lanz E, Herman P, Schoner W. Fluoresceinyl-ethylenediamine-ouabain detects an acidic environment in the cardiac glycoside binding site of Na+/K+-ATPase. Eur J Biochem. 1997;249:301–8. doi: 10.1111/j.1432-1033.1997.t01-2-00301.x. [DOI] [PubMed] [Google Scholar]
- 25.Canessa CM, Horisberger JD, Louvard D, Rossier BC. Mutation of a cysteine in the first transmembrane segment of Na,K-ATPase alpha subunit confers ouabain resistance. EMBO J. 1992;11:1681–7. doi: 10.1002/j.1460-2075.1992.tb05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canessa CM, Horisberger JD, Rossier BC. Mutation of a tyrosine in the H3–H4 ectodomain of Na,K-ATPase alpha subunit confers ouabain resistance. J Biol Chem. 1993;268:17722–6. [PubMed] [Google Scholar]
- 27.Croyle ML, Woo AL, Lingrel JB. Extensive random mutagenesis analysis of the Na+/K+-ATPase alpha subunit identifies known and previously unidentified amino acid residues that alter ouabain sensitivity--implications for ouabain binding. Eur J Biochem. 1997;248:488–95. doi: 10.1111/j.1432-1033.1997.00488.x. [DOI] [PubMed] [Google Scholar]
- 28.Forbush B, 3rd, Kaplan JH, Hoffman JF. Characterization of a new photoaffinity derivative of ouabain: labeling of the large polypeptide and of a proteolipid component of the Na, K-ATPase. Biochemistry. 1978;17:3667–76. doi: 10.1021/bi00610a037. [DOI] [PubMed] [Google Scholar]
- 29.Lingrel JB, Orlowski J, Price EM, Pathak BG. Regulation of the alpha-subunit genes of the Na,K-ATPase and determinants of cardiac glycoside sensitivity. Soc Gen Physiol Ser. 1991;46:1–16. [PubMed] [Google Scholar]
- 30.Middleton DA, Rankin S, Esmann M, Watts A. Structural insights into the binding of cardiac glycosides to the digitalis receptor revealed by solid-state NMR. Proc Natl Acad Sci USA. 2000;97:13602–7. doi: 10.1073/pnas.250471997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa H, Shinoda T, Cornelius F, Toyoshima C. Crystal structure of the sodium-potassium pump (Na+,K+-ATPase) with bound potassium and ouabain. Proc Natl Acad Sci USA. 2009;106:13742–7. doi: 10.1073/pnas.0907054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price EM, Rice DA, Lingrel JB. Site-directed mutagenesis of a conserved, extracellular aspartic acid residue affects the ouabain sensitivity of sheep Na,K-ATPase. J Biol Chem. 1989;264:21902–6. [PubMed] [Google Scholar]
- 33.Qiu LY, Krieger E, Schaftenaar G, Swarts HG, Willems PH, De Pont JJ, Koenderink JB. Reconstruction of the complete ouabain-binding pocket of Na,K-ATPase in gastric H,K-ATPase by substitution of only seven amino acids. J Biol Chem. 2005;280:32349–55. doi: 10.1074/jbc.M505168200. [DOI] [PubMed] [Google Scholar]
- 34.Sandtner W, Egwolf B, Khalili-Araghi F, Sanchez-Rodriguez JE, Roux B, Bezanilla F, Holmgren M. Ouabain binding site in a functioning Na+/K+ ATPase. J Biol Chem. 2011;286:38177–83. doi: 10.1074/jbc.M111.267682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stürmer W, Apell HJ. Fluorescence study on cardiac glycoside binding to the Na,K-pump. Ouabain binding is associated with movement of electrical charge. FEBS Lett. 1992;300:1–4. doi: 10.1016/0014-5793(92)80151-6. [DOI] [PubMed] [Google Scholar]
- 36.Yatime L, Laursen M, Morth JP, Esmann M, Nissen P, Fedosova NU. Structural insights into the high affinity binding of cardiotonic steroids to the Na+,K+-ATPase. J Struct Biol. 2011;174:296–306. doi: 10.1016/j.jsb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Laursen M, Yatime L, Nissen P, Fedosova NU. Crystal structure of the high-affinity Na+K+-ATPase-ouabain complex with Mg2+ bound in the cation binding site. Proc Natl Acad Sci USA. 2013;110:10958–63. doi: 10.1073/pnas.1222308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanai R, Ogawa H, Vilsen B, Cornelius F, Toyoshima C. Crystal structure of a Na+-bound Na+,K+-ATPase preceding the E1P state. Nature. 2013;502:201–6. doi: 10.1038/nature12578. [DOI] [PubMed] [Google Scholar]
- 39.Olesen C, Picard M, Winther AM, Gyrup C, Morth JP, Oxvig C, Moller JV, Nissen P. The structural basis of calcium transport by the calcium pump. Nature. 2007;450:1036–42. doi: 10.1038/nature06418. [DOI] [PubMed] [Google Scholar]
- 40.Cornelius F, Mahmmoud YA. Interaction between cardiotonic steroids and Na,K-ATPase. Effects of pH and ouabain-induced changes in enzyme conformation. Biochemistry. 2009;48:10056–65. doi: 10.1021/bi901212r. [DOI] [PubMed] [Google Scholar]
- 41.Nitz M, Sherawat M, Franz KJ, Peisach E, Allen KN, Imperiali B. Structural origin of the high affinity of a chemically evolved lanthanide-binding peptide. Angew Chem Int Ed Engl. 2004;43:3682–5. doi: 10.1002/anie.200460028. [DOI] [PubMed] [Google Scholar]
- 42.Colina C, Rosenthal JJ, DeGiorgis JA, Srikumar D, Iruku N, Holmgren M. Structural basis of Na+/K+-ATPase adaptation to marine environments. Nat Struct Mol Biol. 2007;14:427–31. doi: 10.1038/nsmb1237. [DOI] [PubMed] [Google Scholar]
- 43.Selvin PR. Principles and biophysical applications of lanthanide-based probes. Annu Rev Biophys Biomol Struct. 2002;31:275–302. doi: 10.1146/annurev.biophys.31.101101.140927. [DOI] [PubMed] [Google Scholar]
- 44.Sandtner W, Bezanilla F, Correa AM. In vivo measurement of intramolecular distances using genetically encoded reporters. Biophys J. 2007;93:L45–7. doi: 10.1529/biophysj.107.119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marti-Renom MA, Stuart AC, Fiser A, Sanchez R, Melo F, Sali A. Comparative protein structure modeling of genes and genomes. Annu Rev Biophys Biomol Struct. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- 46.Huang L, Roux B. Automated Force Field Parameterization for Non-Polarizable and Polarizable Atomic Models Based on Target Data. J Chem Theo Comp. 2013;9 doi: 10.1021/ct4003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacKerell AD, Jr, Bashford D, Bellot M, Dunbrack RL, Jr, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.