Abstract
The importance of sleep to hormones and glucose metabolism was first documented more than four decades ago. Since then, sleep curtailment has become an endemic behavior in modern society. In addition, the prevalence of sleep disorders, particularly obstructive sleep apnea (OSA), has increased. OSA is very common in endocrine and metabolic disorders, but often remains undiagnosed. This Review summarizes the laboratory and epidemiologic evidence that suggests how sleep loss, either behavioral or disease-related, and poor quality of sleep might promote the development of obesity and diabetes mellitus, and exacerbate existing endocrine conditions. Treatment of sleep disorders has the potential to improve glucose metabolism and energy balance. Screening for habitual sleep patterns and OSA might be critically important for patients with endocrine and metabolic disorders.
Introduction
More than four decades ago, multiple studies demonstrated that sleep has a major role in the regulation of endocrine functions and glucose metabolism. In healthy adults, reproducible changes in the release of pituitary hormones and pituitary-dependent hormones follow the wake–sleep transition. The effect of sleep on hormone secretion is dependent on the occurrence of specific stages of sleep.1,2
Human sleep is composed of rapid-eye-movement (REM) sleep and non-REM sleep. Deep non-REM sleep is characterized by ‘slow waves’ in the electroencephalogram, which reflect a mode of synchronous firing of thalamocortical neurons. During slow-wave sleep, the brain uses less glucose, sympathetic nervous activity is decreased and vagal tone is increased, relative to both wakefulness and REM sleep. Slow-wave sleep is also associated with robust elevations in levels of growth hormone, while the activity of the pituitary–adrenal axis is inhibited.2 Because of the effect of slow-wave sleep on cerebral glucose metabolism, sympathovagal balance and counter-regulatory hormones’ release, this type of sleep is thought to have a major role in total-body glucose regulation and, more generally, in the restorative effect of sleep.
The 1998 discovery of orexin A and orexin B, two peptides synthesized by neurons which are concentrated in the lateral hypothalamus, demonstrated a molecular link between wake–sleep regulation and the neuroendocrine control of appetite. Orexin-containing neurons have a central role in the maintenance of arousal, but also increase food intake,3,4 particularly at a time when normal food intake is low. Feeding requires the maintenance of wakefulness and the orexin system seems to have a key role in the interaction between feeding and arousal. Orexin-containing neurons are active during wakefulness and quiescent during sleep. Deficiencies in the orexin system are associated with sleep disorders that involve chronic, excessive daytime sleepiness, including narcolepsy and obstructive sleep apnea (OSA). By contrast, when sleep deprivation is behaviorally enforced, the orexin system is overactive—most likely to maintain wakefulness against the increased pressure to sleep. Increased orexin activity during periods of sleep deprivation has been shown in rats, dogs and squirrel monkeys.5–7 A profound effect of sleep deprivation and/or poor-quality sleep on glucose metabolism and appetite regulation should, therefore, be expected.
Remarkably, the adverse effects of chronically reduced sleep duration and/or quality have only begun to be evaluated in the past few years. Voluntary sleep curtailment has, however, become a common behavior in modern society. Data from the 2008 ‘Sleep in America’ poll indicate that, although working adults report that they need an average 7 h 18 min of sleep to function at their best, 44% of them sleep less than 7 h, and 16% sleep less than 6 h on a typical weeknight.8 Sleep duration in European countries seems to follow a similar trend.9 The cumulative sleep loss per working week of a substantial portion of the adult population may correspond to as much as one full night of sleep deprivation. Chronic sleep loss might also be the consequence of a pathological condition—particularly the most common sleep disorder, OSA, which is characterized by repetitive breathing disturbances during sleep and by poor-quality sleep. The current epidemic of obesity in industrialized countries is paralleled by an epidemic of OSA.
The following two sections summarize the laboratory and epidemiologic evidence that links short-duration sleep and/or poor-quality sleep with an increased risk of diabetes mellitus and obesity. The evidence on adverse effects of poor-quality sleep is based on studies that involved an experimental reduction of sleep quality, and on population studies in which sleep quality was self-reported. Indeed, no systematic evaluations have been published to date on endocrine or metabolic disturbances in any sleep disorder, except in OSA. Reciprocally, associations between metabolic or hormonal conditions and the prevalence or severity of sleep disorders have only been examined for OSA. The last section of this review, therefore, focuses on the links between OSA and metabolic disorders, with a particular focus on diabetes mellitus, obesity and polycystic-ovary syndrome (PCOS).
Poor and short sleep and diabetes
Evidence from laboratory studies
Short-duration sleep and glucose regulation
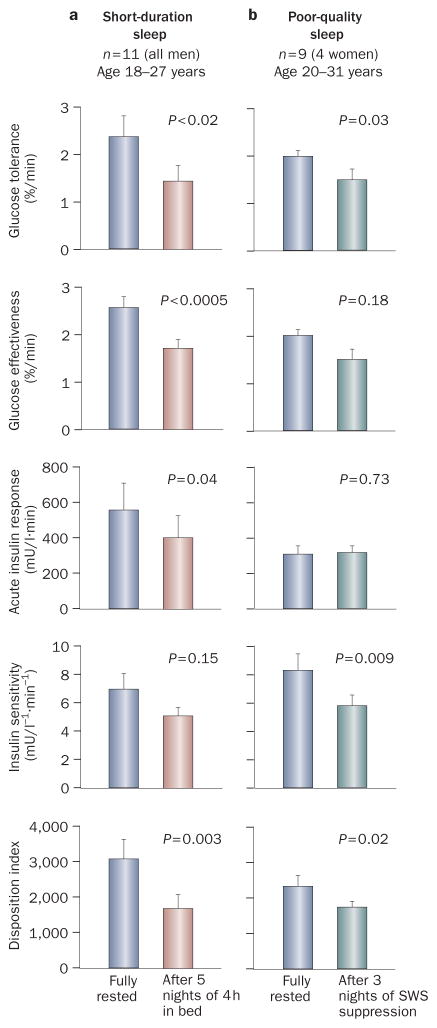
The first study that assessed the consequences of recurrent, partial sleep loss on hormonal and metabolic variables involved restriction of participants’ (healthy young men) 10 sleep time to 4 h per night for 6 consecutive nights, and then extending it to 12 h for 6 nights. Figure 1a shows the results from a frequently sampled, intravenous glucose-tolerance test performed on the fifth day of both study periods. Glucose tolerance, estimated as the rate of decrease of glucose levels, was 40% lower after sleep restriction than after sleep extension, and reached a range that is typical of aging people who have impaired glucose tolerance.10 This decrease in glucose tolerance was the combined consequence of a 30–40% decrease in glucose effectiveness (which is a measure of noninsulin-dependent glucose utilization) and a near 30% reduction in the acute insulin response to glucose, despite a trend for decreased insulin sensitivity. The product of insulin sensitivity and acute insulin response to glucose (that is, the disposition index, a validated marker of diabetes risk),11 was decreased by nearly 40% in the state of sleep debt.
Figure 1.
Results from intravenous glucose-tolerance tests in healthy individuals when fully rested and after sleep manipulations. a | Results when fully rested and after 5 nights of 4 h in bed;10 b | Results during baseline sleep and after 3 nights of suppression of slow-wave sleep.14 Abbreviation: SWS, slow-wave sleep. Permission for part a was obtained from Elsevier Ltd © Spiegel, K. et al. Impact of sleep debt on metabolic and endocrine function. Lancet 354, 1435–1439 (1999). Part b was adapted from Tasali, E. et al. Proc. Natl Acad. Sci. USA 105, 1044–1049 (2008).
The glucose and insulin responses to a high-carbohydrate breakfast were assessed on the sixth day of both sleep conditions. Both peak glucose level and the overall glucose response were increased following sleep restriction, which confirmed a decrease in glucose tolerance. When the integrated glucose and insulin responses were examined with the homeostasis model-assessment index (the normalized product of insulin and glucose levels), a greater than 50% increase of homeostasis model-assessment values was observed after sleep restriction, as compared to the fully rested state; these results are consistent with a reduction in insulin sensitivity. In a subsequent study, 12 healthy men were assessed after two 10 h nights versus two 4 h nights, in randomized order.12 Similarly to the findings of the previous study, after only two nights of short-duration sleep, morning levels of glucose were higher than normal, whereas insulin levels tended to be lower than after two nights of long sleep. Preliminary data from an independent laboratory have confirmed and extended these findings: 1 week of sleep that is restricted to 5 h per night in healthy men resulted in a marked reduction in insulin sensitivity, as assessed by the hyperinsulinemic euglycemic clamp.13
Poor-quality sleep and glucose regulation
The initiation of slow-wave sleep is associated with a decrease in use of glucose by the brain, stimulation of growth-hormone release, inhibition of cortisol secretion, decreased sympathetic nervous system activity and increased vagal tone. All these correlates of slow-wave sleep affect total-body glucose homeostasis; therefore, low amounts of slow-wave sleep, which normally occur in aging individuals and in those who have sleep disorders, might be associated with decreased glucose tolerance.
A recent study directly tested this hypothesis by selectively suppressing slow-wave sleep in healthy young adults and examining the effect on glucose tolerance.14 Suppression of slow-wave sleep was achieved by delivery of acoustic stimuli that were designed to replace slow-wave sleep with shallow sleep, but to avoid full awakenings. The amount of slow-wave sleep was reduced by nearly 90%—a similar reduction to that associated with four decades of aging. Such low levels of slow-wave sleep are also typical of moderate to severe OSA. Importantly, this intervention did not reduce total sleep duration. Figure 1b shows the results from an intravenous glucose-tolerance test performed after 2 nights of undisturbed sleep and after 3 nights of slow-wave sleep suppression. After suppression of slow-wave sleep, insulin sensitivity had decreased by around 25% and reached the level reported in aging individuals and in populations at high risk of diabetes mellitus.15 This decrease in insulin sensitivity was not compensated for by an increase in the acute insulin response to glucose; consequently, the disposition index decreased by around 20% after slow-wave sleep suppression. Consistent with an increased risk of diabetes mellitus, glucose tolerance was reduced by around 23%, which approaches the range typical of impaired glucose tolerance.14 Importantly, the decrease in insulin sensitivity was strongly correlated to the decrease in slow-wave sleep. These laboratory findings demonstrate unequivocally that poor-quality sleep may adversely affect glucose regulation.
Evidence from epidemiologic studies
A number of cross-sectional, as well as prospective, epidemiologic studies,16,17 have provided evidence of an association between self-reported short-duration and/ or poor-quality sleep and the prevalence or incidence of diabetes mellitus, after age, BMI and various other confounding variables are taken into account. Studies that have assessed sleep at baseline and incidence of diabetes mellitus over a follow-up period provide some indication of the direction of causality. Six studies have examined the effect of sleep duration, and an equal number of studies have addressed the effect of sleep quality as determined by self-reported problems, such as difficulty initiating or maintaining sleep, use of sleeping pills, or presence of insomnia (reviewed elsewhere18). Short sleep duration was found to predict an increased incidence of diabetes in four of the six studies, whereas poor-quality sleep was associated with an increased risk of diabetes mellitus in five of the six studies.18
Poor or short sleep and obesity
Evidence from laboratory studies
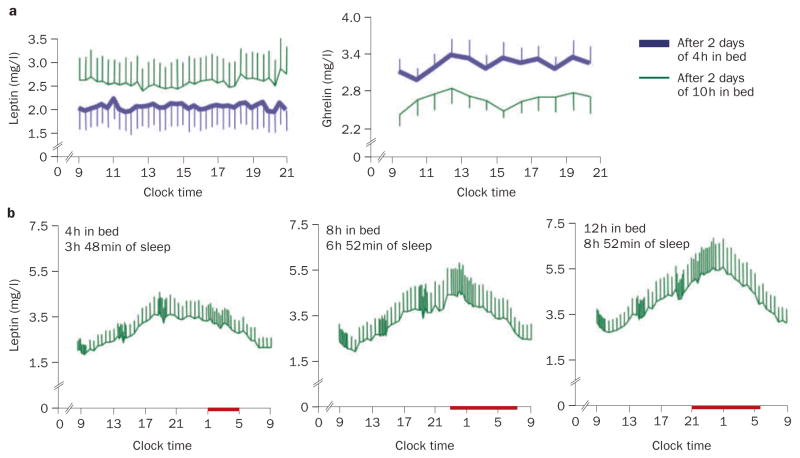
In a randomized, crossover study that involved two nights of 4 h in bed versus two nights of 10 h in bed, the daytime profiles of the satiety hormone, leptin, and the appetite-stimulating hormone, ghrelin, were measured and the participants completed validated scales for hunger and appetite for various food categories (Figure 2a).19 Overall leptin levels decreased by an average of 18%, while ghrelin levels increased by 28%; the ghrelin:leptin ratio increased by more than 70%. Hunger increased by 23%, and appetite for nutrients with a high carbohydrate content was increased by more than 30% when sleep was restricted. If this increase in hunger during sleep restriction were to translate into a commensurate increase in food intake, weight gain would be expected to occur over time. A randomized, crossover laboratory study of overweight, middle-aged adults who underwent 2 weeks of sleep extension (+1.5 h per night) and 2 weeks of restriction (–1.5 h per night) has shown an increase in food intake from snacks during the short-sleep condition.20 However, the participants gained weight under both sleep conditions, and no differences were detected in leptin or ghrelin levels between the two study conditions.
Figure 2.
Effect of sleep duration on leptin and ghrelin levels. a | Mean (±SEM) leptin and serum plasma ghrelin levels in healthy individuals after 2 days with 4 h or 10 h sleep periods. b | Mean (SEM) 24 h serum leptin profiles after 6 days of 4 h, 8 h and 12 h in bed in nine healthy, lean men, studied at bed rest who ate three identical carbohydrate-rich meals. At the end of these study periods, the participants slept an average of 3 h 48 min in the 4 h in bed group, 6 h 52 min in the 8 h in bed group, and 8 h 52 min in the 12 h in bed group. All characteristics of the 24 h leptin profile increased from the 4 h to the 12 h bedtime condition. The bars represent sleep periods. Permission for panel a was obtained from the American College of Physicians © Spiegel K et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 141, 846–850 (2004). Permission for panel b was obtained from The Endocrine Society © Spiegel K et al. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin. Endocrinol. Metab. 89, 5762–5771 (2004).
In a separate study, a clear, dose-response relationship was observed between sleep duration and the 24 h serum leptin profile.21 Figure 2b shows the 24 h leptin profiles that were obtained after 6 days of 4 h, 8 h, and 12 h periods of sleep in healthy, lean young men. All the characteristics of the 24 h leptin profile (overall mean level, nocturnal maximum, amplitude) gradually increased from the 4 h to the 12 h sleep-period condition. Importantly, these differences in 24 h regulation of leptin levels occurred despite identical amounts of caloric intake, similar sedentary conditions, and stable weight. Of note, the reduction in peak leptin levels (average of 26%) between the 4 h and the 12 h bedtime conditions was similar to that reported in healthy volunteers who were fed only 70% of their energy requirement during 3 consecutive days. These findings confirmed and extended the observations of an earlier study that assessed leptin levels 6 times during the 24 h cycle in volunteers studied after 7 days of sleep that was restricted to 4 h per night and reported a decrease in peak leptin levels.22 Another study, which involved total sleep deprivation, or 4.5 h or 7 h of sleep for one night, also reported dose-response relationships between the duration of sleep, hunger ratings and ghrelin levels.23 In this study, leptin levels were not affected.
The effect of sleep restriction on appetite regulation seems to be similar in the short term (2–6 days)19,21 and in chronic conditions. Indeed, two epidemiologic studies have shown reduced leptin levels after controlling for BMI or adiposity in habitual short-duration sleepers.24,25 High ghrelin levels were also associated with short-duration sleep.24 A subsequent, small study, which involved only postmenopausal women, did not confirm the link between sleep duration, leptin and ghrelin levels;26 however, very few participants in this study had short sleep durations.
Evidence from epidemiologic studies
An ever-growing number of cross-sectional, epidemiological studies (52 in September 2008) have provided evidence of an association between short-duration and/or poor-quality sleep and risk of obesity. Two meta-analyses, which included a total of more than 600,000 adults and 30,000 children from all over the world, attempted to quantify the link between short-duration sleep and obesity risk. In the first study, the pooled odds ratio (OR) that linked short-duration sleep to obesity was 1.89 (95% CI 1.46–2.43, p <0.0001) in children and 1.55 (95% CI 1.43–1.68, p <0.0001) in adults.27 The second study reported an OR of 1.58 (95% CI 1.26–1.98) in children with short sleep duration, and an OR of 1.92 (95% CI 1.15–3.2) in children with the shortest sleep duration; these results suggest existence of a dose-response relationship between sleep duration and risk of obesity.28 A third systematic review similarly concluded that short sleep duration seems to be independently associated with weight gain, particularly in young age groups.29 Whereas these analyses suggest that the effect of sleep duration on weight may be less robust in aging populations than in children and young adults, a cross-sectional analysis that used wrist actigraphy to objectively assess sleep duration objectively in more than 6,000 men and women aged 67–99 years, provided strong evidence to the contrary.30 Compared with sleeping 7–8 h per night, sleeping less than 5 h was associated with a BMI that was, on average, more than 2.5 kg/m2 in men and 1.8 kg/m2 in women, after adjustments were made for multiple potentially confounding variables.
Of the 10 longitudinal studies on sleep duration and obesity risk in children and adults that have been performed, 9 reported that reduced sleep durations are associated with an increased risk of being overweight or obese a few years later.17,31 This pattern is particularly consistent in children.16
This body of epidemiologic evidence supports the hypothesis that sleep curtailment may be a plausible ‘nontraditional’ lifestyle factor that contributes to the epidemic of obesity.32 Increasing the duration of sleep for those who regularly curtail it has been suggested as a means to improve the health of the population as a whole.33 Critics have argued that the effect of short-duration sleep in longitudinal studies is small (short-duration sleepers gain excess weight of 1–7 kg over 10 years) and that the number of short-duration sleepers (less than or equal to 5 h) in the general population is low.34 Yet, the difference in weight gain between short-duration and normal-duration sleepers is well within the range of weight loss that can be achieved with pharmacological interventions, and in the two epidemiologic studies that assessed sleep duration objectively by monitoring wrist activity, more than 10% of the participants slept for fewer than 5 h per night.30,35
A limitation of nearly all of these epidemiologic studies is that they did not simultaneously assess sleep quality. Thus, whether short-duration sleep in obese individuals is the result of sleep-time curtailment, or the presence of a sleep disorder remains to be determined. A large study,36 in which the participants reported sleep duration, subjective sleep disturbances (for example, insomnia, excessive daytime sleepiness, sleep difficulty) and a measure of chronic emotional stress, concluded that self-reported short-duration sleep in obese adults may be a surrogate marker of sleep disturbance and psychosocial stress. This hypothesis is consistent with the existence of a ‘vicious circle’, in which short-duration sleep may initially promote weight gain; the resultant excess adiposity would then induce sleep disturbances and psychological stress, with a net further decrease in total sleep time.
OSA and metabolic disorders
Prevalence of OSA
OSA is a highly prevalent sleep condition that is associated with increased morbidity and mortality. OSA is characterized by repetitive episodes of upper-airway obstruction that lead to intermittent hypoxemia and/or hypercapnia and sleep fragmentation. Diagnosis of OSA is based on the apnea–hypopnea index (AHI), which measures the total number of apnea plus hypopnea episodes per hour of sleep; individuals with an AHI of greater than 5 by polysomnography are considered to have OSA. Table 1 summarizes the prevalence of OSA in the general population and in populations with metabolic or endocrine disorders. Obesity is a major risk factor for OSA, and the prevalence of OSA in the morbidly obese population is strikingly high—(50% to 98%).37
Table 1.
Prevalence of OSA in various populations
| Population of patients | Prevalence of OSA (%) |
|---|---|
| General population | |
| Patients with OSA (AHI >5) and excessive daytime sleepiness81 | 2–7 |
| Patients with OSA (AHI >5)82 | 17 |
| Patients with endocrine disorders | |
| Obese patients82 | 41–58 |
| Morbidly obese patients37 | 50–98 |
| Patients with diabetes mellitus38–40 | 17–97 |
| Patients with polycystic-ovary syndrome41,42 | 44–70 |
| Patients with acromegaly43 | 19–23 |
| Patients with hypothyroidism43 | 50–100 |
| Patients with Cushing syndrome43 | 18–32 |
Abbreviations: AHI, apnea–hypopnea index; OSA, obstructive sleep apnea.
The prevalence of OSA in metabolic and endocrine disorders is very high. In patients with diabetes mellitus, the prevalence of OSA is between 17%38 and 48%.39 In a preliminary report, which involved only obese patients with diabetes mellitus, undiagnosed OSA was found in 97% of the participants.40 This evidence for an exceptionally high rate of OSA in individuals with diabetes mellitus might have important implications, including a need for systematic evaluation and treatment of OSA in this group. Polycystic-ovary syndrome (PCOS), the most common endocrine disorder of premenopausal women, involves obesity, insulin resistance and a substantially elevated risk of early-onset, impaired glucose tolerance and diabetes mellitus. Whereas the risk of OSA in healthy young women, even if they are overweight or obese, is less than 10%, in women with PCOS, recent reports have found a prevalence of OSA of 44–70%.41,42 In acromegaly, hypothyroidism and Cushing syndrome, the prevalence of OSA is 19%–23%, 50%–100%, and 18%–32%, respectively.43 The pathophysiology that underlies the high prevalence of OSA in endocrine disorders is likely to be multifactorial and include anatomic, functional and hormonal factors.
OSA involves respiratory disturbances, hypoxic stress, poor-quality sleep (owing to sleep fragmentation and low levels of slow-wave sleep) and reduced total sleep time. The alterations in glucose regulation and/or appetite regulation observed with experimentally reduced sleep duration and quality10,19 suggest that poor-quality and short-duration sleep, in addition to hypoxia, could contribute to altered glucose homeostasis and weight gain in patients with OSA.
OSA and diabetes mellitus
Cross-sectional studies demonstrate an association between OSA and diabetes mellitus, independent of confounding factors.44–47 By contrast, no evidence supports a causal role for OSA in the development of diabetes mellitus. Only one prospective study used polysomnography to assess OSA and its relationship to diabetes risk, but the results failed to show a significant association between incident diabetes mellitus and OSA at 4-year follow-up (OR 1.62, p = 0.24).48
A review of studies that assessed the effect of continuous positive airway pressure (CPAP) on glucose regulation suggests that the treatment gives more consistently positive results in patients with diabetes mellitus than in non-diabetic populations. Table 2 summarizes the findings from full reports in international journals that involved more than one night of CPAP treatment in adult populations. Of six studies that assessed glycemic control in a total number of 150 diabetic patients with OSA, five reported a positive effect (Tables 2 and 3).49–53 Notably, the one study that did not find such a correlation reported an average nightly therapeutic CPAP use of only 3.6 h.54 By contrast, in patients with OSA who do not have diabetes mellitus, a beneficial effect of CPAP on parameters of glucose regulation, including various measures of insulin sensitivity, has only been found in 455–58 of a total 11 studies,55–65 which together involved more than 300 individuals.
Table 2.
Effect of CPAP treatment on glucose metabolism
| Population of patients | Positive effect reported | No effect reported |
|---|---|---|
| Patients with T2DMa | ||
| Total number of studies | 549–53 | 154 |
| Total number of patients | 102 | 42 |
| Nondiabetic patientsb | ||
| Total number of studies | 455–58 | 759–65 |
| Total number of patients | 109 | 225 |
Improvement of glucose metabolism in patients with T2DM was defined as decreased HbA1C level and/or decreased postprandial glucose level by continuous glucose monitoring, and/or improved insulin sensitivity by hyperinsulinemic euglycemic clamp, and/or improved insulin sensitivity by fasting HOMA index.
Improvement of glucose metabolism in nondiabetic patients was defined as improved insulin sensitivity by hyperinsulinemic euglycemic clamp, and/or improved insulin sensitivity by fasting HOMA index, and/or decreased fasting glucose and insulin levels.
Abbreviations: CPAP, continuous positive airway pressure; HOMA, homeostasis model assessment; T2DM, type 2 diabetes mellitus.
Table 3.
Effect of CPAP on leptin/ghrelin levels and weight
| Outcome measure | Positive effect reported | No effect reported |
|---|---|---|
| Decrease in leptin level | ||
| Total number of studies | 659,60,63,68,70,71 | 272,73 |
| Total number of patients | 183 | 127 |
| Decrease in ghrelin level | ||
| Total number of studies | 268,74 | NA |
| Total number of patients | 51 | NA |
| Decreased body weight or visceral adiposity | ||
| Total number of studies | 363,70,75 | 365,76,77 |
| Total number of patients | 83 | 230 |
Abbreviations: CPAP, continuous positive airway pressure; NA, no data available.
These inconsistent results may be partly attributed to differences in sample sizes, study populations, durations of therapy, adherence to therapy, and changes in body composition during the study period. Importantly, most studies did not report objective data on CPAP usage, and in those that reported compliance, use of CPAP for greater than or equal to 4 h per night was considered ‘compliant’.
OSA and regulation of food intake and weight
Patients with OSA seem to be more predisposed to weight gain than control individuals with similar levels of obesity who do not have OSA.66,67 Consistent with the upregulation of ghrelin that is observed during short-duration sleep in healthy individuals,19,23,24 patients with OSA have high ghrelin levels, which decrease after as little as 2 days of CPAP treatment.68 By contrast, the decreased leptin levels that follow sleep restriction in normal individuals19,21,22 are not consistent with the hyperleptinemia observed in OSA.67 Whereas leptin levels are reduced in individuals with chronic short-duration sleep without OSA, independently of BMI and adiposity,24,25 patients with OSA display higher leptin levels than BMI-matched controls.67,69
Tables 2 and 3 summarize the studies that have examined the effect of CPAP treatment on leptin levels.59,60,63,68,70–73 Although the positive findings of these studies outnumber negative findings, the overall evidence is inconclusive. The hyperleptinemia in patients with OSA is thought to reflect leptin resistance. Thus, a putative reduction in leptin resistance after CPAP treatment would be expected to result in weight loss in these patients. Only two studies have measured post-CPAP ghrelin levels in patients with OSA, and both reported a decrease in ghrelin levels,68,74 which should also lead to decreased hunger and a possible beneficial effect on weight (Table 3). However, findings on the effect of CPAP on body weight and/or visceral adiposity are mixed (Table 3). One study reported weight loss after 6 months of CPAP,75 whereas another study found no weight loss after 1 year of treatment with CPAP.76 Finally, 6 months of CPAP therapy added to a weight-reduction program have not resulted in increased weight loss.77 From a metabolic point of view, loss of visceral fat is far more relevant than overall weight loss. Again, relevant studies are scarce and provide conflicting results; two63,70 of only three studies63,65,70 have reported a beneficial effect of CPAP on visceral adiposity.
OSA and polycystic-ovary syndrome
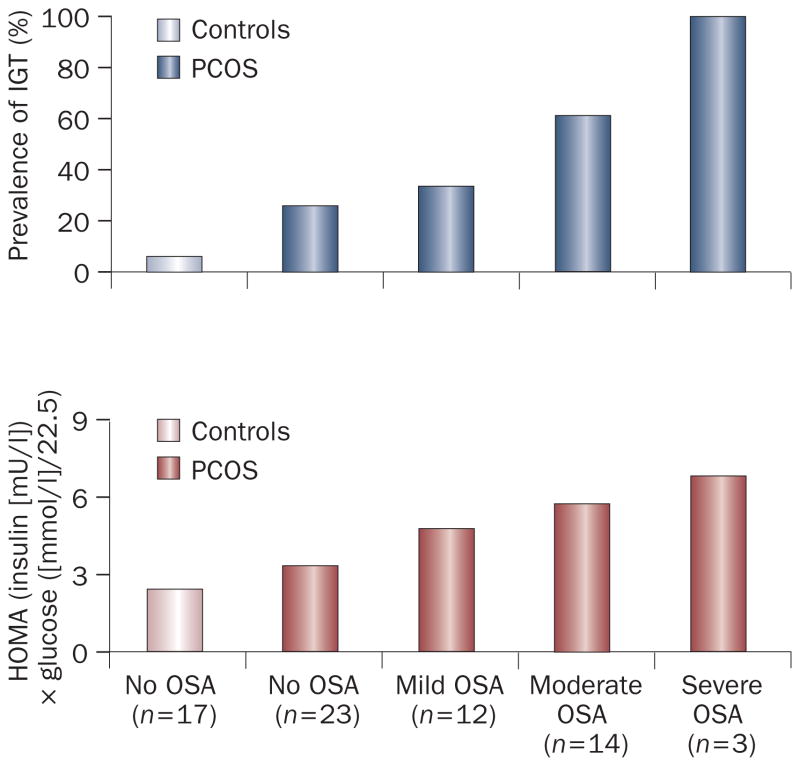
Polycystic-ovary syndrome (PCOS), the most common endocrine disorder of premenopausal women, is characterized by chronic hyperandrogenism, oligo-ovulation and anovulation, obesity, insulin resistance and a substantially elevated risk of early-onset impaired glucose tolerance and diabetes mellitus. Insulin resistance is often referred to as a ‘hallmark’ of PCOS. OSA is highly prevalent in women with this syndrome. One study reported an AHI of greater than 5 in 56% of women with PCOS, compared with 19% of age-matched and weight-matched controls.78 This study was the first to examine metabolic disturbances in women with PCOS after taking the presence and severity of OSA into account. The findings indicated that insulin resistance and reduced glucose tolerance in women with PCOS are largely the result of OSA.78 Figure 3 shows the prevalence of impaired glucose tolerance and the degree of insulin resistance in control women without OSA, women with PCOS but without OSA, and women with PCOS and mild, moderate, or severe OSA. The prevalence of impaired glucose tolerance and the degree of insulin resistance increased in direct proportion to the severity of OSA. Furthermore, when women with PCOS who had preserved normal glucose tolerance were examined, they were no more insulin-resistant than control women who did not have PCOS.
Figure 3.
Prevalence of impaired glucose tolerance and degree of insulin resistance, as assessed by the HOMA index, in control women without OSA, women with PCOS and without OSA, and women with PCOS and mild (5<AHI<15, moderate (15<AHI<30), and severe (AHI≥15) OSA. As expected, women who had PCOS with or without OSA displayed a higher prevalence of IGT and greater insulin resistance than controls. Among women with PCOS, the prevalence of IGT and degree of insulin resistance increased in direct proportion to the severity of OSA. Abbreviations: AHI, apnea–hypopnea index; HOMA, homeostasis model assessment; IGT, impaired glucose tolerance; OSA, obstructive sleep apnea. Permission obtained from The Endocrine Society © Tasali, E. et al. Impact of obstructive sleep apnea on insulin resistance and glucose tolerance in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 93, 3878–3884 (2008).
Thus, PCOS seems to be composed of two subphenotypes: PCOS with OSA, and PCOS without OSA; the latter represents less than 50% of women with PCOS. PCOS with OSA is clearly associated with a higher risk of diabetes mellitus than PCOS without OSA. As insulin resistance is thought to contribute to both androgen overproduction and metabolic disturbances in PCOS, assessment of OSA in PCOS is highly recommended; the correction of OSA might greatly improve these women’s prognosis. A quick and easy screen for OSA can be performed with the Berlin Questionnaire, a well-validated survey that identifies patients who have a high risk of OSA.79 Unfortunately, most clinicians who treat women with PCOS today are not yet aware of the high risk of OSA in this group of patients.80
Conclusions
Restorative sleep is essential for well-being, but sleep curtailment has become a common behavior in modern society. In addition, sleep disorders, particularly OSA, are very common in individuals with metabolic and endocrine disorders, but often remain undiagnosed. The accumulated evidence for a deleterious effect of short-duration or poor-quality sleep on metabolic and endocrine function supports the hypothesis that chronic, voluntary sleep curtailment and sleep disorders such as OSA may adversely affect the course of disease in patients with metabolic and endocrine disorders. Treatment of OSA by CPAP has the potential to improve glucose metabolism and appetite regulation. Screening for habitual sleep patterns and OSA—for which simple and inexpensive tools are available—such as sleep logs to characterize habitual sleep patterns and the Berlin Questionnaire, may be critically important in patients with endocrine and metabolic disorders.
Key points.
Sleep loss, be it behavioral or related to sleep disorders, is an increasingly common condition in modern society
Experimental reduction of the duration or quality of sleep has a deleterious effect on glucose metabolism
Experimental reduction of sleep duration downregulates the satiety hormone, leptin, upregulates the appetite-stimulating hormone, ghrelin, and increases hunger and appetite
Numerous cross-sectional and prospective, epidemiologic studies have provided evidence of an association between short-duration and/or poor-quality sleep and the prevalence or incidence of diabetes mellitus or obesity
Effective treatment of obstructive sleep apnea, a sleep disorder that is highly prevalent in metabolic and endocrine disorders, has the potential to improve glucose metabolism and energy balance
Screening for habitual sleep patterns and obstructive sleep apnea might be critically important for patients with endocrine and metabolic disorders
Review criteria.
A search for original and review articles that focus on sleep, hormones and metabolism was performed in PubMed. The search terms used were “sleep”, “OSA”, “sleep apnea”, “CPAP”, “obesity”, “leptin”, “ghrelin”, “weight”, “visceral”, “diabetes”, “glucose”, “insulin”, “metabolic”, “endocrine”, “acromegaly”, “hypothyroidism”, “polycystic ovary syndrome”, and “PCOS”. We also searched the reference lists of identified articles for further papers. Articles were restricted to human studies. In order to limit the number of references, we selected, whenever possible, a recent review complemented by original papers published after the review.
Acknowledgments
Some research described in this article was supported by US National Institute of Health grants P01 AG-11412, R01 HL-075079, P60 DK-20595, R01 DK-0716960, R01 HL-075025 and M01 RR000055, by US Department of Defense award W81XWH-07-2-0071, by AASM/Pfizer Scholars Grant in Sleep Medicine (E Tasali), by Belgian ‘Fonds de la Recherche Scientifique Médicale’ (FRSM-3.4583.02), ‘Fonds National de la Recherche Scientifique’ (FNRS) and ‘CARE Foundation’ grants, by INSERM U628, and by Claude Bernard University of Lyon, France.
Footnotes
Competing Interests
E. Van Cauter has declared associations with the following companies: Actelyon and Sanofi-Aventis. See the article online for full details of the relationships. R. Leprout, K. Spiegel and E. Tasali declared no competing interests.
References
- 1.Gronfier C, Brandenberger G. Ultradian rhythms in pituitary and adrenal hormones: their relations to sleep. Sleep Med Rev. 1998;2:17–29. doi: 10.1016/s1087-0792(98)90051-x. [DOI] [PubMed] [Google Scholar]
- 2.Van Cauter E. Endocrine physiology. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4. Elsevier–Saunders; Philadelphia: 2005. pp. 266–282. [Google Scholar]
- 3.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 4.Adamantidis A, de Lecea L. The hypocretins as sensors for metabolism and arousal. J Physiol. 2009;587:33–40. doi: 10.1113/jphysiol.2008.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu MF, John J, Maidment N, Lam HA, Siegel JM. Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1079–R1086. doi: 10.1152/ajpregu.00207.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estabrooke IV, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeitzer JM, Buckmaster CL, Lyons DM, Mignot E. Increasing length of wakefulness and modulation of hypocretin-1 in the wake-consolidated squirrel monkey. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1736–R1742. doi: 10.1152/ajpregu.00460.2007. [DOI] [PubMed] [Google Scholar]
- 8.National Sleep Foundation. [accessed 19 January 2009];“Sleep in America” poll, summary of findings. 2008 :1–45. online 19 Jan 2009 http://www.sleepfoundation.org/atf/cf/%7Bf6bf2668-a1b4-4fe8-8d1a-a5d39340d9cb%7D/2008%20POLL%20SOF.pdf.
- 9.Institut National de Prévention et d’Education pour la Santé. [accessed 19 January 2009];Les français et leur sommeil. :1–12. [French] [online 19 Jan 2009] http://www.inpes.sante.fr/70000/dp/08/dp080310.pdf.
- 10.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 11.Bergman RN. Minimal model: perspective from 2005. Hormone Res. 2005;64(Suppl 3):S8–S15. doi: 10.1159/000089312. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 13.Buxton OM, Pavlova MK, Reid E, Simonson DC, Adler GK. [accessed 27 January 2009];Sleep restriction for one week reduces insulin sensitivity measured using the eugylcemic hyperinsulinemic clamp technique. online 12 June 2008 http://www.journalsleep.org/PDF/AbstractBook2008.pdf.
- 14.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergman RN. Toward physiological understanding of glucose tolerance. Minimal model approach. Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- 16.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann NY Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Cauter E, Knutson K. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol. 2008;159(Suppl 1):S59–S66. doi: 10.1530/EJE-08-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tasali E, Leproult R, Spiegel K. Reduced sleep duration or quality: relationships with insulin resistance and type 2 diabetes. Prog Cardiovasc Dis. doi: 10.1016/j.pcad.2008.10.002. in press. [DOI] [PubMed] [Google Scholar]
- 19.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 20.Nedeltcheva A, et al. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2008;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiegel K, et al. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 22.Guilleminault C, et al. Preliminary observations on the effects of sleep time in a sleep-restriction paradigm. Sleep Med. 2003;4:177–184. doi: 10.1016/s1389-9457(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 23.Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008;17:331–334. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 24.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body-mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec family study. Obesity (Silver Spring) 2007;15:253–261. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 26.Littman AJ, et al. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int J Obes (Lond) 2007;31:466–475. doi: 10.1038/sj.ijo.0803438. [DOI] [PubMed] [Google Scholar]
- 27.Cappuccio FP, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–274. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 29.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel SR, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond) 2008;32:1825–1834. doi: 10.1038/ijo.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berkey CS, Rockett HR, Colditz GA. Weight gain in older adolescent females: the internet, sleep, coffee, and alcohol. J Pediatr. 2008;153:635–639. doi: 10.1016/j.jpeds.2008.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keith SW, et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes (Lond) 2006;30:1585–1594. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- 33.Young T. Increasing sleep duration for a healthier (and less obese?) population tomorrow. Sleep. 2008;31:593–594. doi: 10.1093/sleep/31.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horne J. Too weighty a link between short sleep and obesity? Sleep. 2008;31:595–596. doi: 10.1093/sleep/31.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lauderdale DS, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 36.Vgontzas AN, et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes (Lond) 2008;32:801–809. doi: 10.1038/ijo.2008.4. [DOI] [PubMed] [Google Scholar]
- 37.Sanders M. Sleep breathing disorders. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. W.B Saunders Company; Philadelphia: 2005. pp. 969–1157. [Google Scholar]
- 38.West SD, Nicoll DJ, Stradling JR. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax. 2006;61:945–950. doi: 10.1136/thx.2005.057745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Einhorn D, et al. Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocr Pract. 2007;13:355–362. doi: 10.4158/EP.13.4.355. [DOI] [PubMed] [Google Scholar]
- 40.Foster GE, et al. Sleep apnea in obese adults with type 2 diabetes: baseline results from sleep AHEAD study. [accessed 16 February 2009];Sleep. 2005 28(Suppl):A204. http://www.journalsleep.org/pdf/Abstractbook2005.pdf. [Google Scholar]
- 41.Tasali E, Van Cauter E, Ehrmann D. Polycystic-ovary syndrome and obstrcutive sleep apnea. Sleep Med Clin. 2008;3:37–46. doi: 10.1016/j.jsmc.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vgontzas AN, et al. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab. 2001;86:517–520. doi: 10.1210/jcem.86.2.7185. [DOI] [PubMed] [Google Scholar]
- 43.Bottini P, Tantucci C. Sleep apnea syndrome in endocrine diseases. Respiration. 2003;70:320–327. doi: 10.1159/000072019. [DOI] [PubMed] [Google Scholar]
- 44.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 45.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5:207–217. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 46.Seicean S, et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care. 2008;31:1001–1006. doi: 10.2337/dc07-2003. [DOI] [PubMed] [Google Scholar]
- 47.Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179:235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dawson A, et al. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med. 2008;4:538–542. [PMC free article] [PubMed] [Google Scholar]
- 50.Hassaballa HA, Tulaimat A, Herdegen JJ, Mokhlesi B. The effect of continuous, positive airway pressure on glucose control in diabetic patients with severe obstructive sleep apnea. Sleep Breath. 2005;9:176–180. doi: 10.1007/s11325-005-0033-y. [DOI] [PubMed] [Google Scholar]
- 51.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 52.Harsch IA, et al. The effect of continuous, positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration. 2004;71:252–259. doi: 10.1159/000077423. [DOI] [PubMed] [Google Scholar]
- 53.Brooks B, et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effects of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79:1681–1685. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 54.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–974. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harsch IA, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–162. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 56.Schahin SP, et al. Long-term improvement of insulin sensitivity during CPAP therapy in the obstructive sleep-apnoea syndrome. Med Sci Monit. 2008;14:CR117–CR121. [PubMed] [Google Scholar]
- 57.Lindberg E, Berne C, Elmasry A, Hedner J, Janson C. CPAP treatment of a population-based sample—what are the benefits and the treatment compliance? Sleep Med. 2006;7:553–560. doi: 10.1016/j.sleep.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of CPAP on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 2008;134:686–692. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- 59.Saarelainen S, Lahtela J, Kallonen E. Effect of nasal CPAP treatment on insulin sensitivity and plasma leptin. J Sleep Res. 1997;6:146–147. doi: 10.1046/j.1365-2869.1997.00034.x. [DOI] [PubMed] [Google Scholar]
- 60.Ip S, Lam K, Ho C, Tsang K, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–586. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 61.Smurra M, et al. CPAP treatment does not affect glucose-insulin metabolism in sleep apneic patients. Sleep Med. 2001;2:207–213. doi: 10.1016/s1389-9457(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 62.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese men with OSA. Eur Respir J. 2007;29:720–727. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 63.Trenell MI, et al. Influence of constant, positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab. 2007;9:679–687. doi: 10.1111/j.1463-1326.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 64.Teramoto S, et al. Cardiovascular and metabolic effects of CPAP in obese obstructive sleep apnoea patients. Eur Respir J. 2008;31:223–225. doi: 10.1183/09031936.00105707. [DOI] [PubMed] [Google Scholar]
- 65.Vgontzas AN, et al. Selective effects of CPAP on sleep apnoea-associated manifestations. Eur J Clin Invest. 2008;38:585–595. doi: 10.1111/j.1365-2362.2008.01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phillips BG, et al. Recent weight gain in patients with newly diagnosed obstructive sleep apnea. J Hypertens. 1999;17:1297–1300. doi: 10.1097/00004872-199917090-00009. [DOI] [PubMed] [Google Scholar]
- 67.Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279:H234–H237. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 68.Harsch IA, et al. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003;22:251–257. doi: 10.1183/09031936.03.00010103. [DOI] [PubMed] [Google Scholar]
- 69.Pillar G, Shehadeh N. Abdominal fat and sleep apnea: the chicken or the egg? Diabetes Care. 2008;31(Suppl 2):S303–S309. doi: 10.2337/dc08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chin K, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–712. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 71.Sanner BM, Kollhosser P, Buechner N, Zidek W, Tepel M. Influence of treatment on leptin levels in patients with obstructive sleep apnoea. Eur Respir J. 2004;23:601–604. doi: 10.1183/09031936.04.00067804. [DOI] [PubMed] [Google Scholar]
- 72.Rubinsztajn R, Kumor M, Byskiniewicz K, Chazan R. The influence of 3 weeks therapy with continuous positive airway pressure on serum leptin and homocysteine concentration in patients with obstructive sleep apnea syndrome [Polish] Pneumonol Alergol Pol. 2006;74:63–67. [PubMed] [Google Scholar]
- 73.Drummond M, et al. Autoadjusting-CPAP effect on serum leptin concentrations in obstructive sleep apnoea patients. BMC Pulm Med. 2008;8:21. doi: 10.1186/1471-2466-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takahashi K, et al. Acylated ghrelin level in patients with OSA before and after nasal CPAP treatment. Respirology. 2008;13:810–816. doi: 10.1111/j.1440-1843.2008.01357.x. [DOI] [PubMed] [Google Scholar]
- 75.Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight loss in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97:896–897. doi: 10.1016/s0002-8223(97)00220-4. [DOI] [PubMed] [Google Scholar]
- 76.Redenius R, Murphy C, O’Neill E, Al-Hamwi M, Zallek SN. Does CPAP lead to change in BMI? J Clin Sleep Med. 2008;4:205–209. [PMC free article] [PubMed] [Google Scholar]
- 77.Kajaste S, Brander PE, Telakivi T, Partinen M, Mustajoki P. A cognitive-behavioral weight-reduction program in the treatment of obstructive sleep apnea syndrome, with or without initial nasal CPAP: a randomized study. Sleep Med. 2004;5:125–131. doi: 10.1016/j.sleep.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 78.Tasali E, et al. Impact of obstructive sleep apnea on insulin resistance and glucose tolerance in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:3878–3884. doi: 10.1210/jc.2008-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Subramanian S, Desai A, Joshipura M, Surani S. Practice patterns of screening for sleep apnea in physicians treating PCOS patients. Sleep Breath. 2007;11:233–237. doi: 10.1007/s11325-007-0120-3. [DOI] [PubMed] [Google Scholar]
- 80.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep-apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 81.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]