Abstract
Pancreatic cancer, often considered a metastatic disease at the time of clinical diagnosis due to lack of any reliable early diagnostic marker(s), is refractory to conventional chemo- and radiotherapy and has a dismal 5-year survival rate of only 6%. Although surgical removal of the primary tumor is considered to be curative, the 5-year survival rate is no more than 20% even in patients with clear resection margins (R0). The recurrence of local and metastatic disease (primarily liver metastasis) post resection is considered to be the leading cause of mortality in these patients. In addition, instances of metastatic disease without any local recurrence post resection have also been observed. Cancer metastasis is the primary cause of mortality in cancer patients and is classically viewed as a late event during the progression of the disease, which is supported by the genetic studies used to understand the evolution of pancreatic cancer. However, this view has recently been challenged by studies using mathematical modeling and genetically labeled mouse models of pancreatic cancer to understand the dynamics of tumor cell dissemination and epithelial to mesenchymal transition (EMT) of tumor cells well before the primary tumor is formed. Given that EMT is a hallmark process that initiates the metastatic seeding of cancer cells and the dismal prognosis of pancreatic cancer patients even after efficient removal of the primary tumor (99.9%), an early dissemination hypothesis of cancer cells cannot be undermined. In this review, we will discuss the current views regarding pancreatic cancer metastasis with particular emphasis on the epithelial to mesenchymal transition, its influence on the selection of patients for surgical resection and the therapeutic intervention.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC), the fourth leading cause of cancer-associated deaths in the United States, confers a near 100% mortality with a dismal 5-year survival of only 6% and a median survival time of 5–8 months [1]. Of all patients, only 10–15% are eligible for surgical resection, ~ 40% are diagnosed with locally advanced unresectable disease and ~ 45% with metastatic disease [2, 3]. While surgical resection offers the best prognosis compared to chemo/radiotherapy for locally advanced and metastatic diseases, survival of patients who undergo surgical resection depends on factors such as margin of resection (R0 vs. R1 vs. R2), size and stage of the primary tumor, involvement of lymph node, etc. [2–5]. Surgical resection without any margin (R0, curative intent) offers a median survival time of 22 months, 11–14 months with positive margins (R1/R2, palliative intent), 6–11 months for patients with locally advanced disease and 3–6 months for the metastatic disease [2]. Although surgical resection is believed to be curative, the 5-year survival is still in the ballpark of 15–20% and even complete removal of the primary tumor (99.9%) without any systemic therapy does not have significant effect on the survival [5, 6]. Therefore, use of adjuvant and neo-adjuvant chemotherapy following resection of pancreatic tumor provides better survival benefits compared to those without chemotherapy [6, 7]. Such dismal survival benefits after curative surgery are due to local recurrence of pancreatic tumor, metastasis to the liver or both [7–10]. Although liver metastasis is the primary site of recurrence, peripancreatic or retroperitoneal recurrence, peritoneal metastasis and metastases to other distant sites is also observed following surgical resection [11].
Cancer metastasis, defined as the focal growth of tumor cells (same as the primary tumor type) at site(s) anatomically distinct from the primary tumor site [12], is responsible for approximately 90% of cancer-associated deaths [13, 14]. Therefore, our understanding of the basic processes involved in establishing the metastatic disease is critical in dictating how we approach the disease in the clinic. Primarily two models have been proposed for the establishment of the metastatic disease [13, 15]: (i) the classical late dissemination model (also known as linear progression model), which posits that metastasis is the last step in the evolution of the primary tumor in which the tumor cells have undergone repeated fitness testing, acquiring multiple mutations, enabling them to survive in an otherwise harsh environment (secondary site), (ii) the early dissemination model (also known as parallel progression model), on the other hand, suggests early departure of tumor cells from the primary site even before a critical mass of the primary tumor is achieved. Although both the models have recently been reviewed in the context of breast cancer metastasis [13], recent studies using high-resolution genomic sequencing [16, 17], mathematical modeling [6] and faithfully recapitulated mouse models [15] have argued both in favor and against the two metastatic models in pancreatic cancer. An inclination towards one of the models will significantly alter our outlook towards the disease particularly with respect to the diagnosis and therapy. Therefore, extreme caution should be exercised, and further in-depth studies should be carried out to decide which side tips the balance.
Epithelial to mesenchymal transition (EMT) is a developmental program that was initially identified during embryogenesis and has recently been implicated in the metastatic dissemination of cancer cells, where the polarized epithelial cells lose their junctional complexes responsible for establishing contacts between them and acquire a non-polarized motile phenotype losing contact with the neighboring cells that help them to invade the extracellular matrix [18, 19]. Although the phenomenon of EMT has been demonstrated in vitro, its existence and role in metastasis in vivo has remained elusive, partly due to (i) the inability to capture the quasi-epithelial and/or quasi-mesenchymal state(s) that exist between the epithelial and the mesenchymal phenotype using the EMT markers, (ii) inability to distinguish between the mesenchymal cells (EMTed) and mesenchymal nature of the stromal cells around the epithelial cells and (iii) the apparent epithelial nature of the tumor cells at the metastatic site [18–21]. However, a recent study using lineage tracing in the genetically engineered mouse model of pancreatic cancer demonstrated the in vivo existence of mesenchymal state that had transitioned from the epithelial cells (rules out the concerns of stromal cells being misjudged to be the EMTed mesenchymal type) [15]. Findings from this study and a mathematical model for understanding the dynamics of pancreatic cancer metastasis developed by Haeno et al., [6] using the patient data sets have challenged the classical late dissemination model of pancreatic cancer metastasis. In this review, we discuss the process of EMT and the early and late dissemination models of pancreatic cancer metastasis and its potential implication on the clinical practice.
PLASTICITY OF EPITHELIAL TO MESENCHYMAL TRANSITION AND METASTASIS
The metastatic process during tumorigenesis is a complex and multistep process by which the tumor cells from the primary location travel to distant site(s) for colonization and subsequent growth. This is primarily achieved in five distinct steps, each step requiring impeccable coordination with the others and the system under consideration [18, 19]. The first step in this process is invasion; where the otherwise polar (apical-basal polarity) and non-motile epithelial cells become motile by losing the junctional protein complexes (particularly adherens junction protein, E-cadherin) thereby losing contact with the neighboring cells and acquiring a front-back end polarity that helps in invading the extracellular matrix (ECM) and enable them to move beyond their own niche [18, 19]. Since this process of acquisition of motile phenotype by changing the overall structural framework of the cells closely resembles that of cellular movements observed during implantation and embryonic development (type-I EMT) and inflammation and fibrosis during wound healing (type-II EMT), this has been termed as the epithelial to mesenchymal transition (EMT) during tumorigenesis and metastasis (type-III EMT) [22]. Although the type-I and type-II EMTs have been well documented and validated, the in vivo existence and significance of type-III EMT is still being challenged [18–20, 22]. In spite of the doubts, EMT is still considered to be critical in initiating the process of metastasis in epithelial malignancies. The second step in the process of metastasis is extravasation; where the tumor cells that have escaped from the primary site enter the blood and lymphatic vessels in the adjoining areas making their way through the endothelial cells lining these vessels into the systemic circulation. The next step in this process is a daunting one, where the majority of the tumor cells find it extremely challenging to survive the insults in the systemic circulation, termed as systemic transport. The fourth step in this series is extravasation, in which the tumor cells that have managed to survive in the systemic circulation make their way out of the blood vessels through the endothelial cells of the capillaries into the parenchyma of distant tissue site(s). The final step in the process of metastasis is the colonization at the distant site termed as metastatic colonization, an extremely inefficient and critical step (rate limiting) where the tumor cells that have successfully completed the rigors of the previous steps prepare for the final hurdle to survive the microenvironment of the distant organ. Once they have succeeded, these cells (presumably mesenchymal type) need to reinitiate their colonizing ability (by converting to epithelial type) by a process termed as mesenchymal to epithelial transition (MET) that will lead to the formation of micro followed by macrometastasis and then the life-threatening metastatic disease. Although metastasis is considered to be a highly inefficient process resulting in only successful colonization of 0.01% of tumor cells released into the circulation, metastasis is the major cause of cancer-associated deaths [14, 15, 18–20, 22].
Considering the dynamic and reversible nature of the epithelial to mesenchymal transition (EMT-MET) that fits the scheme of conceptual understanding of metastasis, it ought to be fine-tuned and precisely regulated. The regulators of this plastic process, broadly classified into EMT-effectors, EMT-core regulators and EMT-inducers are not in the scope of this review and have been reviewed elsewhere [19, 23]. Of all the steps of the metastatic cascade, the mesenchymal state has been proposed to be advantageous during intravasation [24, 25], systemic transport [15, 26, 27] and extravasation [28, 29] without the need for actively switching on or off the transition program. However, during the process of invasion (first step) and metastatic colonization (last step), an active transition program needs to be initiated from epithelial to mesenchymal (EMT) at the primary site and mesenchymal to epithelial (MET) at the metastatic site respectively. Besides, cells that have undergone EMT have been proposed to harbor traits of cancer stem cells and are also implicated in drug resistance [19]. Although the concept of EMT/MET in tumorigenesis has drawn a lot of criticism due to apparent lack of evidence in an in vivo setting, lack of specific marker(s), the potential of confusing the mesenchymal cells with fibroblasts-like cells, and the epithelial morphology of the metastatic tumor which once was believed to be mesenchymal during the transit from the primary tumor, etc. [19, 20, 22, 30], recent lineage tracing experiments using genetically engineered mouse models strongly argues in favor of the existence of such cells in vivo [15]. However, further in-depth studies using high-resolution intravital microscopy [31, 32] and genetic labeling studies [15] would be informative in this regard. Nonetheless, the concept of EMT has resulted in a paradigm shift in our understanding of the cancer metastasis. Therefore, a thorough understanding of the involvement of the EMT/MET in regulating various steps of the metastatic cascade is critical in designing effective therapeutic strategy for the metastatic disease.
EPITHELIAL TO MESENCHYMAL TRANSITION AND PANCREATIC CANCER METASTASIS
Pancreatic cancer is primarily considered to be a metastatic disease because only 10–15% patients present themselves with the resectable disease with another 85–90% as locally advanced (potentially positive for micrometastasis) and metastatic (gross metastatic lesions) [1–3]. As mentioned previously, EMT is considered to be the initiating and critical step in the invasion-metastasis cascade. However, studies carried out to understand the relevance of EMT in pancreatic cancer remain inconclusive using various markers of EMT in human pancreatic cancer tissue samples [33], possibly due to the inability to capture the plasticity of the transition process by a static snapshot using immunohistochemical analyses.
Although pancreatic cancer does not display a very long latency for the development of metastatic disease unlike breast cancer and is primarily considered to be a metastatic disease when presented in the clinic, this is one of the few tumor types where the premalignant lesions are very well defined and characterized in patient samples, as well as genetically engineered mouse models. A model system (first proposed by Hruban et al., [34, 35] and subsequently validated [36, 37]) has emerged in recent years, which identifies the premalignant lesions (carcinoma in situ) that are considered precursors of invasive adenocarcinoma. These lesions termed pancreatic intraepithelial neoplasia (PanINs) are defined as microscopic, non-invasive epithelial neoplasm of the pancreatic duct system [36]. PanINs can be either papillary or flat, and are composed of columnar to cuboidal cells with varying amounts of mucin. PanINs are classified according to a four-tier classification system, PanIN-1A, PanIN-1B (low-grade PanINs), PanIN-2 (intermediate grade Pan-INs), and PanIN-3 (high-grade PanIN), reflecting a progressive increase in histologic grade culminating ultimately in invasive neoplasia. The lowest grade PanIN lesions are either flat (1A) or papillary (1B) and are characterized by the absence of nuclear atypia and retain nuclear polarity. PanIN-2 lesions are architecturally slightly more complex than PanIN-1 and have more nuclear changes, including loss of nuclear polarity, nuclear crowding, nuclear size variation (pleomorphism), nuclear hyperchromasia, and pseudostratification. However, in contrast to carcinoma, mitoses are rarely seen. In contrast, PanIN-3 lesions are referred to as “carcinoma-in-situ,” owing to the presence of loss of apical polarity, increased nuclear atypia, and frequent mitoses. However, being a pre-invasive lesion, PanIN-3 is still contained within the basement membrane [34–37] (Fig. 1). The development of pancreatic ductal adenocarcinoma (PDAC) from the non-neoplastic pancreatic epithelial cells involves a series of well-defined genetic alterations [38] primarily in K-RAS (~ 90–95%) [39], p16INK4A (~ 90%) [40, 41], SMAD4 (~ 55%) [42] and p53 (~ 50–75%) [43]. Since K-RAS is the most prevalent genetic mutation (K-RASG12D) observed in pancreatic cancer patients, Hingorani et al., [44] generated a genetically engineered mouse model where the pancreas specific expression of K-rasG12D from its endogenous promoter (Pdx1-Cre; LSL-KrasG12D, also known as KC model) results in the formation of preinvasive PanINs and invasive pancreatic cancer (long latency and incomplete penetrance) that faithfully recapitulates the human disease.
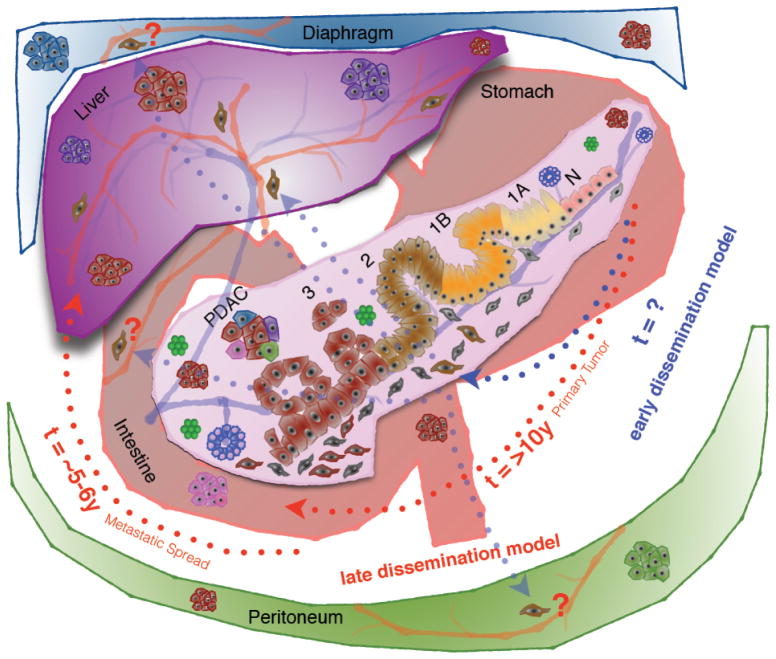
Fig. 1. Late dissemination (linear progression) and early dissemination (parallel progression) model of pancreatic cancer metastasis.
Pancreatic cancer progression model is depicted inside the pancreas. Normal pancreatic epithelial cells (N) by acquiring several genetic alterations (primarily K-RAS, p16INK4A, SMAD4 and p53) progress through a step-wise histological abnormality characterized by columnar epithelial cells with a gradual increase in nuclear atypia, loss of polarity, nuclear crowding and hyperchromasia and pseudostratification known as pancreatic intra epithelial neoplasia (PanIN-1A, 1B, 2 & 3) and finally culminating in pancreatic ductal adenocarcinoma (PDAC).
Late dissemination model: Proponents of the late dissemination (also known as linear progression) model suggest it takes >10 years for the non-metastatic primary tumor to be formed from the time of the initiating mutation followed by another 5–6 years for the development of metastatic subclones (red dotted arrows). According to this model, pancreatic cancer metastasis is a late event, and this presents with a window of opportunity of more than 10 years to catch the disease early. Once the metastatic subclones are developed, it takes another 2–3 years to develop the metastatic disease, which ultimately kills the patient.
Early dissemination model: On the other hand, proponents of early dissemination model (also known as parallel progression model) suggest tumor cells as early as PanIN-2 lesions (brown colored cells) activate the epithelial to mesenchymal transition (EMT) program, invade the basement membrane, enter the systemic circulation and seed at anatomically distant organ site(s) (blue dotted arrows). Although these solitary cells have been seen only in the liver, they could be present any organ (Lungs, Peritoneum, Diaphragm etc.) where pancreatic cancer metastasizes. At this point, experimental validation is lacking for the cells that disseminated from early precancerous state for metastatic colonization. Besides, the kinetics of this dissemination is also unknown.
Using variants of the aforementioned mouse model (Pdx1-Cre; LSL-KrasG12D; p53fl/+; Rosa-LSL-YFP also known as PKCY and Pdx1-Cre; LSL-KrasG12D; p16/p19fl/+; Rosa-LSL-YFP also known as IKCY) where only the pancreatic cells of epithelial origin were genetically labeled with yellow fluorescent protein (YFP), Rhim et al., [15] have demonstrated the in vivo existence of mesenchymal cells i.e. cells that have successfully undergone epithelial to mesenchymal transition (EMTed) and, therefore, express both YFP (genetic trace for epithelial cells) and mesenchymal marker such as Zeb1 or Fsp1. While the proportions of the cells that have undergone EMT (YFP+ and Zeb1+) were much higher (42%) in PKCY mice that had developed PDAC, mice with preinvasive lesions i.e. as early as PanIN-2 and PanIN-3 also displayed characteristic mesenchymal phenotype (YFP+ and Zeb1+), though with much lower frequency. To further validate their mesenchymal nature, sorted YFP+ cells were demonstrated to have activated the transcriptional program to induce EMT by up regulating mesenchymal markers such as Zeb1, Fsp1 and N-cadherin. Since invasion into the basement membrane constitutes the first step in the metastatic cascade, examination of the surrounding stroma for the presence of YFP+ cells with mesenchymal phenotype (Zeb1+ and the fibroblastic appearance) demonstrated that these cells have not only activated the EMT program, but have also initiated the first prerequisite step of the metastatic cascade. Next, the second and third steps of the metastatic cascade i.e. intravasation and systemic transport were also evident as YFP+ cells were detected in the blood circulation of mice bearing PDAC as well as PanIN lesions and a large fraction of the tumor cells maintained the mesenchymal phenotype in the circulation. Although distant metastases were evident in the tumor (PDAC) bearing mice (i.e. demonstrated the ability for extravasation and metastatic colonization), solitary YFP+ tumor cells were observed near the blood vessels of the liver in mice bearing PanIN lesions (indicating extravasation) without any evident macro- or micro-metastases (suggesting their inability for metastatic colonization). This reiterates the fact that among all the steps required for metastatic spread of the disease, metastatic colonization is indeed the rate-limiting step. Further, the YFP+ cells isolated from the circulation (CPCs) of PanIN and PDAC mice exhibited increased expression of cancer stem cell markers CD24+CD44+ (possess tumor initiating capabilities) and enhanced survival and self-renewal properties in vitro (pancreatosphere formation assay) compared to the YFP+ cells isolated from the pancreatic tumor of the same mice. This demonstrates that the circulatory pancreatic tumor cells (CPCs) isolated from the preneoplastic (PanIN) state of the disease possess increased survival and self-renewal properties that is indistinguishable from the CPCs isolated from mice with full blown PDAC. However, whether the CPCs isolated from the PanIN mice can form metastatic tumors in vivo, remains to be established. In an effort to demonstrate the tumor-initiating properties of the EMTed cells in vivo, YFP+E-cad− (mesenchymal cells) and YFP+E-cad+ (epithelial cells) isolated from the primary tumor of mice bearing PDAC or PanIN lesions were orthotopically implanted into the pancreas of NOD/SCID mice. While the cells from the PDAC mice developed large tumors and local and distant metastasis irrespective of the E-cad status by 3 weeks of implantation, YFP+E-cad− cells from PanIN mice led to pancreatic formation in approximately 67% of mice opposed to none (0%) in case of YFP+E-cad+ cells from PanIN mice after 2 months of implantation. This demonstrates the cells from the PanIN mice that have undergone EMT possess increased potential to initiate tumorigenesis than those that have not undergone EMT.
This is a landmark study demonstrating EMT in pancreatic cancer in two ways. First, while there are conflicting reports of EMT in pancreatic cancer in literature, it demonstrated the existence of EMT in vivo using a sophisticated lineage-tracing model. In addition, all the steps of the metastatic cascade were elegantly demonstrated along with the EMT-MET plasticity and establishment of the metastatic disease. Second and more importantly, it provides experimental evidence for the concept of early dissemination (parallel progression) model of cancer metastasis, which will have significant impact the way we should address the lethal disease such as pancreatic cancer. Although this study has unequivocally established the importance of EMT-MET plasticity in the metastatic disease, questions still remain (i) Are the cells disseminated early on capable of establishing the metastatic disease? (ii) Are these solitary cells observed in organs (lungs and peritoneum, other common sites of pancreatic cancer metastasis) other than the liver (Figure)? Total pancreatectomy of 8–10 week old genetically labeled PKCY mice (PanIN stage), when there are cells that have already disseminated and seen at a distant site (i.e. liver) could provide an interesting insight into the ability of these cells in establishing the final step of the metastatic cascade i.e. metastatic colonization without further dissemination from the primary tumor.
LATE DISSEMINATION (LINEAR PROGRESSION) VS. EARLY DISSEMINATION (PARALLEL PROGRESSION) MODEL OF PANCREATIC CANCER METASTASIS
Metastasis is the primary cause of cancer-associated deaths accounting for approximately 90% of cancer related mortality [45]. Therefore, our understanding of the process of metastasis is critical for devising any treatment modalities for cancer patients, be it surgical resection, chemotherapy or radiotherapy either alone or in combination. Primarily two fundamental models of metastasis have been proposed to explain the clinical findings in breast cancer [46], which are also supported by the recent studies in pancreatic cancer [6, 15–17]. In this section, we briefly describe each model and evaluate them in the light of recent studies in pancreatic cancer. The merits and demerits of each model have been extensively reviewed elsewhere [45, 46], which is beyond the scope of this review.
Late Dissemination (Linear Progression) Model
This is also known as the classical model that proposes that the development of metastasis is an evolutionary process similar to Darwinian selection where the tumor cells undergo successive rounds of mutations and selection at the primary site before being deemed fit to be disseminated for metastatic colonization. According to this model, the best-fit cells that possess the ability to grow autonomously at a competitive rate would further be selected and expanded into metastatic subclones within the primary tumor and, therefore, the landscapes of genetic alterations in the primary tumor and metastatic subclones is thought to similar. Since it is time consuming for the metastatic clones to develop within the primary tumor, metastasis is considered to be a very late event in this evolutionary process and the dissemination efficiency is proportional to the primary tumor size [15, 45, 46].
Recent high-resolution genetic studies using low-passage cell lines and matched primary and metastatic pancreatic tumors demonstrate that most of the genetic alterations observed in metastatic tumors are also present in their corresponding primary tumor suggesting that the metastasis is a late event during the evolution of pancreatic cancer [16, 17]. Using the high-throughput sequencing information Yachida et al., [17] proposed that it takes approximately >10 years from the time of initiating mutation to the emergence of a non-metastatic parental clone required for the formation of pancreatic tumor. Further, another 5–6 years will be required to for the establishment of the metastatic subclones that will initiate the metastatic seeding followed by another 2–3 years for the manifestation of the metastatic disease and subsequent death of pancreatic cancer patients. This time scale fits well with the late dissemination model of metastasis (Figure). However, this model does not explain all the clinical observations seen in pancreatic cancer patients. As mentioned earlier, even with curative surgical resection of primary pancreatic tumor with no margin (R0) and no evidence of metastasis at resection, 75% of the patients die of metastatic disease within 5-years post resection [7]. The primary reason for death of patients who have undergone surgical resection is believed to be local recurrence and/or hepatic metastasis. A study carried out by Westerdahl J et al., [8] demonstrated that of 74 patients who had undergone either total or sub-total pancreatectomy, six patients had only local recurrence without liver metastasis, ten had liver metastasis without any sign of local recurrence and 58 patients had both local recurrence and liver metastasis. Altogether 64 patients developed local recurrence and 68 developed hepatic metastases. The late dissemination model does not explain the findings of hepatic metastasis without any local recurrence of the primary tumor. Finally, the evidence of metastatic pancreatic cancer in patients who underwent pancreatectomy for chronic pancreatitis argues against the late dissemination model of pancreatic cancer metastasis [47].
Early Dissemination (Parallel Progression) Model
Due to the inability of the late dissemination model to explain all the clinical observations in multiple tumor types, an alternative early dissemination model has attracted interest from the scientific community recently, although it dates back to the 1950s [13–15]. This model proposes early dissemination of tumor cells in the preneoplastic stage even before the primary tumor is formed. According to the model, since the primary tumor and the metastatic tumor will be developed in parallel (i) the genetic and epigenetic alterations are likely to be different between primary and the metastatic tumors, (ii) the disseminated cells might undergo genetic diversification and clonal expansion in the metastatic niche itself which might prove to be advantageous than the ones developed at the primary site and (iii) metastasis to multiple organ sites might develop simultaneously. However, this does not contradict the clonal selection and competitive fitness of tumor cells during the development of the primary tumor [13, 14].
Evidence in pancreatic cancer that argue in favor of this model stems from the clinical observations mentioned earlier that were not explained by the linear progression model [7, 8, 47]. In addition, a recent mathematical modeling study [6] using the pathological (autopsy cohort) and chemo/radiological (adjuvant cohort) data from pancreatic cancer patient samples predicted the existence of cells that are capable of establishing metastasis (not necessarily metastatic disease) even when the size of the primary tumor is small. Another line of direct evidence of early dissemination comes from a study by Rhim et al., [15] using lineage tracing in a genetically engineered mouse model (described in the previous section). With respect to early dissemination model, the tumor cells from PanIN-2 and 3 precursor lesions were shown to undergo EMT, cross the basement membrane and enter systemic circulation, harbor the self-renewal and tumor properties of cancer stem cells, extravasate and seed in the liver near to the blood vessels (Figure). Though this study did not address whether these cells were capable of forming metastatic disease or not, it elegantly demonstrates support for the early dissemination model of pancreatic cancer metastasis.
There are two major conceptual challenges for this model. The first one comes from the rate-limiting step of the metastatic process i.e. metastatic colonization. As mentioned earlier, metastasis is a highly inefficient process with < 0.01% of the cells entering the systemic circulation capable of successful colonization at a distant site. Since these cells depart the primary tumor site early, they may not be fully equipped with the requisite genetic and epigenetic alterations to colonize a distant site when fully equipped neoplastic cells struggle to survive. Second, several studies have shown that gene expression and mutational signature (molecular alterations) of primary and matched metastatic sites are quite similar [16, 17, 48–50] (supporting linear progression model), which does not fit the basic presumptions of parallel progression model. However, there are studies that have demonstrated molecular disparity between primary and disseminated tumor cells (DTCs) or matched metastatic tumors arguing in support of early dissemination [51–55]. While most of the above-mentioned studies were carried out in breast, colorectal, melanoma and renal cell carcinoma, there is a single report in pancreatic cancer where single molecule RNA-sequencing of circulatory tumor cells (CTCs) from genetically engineered mouse models of pancreatic cancer. In this study, an enrichment of non-canonical WNT pathway gene Wnt2 was observed in the CTCs, which was also found in the metastatic cells in the mouse ascites [56]. However, expression of Wnt2 was observed in only very small localized cluster of cells suggesting the close relatedness of the CTCs with the metastatic site than the primary site. WNT2 expression was also confirmed in the CTCs isolated from PC patients [56]. Although, this study provides some insight into the relatedness of CTCs with the metastatic cells from the ascites in a mouse model, further systematic analysis of the broad expression and mutational landscape of CTCs with the primary and metastatic PC tissues from patients will be more informative with respect to kinetics of metastasis.
It is tempting to speculate explanations for the aforementioned conceptual challenges of the early dissemination model in the light of tumor self-seeding hypothesis. First, according to self-seeding model, metastatic tumor cells are capable of infiltrating their primary tumor of origin and promote their growth [57, 58]. Although the concept of self-seeding has not been applied to cancer progression and metastasis, one could argue that the cancer cells disseminated very early on could return to their home (primary tumor) to acquire further genetic and epigenetic alterations as they are already primed for metastasis (i.e. already had exposure to the metastatic niche). It could be possible that after few rounds of seeding and reseeding, these cells might undergo clonal expansion either at the primary or the metastatic site(s) and could be considered more adaptable (sinister) compared to the cells that have been undergoing successive rounds of mutations and selections only at the primary site without being exposed to the metastatic site at all. However, this may not be an absolute requirement as instances of metastatic recurrences have been observed even after complete resection of the primary tumor (self-seeding can’t take place in the absence of a tangible primary tumor). Second, the similarity in the molecular alterations observed between the primary and matched metastatic sites could have been due to infiltration of the metastatic clones into the primary site either to augment the growth of the primary tumor or to hone their capability of metastatic colonization [14, 57, 58]. However, these ideas need to be experimentally validated.
CLINICAL IMPLICATIONS
Since metastasis contributes to 90% of cancer related mortality [14], understanding the true nature of the metastatic dissemination will significantly impact our outlook towards pancreatic cancer therapy. According to late dissemination model, we have a window of opportunity of >10 years to intervene before the metastatic disease is formed. However, the clinical data where patients have undergone curative surgery with a clear margin of resection (R0) still die of the disease soon after, contradicts the model. On the other hand, use of adjuvant chemo- or radiation therapy following surgery has shown to prolong the survival of patients compared to those with surgery alone [59]. Further, use of neo-adjuvant therapy has shown better survival compared to adjuvant therapy following surgery [60] suggesting it is critical to reduce both the primary and metastatic tumor burden than to remove the primary tumor only. Both these studies speak in favor of the early dissemination model under the assumption that patients with primary pancreatic tumor might harbor the metastatic disease (proof of concept), therefore, the neo-adjuvant and adjuvant therapy have proved to be useful. A simultaneous attack at the primary (by curative resection) and metastatic site(s) (by chemotherapy) would be a better strategy than surgery alone in prolonging the survival of patients eligible for resection. Therefore, clinical trials with prior neo-adjuvant and anti-metastatic therapies should accompany the curative resection to further evaluate the concept of early dissemination [38].
Chronic pancreatitis is one of the major risk factors for pancreatic cancer and particularly patients with hereditary and tropical pancreatitis are approximately at 50-fold increased risk of developing pancreatic cancer compared to the general population [61, 62]. Further support stems from experimental inflammation induced by cholecystokinin analog cerulein that has been shown to hasten the oncogenic effects of K-ras using genetically engineered mouse model of pancreatic cancer [15, 63]. Further, use of the anti-inflammatory agent dexamethasone resulted in a significant reduction in the PanIN lesions and circulatory YFP+ cells in the mouse model, suggesting the importance of inflammatory reaction in maintaining the premalignant lesions and early dissemination [15]. A recent study [64] assessed the effect of the long-term use of non-steroidal anti-inflammatory drug aspirin on the risk of cancer related deaths demonstrating its beneficial effect in prolonging the life of cancer patients. Therefore, use of anti-inflammatory agents could act as a prophylactic measure in high-risk patients with pancreatitis, a concept that emerges from the early dissemination theory [15]. Although further in-depth studies in the basic science as well as translational science laboratories would be critical in evaluating these models of metastatic dissemination, our belief in the late dissemination model might prevent us from taking preemptive measures against this lethal disease i.e. pancreatic cancer.
CONCLUSIONS AND PERSPECTIVES
Based on the clinical findings in pancreatic cancer patients, mathematical modeling using patient data sets and use of lineage tracing in genetically engineered mouse models of pancreatic cancer [6–8, 15, 47], we believe that the balance is tipped in favor of the early dissemination theory of pancreatic cancer metastasis. In addition, studies using neo-adjuvant and adjuvant therapy to bolster the benefits of resection and long-term benefits of anti-inflammatory drugs in prolonging the survival and reducing the cancer related deaths respectively in pancreatic cancer patients are some of the proof of concept studies that argue in favor of this model [59, 60, 64]. Monitoring the primary tumor for therapeutic response and the belief in a larger window of opportunity to intervene before the metastatic disease is formed, as believed in the late dissemination model, are probably a few reasons for the dismal therapeutic progress in the recent years [13, 17]. On the other hand, the early dissemination model reposes faith in catching the metastatic disease early with less time for effective intervention. This could be achieved by devising and fine-tuning ways to detect the disease early (using biomarkers) or the disseminated cells (DTCs or CTCs) bearing characteristics of cancer stem cells in the circulation and to look for these cells in unconventional places and identification of new anti-metastasis therapies along with the conventional chemotherapy [13]. The paucity of samples from patients at an early stage of the disease thwarts efforts to identify early diagnostic markers, which ironically, exacerbates the low rate of early diagnosis. A recent study by Huch et al., [65] demonstrated the potential of long term organoid culture of normal pancreatic ductal cells using a 3D culture system, suggesting the same can be applied towards the ducts from preinvasive (PanINs) as well as invasive stages. If successful, comparative molecular characterization of their genomic, proteomic (intracellular, transmembrane and secretory) and glycomic profiles would result in plethora of useful information, which can then be used for validation of biomarker(s) as well as novel therapeutics. In the same line of thoughts, Kim et al., [66] generated a induced pluripotent stem cell (iPSCs) line using the tumor epithelial cells from a patient and were able to differentiate them back to pancreatic tissue resulting in the formation of precursor lesions (PanINs) in a xenograft model. These studies open up entirely new avenues of investigation for studying the early stages of human pancreatic cancer and might prove extremely beneficial in identification of novel biomarker(s) for early diagnosis, pathways for therapeutic targeting etc. Once mastered, these organoid cultures can also be used for the preclinical drug evaluation for personalized cancer therapy. Therefore, harnessing the potential of unprecedented advances in technology (such as high-resolution microscopy, genomic sequencing technologies, proteomics, organoid culture to capture the early stages of human disease etc.), as well as murine models (compound models with lineage tracing capabilities), is expected to pay dividends in the future.
Acknowledgments
The authors on this work, in part, are supported by grants from the Department of Defense BC101014 and NIH (TMEN U54 CA163120, EDRN UO1, CA111294, SPORE P50 CA127297, and RO3 CA167342).
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg. 2013;257:731–6. doi: 10.1097/SLA.0b013e318263da2f. [DOI] [PubMed] [Google Scholar]
- 3.Richter A, Niedergethmann M, Sturm JW, et al. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–9. doi: 10.1007/s00268-002-6659-z. [DOI] [PubMed] [Google Scholar]
- 4.Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Torre M, Nigri G, Ferrari L, et al. Hospital volume, margin status, and long-term survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2012;78:225–9. [PubMed] [Google Scholar]
- 6.Haeno H, Gonen M, Davis MB, et al. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148:362–75. doi: 10.1016/j.cell.2011.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 8.Westerdahl J, Andren-Sandberg A. Ihse I Recurrence of exocrine pancreatic cancer--local or hepatic? Hepatogastroenterology. 1993;40:384–7. [PubMed] [Google Scholar]
- 9.Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21:195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- 10.Hishinuma S, Ogata Y, Tomikawa M, et al. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006;10:511–8. doi: 10.1016/j.gassur.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Paik KY, Choi SH, Heo JS, Choi DW. Analysis of liver metastasis after resection for pancreatic ductal adenocarcinoma. World J Gastrointest Oncol. 2012;4:109–14. doi: 10.4251/wjgo.v4.i5.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida BA, Sokoloff MM, Welch DR, Rinker-Schaeffer CW. Metastasis-suppressor genes: a review and perspective on an emerging field. J Natl Cancer Inst. 2000;92:1717–30. doi: 10.1093/jnci/92.21.1717. [DOI] [PubMed] [Google Scholar]
- 13.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–12. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 14.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–13. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–29. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng F, Wu G. The rejuvenated scenario of epithelial-mesenchymal transition (EMT) and cancer metastasis. Cancer Metastasis Rev. 2012;31:455–67. doi: 10.1007/s10555-012-9379-3. [DOI] [PubMed] [Google Scholar]
- 21.Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19:1438–49. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drake JM, Strohbehn G, Bair TB, Moreland JG, Henry MD. ZEB1 enhances transendothelial migration and represses the epithelial phenotype of prostate cancer cells. Mol Biol Cell. 2009;20:2207–17. doi: 10.1091/mbc.E08-10-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ota I, Li XY, Hu Y, Weiss SJ. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc Natl Acad Sci USA. 2009;106:20318–23. doi: 10.1073/pnas.0910962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Husemann Y, Geigl JB, Schubert F, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–36. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shibue T, Brooks MW, Inan MF, Reinhardt F, Weinberg RA. The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov. 2012;2:706–21. doi: 10.1158/2159-8290.CD-11-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoletov K, Kato H, Zardouzian E, et al. Visualizing extravasation dynamics of metastatic tumor cells. J Cell Sci. 2010;123:2332–41. doi: 10.1242/jcs.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chui MH. Insights into cancer metastasis from a clinicopathologic perspective: Epithelial-Mesenchymal Transition is not a necessary step. Int J Cancer. 2013;132:1487–95. doi: 10.1002/ijc.27745. [DOI] [PubMed] [Google Scholar]
- 31.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. 2011;11:512–22. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beerling E, Ritsma L, Vrisekoop N, Derksen PW, van Rheenen J. Intravital microscopy: new insights into metastasis of tumors. J Cell Sci. 2011;124:299–310. doi: 10.1242/jcs.072728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maier HJ, Wirth T, Beug H. Epithelial-mesenchymal transition in pancreatic carcinoma. Cancers (Basel) 2010;2:2058–83. doi: 10.3390/cancers2042058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–72. [PubMed] [Google Scholar]
- 35.Hruban RH, Wilentz RE, Kern SE. Genetic progression in the pancreatic ducts. Am J Pathol. 2000;156:1821–5. doi: 10.1016/S0002-9440(10)65054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intra-epithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–86. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–87. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 38.Tuveson DA, Neoptolemos JP. Understanding metastasis in pancreatic cancer: a call for new clinical approaches. Cell. 2012;148:21–3. doi: 10.1016/j.cell.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 39.Maitra A, Fukushima N, Takaori K, Hruban RH. Precursors to invasive pancreatic cancer. Adv Anat Pathol. 2005;12:81–91. doi: 10.1097/01.pap.0000155055.14238.25. [DOI] [PubMed] [Google Scholar]
- 40.Maitra A, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:211–26. doi: 10.1016/j.bpg.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Schutte M, Hruban RH, Geradts J, et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res. 1997;57:3126–30. [PubMed] [Google Scholar]
- 42.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21. 1. Science. 1996;271:350–3. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 43.Redston MS, Caldas C, Seymour AB, et al. p53 mutations in pancreatic carcinoma and evidence of common involvement of homo-copolymer tracts in DNA microdeletions. Cancer Res. 1994;54:3025–33. [PubMed] [Google Scholar]
- 44.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 45.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–12. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 47.Sakorafas GH, Sarr MG. Pancreatic cancer after surgery for chronic pancreatitis. Dig Liver Dis. 2003;35:482–5. doi: 10.1016/s1590-8658(03)00221-4. [DOI] [PubMed] [Google Scholar]
- 48.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 49.Navin N, Kendall J, Troge J, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–4. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones S, Chen WD, Parmigiani G, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci USA. 2008;105:4283–8. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt-Kittler O, Ragg T, Daskalakis A, et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci USA. 2003;100:7737–42. doi: 10.1073/pnas.1331931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albanese I, Scibetta AG, Migliavacca M, et al. Heterogeneity within and between primary colorectal carcinomas and matched metastases as revealed by analysis of Ki-ras and p53 mutations. Biochem Biophys Res Commun. 2004;325:784–91. doi: 10.1016/j.bbrc.2004.10.111. [DOI] [PubMed] [Google Scholar]
- 53.Kuukasjarvi T, Karhu R, Tanner M, et al. Genetic heterogeneity and clonal evolution underlying development of asynchronous metastasis in human breast cancer. Cancer Res. 1997;57:1597–604. [PubMed] [Google Scholar]
- 54.Bissig H, Richter J, Desper R, et al. Evaluation of the clonal relationship between primary and metastatic renal cell carcinoma by comparative genomic hybridization. Am J Pathol. 1999;155:267–74. doi: 10.1016/S0002-9440(10)65120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katona TM, Jones TD, Wang M, et al. Genetically heterogeneous and clonally unrelated metastases may arise in patients with cutaneous melanoma. Am J Surg Pathol. 2007;31:1029–37. doi: 10.1097/PAS.0b013e31802b3488. [DOI] [PubMed] [Google Scholar]
- 56.Yu M, Ting DT, Stott SL, et al. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature. 2012;487:510–3. doi: 10.1038/nature11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–26. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leung CT, Brugge JS. Tumor self-seeding: bidirectional flow of tumor cells. Cell. 2009;139:1226–8. doi: 10.1016/j.cell.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 59.Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981–90. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Artinyan A, Anaya DA, McKenzie S, Ellenhorn JD, Kim J. Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma. Cancer. 2011;117:2044–9. doi: 10.1002/cncr.25763. [DOI] [PubMed] [Google Scholar]
- 61.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–7. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 62.Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349–58. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 63.Guerra C, Collado M, Navas C, et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–39. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rothwell PM, Fowkes FG, Belch JF, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 65.Huch M, Bonfanti P, Boj SF, et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013;32:2708–21. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim J, Hoffman JP, Alpaugh RK, et al. An iPSC line from human pancreatic ductal adenocarcinoma undergoes early to invasive stages of pancreatic cancer progression. Cell Rep. 2013;3:2088–99. doi: 10.1016/j.celrep.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]