Abstract
We developed a method to measure hemoglobin synthesis rate (SynHb) in humans, assuming that free glycine in the red blood cell (RBC) represents free glycine in bone marrow for hemoglobin synthesis. The present rat study examines this assumption of the method and quantifies SynHb in rats. Sprague–Dawley rats (n = 9) were studied, [2-13C]glycine was intravenously infused over 24 h (2.5 mg kg−1 h−1), blood was drawn for glycine and heme isolation, and bone marrow was harvested for glycine isolation. Isotopic enrichments of glycine and heme were measured, fractional hemoglobin synthesis rate (fSynHb% day−1) was calculated, and from this a value for SynHb (mg g−1 day−1) was derived. Mean body weight was 446 ± 10 g (mean ± SE) and hemoglobin concentration was 14 ± 0.5 g dl−1. At 24 h, the mean isotopic enrichment, atom percentage excess (APE), of the RBC free glycine (1.56 ± 0.18 APE) was similar to the bone marrow (1.68 ± 0.15 APE). The rate of incorporation of 13C into heme increased over time from 0.0004 APE/h between 6 and 12 h, to 0.0014 APE/h between 12 and 18 h, and 0.0024 APE/h between 18 and 24 h. Consequently, fSynHb (1.19 ± 0.32, 2.92 ± 0.66, and 4.22 ± 0.56% day−1, respectively) and SynHb (0.11 ± 0.03, 0.28 ± 0.05, and 0.42 ± 0.05 mg g−1 day−1, respectively) showed similar patterns over the 24-h study period. We conclude that (1) enrichment of free glycine in the circulating RBC approximates enrichment of bone marrow free glycine for heme formation and (2) this pattern of hemoglobin synthesis rate is reflecting the characteristic release and gradual maturation of reticulocytes in the circulation.
Keywords: hemoglobin synthesis, anemia, glycine, bone marrow, rats
Hemoglobin metabolism is likely to be adversely affected in nutritional anemias and blood diseases of genetic or nongenetic origin. Many attempts have been made to obtain basic information about blood diseases by estimating the life span of the red blood cell (RBC).2 While the determination of RBC life span gives an indication of red cell destruction, it does not measure production. Furthermore, although a number of different clinical methods have been used, most are unreliable and none is based on the direct in vivo measurement of hemoglobin synthesis rate. The current standard clinical method to measure RBC mean life span and age distribution at death, requires tagging the RBC with chromium 51 (51Cr), in vitro (1). There are a number of difficulties with this method: (a) the dynamics of hemoglobin and RBC metabolism (e.g., heme synthesis and destruction) cannot be measured using the standard 51Cr method; (b) 51Cr is eluted from the RBC at a rate which significantly affects estimates of mean red cell life span (1)—although elution is fairly constant in normal blood and an appropriate correction factor can be applied, variation of elution rates in individuals with blood diseases can seriously affect the accuracy of the estimates of mean RBC life; (c) removal of the RBC for in vitro tagging with 51Cr and then reintroduction of the cells to the subject may cause other metabolic perturbations which are not measured independently; and (d) the use of radioisotopes cause reluctance in using the test with pregnancy, infants, and children.
The amino acid glycine is the sole nitrogenous precursor for the synthesis of the protoporphyrin of hemoglobin (2). The synthesis of one heme ring requires eight molecules of glycine (3, 4) which contribute all four nitrogen atoms plus five carbon atoms. The heme is quantitatively lost from the body as bilirubin and represents a persistent drain on the glycine pool of the body (5 mg kg−1 day−1). Work described by Shemin and Rittenberg (5) showed that the stable isotope [15N]glycine fed to a normal man resulted in the production of RBCs containing labeled heme. The curve of 15N concentration of heme versus time enabled the determination of the average life span of the RBC (5). The results obtained by this method agreed with the first reproducible results previously obtained in normal men, by using the method of differential agglutination of transfused blood (6). Subsequently, London et al. (7) studied two normal subjects, one male and one female, and three subjects with pernicious anemia, sickle cell anemia, and polycythemia vera. They confirmed the previous value of a 120-day RBC life span for the normal man, demonstrated a slightly lower value for the normal woman (109 days), and demonstrated much reduced values for the first two of the three blood diseases (85 and 42 days, respectively). The remaining subject with the blood disease polycythemia vera had normal red cell life span and pattern of RBC destruction, but the calculated rate of hemoglobin production for this individual was 2.5 times the values for the normal subjects. This result indicated the need for a direct measurement of hemoglobin production and disposal rates, in addition to estimating the life of the red cell. The method of London et al. (7) has not been used or developed since the 1940s.
We developed a method to measure fractional hemoglobin synthesis (fSynHb) rate (8) in vivo in humans. To reduce the study period to approximately 1 day, a value was required for incorporation of labeled glycine into the precursor pool (the bone marrow) for hemoglobin synthesis. Since the bone marrow was not easily accessible for sampling, we assumed that a value for the intracellular free glycine pool of the RBC could approximate the value for the bone marrow pool. The objectives of this present study were (a) to determine if the RBC free glycine pool provides a reasonable estimate for free glycine in the bone marrow and (b) to measure fSynHb rate in vivo in rats.
MATERIALS AND METHODS
Animals
The Institutional Animal Care and Use Committee at Virginia Commonwealth University approved the protocol. Nine male Sprague–Dawley rats fed a diet of regular rat chow (ICN Biomedicals, Inc., Costa Mesa, CA) were studied. On test days the animals were surgically cannulated at the jugular vein and carotid artery, to allow simultaneous intravenous infusion and blood sampling.
Surgery
Animals were anesthetized by administering intramuscular injections of 1.0 to 1.5 μg midazolam HCl (versed), followed by 25 mg of xylazine and 250 mg of ketamine HCl. The versed counteracted cataleptic effects while retaining the anesthetic properties of the ketamine. After anesthesia aseptic surgery was used to expose the carotid artery and jugular vein through a lateral neck incision. Both vessels were cannulated with 0.50 by 0.80-mm polyvinyl tubing (Critchley Electric, Australia) inserted 3 cm into the artery and 2 cm into the vein. Both catheters were secured to the surrounding muscle tissue with 4-O softsilk and tunneled subcutaneously to exit at the nape of the neck. The incisions were closed with 2-O softsilk and the animals were kept on a heating pad for 30 min after surgery, to avoid hypothermia. The infusion studies were conducted with the animals in secure cages and having free access to food and water. Movement was restricted by cage size and by using a swivel with the infusion system. The carotid line was used for blood sampling and the jugular line was connected to an infusion pump system supplying [2-13C]glycine (Cambridge Isotopes Laboratories, Andover, MA).
Experimental Design
The study period lasted 24 h and the timing of the measurement was standardized to begin at 1:00 PM, because of the possible circadian rhythm of heme formation (9). Following recovery from anesthesia a baseline (0 h) blood specimen was drawn, and then the intravenous infusion of the [2-13C]glycine was given at a rate of 2.5 mg kg−1 h−1 for 24 h. Subsequent blood samples were drawn at 6, 12, 18, and 24 h, to follow the rise of isotopic enrichment of glycine in plasma, in the RBC free glycine pool, and in heme isolated from the RBC. The hematology laboratory also measured the hemoglobin concentration of the blood. An infusion of normal saline was given immediately after each blood sampling to replenish twice the shed volume. After the 24-h blood draw the animals were killed with a lethal dose of xylazine (700 mg). All of the long bones were removed and the bone marrow was harvested using an 18-gauge needle to flush with normal saline. A pooled bone marrow sample from eight animals not infused with isotope was used to measure the natural abundance of [13C]glycine at 0 h. Blood and marrow samples were centrifuged at 3000 rpm for 10 min, plasma and RBC were separated, residual plasma was removed from the RBC by washing twice with normal saline and once with Kreb’s solution, and then the RBC was hemolyzed with an equal volume of distilled water and vigorous stirring. The saline was removed from the bone marrow samples prior to storage. Plasma and hemolyzed RBC samples were stored frozen at −20°C and bone marrow at −80°C until analysis.
Analytical Procedures
Heme analysis
Heme was isolated from 1 to 2 ml of hemolyzed blood by a previously published method (10). The 13C enrichment of the resulting dried heme crystals was measured by combustion and continuous flow isotope ratio mass spectrometry (ANCA, Europa Scientific Inc., Vandalia, OH).
Glycine analysis
The proteins were precipitated from 0.5 ml of the RBC and all of the bone marrow by adding twice the volume of a cold 10% trichloroacetic acid (TCA) solution in a centrifuge tube with vigorous mixing. The resulting mixture was spun cold at 3000 rpm for 10 min, to obtain a clear extract containing the free amino acid pool. The amino acids were isolated from the TCA extracts by column chromatography as previously described (11, 12) and the eluate was esterified and derivatized in preparation for analysis by gas chromatography mass spectrometry (GC5890E, MSD5972A, Hewlett–Packard, Wilmington, DE). The mass spectrometry measurements were made by adapting the method of Jahoor et al. (11) to the positive chemical ionization mode. The gas chromatograph used a 30 m × 0.25 μm HP-5MS siloxane capillary column. The oven was programmed for a temperature gradient from 80 to 150°C at 40°C/min and the retention time of the glycine derivative was approximately 3.5 min, at a helium flow rate of 1.59 ml/min. Ions were monitored at m/z 272 to 273 and the instrument was calibrated with suitable standards ranging from 0 to 1.64% of [13C]glycine.
Calculations
The fSynHb was calculated according to the precursor–product relationship (11) by the equation
where IRhemet2 − IRhemet1 is the increase in the isotopic ratio of the RBC heme isolates over the period t2 − t1 of the infusion, after the isotopic ratio of the RBC free glycine, RBCgly, had reached a steady state at plateau. Plateau was defined as previously described (11). The isotopic ratios were corrected for natural abundance of the 13C isotope measured in samples obtained before the infusion.
Absolute hemoglobin synthesis rate, SynHb, was calculated as the product of total circulating hemoglobin (mg) and the fSynHb. The units of SynHb are expressed as milligram per gram body weight per day.
Statistical Analysis
Results are presented as means ± SE. Isotope enrichment values of plasma and RBCgly were analyzed by linear regression against time of infusion and plateau was verified if the slope of the line was not significantly different from zero (11). The rate of isotope incorporation over time into plasma, RBCgly and heme was analyzed by repeated measures ANOVA, as were values obtained for SynHb using RBCgly measurement for 6–12, 12–18, and 18–24 h. The difference between the enrichment of free glycine in the RBC and the bone marrow at 24 h was tested by paired t test. Significant difference was assumed at P = 0.05. SAS software package was used for analysis.
RESULTS
The nine animals had a mean body weight of 446 ± 10 g (mean ± SE) and hemoglobin concentration of 14 ± 0.5 g dl−1.
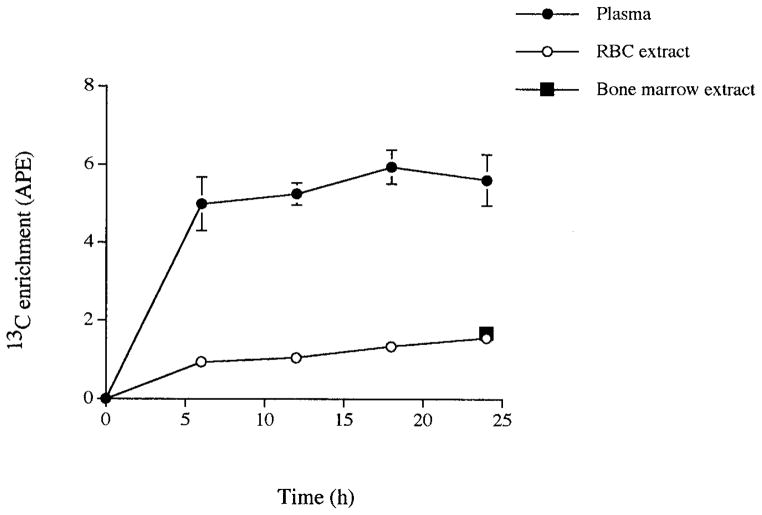
Isotopic enrichment, expressed as atom percentage excess (APE), increased rapidly in plasma during the infusion and reached a plateau by 6 h (Fig. 1). Linear regression analysis of the values from 6 to 24 h resulted in no significant difference from zero. Further, the results of repeated measures ANOVA over time showed no significant differences in rate of isotope incorporation into plasma between 6 and 24 h. Enrichment of the RBC extract followed a similar pattern of rise (Fig. 1) with values at approximately one-quarter of those obtained in plasma during the infusion. Although visual inspection of the pattern of rise in enrichment of the RBC extract suggested that a plateau had been achieved, linear regression analysis demonstrated some significant difference from zero for the values between 6 and 24 h (Fig. 1). Repeated measures ANOVA revealed a significant difference in rate of isotope incorporation between 12 and 18 h of infusion (P < 0.005). At the end of the infusion period (Fig. 1), the enrichment of the RBC free glycine extract (1.56 ± 0.17) was not significantly different from the bone marrow free glycine extract (1.68 ± 0.15, P > 0.1).
FIG. 1.
Rise of 13C enrichment of plasma, RBC acid extract, and bone marrow acid extract during 24-h infusion of [13C]glycine in nine Sprague–Dawley rats. Values are means ± SE.
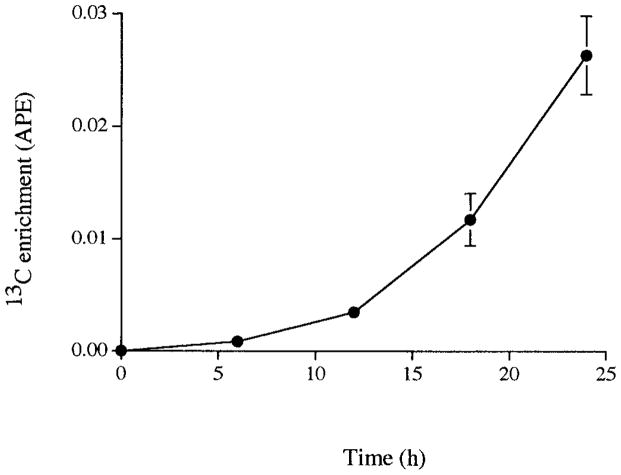
Figure 2 shows the pattern of rise in isotopic enrichment of heme in these animals. 13C enrichment was low in heme at 6 h (0.001 ± 0.000 APE) and levels were rapidly increasing at 24 h, by which time values were raised 26-fold above the 6-h measurement. Repeated measures ANOVA revealed significant differences in rate of isotope incorporation into heme between 6 to 12, 12 to 18, and 18 to 24 h (P < 0.005). Fractional hemoglobin synthesis rate (% day−1) and SynHb (mg g−1 day−1) were calculated according to the pattern observed for the rate of isotope incorporation into heme. Both fSynHb (1.20 ± 0.32, 2.92 ± 0.66, and 4.22 ± 0.56, % day−1) and SynHb (0.11 ± 0.03, 0.28 ± 0.05, and 0.42 ± 0.05, mg g−1 day−1) increased fourfold between 6 and 24 h (Table 1).
FIG. 2.
Rise of 13C enrichment of heme during 24-h infusion of [13C]glycine in nine Sprague–Dawley rats. Values are means ± SE.
TABLE 1.
Fractional Hemoglobin Synthesis Rate and Hemoglobin Synthesis Rate of Nine Rats Calculated According to the Pattern of 13C Isotope Incorporation into Heme
| Time period (h) | fSynHb (% day−1) | SynHb (mg g−1 day−1) |
|---|---|---|
| 6–12 | 1.20 ± 0.32 | 0.11 ± 0.03 |
| 12–18 | 2.92 ± 0.66 | 0.28 ± 0.05 |
| 18–24 | 4.22 ± 0.56 | 0.42 ± 0.05 |
Note. Values are means ± SE.
DISCUSSION
The finding by London et al. (7) that a blood disease can present with normal red cell life span yet elevated rates of hemoglobin synthesis prompted our specific interest in developing an accurate and practical method to measure fractional hemoglobin synthesis rate directly, in health and disease. The original method required large doses of the glycine isotope given over 48 h and for participants to be followed for a minimum of 200 days. We considered that for practical reasons, both the study time and the quantity (hence cost) of the isotope would need to be reduced. We easily reduced the quantity of isotope from 36–48 g used by London et al. (7), to 0.8–1.2 g (8) which gave measurable enrichments using a modern mass spectrometer at least 35 times more sensitive than used for the original method. Reducing the study time to 24 h, however, required a value for the level of isotopic enrichment in the free glycine available from the bone marrow, the precursor pool for hemoglobin synthesis. Since it is impractical to sample bone marrow routinely in humans, this present study investigated the extent to which the enrichment of free glycine in the circulating RBC was a reasonable alternative.
After 24 h of isotope infusion the mean enrichment of free glycine in the RBC for these rats was only 7% lower than the enrichment of the bone marrow free glycine. This result is encouraging, as one could now assume an approximation to the precursor pool from sampling the RBC free glycine pool. Indeed, the ability to measure the true enrichment of the precursor pool for body protein synthesis has been recognized as a limitation of several methods available to measure the kinetics of body proteins (13, 14). The analytical variability for the isotope enrichment measurements is small. The mean coefficient of variability (CV%) for quadruplicate measurements of glycine enrichment by this gas chromatography mass spectrometry method is 0.4 ± 0.04 (26 samples analyzed) and mean CV% of 0.4 ± 0.08 is obtained for quadruplicate measurements of heme enrichments by combustion and continuous flow mass spectrometry (25 samples analyzed).
Blood was drawn every 6 h to examine the pattern of rise of the isotope in plasma, RBC free glycine, and heme over time and to determine when plateau enrichment was achieved (Fig. 1). Plasma had reached plateau value for isotopic enrichment by 6 h. However, RBC free glycine enrichment showed a significant increase between 12 and 18 h, but not between 6 and 12 or between 18 and 24 h. To obtain a steady plateau value in the RBC free glycine pool will require a longer infusion time or use of a priming dose to shorten the time to plateau (15). The isotopic enrichment in heme (Fig. 2) which was only just measurable at 6 h had by 24 h increased at three distinctly different rates, to approximately 26 times the 6-h value. We believe that the pattern of isotope incorporation into heme is reflecting the characteristic release and gradual maturation of reticulocytes in the circulation (16). The development from circulatory reticulocytes to mature red blood cells has been reported to take 24 to 29 h in man (17). During this time heme formation continues until a critical hemoglobin concentration is reached, approximately 30 to 36% of red cell volume (16). Furthermore, there is evidence for a circadian rhythm in protein turnover, with particular reference to bone marrow and glycine metabolism (18, 19). Therefore, it was not entirely surprising that we calculated increasing values for fSynHb over the study period (Table 1). Clearly this model is more complex than a direct relationship between precursor and product. The precise lag time to first appearance of the label in the circulation and the time to mature RBCs will need to be investigated further in order to hone the method. It will be necessary to incorporate such findings into future calculations of heme synthesis rate by this model. Indeed, isolation of the reticulocyte fraction and other RBC fractions by age may lead to an even more suitable substitute index for the precursor pool enrichment.
Based on the mean total hemoglobin content of 4.38 ± 0.2 g and the derived SynHb (g day−1) over the 24-h period, we calculated the number of days to replace the hemoglobin pool in these animals. The result was a mean of 49 days. Alternatively, RBC life span calculated as the reciprocal of fSynHb returned a value of mean 47 days. These values are within the range of 45 to 68 days reported in the literature for mean RBC life span in rats (16). This result demonstrates that a study time of at least 24 h may be feasible for measuring hemoglobin synthesis rate by this method.
The rate of protein synthesis in healthy rats approximates 22 g kg−1 day−1 (20). Therefore, heme synthesis accounted for 0.10% of total body protein synthesis in these rats. In a rat model of hemolytic anemia, the heme synthesis is likely to account for quantitatively more of the total body protein synthesis. For example, a theoretical estimate for hemoglobin synthesis of 2–4% of total body synthesis has been made for healthy adult men compared with as much as 12% in the sickle cell patient (21). In the case of the sickle cell patient, this elevated proportion for hemoglobin synthesis rate may be indicating a harmful shortage of substrates for other important protein synthetic pathways. Hence, the ability to estimate the contribution of heme synthesis to whole body protein synthesis will be useful for characterizing a variety of blood diseases in which hemoglobin metabolism may be affected.
Acknowledgments
We thank Professor Alan Jackson and Dr. Farook Jahoor for their support. The project was funded by a grant from the Thomas F. Jeffress and Kate Miller Jeffress Memorial Trust.
Footnotes
Abbreviations used: RBC, red blood cell; SynHb, hemoglobin synthesis rate; fSyn Hb, fractional hemoglobin synthesis rate; APE, atom percent excess; TCA, trichloroacetic acid, IRheme, isotope ratio of heme; RBCgly, RBC free glycine; CV%, coefficient of variability.
References
- 1.International Committee for Standardization in Haematology. Recommended method for radioisotope red cell survival studies. Br J Haematol. 1980;45:659–666. doi: 10.1111/j.1365-2141.1980.tb07189.x. [DOI] [PubMed] [Google Scholar]
- 2.Shemin D, Rittenberg D. The utilization of glycine for the synthesis of a porphyrin. J Biol Chem. 1945;159:567–568. [Google Scholar]
- 3.Muir HM, Neuberger A. The biosynthesis of porphyrins: The distribution of 15N in the ring system. Biochem J. 1949;45:163. [PubMed] [Google Scholar]
- 4.Muir HM, Neuberger A. The biosynthesis of porphyrins 2: The origins of the methyne carbon atoms. Biochem J. 1950;47:87. doi: 10.1042/bj0470097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shemin D, Rittenberg D. The life span of the human red blood cell. J Biol Chem. 1946;166:627–636. [PubMed] [Google Scholar]
- 6.Calender ST, Powell OE, Witts LJ. The life span of the red cell in man. J Pathol Bacteriol. 1945;57:129–139. [Google Scholar]
- 7.London IM, Shemin D, West R, Rittenberg D. Heme synthesis and red blood cell dynamics in normal humans and in subjects with polycythemia vera, sickle cell anemia, and pernicious anemia. J Biol Chem. 1949;179:463–484. [PubMed] [Google Scholar]
- 8.Hibbert JM, Swerdlow PS, Gore DC, Wolfe L, Jahoor F, Jackson AA. A method to measure haemoglobin turnover rate. Proc Nutr Soc. 1998;57:37A. [Abstract] [Google Scholar]
- 9.Smaaland R, Laerum OD. In: Biologic Rhythm in Clinical and Laboratory Medicine. Touitou, Haus, editors. Springer Verlag; Berlin: 1992. pp. 527–546. [Google Scholar]
- 10.Labbe RF, Nishida G. A new method of hemin isolation. Biochim Biophys Acta. 1957;26:437. doi: 10.1016/0006-3002(57)90033-1. [DOI] [PubMed] [Google Scholar]
- 11.Jahoor F, Wykes LJ, Reeds PJ, Henry JF, Del Rosario MP, Frazer ME. Protein deficient pigs cannot maintain reduced glutathione homeostasis when subjected to the stress of inflammation. J Nutr. 1995;125:1462–1472. doi: 10.1093/jn/125.6.1462. [DOI] [PubMed] [Google Scholar]
- 12.Patterson BW, Hachey DL, Cooke GL, Amann JM, Klein PD. Incorporation of a stable isotopically labeled amino acid into multiple human apolipoproteins. J Lipid Res. 1991;32:1063–1072. [PubMed] [Google Scholar]
- 13.Bier DM. Intrinsically difficult problems: The kinetics of body proteins and amino acids in man. Diabetes Metab Rev. 1989;5:111–132. doi: 10.1002/dmr.5610050203. [DOI] [PubMed] [Google Scholar]
- 14.Rennie MJ, Smith K, Watt PW. Measurement of human protein synthesis: An optimal approach. Am J Physiol. 1994;266:E298–E307. doi: 10.1152/ajpendo.1994.266.3.E298. [DOI] [PubMed] [Google Scholar]
- 15.Matthews DE, Downey RS. Measurement of urea kinetics in humans: A validation of stable isotope tracer methods. Am J Physiol. 1984;246:E519–E527. doi: 10.1152/ajpendo.1984.246.6.E519. [DOI] [PubMed] [Google Scholar]
- 16.Schalm OW, Jain NC, Carroll EJ. Veterinary Hematology. Lea and Febiger; Philadelphia: 1975. pp. 356–404. [Google Scholar]
- 17.Finch CA. Some quantitative aspects of erythropoiesis. Ann N Y Acad Sci. 1959;77:410–416. doi: 10.1111/j.1749-6632.1959.tb36917.x. [DOI] [PubMed] [Google Scholar]
- 18.Smaalamd R, Abrahamsen JF, Svardal AM, Lote K, Ueland PM. DNA cell cycle distribution and glutathione (GSH) content according to circadian stage in bone marrow of cancer patients. Br J Cancer. 1992;66:39–45. doi: 10.1038/bjc.1992.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson AA, Soares MJ, Grove G, Waterlow JC. Enrichment in urinary ammonia and urea with hourly oral doses of [15N]glycine: Evidence for a step function and a circadian rhythm in protein turnover. Clin Sci. 1997;93:265–271. doi: 10.1042/cs0930265. [DOI] [PubMed] [Google Scholar]
- 20.Reeds PJ, Harris C. In: Nitrogen Metabolism in Man. Waterlow, Stephen, editors. Appl. Sci; London: 1981. pp. 391–408. [Google Scholar]
- 21.Badaloo A, Jackson AA, Jahoor F. Whole body protein turnover and resting metabolic rate in homozygous sickle cell disease. Clin Sci. 1989;77:93–97. doi: 10.1042/cs0770093. [DOI] [PubMed] [Google Scholar]