Abstract
Cell migration is fundamental to establishing and maintaining the proper organization of multicellular organisms. Morphogenesis can be viewed as a consequence, in part, of cell locomotion, from large-scale migrations of epithelial sheets during gastrulation, to the movement of individual cells during development of the nervous system. In an adult organism, cell migration is essential for proper immune response, wound repair, and tissue homeostasis, while aberrant cell migration is found in various pathologies. Indeed, as our knowledge of migration increases, we can look forward to, for example, abating the spread of highly malignant cancer cells, retarding the invasion of white cells in the inflammatory process, or enhancing the healing of wounds. This article is organized in two main sections. The first section is devoted to the single-cell migrating in isolation such as occurs when leukocytes migrate during the immune response or when fibroblasts squeeze through connective tissue. The second section is devoted to cells collectively migrating as part of multicellular clusters or sheets. This second type of migration is prevalent in development, wound healing, and in some forms of cancer metastasis.
Single-Cell Migration
In this section, some representative migrating cells will be introduced citing appropriate reviews, as there is by now a vast literature on cell migration. As examples, we will focus on fibroblast migration, the unusual movement of fish or amphibian keratocytes, and amoeboid locomotion as exemplified by leukocytes. Generally, in cell migration, cells must first adhere at some point. In this review, we will focus on various types of cell adhesions, highlighting some of the structural and signaling proteins involved. It is through adhesions that the tractions required for movement are applied to the substrate and we will outline the measurement of tractions in single, migrating cells. While, we focus on migration principles for cells moving on two-dimensional (2D) substrates, there is now a great deal of interest in single-cell movement in three-dimensional (3D) tissue environments (79, 99). However, detailed mechanisms are more difficult to dissect in these environments as the imaging tools available provide lower resolution at this juncture.
Types of cell migration and related phenomena
Fibroblasts
In vivo, fibroblasts are typically found in connective tissue where they synthesize collagens, glycosaminoglycans, and other important glycoproteins of the extracellular matrix (ECM) including fibronectin, for example. In vitro, these cells have been objects of extensive study because of the ease of culturing them (Fig. 1A). Fibroblasts cultured on glass have a spread or spindle-shaped morphology, often characterized by several extending processes (2, 215). In cell culture, fibroblasts move slowly with an average speed less than 1 μm/min and often change direction. It is from fibroblast cell migration that the textbook paradigm for the classic steps of locomotion is derived. The locomotory cycle (e.g., Alberts et al., pp 965–1051. Molecular Biology of the Cell, 5th Edition) consists of cells protruding and subsequently adhering at the leading margin, developing contractile forces between the front and trailing margins, and finally releasing trailing adhesions due to the applied tension and/or enzymatic action. Retraction generates excess dorsal surface to sustain the protrusion in a process termed retraction induced spreading (41, 60). Over the past several decades, considerable work has been devoted to understanding the mechanistic steps of cell migration as exemplified by fibroblasts (213, 221).
Figure 1.
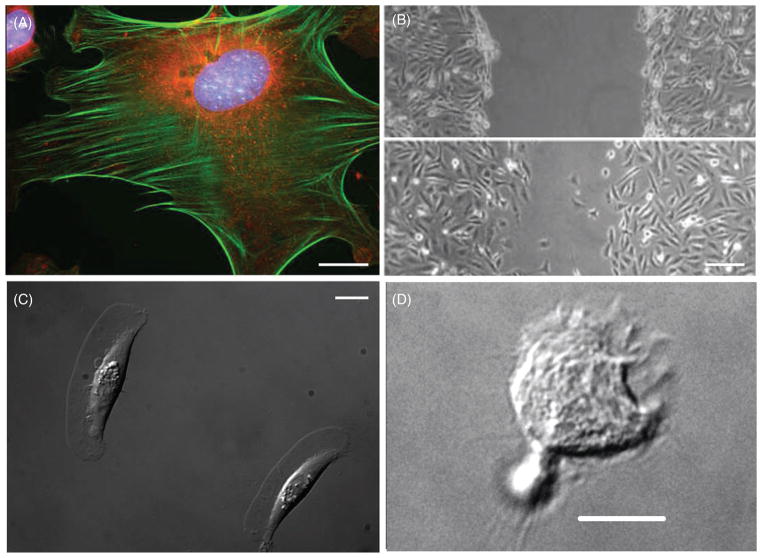
Different types of cell migration. (A) A stationary, spread C3H10T1/2 fibroblast triple stained with DAPI (blue) for DNA, MitoTracker (red) for mitochondria, and Alexa Fluor phalloidin (94) for F-actin. (B) Fibroblasts migrating into wound. Top: initially, a wound was made in a confluent monolayer of MDA-MB-231cells by scratching using a pipette tip. Bottom: after 15 h, migrating cells began to fill in the wound (120). (C) Migrating zebrafish keratocytes with large fan-like lamellipodia. (D) An HL-60 cell (human promyelocytic leukemia cell) migrating on a glass substrate after differentiation with dimethyl sulfoxide (DMSO) to exhibit leukocyte-like behavior on glass substrate. (Image in 1A and 1D are courtesy of Bing Yang and Zenon Rajfur, respectively.) Scale bars in A, C, and D are 10 μm, in B is 100 um.
Fibroblasts play a critical role in wound healing. In vivo (175), and in vitro (236), fibroblasts migrate into wounds, in the process cell acquiring cues that enable them to secrete ECM proteins and proliferate. However, they migrate in vitro with different speeds and morphology when compared to single fibroblasts in cell culture. Fibroblasts migrating into a wound tend to have a large lamellipodium extending into the wound with few stress fibers in the cell; by contrast, stationary fibroblasts have smaller lamellipodia, and are characterized by multiple stress fibers. A typical wound healing assay is shown in Figure 1B. It is known that many of the growth factors presented at a wound site act either as mitogens or as chemotactic factors for fibroblasts (282); these include, for example, epidermal growth factor (EGF) (276) and platelet-derived growth factor (PDGF) (248). Stimulation by growth factors can increase single fibroblast migration speed up to 3-fold, at the same time increasing changes in cell migration direction (276).
Keratocytes
At the other end of the spectrum of cell locomotion, fish or amphibian keratocytes migrate in rapid, highly persistent mode in which protrusion, contraction, and retraction are smoothly coordinated so that the cell maintains a nearly constant shape. Keratocytes are terminally differentiated epithelial cells in fish and amphibians that make good models for several aspects of migrating cells. In primary cultures of scales, keratocytes from goldfish (101, 216) were found to move away from the scale with high velocities (typically 10–15 μm/min but occasionally up to 60 μm/min). The highly directional movement of isolated keratocytes may originate from their ability to move as sheets to close wounds at the surface of the scale. Indeed, they are robust migration machines, migrating for days under proper culture conditions. Even keratocytes lacking the nucleus and microtubules (MTs) can migrate following a stimulus (268).
Lamellipodium structure
Keratocytes have a large fan-like lamellipodium (Fig. 1C). The cell body at the base of lamellipodium is pulled (laterally) into to an elongated shape by actin bundles; in keratocytes MTs and intermediate filaments do not penetrate the thin, actin-rich lamellipodium but are confined to the perinuclear region. Light, fluorescence, and electron microscope images of f-actin in the lamellipodium can be reconciled and all show an oriented f-actin network (267); this presumably reflects the underlying branched actin network as described by the dendritic nucleation model (213). However, the issue of the predominant f-actin structure is not completely settled, and an alternate view is offered by Urban et al. (262).
Cytoskeletal dynamics and migration
Considerable work has been devoted to the cytoskeletal mechanisms involved in keratocyte migration (73, 244, 249). Actin polymerization, treadmilling, retrograde actin network flow, and myosin II-based contractility all play major roles in migrating keratocytes (244,249). Indeed, the force required to stall a protruding keratocyte is consistent with an actin polymerization ratchet model (214); however, the shape of the force-velocity curve is indicating additional factors come into play when the elastic ratchet model (182,183) is placed in a cellular context. Careful examination of actin flows using fluorescence speckle microscopy (FSM) reveals retrograde actin flow, smaller at the leading edge and larger at the wings (sides) of the keratocyte (265). The difference between protrusion and retrograde actin flow rates represents the net actin polymerization rate that is highest at center of the leading margin and falls off toward the wings. These flows are related to tractions exerted on the substratum (see later).
Interestingly, the myosin II network moves relative to the actin network (235). Since the myosin II inhibitor, blebistatin, reduces keratocyte locomotion, cell body translocation involves both actomyosin contraction as well as actin assembly. In fact, Theriot and co-workers (283) demonstrated a novel role for myosin II in addition to its well-known role powering contraction: by accelerating network disassembly, myosin II activity leads to network shrinkage via tension induced actin filament breakage. This action will not only directly lead to retraction but it also recycles monomeric actin for new polymerization at the front.
One effect of myosin II-based contraction is to drive a forward flow of cytoplasm in migrating keratocytes (142). By measuring the front to rear gradient (higher in the front) in the concentration of quantum dots that had been introduced into the cytoplasm and fitting this data to a simple model for flow driven accumulation at the front, anterograde flow velocities in the cell frame of reference that were about 1/3 that of the keratocyte velocity (~0.1 μm/s vs. ~0.3 μm/s) were obtained. Such flows could augment migration by feeding more actin monomer to the growing network at the leading edge and perhaps even providing pressure on the cell surface at the leading margin making network growth via actin polymerization more facile.
Shape and migration
Recently, the shape and movement of keratocytes has been described in detail following an initial description by Lee et al. (157) termed the graded radial extension model. Based on a shape and speed analysis of hundreds of cells, Theriot and co-workers proposed a model for observed keratocyte morphology and crawling behavior (142). Their model is based on the notion that actin polymerization and treadmilling drives migration but is it is resisted by the constant tension of an inextensible membrane surrounding cells of constant area. Spatial differences in the density of growing actin filament network, namely, that the density of filaments is graded with highest values at the center of the leading edge, give rise to characteristic shape of the dominant modes of keratocyte locomotion. Thus, cells with higher actin density at the center than at the sides will have a larger aspect ratio defined as the ratio of the long axis (width) to short axis (length) of the keratocyte. In this model, global integration of spatially varying actin polymerization powered protrusion is provided by membrane tension to specify cell shape. In addition, the model predicts that cell speed will be positively related to the aspect ratio of the cells; thus, canoe-shaped keratocytes, with a larger aspect ratio, move faster than D-shaped cells, with a smaller aspect ratio.
Mogilner and colleagues (227) have constructed in silico models of keratocyte locomotion in which several qualitative notions are incorporated mathematically. At the front of the cell, the dendritic nucleation model (213) is responsible for protrusion while at the rear, the dynamic network contraction model (249) is responsible for retraction. Recently, a model of a viscoelastic lamellipodium was generated using a realistic geometry that correctly predicts measured centripetal flow of the actin network and the positive gradient of myosin II going from front to rear (226).
Leukocytes
Leukocytes, or white blood cells (WBCs), are cells of the immune system defending the body against infecting organisms and foreign materials. They are highly motile cells found throughout the body, including tissues, blood, and the lymphatic system. The recruitment of leukocytes to the site of bacterial and viral infection involves initial attachment to vascular endothelium, rolling, weak and firm adhesion, transendothelial migration, and chemotaxis (128). Leukocyte chemotaxis in vivo and in vitro occurs at speeds around 4 μm/min (151). Leukocytes migrate on different substrates through adhesions that involve the integrins β2 and α4β1 (154). However, recently it has been reported that leukocytes can adhere and migrate in an integrin-independent manner (151), indicating that leukocytes employ additional mechanisms for adhesion and migration. A view of differentiated migrating HL-60 leukemia-like cell is shown in Figure 1D.
Single-cell migration in three dimensions
Although cell migration has been studied extensively in essentially 2D cell culture conditions where cells grow on a substrate, increasing attention has been paid to the movement of cells in 3D environments. The 3D matrix acts as a scaffold that produces physical support for cells that can affect cell morphology and induce cell growth or migration (67, 75). In addition, the matrix can induce variation in signaling cascades in cells via adhesions and tensile forces (see, for example, reference 9).
Cell morphology and migration in 3D environments
Most migration modes previously observed in 2D environments also occur in 3D tissue environments. However, because the distribution of ligands in 2D is generally much more uniform than in 3D matrix models where, for example, clustered ligands may exist on fibrils, cell morphology is quite different in the two environments (67). In 2D cell culture, fibroblasts have large lamellipodia and filopodia. By contrast, fibroblasts in 3D collagen gels exhibit both smaller and fewer lamellipodia and filopodia (112). Due to extensive adhesion to a flat substratum, cells in 2D show very broad, flat and thin lamellipodia whereas cells in 3D show a less exaggerated appearance. Three motile morphologies can be delineated in a 3D matrix (75): amoeboid blebby (macrophages, some stem cells on soft/loose connective tissue); amoeboid pseudopodal (leukocytes, dictyostelium on loose connective tissue); and mesenchymal (fibroblasts, and some cancer cells on loose or dense connective tissue).
Regulation of cell migration in 3D matrices
Three important factors regulate 3D cell migration: cell-matrix adhesions, the Rho family of small GTPases, and proteases. In 2D culture, integrins are primarily responsible for cell adhesions to ECM in the form of focal adhesions (FAs), focal contacts, podosomes, etc. However, in 3D cell culture, a reduction in the number of FAs and their component integrins occurs. Thus, for example, αVβ3 integrin, which is highly expressed in 2D cell culture, was not detected in the 3D-matrix adhesions of fibroblasts, and the level of FA kinase (FAK) phosphorylation was reduced (38). Changes in the nature and strength of adhesions in 3D and 2D environments will result in differences in cell tension, morphology, and migration type (148).
The Rho family of small GTPases plays a prominent role in regulating cell migration in 3D. Leukocytes employ amoeboid migration that is based on the Rho/Rho-associated protein kinase (ROCK) pathway maintaining contractility at the posterior end and Rac1 mediating protrusion at the leading margin (232). However, other reports indicate that Rac1 activity is suppressed in fibroblasts and neurons in 3D culture, thus decreasing leading edge ruffling and axonal branching, respectively (117, 206).
The role of proteolysis in 3D migration in tissue has been actively investigated. Multiple proteases have collagenolytic activity but the emphasis has been on matrix-metallo proteases (MMPs) and these have been reported to affect both normal and cancer cell migration in vitro (see also collective cell migration later). However, clinical trials of MMP inhibitors did not impair metastasis suggesting that metastatic cells may switch from mesenchymal to amoeboid locomotion (195,285, 286).
Adhesions in migrating cells
Cells adhere to ECM or other cells by both nonspecific electrostatic interactions and specific binding of cell adhesion molecules such as selectins, integrins, and cadherins to ECM ligands and to cadherins on other cells. We will focus on cell-ECM adhesions, and divide such adhesions into FAs, podosomes, focal complexes, and close contacts.
Focal adhesions: Composition and structure
FAs were first identified in chicken heart fibroblasts by electron microscopy, as dense plaques between the cell’s ventral surface and the substrate (2). FAs are usually found at the ends of stress fibers; they have a dimension on the order of a micron, and a lifetime ranging between minutes and hours. They have been visualized by epifluorescence microscopy (Fig. 2A), by total internal fluorescence microscopy (TIRFM), or by interference reflection contrast microscopy (IRM) (Fig. 2B). In the past, terms such as adhesion plaques (2), or focal contacts (132) were employed, but now the field appears to have settled on the term FA (295). FA components can be divided into four general categories: (1) ECM components, of which fibronectin, laminin, vitronectin, and the collagens are important examples; (2) transmembrane proteins, of which integrins are the most prominent class; (3) structural proteins that both stabilize the FA and provide scaffolding functions; and (4) signaling proteins (166, 294). The number of proteins found in FAs is now exceeds 160, and the possible interactions between these components is described in what is colloquially called the “Geiger diagram” (295) which evolves as new components are identified (92, 294).
Figure 2.
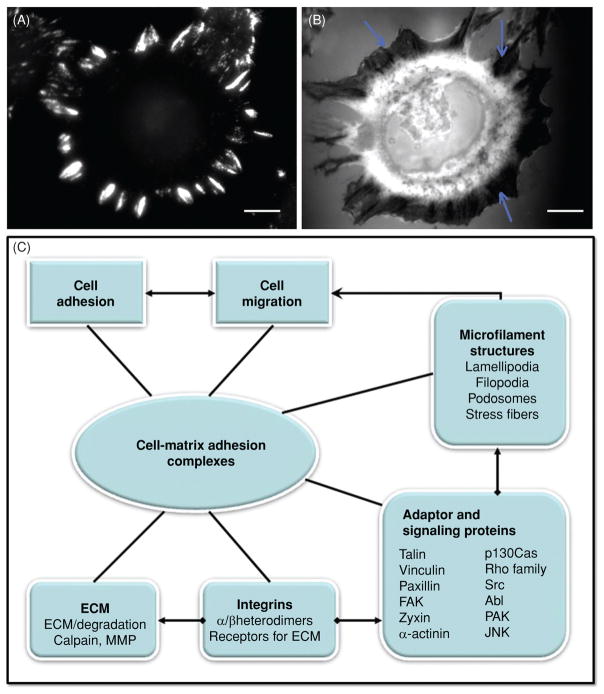
Adhesion structure and function in cells. (A) An immunofluorescence image of focal adhesions (FAs) in an NIH 3T3 cell stained with antipaxillin; (B) an interference reflection microscopy (IRM) image of FAs in a similar NIH 3T3 fibroblast on a fibronectin (FN)-coated substrate; the very dark regions (arrows) are FAs; and (C) schematic figure for the relationship between cell adhesion, cell migration, and some of the corresponding adaptor and signal proteins. Cell matrix adhesion complexes are depicted a key component in single-cell adhesion and migration. After activation, integrins bind extracellular matrix (ECM) and provide a link to the actin cytoskeleton. Cytoplasmic adaptor proteins bind integrin cytoplasmic domains, stabilize FA, and provide scaffolding functions. Integrin activation also initiates downstream signaling. Such signaling may regulate cell adhesion turnover, internal force development, and cytoskeletal rearrangements including formation of stress fibers, lamellipodia, filopodia, and podosomes. Cell migration also involves both ECM degradation and proteolysis and adhesion complex internalization (see section on focal adhesion dynamics). Scale bars in A and B are 10 μm.
Integrins are the transmembrane proteins that recognize ECM proteins containing short amino acid sequences, such as the arginine-glycine-aspartic acid (RGD), Asp-Gly-Glu-Ala (DGEA), and glycine-phenylalanine-hydroxyproline-glycine-glutamate-arginine (GFOGER) motifs (64, 220). Functional integrins are heterodimers containing two distinct (α and β) subunits. Currently, there are more than 24 types of α and β integrin subunits characterized in mammals (15,124). Each type of integrin heterodimer binds distinct ligands, for example, α5β1 integrin binds fibronectin, and α3β1 bind to laminin (253). FAs in different fibroblasts and epithelial cells that are adherent to distinct ECM materials contain integrins with various combinations of α- and β-subunits (6).
One function of cytoskeletal proteins, including talin (27), α-actinin (44), filamin (186), and tensin (167), is to link inte-grins to the actin cytoskeleton. Other adaptor proteins directly or indirectly interact with integrin cytoplasmic tails and form protein complexes; examples include FAK (181, 207)], vin-culin (Vn) (72), paxillin (Pax) (234, 261), dynamin (33), and Ena/vasodilator-stimulated phosphoprotein (VASP) (149). As an example, an epifluorescence image of antibody labeled Pax is given in Figure 2A, and shows the extensive array of FAs in murine fibroblasts adherent to a serum coated glass substrate.
Signaling proteins are recruited to FA and regulate their assembly and disassembly; examples include the Src family of nonreceptor tyrosine kinases (NRPTKs) (127), the Abl family NRPTK (114) and the Rho family of small GTPases (108), and p21-activated kinase (62). In addition, phospho-rylation of Pax by c-Jun amino-terminal kinase (JNK) or cdk 5 has been found essential for maintaining the labile adhesions required for rapid migration in both fibroblasts and neurons (119,120). Some proteins and signaling pathways involved in FA structure and regulation and their relationship to cell adhesion and migration are diagrammed schematically in Figure 2C.
FA appears to be an amorphous collection of interacting proteins making 3D structure determinations difficult be either light or electron microscopy. However, recently progress has been made employing photoactivation localization microscopy (PALM) in 2D (239, 240) and by iPALM, in 3D (241). Such studies are revealing the 3D organization of individual FA proteins (139).
Focal adhesion dynamics
FAs are dynamic structures that undergo cycles of assembly and disassembly; indeed, regulated FA turnover is integral to cell migration. Thus, here we will review some of the key aspects of FA dynamics.
Focal adhesion assembly
The role of integrin activation in FA assembly and in initiating downstream signaling has been extensively investigated. With stimulation, for example, by growth factors, integrin β-subunit cytoplasmic domains bind the talin phosphotyrosine-binding (PTB) domain causing integrin activation (145, 280). Activated integrins then bind ECM components and the cytoplasmic domain recruits signaling proteins; this process initiates downstream signaling, including FAK phosphorylation, mitogen-activated protein kinase (MAPK) activation, Pax binding, and the formation of a complex containing Vn, FAK, α-actinin, Wiskott-Aldrich syndrome protein (WASP), tensin, Src, and zyxin (155,279,294). Knockouts of key recruited signaling components have demonstrable effects on cell adhesion and migration. Thus, for example, FAK null fibroblasts exhibit increased numbers of adhesions and consequent reduced cell motility (130). In addition, kinase dead Src mutants promoted both the number and size of cell adhesions, reducing the speed of cell migration. Webb et al. found that Src, Pax, and FAK formed complexes in vitro and in vivo; in this study, FAK and Src were speculated to regulate cell adhesion disassembly via Pax and the downstream extracellular-signal-regulated kinase (ERK) and myosin light-chain kinase (MLCK) pathways (278). Abl knockdown cells also exhibited an increase in cell adhesion size and stability, and rescue of Abl kinase activity restored the cell adhesion disassembly rate (16). Rho family GTPases have also been reported as key regulators of FA dynamics, for example, active RhoA changed small peripheral adhesions (focal complexes) into elongated FAs (245). External stretch induced nascent adhesions to mature into FAs via a RhoA-ROCK pathway (245).
Focal adhesion disassembly
Compared with extensive studies on FA formation, the disassembly process is not as clear. Several related pathways may contribute to FA disassembly: (i) adhesion release produced by ECM degradation; (ii) adhesion turnover mediated by the cytoskeleton and internalization; and (iii) disassembly mediated by kinases and proteases (24, 118). It has been reported that ECM degradation is, in part, responsible for cell adhesion disassembly, cell migration, and invasion (163); thus, for example, ECM degradation by matrix metalloproteinases (MMPs) could induce the release of cell adhesions resulting in an increase cell motility and invasion (32, 160).
Cytoskeletal components are an important regulatory factor in adhesion disassembly. MTs have been observed to target FAs promoting their disassembly (140, 150). Moreover, MTs have been speculated to induce cell adhesion disassembly via dynamin- and clathrin-dependent integrin endocytosis (69,70). Caveolin-1 was also reported to regulate FA turnover and cell migration directionality possibly via internalization (51,102). In addition, cellular contractile machinery may also induce FA disassembly; for example, RhoA, and myosin II were found to positively regulate adhesion disassembly and cause cell rear detachment (66, 270).
Proteases and kinases have also been reported to regulate cell adhesion. Calpain, a calcium-dependent protease cleaves talin, FAK, and Pax in FA (32). Cleavage of these proteins leads to disassembly of the FA and the detachment of the tail of the cell (126, 288). Moreover, recent studies have demonstrated that, Smurf1, an E3 ubiquitin ligase, degrades the talin head and controls cell adhesion stability (121). Other ubiquitin ligases, including Cbl, Smurf2, HDM2, and BCA2, also play an important role in regulating cell adhesion and migration through ubiquitination of their specific substrates (118).
Methods have been developed to study the dynamics of FAs. Studies using fluorescence recovery after photobleaching (FRAP) and green fluorescent protein (GFP)-fusion proteins or labeled microinjected proteins have shown that protein components of FAs including α-actinin, Vn, and FAK slowly exchange between the cytosol and the adhesion with half-times for recovery on the order of minutes. More recently, Horwitz and co-workers measured adhesion disassembly rates of FP conjugated Pax, FAK, and zyxin; these studies indicated that the FAK-Src complex could interrupt FA maturation by promoting disassembly through the downstream ERK and MLCK pathways (278). Using the techniques of image correlation microscopy, Gratton, Wiseman, Horwitz, and their co-workers measured FAK, Vn, and Pax diffusion and binding to adhesions in mouse embryonic fibroblasts. No FAK, Vn, and Pax complexes were preassembled in cytoplasm, but when the adhesions disassembled, these proteins disassociated in complexes (54, 55). Waterman and colleagues studied FA dynamics using speckle microscopy and advanced image analysis; they found that the retrograde F-actin network velocity is a fundamental regulator of traction force at FAs via the Rho and myosin II pathways (88). These investigators also demonstrated that the interplay between actomyosin and FA dynamics results in a balance between adhesion and contraction to induce maximal migration velocity. Such studies indicated a relationship between force and FA assembly and disassembly, and predicted how under certain circumstances the FA slide (105, 279).
Podosomes
Podosomes are specialized integrin-mediated adhesions often found in highly migratory monocytic cells that mediate the inflammatory response (25, 160). They also have the capacity for matrix degradation. Linking the ECM to the actin cytoskeleton, podosomes have a fairly uniform dimension of around 0.5 μm, a half-life of 2 to 20 min and are abundant (20–100 per cell) (160,161). An image of podosomes is shown in Figure 3B.
Figure 3.
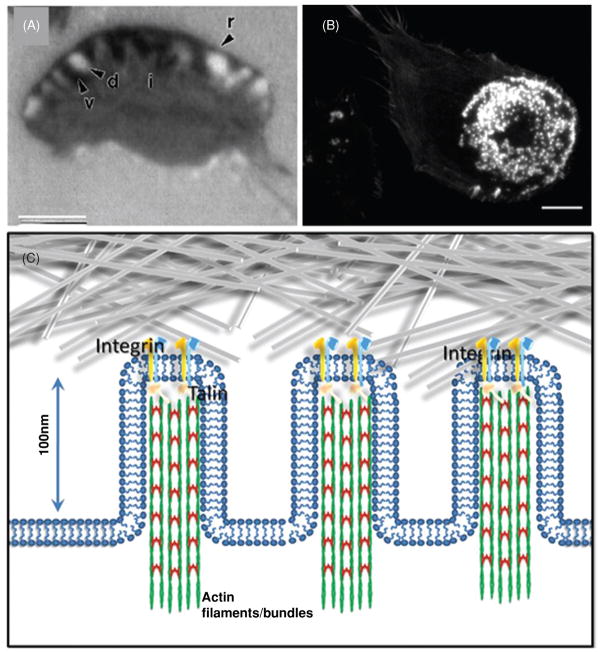
(A) Interference reflection microscopy (IRM) image of close adhesion in migrating fish kera-tocytes, the adhesion pattern consists of an outer rim (r) of very close contact skirting a crescent-shaped band of alternating very close (v) and distant contacts (d). (B) Epifluorescent image of podosomes in a human dendritic cell with F-actin labeling. (C) A hypothetical view of close contacts in which small diameter projections attach to the substrate and serve to draw the ventral surface closer to the substrate such that it appears gray in IRM. Integrin, talin, F-actin have been reported to be in close adhesions [in this schematic, the actin network is depicted like that in a microvillus with parallel actin bundles but it could also be in the form of a dendritic actin network (not shown)]; however, paxillin and focal adhesion kinase (FAK) are not found in initial close contacts. Scale bars are 10 μm. Image in panel A is from Lee and Jacobson (158); image in panel B is courtesy of Aaron Neumann.
Podosomes have a dense actin core surrounded by a rosette-like structure containing integrins, such as αvβ3, FA proteins including talin and Vn that play a major structural role, other actin-associated proteins [gelsolin, alpha-actinin, and actin-related protein 2/3 (Arp2/3)], tyrosine kinases (Src, Pyk2), and phosphoinositide-3 kinase (PI3K), and also the Rho-family GTPases (25). The podosome core also contains proteins involved in regulating actin polymerization including WASP (25, 160). A larger, more stable but related structure, the invadopodia, plays an important role in invasive cancer cells and has been thoroughly reviewed (42, 97, 189).
Focal complexes
The term, “focal complex,” describes small adhesions that form at the leading margin of migrating cells, typically fibroblasts. Focal complexes are significantly smaller in area (<0.25 μm2), and are shorter lived (often <5 min but some have even shorter lifetimes) than FAs (91). Focal complexes contain integrins, talin, and Pax, but fewer actin filaments are associated with them (126, 295). Migrating cells often have a large number of focal complexes at the protruding edge. Most of these focal complexes never mature, and are likely disassembled when the lamellipodium retracts. Some investigators have suggested that focal complexes might be precursors of FA because applied contractile forces can convert focal complexes into larger oval-shape adhesions (18, 87, 223, 225).
Close contacts in migrating cells
Close contacts appear as broad gray areas in IRM (Fig. 3A). The original definition of close contacts was based on IRM images and indicated that the separation between the ventral surface of the cell and the substratum was about 20 to 50 nm (132). By contrast, the ventral surface and substratum is separated by 10 to 15 nm or less in FA. Compared to FA, little is known about these adhesions. They predominate in fast moving cells such as keratocytes (11, 158) although regions of close contact also exist in fibroblasts and epithelial cells in culture (159).
The composition of close contacts was investigated by immunofluorescence staining of fish keratocytes using antibodies against known FA components. The close contact areas at the rim of leading edge were found enriched in β1-integrin and talin, with little Pax and FAK (158). In general, close contacts appear to be mediated by integrins. Forward movement of the Xenopus keratocyte lamella could be halted by adding RGD peptide or an anti-integrin mAb while the rear of cell continued to retract (50).
Anderson and Cross (11) performed a detailed study of more mature Vn-containing adhesions using microinjected fluorescent Vn and combined confocal and IRM imaging. They found that these contacts formed behind the leading edge and matured beneath the lamellipodium and remained stationary while the cell passed over them. By contrast, Vn-containing contacts in the wings of the cell grew larger before sliding inward. These large contacts are presumably transmitting the large lateral traction in keratocytes that are used for retraction of the wings. The actual mechanism for disassembly of released contacts remains an open question.
There are really no structural models for close contacts. A possible model would consist of finger-like projections of a small diameter that contact the surface using the usual repertoire of FAs molecules (Fig. 3C). In this respect, these projections would be a cross between podosomes and filopodia. The net result would be to draw the surface closer to the substratum such that the region appears gray in IRM yet the adhesion itself could be readily remodeled to accommodate rapid cell migration.
Outlook
In addition to the extensive cataloging of adhesion components, there are recent developments in super-resolution microscopy (139) and several live cell fluorescence microscopy methods that promise to enhance our understanding of structure-function relationships in the adhesive structures that enable the cell to exert traction on its environment (39,88,177). Also, recent developments in Rho family biosensors and detailed analysis of such data, promise to provide detailed mapping of the localization and activation pattern of these GTPases in relation to the regulation of dynamic adhesive behavior, tractions, and cell migration (172, 191, 212). Overall, it appears that the next decade will produce important advances in our understanding of cell-substratum adhesions.
Measurements of tractions in single migrating cells
Elastic substrate traction measurements
The effects of tractions exerted by migrating chick heart fibroblasts plated on a deformable silicone substrate (a thin film of silicone cross linked by means of glow discharge) were visualized as visible wrinkles in the film under the cell body and perpendicular to the direction of cell movement (109). Such compression wrinkles qualitatively reflect the strong contractile forces exerted by fibroblasts on their environment but do not give the actual distribution of traction stresses under the cell.
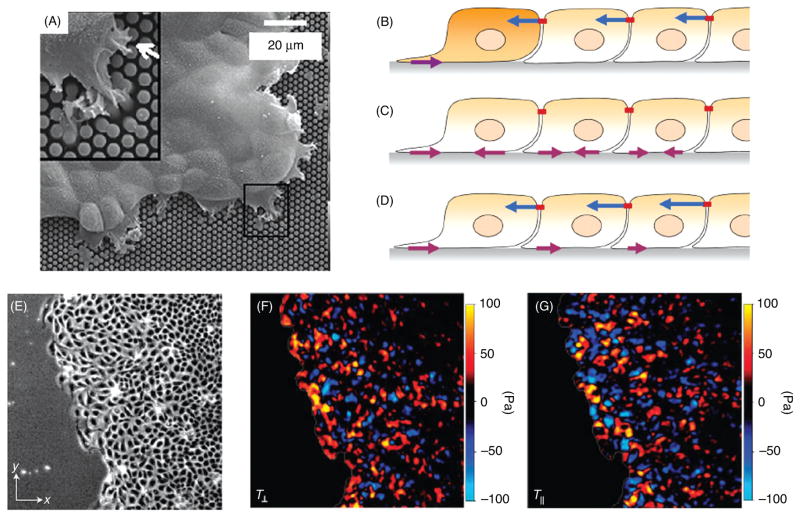
Spatially resolved information on the distribution of tractions has been obtained in the past 15 years by following the displacements of fiduciary markers embedded in deformable substrata (Fig. 4) or the response of individual force-sensing elements. This approach was first applied to fish scale keratocytes migrating on silicone rubber substrata in which small polystyrene latex beads had been embedded (159). When the tractions were calculated from the bead displacements (52), it was found, surprisingly, that the major propulsive tractions were applied in the wings of the keratocyte (203).
Figure 4.
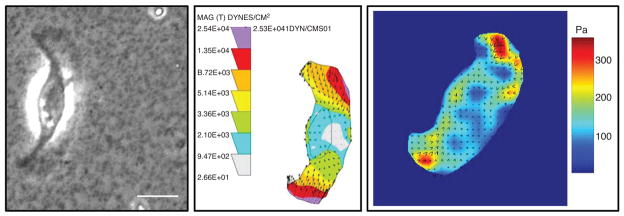
Use of elastic substrates to map tractions in migrating cells. (A) Phase image showing a fish keratocytes crawling on an elastic polyacrylamide substrate. (B) Tractions mapped on the same cell shown in A. The Dembo Boundary element method algorithm (52) was used to calculate the cell traction distribution from the bead displacement map; the units in the map are in Dynes/cm2 (1 dyne = 10−5N). (C) The Fourier-transform traction cytometry (FTTC) algorithm (28) was used to calculate tractions for another keratocyte; the right scale of color bar represents stress in units of Pa (1 Pa = 1 N/m2). Scale bar is 10 μm. Images are courtesy of Zenon Rajfur.
However, with silicone rubber films, matching the compliance to the tractions exerted by the cells and providing a defined surface coating on the film for optimal adhesion is not always easy. These difficulties were circumvented by developing polyacrylamide gel substrates with variable degrees of cross-linking onto which ECM proteins could be conjugated (53, 165, 210, 211, 274, 275). An example of the use of polyacrylamide substrates for examining the tractions exerted by locomoting keratocytes in seen in Figure 4. Moreover, these films are optically tractable so that when fluorescent beads are used as the fiduciary markers in the gel, dual channel fluorescence microscopy permits the correlation of tractions in relation to the spatial localization of fluorescently labeled FA proteins (18).
Another approach employs special microfabricated substrates that contain an array of force-sensing elements. These are flexible cantilevers of known bending stiffness so that the forces exerted by moving cells on these pads can be computed directly from the deflection of the cantilever beams (85, 86, 252). An alternate approach employs an elastomeric silicone substrate that is micropatterned to give rise to a regular array of either surface indentations or projections of sub-micron dimensions (13,223). An algorithm allows the surface distortion of the micropattern caused by cells to be directly translated to the cellular forces. Thus, a number of methods now exist that are similar in overall concept and permit calculation of traction stresses and the correlation of those stresses with the molecular constituents of the force-transmitting adhesive structures.
Force, cell adhesions, and cell migration
There is a clear interplay between contractile force generated by the cell, adhesion to the substrate and the traction applied to the substrate that is beginning to be investigated in detail. As stated previously, force can induce focal complexes to mature into large FAs near the leading edge of migrating cells; at the trailing edge, contractile forces regulate adhesion disassembly and cell detachment. Also, MT-induced adhesion disassembly has been observed as mentioned previously and it was speculated that the growth of stiff MT growth into adhesions can release the force originally exerted by the actomyosin cytoskeleton, thus promoting adhesion disassembly (140, 245).
The relationship among adhesion, traction applied to the substrate, and cell migration is under active investigation. At the outset, it is important to note that the net traction to move the cell through a low viscosity buffer is effectively zero. This leads to the conclusion that the typical tractions measured, which are much larger than what are required to move the cell, must be used to break adhesions in spatiotemporal patterns that dictate both the speed and direction of the cell.
Using keratocytes as a model, Lee and her colleagues reported that slowly migrating keratocytes are more fibroblast-like in their migration and characterized by slipping of adhesions that are coupled with retrograde actin flow; in fast-moving keratocytes, adhesions have more gripping character to sustain the rapid protrusion powered by the fast-paced polymerizing actin network; these cells exhibit a much smaller rearward actin flow (136, 265). Recently, maps of actin-substrate coupling were used to quantify differences in force-transmission efficiency between different cell regions (73). Thus, a more detailed scenario about the substrate adhesion-traction-migration relationship could be proposed: At the leading edge, traction was transmitted in a manner partially independent of actin velocity (gripping) but at the cell flanks, the force transmission was mediated by the high friction between the actin network and the substrate; at the cell body, little traction was transmitted, because of low friction. Undoubtedly, this relationship will be further investigated both experimentally and theoretically (226), as it is key to achieving a global understanding of how cells move.
Collective Cell Migration
Collective cell migration is the prevalent mode of migration during development, wound healing, and tissue regeneration (19, 68, 75, 179, 272). In addition, it is increasingly regarded as a widespread mode of migration during metastasis in epithelial cancers (77,83,171,286). Collectively migrating cells use similar mechanisms as single cells to protrude, polarize, contract, and adhere to the surrounding matrix. However, their ability to interact with each other both chemically and mechanically provides cells within the moving group with additional mechanisms to migrate while (a) maintaining tissue cohesiveness and organization; (b) regulating tissue paracellular permeability; (c) creating large gradients of soluble factors; (d) distributing tasks between specialized mobile and non-mobile cells; (e) propagating mechanical signals via cell-cell junctions; and (f) in the case of cancer, protecting metastatic clusters from an immune assault. The mechanisms underlying collective cell migration are less well understood than those that drive single-cell migration, but improved methods in genomics, proteomics, imaging, and biomechanics are producing rapid advances in this field. In the following sections, we first review the role of collective cell migration in physiology and pathophysiology, and then provide an overview of the basic biological and biophysical mechanisms that drive and regulate collective cell migration (8,19,75,83,156,260,264,286).
Collective cell migration in physiology and pathophysiology
The EMT paradigm
The transition from a static to a motile phenotype that multicellular collectives undergo during embryogenic movements, cancer metastasis, and wound repair is traditionally understood under the rubric of the epithelial to mesenchymal transition (EMT). EMT is a highly conserved cellular program characterized by a number of morphologic, structural, and molecular changes that includes flattening of cell shape, loss of apical-basolateral polarity and cell contacts, formation of a dynamic protrusion at the leading edge, and increased concentration of intermediate filaments (138, 254). The EMT paradigm has been successful in explaining the transition between two well-defined cellular states as in the case of emigration of neural crest cells (35) or gastrulation of mesoderm cells (190). However, many other processes involving cell motility do not follow the guidelines of classic EMT (45, 219). This is illustrated by well-known cases such as migration of the zebrafish lateral line primordium (LLP) or that of border cells in Drosophila egg chamber. In both cases, cells efficiently migrate long distances while keeping a cohesive structure with high levels of E-cadherin and tight junction proteins (19, 156). Thus, rather than being restricted to sharp transitions from well-defined epithelial and mesenchymal states, the cells in tissues can take advantage of both states to fine tune their phenotype. The spatial organization of this finely tuned phenotype within a moving group provides the cell with additional functions that could not be achieved in completely dissociated cell collectives.
Development
From early embryogenesis to postnatal life, the development of living organisms is driven by the motion of cell collectives (281). These collectives move in a variety of geometrical configurations such as sheets, sprouts, strands, tubes, or clusters (224). Given the difficulty of studying the motion of these geometrically diverse cell collectives within higher animals, collective cell migration is commonly studied in relatively simple models such as Drosophila melanogaster, Caenorhabditis elegans, or zebrafish. These model systems offer structural simplicity and genetic accessibility thus allowing the direct visualization of motile groups that selectively express fluorescently tagged proteins (49).
The simplest and best-studied mode of collective cell migration is the advance of 2D epithelial sheets over a basement membrane. Moving cell sheets are typically formed by a relatively large number of cells that remain mostly cohesive as they invade open spaces or surrounding tissues. A paradigmatic example of sheet migration is dorsal closure in Drosophila, a process occurring during the latest stages of embryogenesis. As a consequence of retraction of the germ band, an eye-shaped hole covered by amnioserosa cells is left on the dorsal surface. To seal this hole, two flanks of epidermal cells advance toward each other until they meet at the dorsal midline. This process is driven by at least three mechanisms: (1) active migration of epidermal cells characterized by dynamic extension of filopodia at the leading edge; (2) periodic contraction of the amnioserosa; and (3) zipping of supracellular actin cables at the leading edge (74, 133, 247). A different developmental process also driven both by supracellular purse-string and filopodia-rich migration is ventral enclosure in C. elegans (243). These examples illustrate that even in the simplest cases of collective migration, the motion of the group is not only driven by independent the action of each individual cell within the moving group but also by supracellular mechanisms and by the cooperative action of the surrounding tissue.
The inverse process to epithelial closure is centrifugal expansion of a cell sheet or colony. In some cases, such as the growth of imaginal discs in Drosophila, expansion occurs in the absence of physical constraints that restrict the growth of the colony (208). However, in other cases, cell colonies expand at the expense of the surrounding tissue. During development of the Drosophila abdomen, for example, the larval epithelium of each segment of the abdomen is replaced by proliferation and migration of four pairs of histoblast nests with original sizes ranging from 3 to 18 cells (197) (Fig. 5). These histoblast nests remain growth arrested during larval stages but at the onset of metamorphosis they undergo rapid proliferation and expand radially over each abdomen segment. Expansion occurs against the surrounding larval cells, which undergo apoptosis as they come in contact with the leading edge of the expanding histoblast colony. The mechanism by which histoblasts replace larval cells remains unknown but recent imaging improvements have shown leading histoblasts forming intercalating protrusions between the surrounding larval cells possibly contributing to their apoptosis. These protrusions are highly dynamics and suggestive of active traction generation (196).
Figure 5.
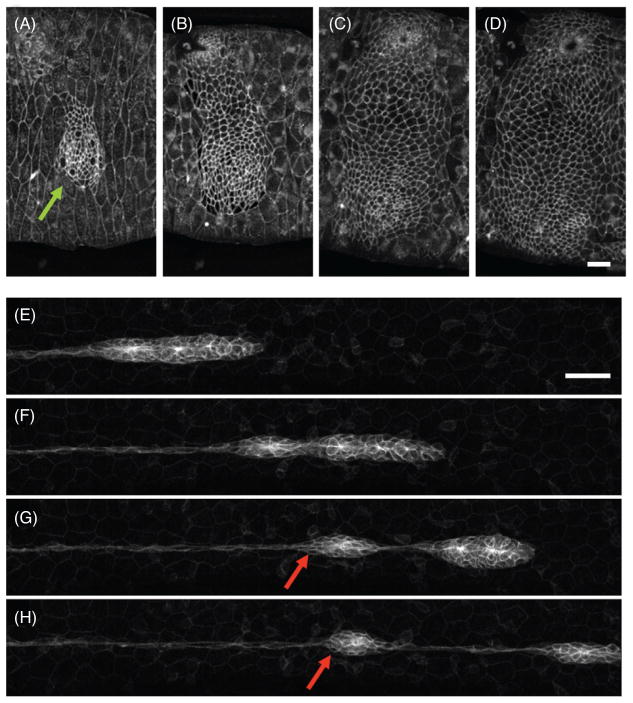
Collective cell migration in development. (A-D) During development of the abdomen of Drosophila melanogaster a cluster of histoblasts (green arrow) grows and migrates radially outward at the expense of the surrounding larval cells. Courtesy of Enrique Martin-Blanco and Carla Prat. (E–F) During development of the sensory system of zebrafish, the lateral line primordium undergoes directed migration from head to tail, leaving behind rosettes (red arrows) at periodic intervals. Scale bars: 60 μm. Courtesy of Hernan Lopez-Schier and Filipe Pinto.
In many processes in development, relatively small cell clusters undergo large collective displacements across the embryo from the location where they are specified to the location where they will ultimately carry out their biological function. A well-studied model for this type of cluster motion is the development of the lateral line system in zebrafish (Fig. 5). The lateral line comprises a series of sensory organs that are arranged in regularly spaced clusters (neuromasts) on each flank of the skin (95). These clusters are deposited by the LLP, a group of 100 cells that migrates from head to tail. As the primordium transverses the animal, cell clusters at its trailing edge become progressively nonmotile and are finally left behind in periodic intervals (107). Another well-studied example of directed cluster motion is the migration of border cells during oogenesis in Drosophila (19, 169). The border cell cluster comprises 6 to 8 migratory border cells and two nonmigratory inner polar cells. After being specified at the anterior end of the egg chamber, the cluster detaches from the follicle and migrates posteriorly toward the oocyte squeezing between the surrounding nurse cells. To do so, all border cells extend protrusions while they exchange positions within the cluster. Remarkably, border cells are not surrounded by ECM and, therefore, they need to adhere directly to nurse cells to generate traction.
Another developmental process in which collective cell migration plays a key role is branching morphogenesis, a process widely used in nature to shape complex organs that require packing of large surfaces into small volumes as in the case of lungs, kidneys, pancreas, mammary glands, salivary glands, and the vasculature (168). All these branched systems are characterized by the presence of a cell monolayer—epithelial, endothelial, or both—that separates two compartments within an organism. This cell layer controls the transport of ions, gases, liquid, solutes, and immune cells between these compartments. The formation of the initial branch commonly starts early in development by invagination of polarized epithelial cell sheets driven by constriction of the apical actomyosin rings (4). Alternatively, tubes can start by formation of a lumen from fusion of vacuoles or by sprouting of an already existing tube. Once the main branch is formed, emergence of new branches occurs either by splitting of one branch at its tip into two branches (bifurcation) or three branches (trifurcation), or by formation of a new branch from the side of an existing one (lateral branching) (277). Collective migration and elongation of tubes appears to be led by extension of filopodia and lamellipodia from tip cells into the surrounding extracellular tissue (26,193,263). Such migration is regulated by the exchange of promigratory [EGF, fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), PDGF, etc.] and inhibitory factors (TGFβ, notch) between the epithelium and the mesenchyme (30, 47, 188, 193). Interestingly, some of these molecular mechanisms are common not only in the morphogenesis of hollow organs but also in branching of the nervous system (153). In addition to these soluble factors, collective migration during branching morphogenesis is heavily regulated by the interaction between migrating cells and the ECM. Indeed, mutations in the cell-ECM adhesion proteins or in proteins involved in ECM degradation, as well as alterations in the composition of the ECM, result in reduced outgrowth of branches (193).
Wound repair
The molecular machinery that governs collective cell migration during development remains largely dormant throughout adult life. However, when a tissue is injured, this machinery is rapidly rescued to repair the wound and restore the viable tissue (237). Wound healing plays a central role in the pathophysiology of virtually every organ (106). For example, in devastating lung diseases such as pulmonary fibrosis, chronic obstructive pulmonary disease, asthma, and acute lung injury, the pulmonary epithelium is injured and often denuded (36). This injury impairs barrier function of the epithelium thereby exposing the lungs to airborne inhaled pathogens and other toxic agents (259). In addition, the damaged airway epithelium prevents key metabolic functions of the airways including fluid and ion transport to the lumen and mucociliary clearance. The ability of the epithelium to rapidly self-repair is thus critical to restore pulmonary function and to prevent further damage.
Wound repair is particularly well understood in the skin but increasing evidence supports that the main stages of the process are conserved across organs (106). The initial physiological response to wounding is the activation of circulating platelets at the site of vascular injury. Such activation is initiated by direct contact between the platelet surface and proteins located at the basement membrane of the endothelium such as collagen, fibronectin, laminin, and von Willebrand factor (22). Activated platelets rapidly aggregate to form stable clots that prevent hemorrhage until the healing process is completed. Platelet aggregates are initially stabilized by a fibrin network that will later serve as a provisional scaffold rich in growth factors on which cells may crawl (200). In parallel with fibrin clotting, damaged cells initiate a stress response that includes the activation of MAPK pathways, the secretion of chemotactic factors, and the recruitment of circulating neutrophils and monocytes to clear pathogens from the injured area (146).
This initial inflammatory response is followed by reepithelialization. During this process, cells surrounding the wound migrate collectively across a provisional matrix rich in fibrin and fibronectin (175). To migrate onto and through this provisional matrix, cells at the first few rows behind the wound margin alter their expression of cell-cell and cell-matrix adhesion proteins (115). Fibrinolytic enzymes such as plasmin and MMPs degrade the matrix to enable rapid cell migration (106, 257). In addition, cells undergo structural changes of their cytoskeleton characterized by the synthesis of transverse stress fibers and by the extension of filopodia and lamellipo-dia into the wound area (204, 287). In striking analogy with development, epithelial cells use two main modes of collective migration during reepithelialization (176). The first mode involves the assembly of a supracellular actin cable at the wound perimeter. Contraction of this actin cable in a “purse string” manner provides efficient closure at the later stages of wound healing. The second mode of migration involves the extension of dynamic lamellipodia and filopodia into the wound area (218). This mechanism appears to be reminiscent of single cell migration although recent studies proved that it also involves strong cooperativity between cells (12, 260).
In addition to playing a central role in reepithelialization, collective cell migration is also involved in wound healing as a primary mediator of angiogenesis (256). Angiogenesis is fundamental during wound healing to provide oxygen and nutrients to the newly assembled tissues and its inhibition impairs wound healing. It is mainly triggered by growth factors such as bFGF, TGFβ, VEGF, and by cytokines such as TNFα secreted by hypoxic macrophages and by damaged endothelial cells (198, 256). In response to these signaling macromolecules, endothelial cells upregulate integrins at the tips of sprouting capillaries to collectively migrate through the surrounding tissue (230). As in the case of reepithelialization, proteolytic enzymes released into the wound tissue degrade the ECM to favor the advance of endothelial cell sprouts.
Cancer
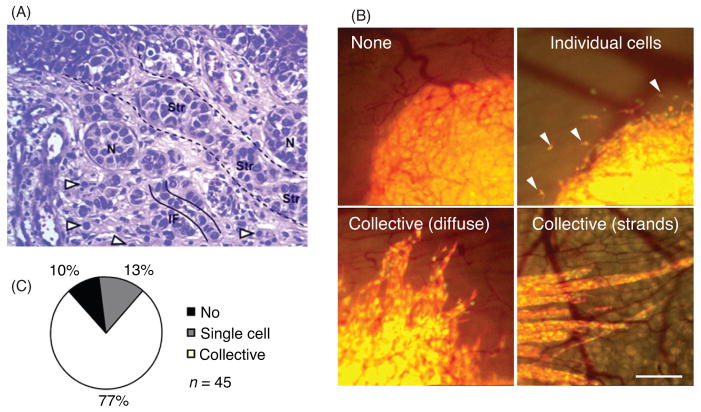
While collective cell migration is crucial in development and tissue repair, it also mediates devastating diseases such as cancer (45,75,228). The traditional view of cancer metastasis is based on the notion that single cells detach from primary tumors, crawl through the stroma, enter the blood and lymphatic vessels, and finally colonize in healthy tissues to form a secondary tumor. However, increasing evidence indicates that tumor dissemination is driven not only by single cells but also by cohesive cell groups (Fig. 6). This notion is supported by the observation that clusters of metastatic cells are often present in the blood and lymphatic vasculature of cancer patients (31, 162). In addition, histopathological sections of breast, colon, ovarian, lung, and other differentiated carcinomas exhibit clusters, chains, and sheets in the stromal areas surrounding primary tumors (141, 171, 185, 291).
Figure 6.
Collective cell migration in cancer. (A) Different invasion patterns in primary melanoma invading the mid-dermis in vivo. Arrowheads indicate scattered individual cells. Collective invasion modes include solid stands (Str), nests (N) representing cross-sectioned strands, and single cell chains (IF, “Indian files”). H&E staining. Image modified, with permission, from Friedl and Wolf (78). (B) Invasion modes in a modified skin-fold chamber model of orthotopic invasion of human HT-1080 fibrosarcoma cells. Patterns include lack of invasion (top, left), disseminating single cells (top, right), and diffuse or compact strand-like collective invasion (lower panels). Bar 250 μm. (C) Frequency of invasion modes displayed in B. Adapted, with permission, from Alexander et al. (7).
One successful strategy to study the role of these cohesive cell aggregates in cancer metastasis has been to analyze the dynamics of neoplastic tissue explants or cell line tumor spheroids in vitro (76,284,286). When embedded in 3D collagen I gels or Matrigel, these cell systems extend multicellular chains or strands into the surrounding matrix. Collective migration of this kind is initiated either by the polarization of a single cell within the cluster or by the activation of fibroblasts from the tumor stroma (84). These leading cells initiate the formation of a migration track by both cleaving and remodeling the surrounding matrix. The cooperative proteolytic activity of leading cells and their followers ultimately results in the generation of large invasive paths into the stroma (286).
Our mechanistic understanding of collective invasion in cancer is currently undergoing rapid progress thanks to the development of intravital microscopy (134). This technique enables the continuous monitoring of the dynamics of tumor tissue implanted in animal models. Typically, the implanted cells are fluorescently labeled with indicators of promoter activity, enzyme activity, or gene expression. Intravital microscopy has demonstrated the coexistence of single and collective cell invasion in a variety of organotypic cancer models. For example, implantation of HT-1080 fibrosarcoma cells in dorsal skin-fold chambers of mice showed that up to 77% of invasive events were collective (7). Cells forming such invasive sheets or strands display heterogeneous phenotypes. While innermost cells in the clusters retain epithelial polarity and cell junctions, marginal cells display mesenchymal traits such as loss of apical-basalolateral polarity, actin-rich protrusions, and proteolytic activity (83, 171). Recent studies using an organotypic model of breast mammary tumor metastasis showed that single-cell migration following dissemination from a primary tumor is relatively fast and capable of creating lung metastases via blood vessel circulation (96). By contrast, collective cell invasion is much slower and mainly invades lymph vessels. The switch from single- and collective cell invasion requires activation of a transcriptional program involving TGFβ and Smad4.
Mechanisms of collective cell migration
Adhesion
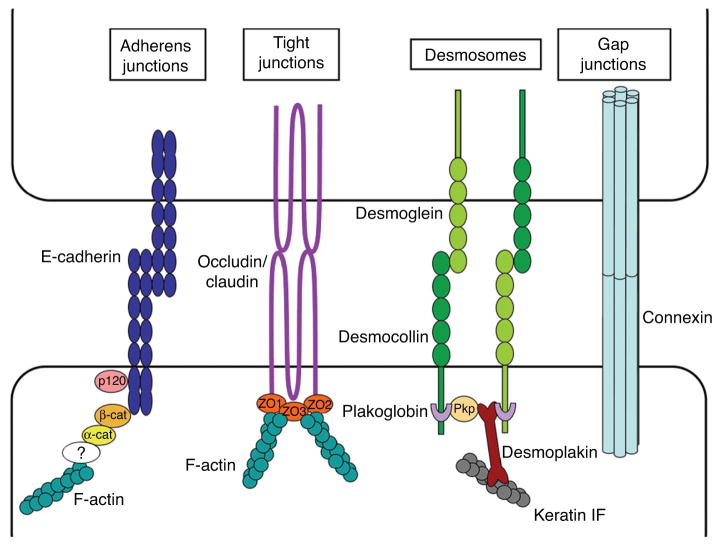
To move as cohesive groups, cells require both cell-matrix and cell-cell adhesions. To a large extent, the molecular basis of cell-matrix adhesion in collective cell migration is analogous to that of single-cell migration (see Section “Single-cell migration”). In this section, we will thus focus on the four major types of cell-cell adhesions: adherens junctions, desmosomes, tight junctions, and gap junctions (Fig. 7).
Figure 7.
Scheme depicting the key molecules that mediate cell-cell adhesion during collective cell migration.
Adherens junctions
Adherens junctions are responsible for a wide range of cellular functions including assembly and maintenance of cell-cell adhesions, stabilization of the actin cytoskeleton, and transcriptional regulation (34, 110). Adherens junctions are based on the generally homophilic interaction between transmembrane glycoproteins of the calcium-dependent cadherin family. Currently more than 350 members of this family have been described in about 30 species, with epithelial (E-) cadherin being the best characterized (123, 266). The extracellular domain of classical cadherins is composed of five domain repeats (EC1-EC5), which bind calcium ions to form parallel homodimers (187). Transpairing of the EC1 domains between cadherins from adjacent cells is required for proper conformational organization of adherens junctions (209), but other EC domains are likely to mediate cell-cell adhesion as well (269).
The cytoplasmic domain of cadherins is formed by two subdomains that mediate junctional stabilization and binding to the actin cytoskeleton (266). The subdomain that lies closer to the cell membrane is termed juxtamembrane domain (JMD). This domain contains a highly conserved octopeptide sequence that binds p120-catenin (293). The binding of p120-catenin and JMD is thought to retain cadherins at the plasma membrane thus providing stronger adhesion to the junction (255, 293). In addition to stabilizing and strengthening junctions, p120-catenin also regulates single and collective cell motility via small GTPases (199). The cytoplasmic subdomain of cadherins that lies furthest from the cell membrane binds to β-catenin with high affinity in a phosphorylation-dependent manner (110). Until recently, β-catenin was thought to provide the physical link between cadherins and the actin cytoskeleton via α-catenin. This notion was supported by evidence showing that α-catenin is able to bind both actin and the β-catenin-cadherin complex (3, 222). However, recent findings ruled out this possibility showing that β-catenin, α-catenin, and actin do not coexist in a single ternary complex (56, 290). This finding raises the question of how adherens junctions are linked to the cytoskeleton. One possibility is that this link is mediated by an additional protein, such as EPLIN, an actin binding protein recently shown to bind the C-terminal domain of monomeric α-catenin (1).
Cohesiveness is usually associated with reduced migration speed. This was clearly illustrated in recent wound-scratch screens which showed that knocking down β-catenin and other key members of the adherens junction family in MCF-10A cells caused acceleration of cell migration (242, 272). For this reason, whenever rapid migration occurs in nature, cells tend to downregulate E-cadherin and dissociate through a complete EMT. However, in many physiological situations, cells undergo an incomplete EMT in which E-cadherin adhesion is weakened to enable dynamic flexibility for each individual cell within the group while keeping a certain degree of cohesiveness. This weakening of adherens junctions is regulated by several signaling networks including those triggered by tyrosine kinases such as hepatocyte growth factor (HGF) receptor (262), epidermal growth factor receptor (EGFR), Eph receptor, Src, and Abl (104). These and other kinases regulate adherens junction strength by cadherin endocytosis, proteolysis, or interaction with other transmembrane proteins (43, 80, 202, 292). Cadherin complexes regulate actin dynamics mainly via α-catenin, which inhibits Arp2/3-mediated branching polymerization (56) and recruits the actin nucleator formin to adherens junctions (147). In addition to their role of providing junctional stability, β-catenin and p120-catenin can act as transcriptional regulators.
Tight junctions
Tight junctions are located apically from adherens junctions both in static monolayers and in migrating epithelia (152, 194). They are thought to play a double role: first, they serve as the intramembranous “fences” that separate the protein content of the apical and basolateral cell membranes; second, they are hydrophobic “barriers” that regulate transport of ions, proteins, and fluids across epithelial and endothelial layers.
Tight junctions comprise three main types of transmembrane proteins: occludins, claudins, and the IgG-like family of junctional adhesion molecules (JAMs). Occludin is a tetra-spanning-transmembrane protein with two extracellular loops that can be phosphorylated at multiple tyrosine, serine, and theonine residues (81, 110, 231). In the absence of phosphorylation, ocludins are localized throughout the basolateral cell membrane and in cytoplasmic vesicles, but phosphorylated occludins are only present at tight junctions (231). Recently, a new protein with a similar structure and role as occludin, tricellulin was found to be enriched only at tricellular tight junctions (129). Despite their ubiquitous presence in tight junctions, a number of studies demonstrated that cells and tissues deficient for occludin display proper barrier function (201). This observation led to the identification of claudins (194). Much like occludins, claudins are also tetra-span-transmembrane proteins with two extracellular loops. The claudin family comprises at least 24 members that are specifically distributed across organs and tissues (82). This distribution selectively tunes the size, strength, and transport specificity of the junctions (10). In addition to tetra-span proteins, tight junctions also contain single spanning transmembrane proteins that mediate homotypic adhesion. These proteins include the IgG-like JAMs (137), which mediate paracellular transmigration of leucocytes (61).
Given that occludins, claudins, and JAMs have not been found to interact directly, it is thought that the integrity of tight junctions is mediated by scaffolding proteins such as ZO-1, ZO-2, and ZO-3 (14). These proteins interact with claudins and occludins though their PDZ domains, and with actin through their C-terminus thus providing a direct connection between the extracellular environment and the cytoskeleton. ZO-1 can bind actin and α-catenin, which has led to the hypothesis that ZO-1 may serve as a link between adherens junctions and tight junctions (194).
Tight junctions are thought to play a central role in finely tuning apical-basolateral polarity within moving groups but mechanism remains poorly understood (68). A wealth of evidence supports that preservation of intact tight junctions prevents tumor dissemination by inhibiting cell proliferation and migration (23). However, several studies have demonstrated the existence of epithelial polarity among invasive tumors suggesting that tight junctions remain functional during certain modes of invasion (45). Tight junction proteins have also been reported to contribute to enhanced invasion and collective cell migration. For example, ZO-1 was found to be upregulated in a high proportion of highly metastatic melanoma cell lines (246). Similarly, overexpression of claudin-3 and claudin-4 in human ovarian epithelial cells resulted in increased collective migration in wound healing experiments (5). Tight junctions also play a central role during collective cell migration in various developmental processes. In Drosophila, mutations in the ZO-1 homologues result in defects in tracheal morphogenesis and in the formation of extrasensory organs (135,250). In zebrafish, the posterior LLP elicits a homogeneous distribution of cadherin among all cell-cell junctions, but ZO-1 is absent in the first few rows behind the leading edge (111). However, toward the trailing edge of the migrating primordium, the emergent proneuromast rosettes display an apical formation of tight junctions before being deposited. These findings indicate that within a cohesive moving group tight junctions can be selectively formed to control apical-basolateral polarity (156).
Desmosomes
Desmosomes are intercellular junctions that connect the intermediate filaments from adjacent cells (89, 94). They are commonly found in tissues that are subjected to substantial mechanical forces such as the epithelia and muscle. Extracellularly, desmosomes are similar to adherens junctions in that extracellular linkers are transmembrane proteins with five domain repeats homologous to classical cadherins. A functional desmosome contains at least one desmosomal cadherin from the desmocollin family and another one from the desmoglein family. The cytoplasmic domains of desmosomal cadherins interact with the armadillo proteins, plakoglobin (gamma-catenin), and plakophilins. The role of plakoglobin in its binding to desmosomal cadherins is reminiscent to that of β-catenin in adherens junctions. Plakoglobin binds to the N-terminal of desmoplakin, the protein that ultimately binds desmosomes to intermediate filaments. The role of plakophilin is more complex than that of plakoglobins as it involves interactions with desmosomal cadherins, plakoglobins, and intermediate filaments. These complex cytoplasmic interactions constitute the molecular clustering that provides strength to desmosomes.
Several lines of evidence indicate that desmosomal adhesion is regulated during collective cell migration. Upon wounding Madin-Darby canine kidney (MDCK) monolayers, desmosomes switch from a Ca2+-independent to a Ca2+-dependent phenotype. This loss of Ca2+ independence is followed by desmosome internalization mediated by protein kinase C (PKC) (90,273). The resulting loss of adhesion provides the epithelium with the flexibility required to achieve fast reepithelialization. It remains unclear, however, if the loss of desmosomal adhesion is complete or partial. In colorectal tumors, two types of desmocollins (Dsc1 and Dsc 2) are expressed de novo, suggesting collective cell invasion (143). In squamous cell carcinomas of the skin, desmoglein 2 is upregulated and the levels of desmoglein activation correlate with risk of metastasis. These findings indicate that desmosomes might remain functional during tumor invasion in some forms of cancer (45).
Gap junctions
Gap junctions are transmembrane channels that connect the cytoplasm of adjacent cells. Each cell contributes to the junction with half a channel (an hemichannel or connexon) formed by six proteins, termed connexins. Each connexin comprises four transmembrane domains connected by two extracellular loops that mediate cell-cell recognition and intercellular docking. Connexins are arranged in a cylindrical patterns that leave a hollow central channel for transit of ions, second messengers, and small metabolites with molecular weights lower than 1 kDa (180).
As central mediators for cell-cell communication, gap junctions have long been implicated in the regulation of collective cell migration during cancer metastasis, wound healing, and morphogenesis. Tumor cells from several human cancers such as skin, lung, gastric, and prostate cancers, exhibit reduced expression of gap junction proteins and reexpression of these proteins appears to play a tumorsuppressive role (63,122). For example, overexpression of Cx26 has been shown to slow down collective migration of the breast cancer cell line MCF-7 and to reverse its malignant phenotype (184). Similarly, exogenous expression of Cx26 and Cx43 also reduced migration of MDA-MB-231 and MDA-MB-435 breast cancer cells (137,178). Contrary to this widespread notion, recent evidence demonstrates that during certain stages of metastasis Cx26 is reexpressed suggesting cell cooperativity during invasion (37, 131). These findings indicate that gap junctions might selectively regulate the transition from single- to collective cell migration during different stages of cancer progression.
Further support for gap junction activity during collective cell migration comes from wound-healing experiments. During epidermal wound healing, the expression of connexins is altered as a function of the distance from the wound (100). Specifically, Cx26 was found to be downregulated at the wound edge, but upregulated away from the wound. By contrast, Cx31.1 and Cx43 were downregulated both at the wound edge and further away from the wound edge. In an in vitro wound-scratch assay using MCF-10A cells, knocking down Cx43 accelerated collective cell migration, whereas silencing Cx26 and Cx40 had no net effect (242). Perhaps the clearest illustration of the pivotal role of gap junctions in regulating collective cell migration can be found in development, where mutations or silencing of genes associated with gap junctions result in abnormal migration. For example, mutations of innexin, the connexin homolog expressed in invertebrates, prevent collective epithelial cell migration during proventriculus organogenesis in Drosophila (17). In mouse models, the levels of expression of Cx43 correlate positively with speed and directionality of neural crest migration (289). Taken together, the studies mentioned previously support the notion that gap junctions play a central role in the regulation of collective cell migration, but the molecular mechanisms underlying this regulation and the interaction between gap junctions and other cell-cell junctions remain largely unknown.
Polarization and guidance
One of the great advantages of collective versus single-cell migration is that each cell within the moving group can exhibit different patterns of expression to carry out specialized functions according to its position within the group. The simplest case of such modular specialization is front-rear polarization in which one subset of leader cells at the front guides a larger group of naïve follower cells at the rear. Polarization of this kind can arise as a consequence of internal genetic programs or external environmental cues. Leader cells typically exhibit a mesenchymal-like phenotype characterized by the extension of lamellipodia and filopodia into the surrounding tissue, a relatively loose cell-cell adhesion, enhanced expression of cell-matrix adhesion proteins, and polarized remodeling of actin filaments and MTs (65, 204). In addition, leader cells moving in 3D are capable of degrading and remodeling the ECM to create channels for the whole cell group to advance cohesively (84, 286). By contrast, followers retain epithelial features such as apical-basolateral polarity and tight junctions and express relatively low levels of guidance receptors.
A clear example of leader/follower polarization occurs during angiogenesis. Tip (leader) cells extend numerous filopodia that probe, guide, and presumably generate tractions to drive motion of the tubes to the avascular area of the embryo (93). In contrast to their follower stalk cells, tip cells are nonlumenized and mostly nonproliferative. In addition, they exhibit a clearly distinct pattern of gene expression with higher levels of expression of VEGF receptor family, which tightly controls the generation of sprouts during angiogenesis (153). While gradients of VEGF guide tip cells, the concentration of VEGF regulates proliferation in stalk cells (93). A central question in angiogenesis is how the tip cell is initially selected from a large pool of endothelial cells exposed to similar VEGF gradients. In other words, why do endothelial cells form sprouts and branches instead of sheets? The answer to these questions lies in the competitive advantage that tip cells gain by signaling their neighbors to become stalk cells. This is accomplished via the Notch signaling pathway. VEGFR2 activation causes upregulation and release of the Notch ligand Dll4 at the tip cells (113). The resulting activation of Notch in the neighboring cells leads to downregulation of VEGFR2 and ultimately the acquisition of stalk phenotype.
Another example of collective cell migration guided by a chemoattractant gradient is border cell migration. In these cells, chemoattractant gradients are sensed by two receptors, EGFR and poliovirus receptor (PVR), each of which can independently guide cell migration (58, 59). During the early phase of border cell migration, these receptors act at the sub-cellular level to drive polarization and guide migration much as in the case of single isolated cells. Each cell individually senses the gradient and acts accordingly resulting in highly persistent directional migration of the cluster. In a later phase, the very same receptors and chemoattractant cues appear to act at a higher level of organization in which the intercellular differences in the levels of signaling from the guidance receptors cells determines the identity of the front cell (19). In this case, cells within the cluster compete to guide the group thus constantly exchanging positions throughout the collective process. This guidance strategy results in slower overall motion, but it offers a broader range of possibilities to probe the cluster environment (19).
Cell groups can also migrate in the absence of chemoattractant gradients by using inherent front/rear polarization. To migrate from the anterior to the posterior regions of the embryo, the posterior LLP follows a track defined by a strip of the chemokine stromal-derived factor, SDF1a (48). Although the precise values of the concentration of SDF1a along the strip remain unknown, it appears that SDF1a is uniformly distributed. This notion is supported by the observation that the primordium is able to perform a U-turn and migrate backward (107). To perform directed migration in the absence of a chemoattractant gradient, the primordium acquires front/rear polarity through the localized activation of two SDF1a receptors, CXCR4 and CXCR7. Both receptors are required for migration of primordium but CXCR4 is active at the leading edge whereas CXCR7 is localized at the trailing edge. Polarization of these receptors is maintained through the interaction of the Wnt/β-catenin, Fgf, and Dkk pathways (8,192). In addition, recent evidence suggests that sequestration of SDF1a by CXCR7 might be a crucial event to determine the persistence of primordium migration (21).
The zebrafish LLP is also a representative model to illustrate that some cell collectives are able to achieve supracellular tissue patterning as they migrate. Roughly behind the leading third of the primordium, a group of 12 to 16 cells organizes in rosettes to form the proneuromasts (Fig. 5). Cells within these rosettes display a marked epithelial phenotype characterized by columnar morphology, apical-basolateral polarization, and the presence of foci of the tight junction protein ZO1 (156). After their formation, rosettes become progressively less motile until they are left behind and deposited at periodic length intervals. Thus the LLP illustrates that moving cell groups may display different levels of collective organization beyond front/rear polarity (264).
Mechanics of collective cell migration
Kinematic observations and models
The kinematics of collective cell migration has been the subject of experimental and theoretical investigation virtually since light microscopy was first applied to the observation of biological processes. Indeed, the first observations of tumor dissemination, growth of epithelial tissues, and wound closure date back more than one century (116, 271). The advent of modern imaging techniques such as confocal microscopy, multiphoton microscopy, and intravital imaging, together with the development of improved fluorescent probes and computational methods now enable us to quantitatively analyze the kinematics of collective cell migration in vivo (229). Outstanding advances in this field include visualization of cancer cells within a collective cluster as they escape a tumor to invade lymphatic vessels (96), or tracking of hundreds of individual cells involved in mesoderm migration during development of the Drosophila embryo (179).
The study of cell kinematics combined with a variety of continuum and discrete physical models has provided substantial advances in our understanding of how cells move collectively. Typical continuum models are governed by reaction-diffusion equations (40, 173):
| (1) |
| (2) |
where n is cell density and c is the concentration of chemotactic signal. The first term on the right hand side of Eq (1) accounts for random cell migration and the second term models chemotaxis (or haptotaxis). In chemotaxis, for example, the chemoattractant is expressed or bound to a matrix, χ is the chemotactic sensitivity, which may be function of c, and the function f describes mitotic generation and natural cell loss. Conversely, the first term on the right hand side of Eq. (2) accounts for diffusion of the tactic agent, and g(n,c) captures its production, uptake, and degradation. This system of reaction diffusion equations can be generalized to any number of cell types and tactic agents present in the system under investigation. Further coupling between equations can be obtained by taking the diffusion coefficient as a function of cell concentration (29, 46, 205).
Continuum models are limited by their relative inability to take into account dynamics of cell adhesion as well as local variability of the cell mechanical properties. These factors are introduced in the so-called vertex models or cellular Potts models, in which cell geometry is discretized and different mechanical and adhesive properties can be associated with each constitutive element of the system (98, 103). In these models, the cell collective explores an energy landscape determined by a 3-term Hamiltonian (H):
| (3) |
The first term on the right-hand side, Hadhesion, embodies the adhesive energy associated with cell-cell and cell-matrix interactions, the second term, Hdeformation, accounts for the deviation of cell shape from a “preferred” geometry, and the third term, Hchemotaxis, models tendency of each cell to move toward maximum concentrations of chemotactic agents. For a given Hamiltonian, the dynamics of the system are obtained by energy minimization using Monte-Carlo strategies. Each element in the system is sampled in a random manner and given a new configuration. If this new configuration decreases the energy of the system the change is always accepted, otherwise it accepted with a given probability. This approach can be coupled to continuum reaction-diffusion equations to take into account the dynamics of diffusive chemicals associated with chemotaxis (233).
The modeling approaches described previously have been successful in reproducing the kinematics of collective cell migration in a variety of biological processes including tumor angiogenesis, wound healing, and cell sorting (40,103,205,238). But even if the kinetics is captured, the underlying mechanisms remain far from being elucidated. Consider, for example, the relatively simple case of 2D wound-scratch assays. The kinematics of this process has been captured by continuum and discrete models in which the key ingredient is the establishment of a chemotactic gradient (205,238). However, other models based on purely mechanical principles in which chemotaxis has been deliberately ignored, have also been able to reproduce experimental data in great detail (20,174). Thus, models of collective cell migration need to formulate further experimentally testable predictions to elucidate the relative contribution of both the biochemical and the biophysical mechanisms that drive collective cell migration.
Dynamics
Our relatively poor understanding of the mechanisms underlying collective cell migration is not so much due to the lack of suitable physical models as it is due to the lack of key experimental information. Perhaps the most important piece of experimental information we are lacking is a physical picture of the forces that drive collective cell migration. Without this information, it is not possible to determine whether collective cell motion is a local process in which each cell is mechanically self-propelled or an integrated process in which physical forces are transmitted across long distances to coordinate the action of each individual cell within a moving group.
The technique that has provided most information about the dynamics of living tissues in vivo is laser microsurgery. This technique is based on the selective ablation of single cells within tissues. The analysis of the resulting tissue relaxation enables the inference of the actual state of stress of the tissue (170). By applying this technique to dorsal closure in Drosophila embryos, Kiehart et al. showed that both the leading edge of the lateral epidermis and the amnioserosa are under tension (144). In a later study, Hutson et al. used straightforward force balance at the leading edge to conclude that the tensile contribution of amnioserosa and the epidermal tissues is of similar magnitude while the contribution of actin ring contraction is 2-fold higher (125). Other findings obtained by laser ablation include the measurement of contractile forces associated with cell apoptosis (258), and the guidance of tissue morphogenesis by anisotropic forces (217).
While laser ablation methods enable the inference of the state of stress of tissues as well as their dynamic relaxation, they do not provide maps of the forces associated with cell migration. The first such maps were obtained by the joint of effort of the groups of B. Ladoux and P. Silberzan (57). The authors seeded epithelial cell colonies on top of a micropillar array and observed the time evolution of the forces exerted by the cells on the pillars. They showed that forces at the leading edge are tensile, thus ruling out that the epithelial tissue advances as a result of pushing forces from submarginal cells (Fig. 8). In addition, the authors remarked that submarginal cells are also able to generate traction forces of substantial magnitude, which is consistent with the observation by Farooqui and Fenteany that submarginal cells extend cryptic lamellipodia under their neighbors at the margin (71). A later study by Trepat et al. showed that not only submarginal cells are able to generate substantial traction forces, but also that these forces are integrated over long distances to generate a stress gradient at cell-cell junctions (260). The existence of such stress gradients combined with mechanotransduction events at cell-cell junctions might provide novel mechanisms of positional sensing within moving groups.
Figure 8.
Mechanics of collective cell migration. (A) The forces exerted by the leading edge of an MDCK epithelial cell sheet migrating on top of a microneedle array are tensile. Adapted, with permission, from reference 57. (B, C, D) Patterns of force generation and transmission in an epithelial cell sheet. (B) An active leader cell generates forces at the leading edge and transmits these forces to follower cells via cell-cell junctions. (C) Each cell within the monolayer generates its own contractile forces. Forces are balanced locally in such a way that there is no force transmission through cell-cell junctions. (D) Tug-of-war force generation and transmission. The local tractions that each cell generates are transmitted through cell-cell junctions to generate a global gradient of tensile stress. (E) Phase contrast image of an MDCK cell sheet advancing on top of a soft polyacrylmide gel (1.2 kPa). In this model, tractions parallel (F) and perpendicular (G) to the leading edge rule out the existence of leader/follower polarity.
Recently, force microscopy technology was improved to enable the measurement of inter- and intracellular forces (164, 177, 251). Starting with tractions at cell-substrate interface and using straightforward force-balance imposed by Newton’s laws, Tambe et al. developed monolayer stress microscopy (MSM) to map the state of stress at any point within a monolayer (251). Using this technology the authors showed that intracellular stresses vary abruptly across a migrating monolayer sheet, and that force transmission through cell-cell junctions expands several cell diameters. In addition, the authors showed in a variety of cell types that cell collectives move along the direction of maximum normal stress—or, equivalently, minimum shear stress. This mode of collective guidance was called plithotaxis, from the Greek πληΘος denoting crowd, swarm, or throng (251).
Outlook
Undoubtedly, the field of cell migration will produce exciting science over the next decade, at the least. Advances in optical techniques to image tissues deep within the organism, combined with expression of FP fusions to key proteins will enable us to perform detailed, real-time studies of the migration of cells in their native physiological environment. Super-resolution microscopy will reveal the 3D molecular architecture of elements of the migratory cell. Biosensors will produce high-resolution spatial and temporal maps of the molecular activities that underlie cell migration. Photomanipulation of proteins involved in cell motility by either photactivation or chromophore assisted laser inactivation of selected proteins will illuminate their precise roles in migration. Structural biology and biophysical tools will identify the structure and mechanics of individual molecules important to cell movement. Multicenter efforts in genomics and proteomics will provide a comprehensive census of migration related proteins, their binding partners, their phosphorylation sites, and their posttranslation modifications in diverse tissues and pathological conditions. This effort will be paralleled by the development of genetically engineered animals, from invertebrates to mammals, to study the functional role of each of these proteins. High throughput screening of chemical libraries will help identify novel compounds that may impair the progression of migration-related diseases such as cancer or chronic inflammation. Multidisciplinary approaches will unravel how the chemical composition and the physical architecture of the ECM regulates the migratory machinery and how this machinery in turn impacts the matrix on which and through which cells migrate.
Each of the tasks highlighted previously is a formidable challenge on its own but perhaps the most daunting goal ahead lies in integrating all of this forthcoming information. The amount high quality data relevant to cell migration will increase by orders of magnitude in the next decade but a proper conceptual framework that integrates this information into comprehensive models with predictive power remains unknown. While this limitation is a general problem in all biology, it is particularly fundamental in the field of cell migration in which any integrative picture must include not only comprehensive networks that are purely chemical in nature, but also the interaction of such networks with physical forces.
Acknowledgments
Xavier Trepat acknowledges support or the Spanish Ministry for Science and Innovation (BFU2009-07595), the European Research Council (Grant Agreement 242993), and the Gener-alitat de Catalunya. Ken Jacobson and Zaozao Chen acknowledge the support of the NIH Cell Migration Consortium grant GM-064346.
References
- 1.Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A. 2008;105:13–19. doi: 10.1073/pnas.0710504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. 3. Movements of particles on the dorsal surface of the leading lamella. Exp Cell Res. 1970;62:389–398. doi: 10.1016/0014-4827(70)90570-7. [DOI] [PubMed] [Google Scholar]
- 3.Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107(Pt 12):3655–3663. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- 4.Abrams EW, Vining MS, Andrew DJ. Constructing an organ: The Drosophila salivary gland as a model for tube formation. Trends Cell Biol. 2003;13:247–254. doi: 10.1016/s0962-8924(03)00055-2. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R, D’Souza T, Morin PJ. Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res. 2005;65:7378–7385. doi: 10.1158/0008-5472.CAN-05-1036. [DOI] [PubMed] [Google Scholar]
- 6.Alberts B, Wilson JH, Hunt T. Molecular Biology of the Cell. New York: Garland Science; 2008. [Google Scholar]
- 7.Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P. Dynamic imaging of cancer growth and invasion: A modified skin-fold chamber model. Histochem Cell Biol. 2008;130:1147–1154. doi: 10.1007/s00418-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 8.Aman A, Piotrowski T. Wnt/beta-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression. Dev Cell. 2008;15:749–761. doi: 10.1016/j.devcel.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Amatangelo MD, Bassi DE, Klein-Szanto AJ, Cukierman E. Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts. Am J Pathol. 2005;167:475–488. doi: 10.1016/S0002-9440(10)62991-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson JM, Van Itallie CM, Fanning AS. Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol. 2004;16:140–145. doi: 10.1016/j.ceb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Anderson KI, Cross R. Contact dynamics during keratocyte motility. Curr Biol. 2000;10:253–260. doi: 10.1016/s0960-9822(00)00357-2. [DOI] [PubMed] [Google Scholar]
- 12.Angelini TE, Hannezo E, Trepat X, Fredberg JJ, Weitz DA. Cell migration driven by cooperative substrate deformation patterns. Phys Rev Lett. 2010;104:168104. doi: 10.1103/PhysRevLett.104.168104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 14.Balda MS, Matter K. Tight junctions at a glance. J Cell Sci. 2008;121:3677–3682. doi: 10.1242/jcs.023887. [DOI] [PubMed] [Google Scholar]
- 15.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baruzzi A, Iacobucci I, Soverini S, Lowell CA, Martinelli G, Berton G. c-Abl and Src-family kinases cross-talk in regulation of myeloid cell migration. FEBS Lett. 2010;584:15–21. doi: 10.1016/j.febslet.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer R, Lehmann C, Fuss B, Eckardt F, Hoch M. The Drosophila gap junction channel gene innexin 2 controls foregut development in response to Wingless signalling. J Cell Sci. 2002;115:1859–1867. doi: 10.1242/jcs.115.9.1859. [DOI] [PubMed] [Google Scholar]
- 18.Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153:881–888. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bianco A, Poukkula M, Cliffe A, Mathieu J, Luque CM, Fulga TA, Rorth P. Two distinct modes of guidance signalling during collective migration of border cells. Nature. 2007;448:362–365. doi: 10.1038/nature05965. [DOI] [PubMed] [Google Scholar]
- 20.Bindschadler M, McGrath JL. Sheet migration by wounded monolayers as an emergent property of single-cell dynamics. J Cell Sci. 2007;120:876–884. doi: 10.1242/jcs.03395. [DOI] [PubMed] [Google Scholar]
- 21.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q, Raz E. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Brass LF. Thrombin and platelet activation. Chest. 2003;124:18S–25S. doi: 10.1378/chest.124.3_suppl.18s. [DOI] [PubMed] [Google Scholar]
- 23.Brennan K, Offiah G, McSherry EA, Hopkins AM. Tight junctions: A barrier to the initiation and progression of breast cancer? J Biomed Biotechnol. 2010;2010:460607. doi: 10.1155/2010/460607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol. 2008;20:85–90. doi: 10.1016/j.ceb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Buccione R, Orth JD, McNiven MA. Foot and mouth: Podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 26.Buechner M. Tubes and the single C. elegans excretory cell. Trends Cell Biol. 2002;12:479–484. doi: 10.1016/s0962-8924(02)02364-4. [DOI] [PubMed] [Google Scholar]
- 27.Burridge K, Connell L. A new protein of adhesion plaques and ruffling membranes. J Cell Biol. 1983;97:359–367. doi: 10.1083/jcb.97.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler JP, Tolic-Norrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol. 2002;282:C595–C605. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 29.Cai AQ, Landman KA, Hughes BD. Multi-scale modeling of a wound-healing cell migration assay. J Theor Biol. 2007;245:576–594. doi: 10.1016/j.jtbi.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 31.Carr I. Experimental lymphatic metastasis. J Microsc. 1983;131:211–220. doi: 10.1111/j.1365-2818.1983.tb04247.x. [DOI] [PubMed] [Google Scholar]
- 32.Carragher NO, Levkau B, Ross R, Raines EW. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J Cell Biol. 1999;147:619–630. doi: 10.1083/jcb.147.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caswell PT, Vadrevu S, Norman JC. Integrins: Masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 34.Cavey M, Lecuit T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb Perspect Biol. 2009;1:a002998. doi: 10.1101/cshperspect.a002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coles EG, Taneyhill LA, Bronner-Fraser M. A critical role for Cadherin6B in regulating avian neural crest emigration. Dev Biol. 2007;312:533–544. doi: 10.1016/j.ydbio.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coraux C, Roux J, Jolly T, Birembaut P. Epithelial cell-extracellular matrix interactions and stem cells in airway epithelial regeneration. Proc Am Thor Soc. 2008;5:689–694. doi: 10.1513/pats.200801-010AW. [DOI] [PubMed] [Google Scholar]
- 37.Cronier L, Crespin S, Strale PO, Defamie N, Mesnil M. Gap junctions and cancer: New functions for an old story. Antioxid Redox Signal. 2009;11:323–338. doi: 10.1089/ars.2008.2153. [DOI] [PubMed] [Google Scholar]
- 38.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 39.Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–1691. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- 40.Chaplain MA, McDougall SR, Anderson AR. Mathematical modeling of tumor-induced angiogenesis. Ann Rev Biomed Eng. 2006;8:233–257. doi: 10.1146/annurev.bioeng.8.061505.095807. [DOI] [PubMed] [Google Scholar]
- 41.Chen W-T. Induction of spreading during fibroblast movement. J Cell Biol. 1979;81:684–691. doi: 10.1083/jcb.81.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen WT, Wang JY. Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization. Ann N Y Acad Sci. 1999;878:361–371. doi: 10.1111/j.1749-6632.1999.tb07695.x. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Gumbiner BM. Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity. J Cell Biol. 2006;174:301–313. doi: 10.1083/jcb.200602062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10:1039–1050. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 46.Dale PD, Maini PK, Sherratt JA. Mathematical modeling of corneal epithelial wound healing. Math Biosci. 1994;124:127–147. doi: 10.1016/0025-5564(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 47.Damm EW, Winklbauer R. PDGF-A controls mesoderm cell orientation and radial intercalation during Xenopus gastrulation. Development. 2011;138:565–575. doi: 10.1242/dev.056903. [DOI] [PubMed] [Google Scholar]
- 48.David NB, Sapede D, Saint-Etienne L, Thisse C, Thisse B, Dambly-Chaudiere C, Rosa FM, Ghysen A. Molecular basis of cell migration in the fish lateral line: Role of the chemokine receptor CXCR4 and of its ligand, SDF1. Proc Natl Acad Sci U S A. 2002;99:16297–16302. doi: 10.1073/pnas.252339399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davies JA. Watching tubules glow and branch. Curr Opin Genet Dev. 2005;15:364–370. doi: 10.1016/j.gde.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 50.de Beus E, Jacobson K. Integrin involvement in keratocyte locomotion. Cell Motil Cytoskeleton. 1998;41:126–137. doi: 10.1002/(SICI)1097-0169(1998)41:2<126::AID-CM4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 51.del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RG, Schwartz MA. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dembo M, Oliver T, Jacobson K. Imaging the traction stresses exerted by locomoting cells with the elastic substratum method. Biophys J. 1996;70:2008–2022. doi: 10.1016/S0006-3495(96)79767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dembo M, Wang Y-L. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Digman MA, Gratton E. Analysis of diffusion and binding in cells using the RICS approach. Microsc Res Tech. 2009;72:323–332. doi: 10.1002/jemt.20655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Digman MA, Wiseman PW, Choi C, Horwitz AR, Gratton E. Stoichiometry of molecular complexes at adhesions in living cells. Proc Natl Acad Sci U S A. 2009;106:2170–2175. doi: 10.1073/pnas.0806036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.du Roure O, Saez A, Buguin A, Austin RH, Chavrier P, Siberzan P, Ladoux B. Force mapping in epithelial cell migration. Proc Natl Acad Sci U S A. 2005;102:2390–2395. doi: 10.1073/pnas.0408482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duchek P, Rorth P. Guidance of cell migration by EGF receptor signaling during Drosophila oogenesis. Science. 2001;291:131–133. doi: 10.1126/science.291.5501.131. [DOI] [PubMed] [Google Scholar]
- 59.Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107:17–26. doi: 10.1016/s0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 60.Dunn GA. Mechanisms of fibroblast locomotion. In: Curtis ASG, Pitts JD, editors. Cell Adhesion and Motility; 3rd Symposium of the British Society for Cell Biology; Cambridge: Cambridge University Press; 1980. pp. 409–423. [Google Scholar]
- 61.Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): More molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 62.Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 63.Eghbali B, Kessler JA, Reid LM, Roy C, Spray DC. Involvement of gap junctions in tumorigenesis: Transfection of tumor cells with connexin 32 cDNA retards growth in vivo. Proc Natl Acad Sci U S A. 1991;88:10701–10705. doi: 10.1073/pnas.88.23.10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddington RC. Structural basis of collagen recognition by integrin alpha2beta1. Cell. 2000;101:47–56. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- 65.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 66.Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol. 2007;9:299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- 67.Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17:524–532. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 68.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–747. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–590. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- 71.Farooqui R, Fenteany G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J Cell Sci. 2005;118:51–63. doi: 10.1242/jcs.01577. [DOI] [PubMed] [Google Scholar]
- 72.Feramisco JR, Smart JE, Burridge K, Helfman DM, Thomas GP. Coexistence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982;257:11024–11031. [PubMed] [Google Scholar]
- 73.Fournier MF, Sauser R, Ambrosi D, Meister JJ, Verkhovsky AB. Force transmission in migrating cells. J Cell Biol. 2010;188:287–297. doi: 10.1083/jcb.200906139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Franke JD, Montague RA, Kiehart DP. Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Curr Biol. 2005;15:2208–2221. doi: 10.1016/j.cub.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 75.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 76.Friedl P, Noble PB, Walton PA, Laird DW, Chauvin PJ, Tabah RJ, Black M, Zanker KS. Migration of coordinated cell clusters in mesenchymal and epithelial cancer explants in vitro. Cancer Res. 1995;55:4557–4560. [PubMed] [Google Scholar]
- 77.Friedl P, Wolf K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 78.Friedl P, Wolf K. Tube travel: The role of proteases in individual and collective cancer cell invasion. Cancer Res. 2008;68:7247–7249. doi: 10.1158/0008-5472.CAN-08-0784. [DOI] [PubMed] [Google Scholar]
- 79.Friedl P, Wolf K. Plasticity of cell migration: A multiscale tuning model. J Cell Biol. 2010;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 81.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: A novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 83.Gaggioli C. Collective invasion of carcinoma cells: When the fibroblasts take the lead. Cell Adh Migr. 2008;2:45–47. doi: 10.4161/cam.2.1.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 85.Galbraith CG, Sheetz MP. A micromachined device provides a new bend on fibroblast traction forces. Proc Natl Acad Sci U S A. 1997;94:9114–9118. doi: 10.1073/pnas.94.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Galbraith CG, Sheetz MP. Keratocytes pull with similar forces on their dorsal and ventral surfaces. J Cell Biol. 1999;147:1313–1324. doi: 10.1083/jcb.147.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gardel ML, Sabass B, Ji L, Danuser G, Schwarz US, Waterman CM. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J Cell Biol. 2008;183:999–1005. doi: 10.1083/jcb.200810060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta. 2008;1778:572–587. doi: 10.1016/j.bbamem.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 90.Garrod DR, Berika MY, Bardsley WF, Holmes D, Tabernero L. Hyperadhesion in desmosomes: Its regulation in wound healing and possible relationship to cadherin crystal structure. J Cell Sci. 2005;118:5743–5754. doi: 10.1242/jcs.02700. [DOI] [PubMed] [Google Scholar]
- 91.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 92.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 93.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- 95.Ghysen A, Dambly-Chaudiere C. The lateral line microcosmos. Genes Dev. 2007;21:2118–2130. doi: 10.1101/gad.1568407. [DOI] [PubMed] [Google Scholar]
- 96.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008;20:235–241. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 98.Glazier JA, Graner F. Simulation of the differential adhesion driven rearrangement of biological cells. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1993;47:2128–2154. doi: 10.1103/physreve.47.2128. [DOI] [PubMed] [Google Scholar]
- 99.Gligorijevic B, Condeelis J. Stretching the timescale of intravital imaging in tumors. Cell Adh Migr. 2009;3:313–315. doi: 10.4161/cam.3.4.9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goliger JA, Paul DL. Wounding alters epidermal connexin expression and gap junction-mediated intercellular communication. Mol Biol Cell. 1995;6:1491–1501. doi: 10.1091/mbc.6.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goodrich HB. Cell behaviour in tissue cultures. Biol Bull. 1924:252–262. [Google Scholar]
- 102.Grande-Garcia A, Echarri A, de Rooij J, Alderson NB, Waterman-Storer CM, Valdivielso JM, del Pozo MA. Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GT-Pases. J Cell Biol. 2007;177:683–694. doi: 10.1083/jcb.200701006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Graner F, Glazier JA. Simulation of biological cell sorting using a two-dimensional extended Potts model. Phys Rev Lett. 1992;69:2013–2016. doi: 10.1103/PhysRevLett.69.2013. [DOI] [PubMed] [Google Scholar]
- 104.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 105.Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 106.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 107.Haas P, Gilmour D. Chemokine Signaling Mediates Self-Organizing Tissue Migration in the Zebrafish Lateral Line. Elsevier; 2006. pp. 673–680. [DOI] [PubMed] [Google Scholar]
- 108.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 109.Harris A, Wild P, Stopak D. Silicone rubber substrata: A new wrinkle in the study of cell locomotion. Science. 1980;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 110.Hartsock A, Nelson WJ. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hava D, Forster U, Matsuda M, Cui S, Link BA, Eichhorst J, Wiesner B, Chitnis A, Abdelilah-Seyfried S. Apical membrane maturation and cellular rosette formation during morphogenesis of the zebrafish lateral line. J Cell Sci. 2009;122:687–695. doi: 10.1242/jcs.032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heath JP, Peachey LD. Morphology of fibroblasts in collagen gels: A study using 400 keV electron microscopy and computer graphics. Cell Motil Cytoskeleton. 1989;14:382–392. doi: 10.1002/cm.970140308. [DOI] [PubMed] [Google Scholar]
- 113.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 114.Hernandez SE, Krishnaswami M, Miller AL, Koleske AJ. How do Abl family kinases regulate cell shape and movement? Trends Cell Biol. 2004;14:36–44. doi: 10.1016/j.tcb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 115.Hertle MD, Kubler MD, Leigh IM, Watt FM. Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest. 1992;89:1892–1901. doi: 10.1172/JCI115794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holmes SJ. The behavior of the epidermis of amphibians when cultivated outside the body. J Exp Zool. 1914;17:281–295. [Google Scholar]
- 117.Hu H, Marton TF, Goodman CS. Plexin B mediates axon guidance in Drosophila by simultaneously inhibiting active Rac and enhancing RhoA signaling. Neuron. 2001;32:39–51. doi: 10.1016/s0896-6273(01)00453-6. [DOI] [PubMed] [Google Scholar]
- 118.Huang C. Roles of E3 ubiquitin ligases in cell adhesion and migration. Cell Adh Migr. 2010;4:10–18. doi: 10.4161/cam.4.1.9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang C, Jacobson K, Schaller MD. A role for JNK-paxillin signaling in cell migration. Cell Cycle. 2004;3:4–6. [PubMed] [Google Scholar]
- 120.Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–223. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- 121.Huang C, Rajfur Z, Yousefi N, Chen Z, Jacobson K, Ginsberg MH. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat Cell Biol. 2009;11:624–630. doi: 10.1038/ncb1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang RP, Fan Y, Hossain MZ, Peng A, Zeng ZL, Boynton AL. Reversion of the neoplastic phenotype of human glioblastoma cells by connexin 43 (cx43) Cancer Res. 1998;58:5089–5096. [PubMed] [Google Scholar]
- 123.Hulpiau P, van Roy F. Molecular evolution of the cadherin superfamily. Int J Biochem Cell Biol. 2009;41:349–369. doi: 10.1016/j.biocel.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 124.Humphries MJ. Integrin structure. Biochem Soc Trans. 2000;28:311–339. [PubMed] [Google Scholar]
- 125.Hutson MS, Tokutake Y, Chang MS, Bloor JW, Venakides S, Kiehart DP, Edwards GS. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science. 2003;300:145–149. doi: 10.1126/science.1079552. [DOI] [PubMed] [Google Scholar]
- 126.Huttenlocher A, Palecek SP, Lu Q, Zhang W, Mellgren RL, Lauffenburger DA, Ginsberg MH, Horwitz AF. Regulation of cell migration by the calcium-dependent protease calpain. J Biol Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- 127.Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 128.Hynes RO, Lander AD. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992;68:303–322. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- 129.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 131.Ito A, Koma Y, Uchino K, Okada T, Ohbayashi C, Tsubota N, Okada M. Increased expression of connexin 26 in the invasive component of lung squamous cell carcinoma: Significant correlation with poor prognosis. Cancer Lett. 2006;234:239–248. doi: 10.1016/j.canlet.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 132.Izzard CS, Lochner LR. Cell-to-substrate contacts in living fibroblasts: An interference reflexion study with an evaluation of the technique. J Cell Sci. 1976;21:129–159. doi: 10.1242/jcs.21.1.129. [DOI] [PubMed] [Google Scholar]
- 133.Jacinto A, Wood W, Balayo T, Turmaine M, Martinez-Arias A, Martin P. Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr Biol. 2000;10:1420–1426. doi: 10.1016/s0960-9822(00)00796-x. [DOI] [PubMed] [Google Scholar]
- 134.Jain RK, Munn LL, Fukumura D. Dissecting tumour pathophysiology using intravital microscopy. Nat Rev Cancer. 2002;2:266–276. doi: 10.1038/nrc778. [DOI] [PubMed] [Google Scholar]
- 135.Jung AC, Ribeiro C, Michaut L, Certa U, Affolter M. Polychaetoid/ZO-1 is required for cell specification and rearrangement during Drosophila tracheal morphogenesis. Curr Biol. 2006;16:1224–1231. doi: 10.1016/j.cub.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 136.Jurado C, Haserick JR, Lee J. Slipping or gripping? Fluorescent speckle microscopy in fish keratocytes reveals two different mechanisms for generating a retrograde flow of actin. Mol Biol Cell. 2005;16:507–518. doi: 10.1091/mbc.E04-10-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kalra J, Shao Q, Qin H, Thomas T, Alaoui-Jamali MA, Laird DW. Cx26 inhibits breast MDA-MB-435 cell tumorigenic properties by a gap junctional intercellular communication-independent mechanism. Carcinogenesis. 2006;27:2528–2537. doi: 10.1093/carcin/bgl110. [DOI] [PubMed] [Google Scholar]
- 138.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146:1033–1044. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kawakami T, Nabeshima K, Hamasaki M, Iwasaki A, Shirakusa T, Iwasaki H. Small cluster invasion: A possible link between micropapillary pattern and lymph node metastasis in pT1 lung adenocarcinomas. Virchows Arch. 2009;454:61–70. doi: 10.1007/s00428-008-0695-5. [DOI] [PubMed] [Google Scholar]
- 142.Keren K, Yam PT, Kinkhabwala A, Mogilner A, Theriot JA. Intracellular fluid flow in rapidly moving cells. Nat Cell Biol. 2009;11:1219–1224. doi: 10.1038/ncb1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Khan K, Hardy R, Haq A, Ogunbiyi O, Morton D, Chidgey M. Desmocollin switching in colorectal cancer. Br J Cancer. 2006;95:1367–1370. doi: 10.1038/sj.bjc.6603453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–490. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- 146.Kobayashi H, Aiba S, Yoshino Y, Tagami H. Acute cutaneous barrier disruption activates epidermal p44/42 and p38 mitogen-activated protein kinases in human and hairless guinea pig skin. Exp Dermatol. 2003;12:734–746. doi: 10.1111/j.0906-6705.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 147.Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kole TP, Tseng Y, Jiang I, Katz JL, Wirtz D. Intracellular mechanics of migrating fibroblasts. Mol Biol Cell. 2005;16:328–338. doi: 10.1091/mbc.E04-06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: Regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 150.Krylyshkina O, Anderson KI, Kaverina I, Upmann I, Manstein DJ, Small JV, Toomre DK. Nanometer targeting of microtubules to focal adhesions. J Cell Biol. 2003;161:853–859. doi: 10.1083/jcb.200301102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 152.Langbein L, Pape UF, Grund C, Kuhn C, Praetzel S, Moll I, Moll R, Franke WW. Tight junction-related structures in the absence of a lumen: Occludin, claudins and tight junction plaque proteins in densely packed cell formations of stratified epithelia and squamous cell carcinomas. Eur J Cell Biol. 2003;82:385–400. doi: 10.1078/0171-9335-00330. [DOI] [PubMed] [Google Scholar]
- 153.Larrivee B, Freitas C, Suchting S, Brunet I, Eichmann A. Guidance of vascular development: Lessons from the nervous system. Circ Res. 2009;104:428–441. doi: 10.1161/CIRCRESAHA.108.188144. [DOI] [PubMed] [Google Scholar]
- 154.Laudanna C, Campbell JJ, Butcher EC. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981–983. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- 155.Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153:1427–1440. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lecaudey V, Cakan-Akdogan G, Norton WH, Gilmour D. Dynamic Fgf signaling couples morphogenesis and migration in the zebrafish lateral line primordium. Development. 2008;135:2695–2705. doi: 10.1242/dev.025981. [DOI] [PubMed] [Google Scholar]
- 157.Lee J, Ishihara A, Theriot JA, Jacobson K. Principles of locomotion for simple-shaped cells. Nature. 1993;362:167–171. doi: 10.1038/362167a0. [DOI] [PubMed] [Google Scholar]
- 158.Lee J, Jacobson K. The composition and dynamics of cell-substratum adhesions in locomoting fish keratocytes. J Cell Sci. 1997;110(Pt 22):2833–2844. doi: 10.1242/jcs.110.22.2833. [DOI] [PubMed] [Google Scholar]
- 159.Lee J, Leonard M, Oliver T, Ishihara A, Jacobson K. Traction forces generated by locomoting keratocytes. J Cell Biol. 1994;127:1957–1964. doi: 10.1083/jcb.127.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Linder S. The matrix corroded: Podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 161.Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci U S A. 1999;96:9648–9653. doi: 10.1073/pnas.96.17.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36:889–894. [PubMed] [Google Scholar]
- 163.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: An imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 164.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lo SH, Chen LB. Focal adhesion as a signal transduction organelle. Cancer Metastasis Rev. 1994;13:9–24. doi: 10.1007/BF00690415. [DOI] [PubMed] [Google Scholar]
- 167.Lo SH, Janmey PA, Hartwig JH, Chen LB. Interactions of tensin with actin and identification of its three distinct actin-binding domains. J Cell Biol. 1994;125:1067–1075. doi: 10.1083/jcb.125.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lu P, Werb Z. Patterning mechanisms of branched organs. Science. 2008;322:1506–1509. doi: 10.1126/science.1162783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Llense F, Martin-Blanco E. JNK signaling controls border cell cluster integrity and collective cell migration. Curr Biol. 2008;18:538–544. doi: 10.1016/j.cub.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 170.Ma X, Lynch HE, Scully PC, Hutson MS. Probing embryonic tissue mechanics with laser hole drilling. Phys Biol. 2009;6:036004. doi: 10.1088/1478-3975/6/3/036004. [DOI] [PubMed] [Google Scholar]
- 171.Macpherson IR, Hooper S, Serrels A, McGarry L, Ozanne BW, Harrington K, Frame MC, Sahai E, Brunton VG. p120-catenin is required for the collective invasion of squamous cell carcinoma cells via a phosphorylation-independent mechanism. Oncogene. 2007;26:5214–5228. doi: 10.1038/sj.onc.1210334. [DOI] [PubMed] [Google Scholar]
- 172.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mantzaris NV, Webb S, Othmer HG. Mathematical modeling of tumor-induced angiogenesis. J Math Biol. 2004;49:111–187. doi: 10.1007/s00285-003-0262-2. [DOI] [PubMed] [Google Scholar]
- 174.Mark S, Shlomovitz R, Gov NS, Poujade M, Grasland-Mongrain E, Silberzan P. Physical model of the dynamic instability in an expanding cell culture. Biophys J. 2010;98:361–370. doi: 10.1016/j.bpj.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 176.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- 177.Maruthamuthu V, Aratyn-Schaus Y, Gardel ML. Conserved F-actin dynamics and force transmission at cell adhesions. Curr Opin Cell Biol. 2010;22:583–588. doi: 10.1016/j.ceb.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McLachlan E, Shao Q, Wang HL, Langlois S, Laird DW. Connexins act as tumor suppressors in three-dimensional mammary cell organoids by regulating differentiation and angiogenesis. Cancer Res. 2006;66:9886–9894. doi: 10.1158/0008-5472.CAN-05-4302. [DOI] [PubMed] [Google Scholar]
- 179.McMahon A, Supatto W, Fraser SE, Stathopoulos A. Dynamic analyses of drosophila gastrulation provide insights into collective cell migration. Science. 2008;322:1546–1550. doi: 10.1126/science.1167094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mese G, Richard G, White TW. Gap junctions: Basic structure and function. J Invest Dermatol. 2007;127:2516–2524. doi: 10.1038/sj.jid.5700770. [DOI] [PubMed] [Google Scholar]
- 181.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: In command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 182.Mogilner A, Oster G. Cell motility driven by actin polymerization. Biophys J. 1996;71:3030–3045. doi: 10.1016/S0006-3495(96)79496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mogilner A, Oster G. Force generation by actin polymerization II: The elastic ratchet and tethered filaments. Biophys J. 2003;84:1591–1605. doi: 10.1016/S0006-3495(03)74969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Momiyama M, Omori Y, Ishizaki Y, Nishikawa Y, Tokairin T, Ogawa J, Enomoto K. Connexin26-mediated gap junctional communication reverses the malignant phenotype of MCF-7 breast cancer cells. Cancer Sci. 2003;94:501–507. doi: 10.1111/j.1349-7006.2003.tb01473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nabeshima K, Inoue T, Shimao Y, Kataoka H, Koono M. Cohort migration of carcinoma cells: Differentiated colorectal carcinoma cells move as coherent cell clusters or sheets. Histol Histopathol. 1999;14:1183–1197. doi: 10.14670/HH-14.1183. [DOI] [PubMed] [Google Scholar]
- 186.Nagano T, Yoneda T, Hatanaka Y, Kubota C, Murakami F, Sato M. Filamin A-interacting protein (FILIP) regulates cortical cell migration out of the ventricular zone. Nat Cell Biol. 2002;4:495–501. doi: 10.1038/ncb808. [DOI] [PubMed] [Google Scholar]
- 187.Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380:360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- 188.Nagel M, Tahinci E, Symes K, Winklbauer R. Guidance of mesoderm cell migration in the Xenopus gastrula requires PDGF signaling. Development. 2004;131:2727–2736. doi: 10.1242/dev.01141. [DOI] [PubMed] [Google Scholar]
- 189.Nakahara H, Otani T, Sasaki T, Miura Y, Takai Y, Kogo M. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells. 2003;8:1019–1027. doi: 10.1111/j.1365-2443.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- 190.Nakaya Y, Sheng G. Epithelial to mesenchymal transition during gastrulation: An embryological view. Dev Growth Differ. 2008;50:755–766. doi: 10.1111/j.1440-169X.2008.01070.x. [DOI] [PubMed] [Google Scholar]
- 191.Nalbant P, Hodgson L, Kraynov V, Toutchkine A, Hahn KM. Activation of endogenous Cdc42 visualized in living cells. Science. 2004;305:1615–1619. doi: 10.1126/science.1100367. [DOI] [PubMed] [Google Scholar]
- 192.Nechiporuk A, Raible DW. FGF-dependent mechanosensory organ patterning in zebrafish. Science. 2008;320:1774–1777. doi: 10.1126/science.1156547. [DOI] [PubMed] [Google Scholar]
- 193.Nelson WJ. Tube morphogenesis: Closure, but many openings remain. Trends Cell Biol. 2003;13:615–621. doi: 10.1016/j.tcb.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Niessen CM. Tight junctions/adherens junctions: Basic structure and function. J Invest Dermatol. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 195.Niggemann B, Drell TLt, Joseph J, Weidt C, Lang K, Zaenker KS, Entschladen F. Tumor cell locomotion: Differential dynamics of spontaneous and induced migration in a 3D collagen matrix. Exp Cell Res. 2004;298:178–187. doi: 10.1016/j.yexcr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 196.Ninov N, Chiarelli DA, Martin-Blanco E. Extrinsic and intrinsic mechanisms directing epithelial cell sheet replacement during Drosophila metamorphosis. Development. 2007;134:367–379. doi: 10.1242/dev.02728. [DOI] [PubMed] [Google Scholar]
- 197.Ninov N, Menezes-Cabral S, Prat-Rojo C, Manjon C, Weiss A, Pyrowolakis G, Affolter M, Martin-Blanco E. Dpp signaling directs cell motility and invasiveness during epithelial morphogenesis. Curr Biol. 20:513–520. doi: 10.1016/j.cub.2010.01.063. [DOI] [PubMed] [Google Scholar]
- 198.Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–1452. [PMC free article] [PubMed] [Google Scholar]
- 199.Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci. 2008;13:3532–3548. doi: 10.2741/2947. [DOI] [PubMed] [Google Scholar]
- 201.Nusrat A, Brown GT, Tom J, Drake A, Bui TT, Quan C, Mrsny RJ. Multiple protein interactions involving proposed extracellular loop domains of the tight junction protein occludin. Mol Biol Cell. 2005;16:1725–1734. doi: 10.1091/mbc.E04-06-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ogata S, Morokuma J, Hayata T, Kolle G, Niehrs C, Ueno N, Cho KW. TGF-beta signaling-mediated morphogenesis: Modulation of cell adhesion via cadherin endocytosis. Genes Dev. 2007;21:1817–1831. doi: 10.1101/gad.1541807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Oliver T, Dembo M, Jacobson K. Separation of propulsive and adhesive traction stresses in locomoting keratocytes. J Cell Biol. 1999;145:589–604. doi: 10.1083/jcb.145.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Rho-dependent formation of epithelial “leader” cells during wound healing. Proc Natl Acad Sci U S A. 2003;100:10788–10793. doi: 10.1073/pnas.1834401100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ouaknin GY, Bar-Yoseph PZ. Stochastic collective movement of cells and fingering morphology: No maverick cells. Biophys J. 2009;97:1811–1821. doi: 10.1016/j.bpj.2009.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pankov R, Endo Y, Even-Ram S, Araki M, Clark K, Cukierman E, Matsumoto K, Yamada KM. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Parsons JT. Focal adhesion kinase: The first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 208.Pastor-Pareja JC, Grawe F, Martin-Blanco E, Garcia-Bellido A. Invasive cell behavior during Drosophila imaginal disc eversion is mediated by the JNK signaling cascade. Dev Cell. 2004;7:387–399. doi: 10.1016/j.devcel.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 209.Patel SD, Ciatto C, Chen CP, Bahna F, Rajebhosale M, Arkus N, Schieren I, Jessell TM, Honig B, Price SR, Shapiro L. Type II cadherin ectodomain structures: Implications for classical cadherin specificity. Cell. 2006;124:1255–1268. doi: 10.1016/j.cell.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 210.Pelham R, Wang Y. High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Mol Biol Cell. 1999;10:933–945. doi: 10.1091/mbc.10.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pelham RJ, Wang Y-L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pertz O, Hahn KM. Designing biosensors for Rho family proteins–deciphering the dynamics of Rho family GTPase activation in living cells. J Cell Sci. 2004;117:1313–1318. doi: 10.1242/jcs.01117. [DOI] [PubMed] [Google Scholar]
- 213.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 214.Prass M, Jacobson K, Mogilner A, Radmacher M. Direct measurement of the lamellipodial protrusive force in a migrating cell. J Cell Biol. 2006;174:767–772. doi: 10.1083/jcb.200601159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Puck TT, Cieciura SJ, Fisher HW. Clonal growth in vitro of human cells with fibroblastic morphology; comparison of growth and genetic characteristics of single epithelioid and fibroblast-like cells from a variety of human organs. J Exp Med. 1957;106:145–158. doi: 10.1084/jem.106.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Radice GP. Locomotion and cell-substratum contacts of Xenopus epidermal cells in vitro and in situ. J Cell Sci. 1980;44:201–223. doi: 10.1242/jcs.44.1.201. [DOI] [PubMed] [Google Scholar]
- 217.Rauzi M, Verant P, Lecuit T, Lenne PF. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol. 2008;10:1401–1410. doi: 10.1038/ncb1798. [DOI] [PubMed] [Google Scholar]
- 218.Redd MJ, Cooper L, Wood W, Stramer B, Martin P. Wound healing and inflammation: Embryos reveal the way to perfect repair. Philos Trans R Soc Lond B Biol Sci. 2004;359:777–784. doi: 10.1098/rstb.2004.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Revenu C, Gilmour D. EMT 2.0: shaping epithelia through collective migration. Curr Opin Genet Dev. 2009;19:338–342. doi: 10.1016/j.gde.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 220.Reyes CD, Garcia AJ. Engineering integrin-specific surfaces with a triple-helical collagen-mimetic peptide. J Biomed Mater Res A. 2003;65:511–523. doi: 10.1002/jbm.a.10550. [DOI] [PubMed] [Google Scholar]
- 221.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 222.Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rorth P. Collective cell migration. Annu Rev Cell Dev Biol. 2009;25:407–429. doi: 10.1146/annurev.cellbio.042308.113231. [DOI] [PubMed] [Google Scholar]
- 225.Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- 226.Rubinstein B, Fournier MF, Jacobson K, Verkhovsky AB, Mogilner A. Actin-myosin viscoelastic flow in the keratocyte lamellipod. Biophys J. 2009;97:1853–1863. doi: 10.1016/j.bpj.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rubinstein B, Jacobson K, Mogilner A. Multiscale two-dimensional modeling of a motile simple-shaped cell. Multiscale Model Simul. 2005;3:413–439. doi: 10.1137/04060370X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sahai E. Mechanisms of cancer cell invasion. Curr Opin Genet Dev. 2005;15:87–96. doi: 10.1016/j.gde.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 229.Sahai E. Illuminating the metastatic process. Nat Rev Cancer. 2007;7:737–749. doi: 10.1038/nrc2229. [DOI] [PubMed] [Google Scholar]
- 230.Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, Davis J, Conn E, Hughes CC. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111:4997–5007. doi: 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol. 1997;137:1393–1401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sanz-Moreno V, Marshall CJ. Rho-GTPase signaling drives melanoma cell plasticity. Cell Cycle. 2009;8:1484–1487. doi: 10.4161/cc.8.10.8490. [DOI] [PubMed] [Google Scholar]
- 233.Savill NJ, Hogeweg P. Modelling morphogenesis: From single cells to crawling slugs. J Theor Biol. 1997;184:229–235. doi: 10.1006/jtbi.1996.0237. [DOI] [PubMed] [Google Scholar]
- 234.Schaller MD. Paxillin: A focal adhesion-associated adaptor protein. Oncogene. 2001;20:6459–6472. doi: 10.1038/sj.onc.1204786. [DOI] [PubMed] [Google Scholar]
- 235.Schaub S, Bohnet S, Laurent VM, Meister JJ, Verkhovsky AB. Comparative maps of motion and assembly of filamentous actin and myosin II in migrating cells. Mol Biol Cell. 2007;18:3723–3732. doi: 10.1091/mbc.E06-09-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Schreier T, Degen E, Baschong W. Fibroblast migration and proliferation during in vitro wound healing. A quantitative comparison between various growth factors and a low molecular weight blood dialysate used in the clinic to normalize impaired wound healing. Res Exp Med (Berl) 1993;193:195–205. doi: 10.1007/BF02576227. [DOI] [PubMed] [Google Scholar]
- 237.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sherratt JA, Martin P, Murray JD, Lewis J. Mathematical models of wound healing in embryonic and adult epidermis. IMA J Math Appl Med Biol. 1992;9:177–196. doi: 10.1093/imammb/9.3.177. [DOI] [PubMed] [Google Scholar]
- 239.Shroff H, Galbraith CG, Galbraith JA, White H, Gillette J, Olenych S, Davidson MW, Betzig E. Dual-color superresolution imaging of genetically expressed probes within individual adhesion complexes. Proc Natl Acad Sci U S A. 2007;104:20308–20313. doi: 10.1073/pnas.0710517105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shroff H, White H, Betzig E. Photoactivated localization microscopy (PALM) of adhesion complexes. Curr Protoc Cell Biol. 2008;4:4.21. doi: 10.1002/0471143030.cb0421s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shtengel G, Galbraith JA, Galbraith CG, Lippincott-Schwartz J, Gillette JM, Manley S, Sougrat R, Waterman CM, Kanchanawong P, Davidson MW, Fetter RD, Hess HF. Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure. Proc Natl Acad Sci U S A. 2009;106:3125–3130. doi: 10.1073/pnas.0813131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Simpson KJ, Selfors LM, Bui J, Reynolds A, Leake D, Khvorova A, Brugge JS. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol. 2008;10:1027–1038. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- 243.Simske JS, Hardin J. Getting into shape: Epidermal morphogenesis in Caenorhabditis elegans embryos. Bioessays. 2001;23:12–23. doi: 10.1002/1521-1878(200101)23:1<12::AID-BIES1003>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 244.Small JV, Herzog M, Anderson K. Actin filament organization in the fish keratocyte lamellipodium. J Cell Biol. 1995;129:1275–1286. doi: 10.1083/jcb.129.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Small JV, Kaverina I. Microtubules meet substrate adhesions to arrange cell polarity. Curr Opin Cell Biol. 2003;15:40–47. doi: 10.1016/s0955-0674(02)00008-x. [DOI] [PubMed] [Google Scholar]
- 246.Smalley KS, Brafford P, Haass NK, Brandner JM, Brown E, Herlyn M. Up-regulated expression of zonula occludens protein-1 in human melanoma associates with N-cadherin and contributes to invasion and adhesion. Am J Pathol. 2005;166:1541–1554. doi: 10.1016/S0002-9440(10)62370-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 2009;137:1331–1342. doi: 10.1016/j.cell.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 248.Suetsugu S, Yamazaki D, Kurisu S, Takenawa T. Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev Cell. 2003;5:595–609. doi: 10.1016/s1534-5807(03)00297-1. [DOI] [PubMed] [Google Scholar]
- 249.Svitkina TM, Verkhovsky AB, McQuade KM, Borisy GG. Analysis of the actin-myosin II system in fish epidermal keratocytes: Mechanism of cell body translocation. J Cell Biol. 1997;139:397–415. doi: 10.1083/jcb.139.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Takahisa M, Togashi S, Suzuki T, Kobayashi M, Murayama A, Kondo K, Miyake T, Ueda R. The Drosophila tamou gene, a component of the activating pathway of extramacrochaetae expression, encodes a protein homologous to mammalian cell-cell junction-associated protein ZO-1. Genes Dev. 1996;10:1783–1795. doi: 10.1101/gad.10.14.1783. [DOI] [PubMed] [Google Scholar]
- 251.Tambe DT, Corey Hardin C, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, Zhou EH, Zaman MH, Butler JP, Weitz DA, Fredberg JJ, Trepat X. Collective cell guidance by cooperative intercellular forces. Nat Mater. 2011;10:469–475. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc Natl Acad Sci U S A. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Tavella S, Bellese G, Castagnola P, Martin I, Piccini D, Doliana R, Colombatti A, Cancedda R, Tacchetti C. Regulated expression of fibronectin, laminin and related integrin receptors during the early chondrocyte differentiation. J Cell Sci. 1997;110(Pt 18):2261–2270. doi: 10.1242/jcs.110.18.2261. [DOI] [PubMed] [Google Scholar]
- 254.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 255.Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Invest Derm Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 257.Toriseva M, Kahari VM. Proteinases in cutaneous wound healing. Cell Mol Life Sci. 2009;66:203–224. doi: 10.1007/s00018-008-8388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Toyama Y, Peralta XG, Wells AR, Kiehart DP, Edwards GS. Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science. 2008;321:1683–1686. doi: 10.1126/science.1157052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Trepat X, Grabulosa M, Buscemi L, Rico F, Farre R, Navajas D. Thrombin and histamine induce stiffening of alveolar epithelial cells. J Appl Physiol. 2005;98:1567–1574. doi: 10.1152/japplphysiol.00925.2004. [DOI] [PubMed] [Google Scholar]
- 260.Trepat X, Wasserman MR, Angelini TE, Millet E, Weitz DA, Butler JP, Fredberg JJ. Physical forces during collective cell migration. Nature Phys. 2009;5:426. [Google Scholar]
- 261.Turner CE. Paxillin interactions. J Cell Sci. 2000;113(Pt 23):4139–4140. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- 262.Urban E, Jacob S, Nemethova M, Resch GP, Small JV. Electron tomography reveals unbranched networks of actin filaments in lamellipodia. Nat Cell Biol. 2010;12:429–435. doi: 10.1038/ncb2044. [DOI] [PubMed] [Google Scholar]
- 263.Uv A, Cantera R, Samakovlis C. Drosophila tracheal morphogenesis: Intricate cellular solutions to basic plumbing problems. Trends Cell Biol. 2003;13:301–309. doi: 10.1016/s0962-8924(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 264.Valentin G, Haas P, Gilmour D. The chemokine SDF1a coordinates tissue migration through the spatially restricted activation of Cxcr7 and Cxcr4b. Curr Biol. 2007;17:1026–1031. doi: 10.1016/j.cub.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 265.Vallotton P, Danuser G, Bohnet S, Meister JJ, Verkhovsky AB. Tracking retrograde flow in keratocytes: News from the front. Mol Biol Cell. 2005;16:1223–1231. doi: 10.1091/mbc.E04-07-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65:3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Verkhovsky AB, Chaga OY, Schaub S, Svitkina TM, Meister JJ, Borisy GG. Orientational order of the lamellipodial actin network as demonstrated in living motile cells. Mol Biol Cell. 2003;14:4667–4675. doi: 10.1091/mbc.E02-10-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Verkhovsky AB, Svitkina TM, Borisy GG. Self-polarization and directional motility of cytoplasm. Curr Biol. 1999;9:11–20. doi: 10.1016/s0960-9822(99)80042-6. [DOI] [PubMed] [Google Scholar]
- 269.Vestweber D, Kemler R. Identification of a putative cell adhesion domain of uvomorulin. EMBO J. 1985;4:3393–3398. doi: 10.1002/j.1460-2075.1985.tb04095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol. 2007;176:573–580. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Virchow R. Uber bewegliche thierische zellen. Arch Path Anat. 1863;28:237–240. [Google Scholar]
- 272.Vitorino P, Meyer T. Modular control of endothelial sheet migration. Genes Dev. 2008;22:3268–3281. doi: 10.1101/gad.1725808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wallis S, Lloyd S, Wise I, Ireland G, Fleming TP, Garrod D. The alpha isoform of protein kinase C is involved in signaling the response of desmosomes to wounding in cultured epithelial cells. Mol Biol Cell. 2000;11:1077–1092. doi: 10.1091/mbc.11.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wang Y-L, Dembo M, Wang H-B, Lo C-M. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wang YL, Pelhan RJ. Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Methods Enzymol. 1998;298:489–496. doi: 10.1016/s0076-6879(98)98041-7. [DOI] [PubMed] [Google Scholar]
- 276.Ware MF, Wells A, Lauffenburger DA. Epidermal growth factor alters fibroblast migration speed and directional persistence reciprocally and in a matrix-dependent manner. J Cell Sci. 1998;111(Pt 16):2423–2432. doi: 10.1242/jcs.111.16.2423. [DOI] [PubMed] [Google Scholar]
- 277.Watanabe T, Costantini F. Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev Biol. 2004;271:98–108. doi: 10.1016/j.ydbio.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 278.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 279.Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells – over and over and over again. Nat Cell Biol. 2002;4:E97–E100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- 280.Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 281.Weijer CJ. Collective cell migration in development. J Cell Sci. 2009;122:3215–3223. doi: 10.1242/jcs.036517. [DOI] [PubMed] [Google Scholar]
- 282.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 283.Wilson CA, Tsuchida MA, Allen GM, Barnhart EL, Applegate KT, Yam PT, Ji L, Keren K, Danuser G, Theriot JA. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature. 2010;465:373–377. doi: 10.1038/nature08994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009;20:931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Brocker EB, Friedl P. Compensation mechanism in tumor cell migration: Mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 287.Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 288.Worthylake RA, Burridge K. Leukocyte transendothelial migration: Orchestrating the underlying molecular machinery. Curr Opin Cell Biol. 2001;13:569–577. doi: 10.1016/s0955-0674(00)00253-2. [DOI] [PubMed] [Google Scholar]
- 289.Xu X, Francis R, Wei CJ, Linask KL, Lo CW. Connexin 43-mediated modulation of polarized cell movement and the directional migration of cardiac neural crest cells. Development. 2006;133:3629–3639. doi: 10.1242/dev.02543. [DOI] [PubMed] [Google Scholar]
- 290.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yamamoto E, Kohama G, Sunakawa H, Iwai M, Hiratsuka H. Mode of invasion, bleomycin sensitivity, and clinical course in squamous cell carcinoma of the oral cavity. Cancer. 1983;51:2175–2180. doi: 10.1002/1097-0142(19830615)51:12<2175::aid-cncr2820511205>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 292.Yap AS, Crampton MS, Hardin J. Making and breaking contacts: The cellular biology of cadherin regulation. Curr Opin Cell Biol. 2007;19:508–514. doi: 10.1016/j.ceb.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zaidel-Bar R, Cohen M, Addadi L, Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans. 2004;32:416–420. doi: 10.1042/BST0320416. [DOI] [PubMed] [Google Scholar]
- 295.Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]