Abstract
NKG2D is an activating receptor expressed on the surface of natural killer (NK) cells, CD8+ T cells, and subsets of CD4+ T cells, iNKT cells, and γδ T cells. In humans NKG2D transmits signals by its association with the DAP10 adapter subunit and in mice alternatively spliced isoforms transmit signals either using DAP10 or DAP12 adapter subunits. Although NKG2D is encoded by a highly conserved gene (KLRK1) with limited polymorphism, the receptor recognizes an extensive repertoire of ligands, encoded by at least 8 genes in humans (MICA, MICB, RAET1E, RAET1G, RAET1H, RAET1I, RAET1L, and RAET1N), some with extensive allelic polymorphism. Expression of the NKG2D ligands is tightly regulated at the level of transcription, translation, and post-translation. In general healthy adult tissues do not express NKG2D glycoproteins on the cell surface, but these ligands can be induced by hyper-proliferation and transformation, as well as when cells are infected by pathogens. Thus, the NKG2D pathway serves a mechanism for the immune system to detect and eliminate cells that have undergone “stress”. Viruses and tumor cells have devised numerous strategies to evade detection by the NKG2D surveillance system and diversification of the NKG2D ligand genes likely has been driven by selective pressures imposed by pathogens. NKG2D provides an attractive target for therapeutics in the treatment of infectious diseases, cancer, and autoimmune diseases.
Introduction
The response of natural killer (NK) cells and T cells to pathogens and tumors is regulated by the integration of signals from numerous receptors expressed on their cell surface that can initiate, enhance, or suppress their effector cell functions. While T-cell recognition and activation are dominated by the antigen-specific T-cell antigen receptors (TCR) that are generated by somatic genetic recombination, NK cells use an extensive repertoire of germline-encoded receptors, many of which are also expressed by T cells. One of the best-characterized receptors shared by NK cells and T cells is NKG2D.
NKG2D genes and proteins
A NKG2D transcript was isolated from a cDNA library prepared from a human NK cell clone and was predicted to encode a type II transmembrane protein with a C-type lectin-like extracellular domain (1). The gene encoding NKG2D, KLRK1, is on human chromosome 12p13.2 flanked on the centromeric side by KLRD1 (CD94) and on the telomeric side by the cluster of KLRC4 (NKG2F), KLRC3 (NKG2E), KLRC2 (NKG2C), and KLRC1 (NKG2A) genes (2). The human KLRK1 gene has limited polymorphism, with only two alleles that differ by a single amino acid. The mouse ortholog, Klrk1, is present on the syntenic region of mouse chromosome 6 and similarly has limited polymorphism (3). Orthologs of KLRK1 are present in the genome of all mammals, as well as in marsupials, indicating that the gene is highly conserved during evolution.
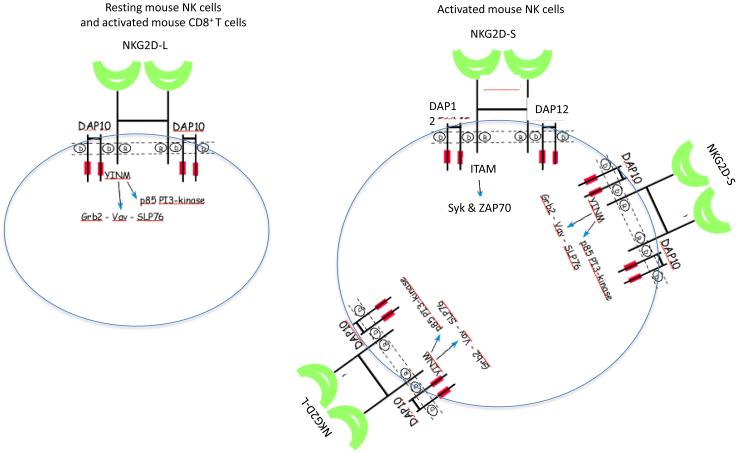
Expression of NKG2D proteins on the cell surface requires its association with adapter proteins to stabilize the receptor complex (Figure 1). Mice express two isoforms of the NKG2D protein as a result of alternative splicing. Resting mouse NK cells express a longer (NKG2D-L) protein that exclusively associates non-covalently with the DAP10 adapter protein, whereas activation of mouse NK cells induces alternative splicing of Klrk1 resulting in a shorter (NKG2D-S) protein isoform that can associate with either the DAP10 or DAP12 adapter protein (4, 5) (Figure 1). The association of NKG2D with DAP10 or DAP12 occurs through interactions between charged residues within the transmembrane regions of the receptor and its adapter subunits (6). Association of NKG2D with DAP12 versus DAP10 has significant consequences for signal transduction in that DAP12 possesses a canonical immunotyrosine-based activation motif (ITAM), which recruits Syk and ZAP70 tyrosine kinases (7), whereas DAP10 has a YINM motif, which recruits a p85 PI3 kinase and Vav-1 signaling complex (6, 8). Each disulfide-bonded NKG2D homodimer associates with two DAP10 disulfide-bonded homodimers to form a hexameric receptor complex (9). Intracellular concentrations of magnesium are critical for the assembly of the NKG2D-DAP10 receptor complex in that patients with a homozygous loss of the magnesium transporter 1 (MAGT1) gene have impaired expression of NKG2D on the surface of their T cells and NK cells, which can be restored by dietary Mg++ supplements (10).
Figure 1.
Mouse NKG2D receptor complex. Mice express two alternatively spliced isoforms of NKG2D. NKG2D-L (long) is expressed as a disulfide-bonded homodimer that can associate with DAP10 homodimers on the surface of resting mouse NK cells and on activated (but not resting) CD8+ T cells. After activation, mouse NK cells express a NKG2D-S (short) isoform that can associate with either homodimers of DAP10 or DAP12. DAP10 contains a YINM motif capable of recruiting a p85 PI3-kinase, Vav-1, Grb2 signaling complex whereas DAP12 contains a canonical ITAM, which can recruit Syk and ZAP70.
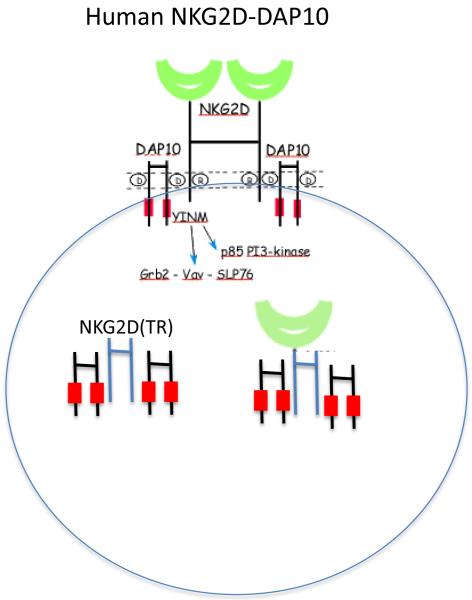
Resting mouse CD8+ T cells do not transcribe Klrk1, but after TCR-induced activation mouse CD8+ T cells express both NKG2D-L and NKG2D-S transcripts; however, activated mouse CD8+ T cell typically express only DAP10 and not DAP12 (4, 5, 11, 12) (Figure 1). In mice, some myeloid cell populations can transcribe (www.immgen.org) and express NKG2D on the cell surface (11, 13). In humans and mice, many γδ T cells and iNKT cells and a small subset of effector or memory CD4+ T cells express NKG2D (11, 14). Unlike CD8+ T cells, TCR-mediated activation is not sufficient to induce NKG2D expression on CD4+ T cells, and the factors responsible for induction of NKG2D on CD4+ T cells are not known. Different than in mice, in humans NKG2D is expressed constitutively on almost all resting CD8+ T cells, and single-positive CD8+ thymocytes, as well as essentially all NK cells, and appears to associate exclusively with DAP10 in both NK cells and T cells (6, 14-16) (Figure 2). In humans, alternative splicing of KLRK1 in NK cells and T cells can generate a truncated protein isoform [NKG2D(TR)] that lacks the extracellular domain, but this truncated protein contains the transmembrane domain and can compete with the full-length NKG2D proteins to sequester the DAP10 signaling proteins, resulting in decreased expression of functional NKG2D receptors on the cell surface (17) (Figure 2). Expression of NKG2D on NK cells and CD8+ T cells can be modulated by cytokines due to their effects on transcription and post-transcriptional processing of NKG2D and DAP10. In humans, IL2, IL7, IL12, and IL15 up-regulate NKG2D expression (18-21), whereas TGFβ (22-24), interferon-β1 (25), and IL21 (26) down-modulate NKG2D.
Figure 2.
Human NKG2D receptor complex. Humans express a single full-length isoform of NKG2D constitutively as a disulfide-bonded homodimer on the cell surface of essentially all NK cells and CD8+ T cells, associated in a hexameric complex with two homodimers of the DAP10 signaling protein. Some individuals express an alternatively spliced NKG2D transcript that lacks the entire extracellular domain, but retains the transmembrane and cytoplasmic domains of NKG2D (NKG2D-TR). NKG2D-TR homodimers and heterodimers of NKG2D-TR and full-length NKG2D apparently associate with DAP10 homodimers, but are retained within the cytoplasm and degraded, thereby diminishing cell surface expression of functional NKG2D complexes.
NKG2D ligand genes and proteins
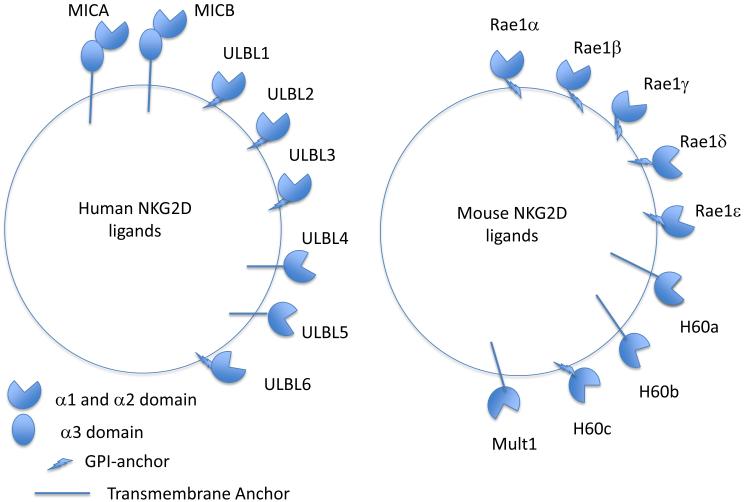
While a single gene with limited polymorphism encodes NKG2D, this receptor recognizes a remarkably diverse array of ligands encoded by numerous genes, some with extensive allelic polymorphism (Figure 3). In humans, NKG2D recognizes proteins encoded by the MICA and MICB locus, which are located within the major histocompatibility complex (MHC) on chromosome 6 near the HLAB locus. Currently, 100 alleles of MICA encoding 79 protein variants and 40 alleles of MICB encoding 26 protein variants (https://www.ebi.ac.uk/ipd/imgt/hla/stats.html) have been identified in the human population. Human NKG2D also binds to another family of glycoproteins encoded by the RAET1 (also known as ULBP) genes located on chromosome 6q24.2–25.3, which comprises 10 genes, RAET1E-N, including 6 loci that encode functional proteins (commonly referred to as ULBP1 (RAET1I), ULBP2 (RAET1H), ULBP3 (RAET1N), ULBP4 (RAET1E), ULBP5 (RAET1G), and ULBP6 (RAET1L)) (27). The RAET1 genes demonstrate less allelic polymorphism than the MICA and MICB genes. MICA, MICB, RAET1E (ULBP4), and RAET1G (ULBP5) are transmembrane-anchored glycoproteins, whereas RAET1I (ULBP1), RAET1H (ULBP2), RAET1N (ULBP3), and RAET1L (ULBP6) are glycophosphatidylinositol (GPI)-anchored, although RAET1H (ULBP2) may be expressed in both transmembrane-anchored and GPI-anchored forms (28) and RAET1G (ULBP5) may be GPI-anchored (29). Mice have orthologs of the human RAET1 genes present on mouse chromosome 10, but none of the mouse ligand genes correspond to MICA or MICB or are encoded within the mouse MHC. The mouse ligands include Rae1α, Rae1β, Rae1γ, Rae1δ, and Rae1ε, MULT1, and H60a, H60b, and H60c, with MULT1, H60a, and H60b being transmembrane-anchored and the others GPI-anchored (30-33). All of the NKG2D ligands have α1 and α2 extracellular domains with homology to MHC class I molecules (MICA and MICB proteins also possess an α3 domain), but none bind peptides or β2-microglobulin. Structures of many of the mouse and human ligands in complex with NKG2D have been reported (reviewed in (34)). NKG2D binds with affinities varying from 10−6 – 10−9 M to these diverse NKG2D ligands, some with only ~25% amino acid homology, by an “adaptive fit” mechanism, rather than inducing conformational changes in the ligands (reviewed in (34)).
Figure 3.
This is a schematic diagram showing all human and mouse NKG2D ligands identified to date.
The NKG2D ligands are regulated by transcriptional, translational, and post-translational mechanisms (reviewed in (35)). Although the mouse Raet1 genes were originally identified as being constitutively expressed in the developing embryo (36), in general, the NKG2D ligands are not expressed on the surface of healthy cells and tissues in adults. However, essentially every cell type, and every type of cancer, is capable of expressing one or more of the NKG2D ligands if appropriately stimulated. Given the existence of several distinct NKG2D ligand genes and extensive allelic polymorphisms at some of these loci, how transcription of these genes in different cell types is induced is not well defined and is likely context-dependent. The induction of NKG2D ligand expression frequently is attributed to cellular “stress”, for example in the case of infection of a cell by pathogens or cells undergoing transformation. However, normal healthy cells undergoing extensive proliferation, for example embryonic tissues, hematopoietic cells rapidly proliferating to repopulate the host after hematopoietic stem cell transplantation, and tissues undergoing wound repair can up-regulate expression of certain NKG2D ligands (36-38). Moreover, aberrant expression of NKG2D ligands has been reported in sites of inflammation and in tissues undergoing autoimmune pathologies, including rheumatoid arthritis, diabetes, celiac disease, Crohn’s disease, atherosclerosis, alopecia, and asthma, although discrepant findings in various mouse model systems and human diseases have highlighted the complexity and heterogeneity of these diseases (reviewed in (39)). NKG2D ligand transcripts can be regulated by RNA degradation and microRNAs, and ligand proteins can be retained and degraded intracellularly by E3 ubiquitin ligases or cleaved from the cell surface by membrane matrix metalloprotease (reviewed in (35)). Thus, the regulation of the NKG2D ligands is a very complex process and there are no general rules that apply for the induction of the different NKG2D ligands in different cell types.
Activation of NK cells and T cells by NKG2D
The prevailing concept is that NKG2D serves as a general sensor for recognition of “induced self” for the detection and elimination of hyper-proliferative cells, transformed cells, or cells infected by pathogens. As with the ligands, signaling by the NKG2D receptor in NK cells and T cells is complex and incompletely understood. While NKG2D is expressed constitutively on essentially all resting human NK cells and CD8+ T cells (40), engagement of NKG2D alone is insufficient to trigger cell-mediated cytotoxicity or cytokine production (41, 42), although the simultaneous engagement of NKG2D and other “costimulatory” receptors, such as CD335 (NKp46) or CD244 (2B4), can trigger cytolytic activity in resting human NK cells (41). However, once human NK cells are “primed” by cultured in IL2 or IL15, engagement of NKG2D alone is sufficient to initiate degranulation and cytokine production. Resting mouse NK cells can be activated directly by crosslinking NKG2D ex vivo (11), and mice can reject normal hematopoietic cells that express a Raet1 transgene without prior priming (37), perhaps because mouse, but not human, NKG2D associates with DAP12 in addition to DAP10. Similarly, although expressed on all naïve human CD8+ T cells, NKG2D fails to co-stimulate TCR-induced activation of resting CD8+ T cells (19, 42, 43), and only augments TCR-dependent activation after the T cells have been activated and cultured for a period of time in vitro (18, 43, 44). After some period of culture, human CD8+ T cells acquire the capacity to kill ligand-bearing target cells in an NKG2D-dependent, TCR-independent fashion. NKG2D-dependent, TCR-independent cytolytic activity has also been observed in intraepithelial intestinal CD8+ T cells isolated from patients with celiac disease, possibly due to exposure to excess IL15 in the inflamed tissue (45). How NK cells and T cells are “primed” to make NKG2D become permissive to trigger effector functions has not been defined, although cytosolic phospholipase A2 has been implicated in the process (46).
NKG2D in immunity to infectious disease and cancer
The NKG2D system likely evolved and has been conserved to provide immunity against pathogens. NKG2D ligands are induced in cells infected with intracellular bacteria and viruses and can be induced on dendritic cells responding to microbial pathogens (reviewed in (35)). Again, there are no general rules for which the numerous NKG2D ligands are induced in various cell types by different pathogens. However, the importance of NKG2D is highlighted by the finding that several viruses have evolved mechanisms to prevent the expression of ligands on the cell surface of infected cells. For example, the RAET1 ligands in humans were discovered because of the ability of the UL16 glycoprotein in human cytomegalovirus (CMV) to bind to RAET1I – hence initially designated “UL16 Binding Protein 1” (ULBP1) –and to bind to MICB (47). Human CMV encodes several proteins that retain and degrade NKG2D ligands, preventing their expression on the cell surface, including UL16 targeting MICB, RAET1I (ULBP1), RAET1H (ULBP2), and RAET1L (ULBP6), US18 and US20 targeting MICA (48), UL142 targeting MICA and RAET1N (ULBP3) (49, 50), and HCMV microRNA-UL112 targeting MICB transcripts (51). Mouse CMV has evolved its own viral proteins to counter NKG2D-dependent immunity. Mouse CMV m152 protein blocks surface expression of Raet1 proteins (52, 53); m155 blocks H60 (54), m145 blocks MULT1 (55), and m138 blocks expression of MULT1 and H60 (56). The Nef protein in HIV-1 inhibits expression of MICA, RAET1I (ULBP1), and RAET1H (ULBP2) (57), as well as inhibiting HLA-A and HLA-B. Similarly, adenovirus E3/19k (58), U21 of human herpesvirus 7 (59), and the K5 ubiquitin ligase encoded by human herpesvirus 8 (60) cause the degradation of MICA and MICB. The cowpox and monkeypox viruses secrete a soluble protein that functions as an NKG2D receptor antagonist, binding to NKG2D ligands on the surface of infected cells with high affinity to prevent detection by T cells and NK cells (61). It seems likely that the diversity of NKG2D ligand genes and their allelic polymorphism is being driven by the pathogens that are devising mechanisms to escape detection by immune cells using NKG2D.
Many cancers of all cell types express one or more of the NKG2D ligands (reviewed in (62)). The factors causing induction of the NKG2D ligand genes likely vary based on intrinsic features of the transformed cells, as well as influences from the microenvironment (reviewed in (35)). In some cases, ligands may be induced due to the genomic instability of the transformed cells, resulting in activation of the ATM and ATR DNA repair pathways (63). Induction of ligands might also be caused by hyper-proliferation of the transformed cell that results in activation of E2F transcription factors (38).
Studies using transplantable mouse tumors have demonstrated that expression of a NKG2D ligand is sufficient to cause rejection of the tumor (64, 65). NKG2D plays a role in immune surveillance against primary tumors as revealed by using NKG2D-deficient TRAMP mice, a transgenic model of prostate adenocarcinoma, and NKG2D-deficient Eμ-myc mice, a transgenic model of B-cell lymphoma (66). Moreover, in these tumor models, higher amounts of NKG2D ligands were present on tumors arising in NKG2D-deficient mice, suggesting NKG2D-mediated immune editing of the tumors (66).
Primary tumors frequently express NKG2D ligands, raising the question of how they avoided detection and elimination by NK cells and T cells. Several mechanisms have been identified that permit the escape of tumors bearing NKG2D ligands. These include systemic release of NKG2D ligands by tumors in cancer patients (67, 68). NKG2D ligands can be secreted from tumor cells, proteolytically cleaved from the cell surface by matrix metalloproteases, or released as exosomes (69-71) (reviewed in (72, 73)). When NK cells or T cells encounter cells bearing NKG2D ligands, it results in down modulation of the receptor. This is observed in vitro when NK cells or T cells are co-cultured with NKG2D ligand-bearing cells and in vivo in mice bearing transgenes of NKG2D ligands. The down-modulation of NKG2D on NK cells and T cells after encounters with ligand-bearing cells likely represents a feedback mechanism to regulate the response of these lymphocytes. The ligand-induced down-modulation of NKG2D is most effectively achieved by interaction with membrane-bound, rather than soluble, NKG2D ligands, presumably because in membrane-bound form the ligands can cluster and cross-link the NKG2D receptors, triggering their internalization in the NK cells and T cells. In addition, tumor-derived factors, such as TGFβ, can cause down-regulation of the NKG2D receptor on NK cells and T cells (22-24). Lactose dehydrogenase 5 released from tumor cells can induce expression of NKG2D ligands on healthy monocytes, allowing them to down-regulate NKG2D on NK cells and T cell and serve as decoys (74). Although it was generally thought that soluble NKG2D ligands released from tumors down-regulated NKG2D on NK cells and T cells, Raulet and colleagues have reported that the high-affinity MULT1 mouse NKG2D ligand can stimulate NK cells and enhance their antitumor activity (75). Cerwenka and colleagues also observed that expression of NKG2D ligands on myeloid-derived suppressor cells can activate NK cells, allowing the NK cells to eliminate tumor cells lacking expression of NKG2D ligands (76). Collectively, these studies highlight the importance of the NKG2D pathway in immune surveillance of cancer.
Therapeutic opportunities
The extent to which viruses and tumors have devised mechanisms to evade detection by NKG2D indicates that therapeutic strategies to restore or enhance NKG2D-dependent activation of NK cells or T cells may be beneficial. Alternatively, in cases where NKG2D serves to exacerbate autoimmune responses, blocking NKG2D or suppressing expression of ligands in inflammation provides an attractive therapeutic target. In the context of cancer therapy, irradiation and chemotherapeutic drugs, for example histone deacetylase inhibitors, have been shown to induce NKG2D ligand expression on some tumor cells, which might synergize with the new generation of immune checkpoint drugs blocking CTLA-4 and PD-1 (reviewed in (62, 73). Similarly, therapeutics that prevent or block the shedding of ligands by tumors or their release of NKG2D ligand-bearing exosomes might restore expression of NKG2D receptors on NK cells and T cells and improve their antitumor activity. Additionally, NKG2D agonists might augment the antitumor activity of NK cells and T cells. Chimeric antigen receptors expressing the extracellular domain of NKG2D linked to the signaling elements of CD3ζ and DAP10 have been constructed and transduced into T cells to test their potential for cancer therapy (reviewed in (77)). As therapeutics in infectious disease, Jonjic and colleagues have introduced a cDNA encoding a NKG2D ligand into the mouse CMV genome and showed that it functions as an effective vaccine for CMV (78). These findings suggest a new strategy for the development of vaccines against pathogens that have been refractory to conventional approaches. Moreover, NKG2D agonists may boost responses against chronic pathogens, allowing their elimination. A better understanding of the cell intrinsic and extrinsic mechanisms that regulate the expression of the NKG2D ligands and the intracellular signals controlling NKG2D-induced responses in T cells and NK cells is needed to take full advantage of this potent immune pathway.
Acknowledgments
Grant Support
L.L.L. is an American Cancer Society Professor and funded by US National Institutes of Health grants AI066897 and AI068129
Footnotes
Disclosure of Potential Conflicts of Interest
L.L.L. and the University of California, San Francisco have licensed intellectual property rights regarding NKG2D for commercial applications.
References
- 1.Houchins JP, Yabe T, McSherry C, Bach FH. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J Exp Med. 1991;173:1017–20. doi: 10.1084/jem.173.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glienke J, Sobanov Y, Brostjan C, Steffens C, Nguyen C, Lehrach H, et al. The genomic organization of NKG2C, E, F, and D receptor genes in the human natural killer gene complex. Immunogenetics. 1998;48:163–73. doi: 10.1007/s002510050420. [DOI] [PubMed] [Google Scholar]
- 3.Ho EL, Heusel JW, Brown MG, Matsumoto K, Scalzo AA, Yokoyama WM. Murine Nkg2d and Cd94 are clustered within the natural killer complex and are expressed independently in natural killer cells. Proc Natl Acad Sci U S A. 1998;95:6320–5. doi: 10.1073/pnas.95.11.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 2002;3:1150–5. doi: 10.1038/ni857. [DOI] [PubMed] [Google Scholar]
- 5.Diefenbach A, Tomasello E, Lucas M, Jamieson AM, Hsia JK, Vivier E, et al. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol. 2002;3:1142–9. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–2. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 7.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–7. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 8.Upshaw JL, Arneson LN, Schoon RA, Dick CJ, Billadeau DD, Leibson PJ. NKG2D-mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells. Nat Immunol. 2006;7:524–32. doi: 10.1038/ni1325. [DOI] [PubMed] [Google Scholar]
- 9.Garrity D, Call ME, Feng J, Wucherpfennig KW. The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proc Natl Acad Sci U S A. 2005;102:7641–6. doi: 10.1073/pnas.0502439102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaigne-Delalande B, Li FY, O'Connor GM, Lukacs MJ, Jiang P, Zheng L, et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341:186–91. doi: 10.1126/science.1240094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich LIR, Ogasawara K, Hamerman JA, Takaki R, Zingoni A, Allison JP, et al. Engagement of NKG2D by cognate ligand or antibody alone is insufficient to mediate costimulation of human and mouse CD8+ T cells. J Immunol. 2005;174:1922–31. doi: 10.4049/jimmunol.174.4.1922. [DOI] [PubMed] [Google Scholar]
- 13.Buhtoiarov IN, Rakhmilevich AL, Lanier LL, Ranheim EA, Sondel PM. Naive mouse macrophages become activated following recognition of L5178Y lymphoma cells via concurrent ligation of CD40, NKG2D, and CD18 molecules. J Immunol. 2009;182:1940–53. doi: 10.4049/jimmunol.0800443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of natural killer cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–30. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Cherwinski H, Spies T, Phillips JH, Lanier LL. DAP10 and DAP12 Form Distinct, but Functionally Cooperative, Receptor Complexes in Natural Killer Cells. J Exp Med. 2000;192:1059–68. doi: 10.1084/jem.192.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen DB, Araki M, Hamerman JA, Chen T, Yamamura T, Lanier LL. A Structural Basis for the Association of DAP12 with Mouse, but Not Human, NKG2D. J Immunol. 2004;173:2470–8. doi: 10.4049/jimmunol.173.4.2470. [DOI] [PubMed] [Google Scholar]
- 17.Karimi MA, Aguilar OA, Zou B, Bachmann MH, Carlyle JR, Baldwin CL, et al. A truncated human NKG2D splice isoform negatively regulates NKG2D-mediated function. J Immunol. 2014;193:2764–71. doi: 10.4049/jimmunol.1400920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts AI, Lee L, Schwarz E, Groh V, Spies T, Ebert EC, et al. Cutting edge: NKG2D receptors induced by IL-15 costimulate CD28- negative effector CTL in the tissue microenvironment. J Immunol. 2001;167:5527–30. doi: 10.4049/jimmunol.167.10.5527. [DOI] [PubMed] [Google Scholar]
- 19.Maasho K, Opoku-Anane J, Marusina AI, Coligan JE, Borrego F. Cutting edge: NKG2D is a costimulatory receptor for human naive CD8+ T cells. J Immunol. 2005;174:4480–4. doi: 10.4049/jimmunol.174.8.4480. [DOI] [PubMed] [Google Scholar]
- 20.Park YP, Choi SC, Kiesler P, Gil-Krzewska A, Borrego F, Weck J, et al. Complex regulation of human NKG2D-DAP10 cell surface expression: opposing roles of the gamma c cytokines and TGF-beta1. Blood. 2011;118:3019–27. doi: 10.1182/blood-2011-04-346825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C, Zhang J, Niu J, Zhou Z, Zhang J, Tian Z. Interleukin-12 improves cytotoxicity of natural killer cells via upregulated expression of NKG2D. Hum Immunol. 2008;69:490–500. doi: 10.1016/j.humimm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U S A. 2003;100:4120–5. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 Secretion and Down-Modulation of NKG2D Underlies Impaired NK Cytotoxicity in Cancer Patients. J Immunol. 2004;172:7335–40. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 24.Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol. 2010;12:7–13. doi: 10.1093/neuonc/nop009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muntasell A, Magri G, Pende D, Angulo A, Lopez-Botet M. Inhibition of NKG2D expression in NK cells by cytokines secreted in response to human cytomegalovirus infection. Blood. 2010;115:5170–9. doi: 10.1182/blood-2009-11-256479. [DOI] [PubMed] [Google Scholar]
- 26.Burgess SJ, Marusina AI, Pathmanathan I, Borrego F, Coligan JE. IL-21 down-regulates NKG2D/DAP10 expression on human NK and CD8+ T cells. J Immunol. 2006;176:1490–7. doi: 10.4049/jimmunol.176.3.1490. [DOI] [PubMed] [Google Scholar]
- 27.Radosavljevic M, Cuillerier B, Wilson MJ, Clement O, Wicker S, Gilfillan S, et al. A cluster of ten novel MHC class I related genes on human chromosome 6q24.2-q25.3. Genomics. 2002;79:114–23. doi: 10.1006/geno.2001.6673. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Messina L, Ashiru O, Aguera-Gonzalez S, Reyburn HT, Vales-Gomez M. The human NKG2D ligand ULBP2 can be expressed at the cell surface with or without a GPI anchor and both forms can activate NK cells. J Cell Sci. 2011;124:321–7. doi: 10.1242/jcs.076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohashi M, Eagle RA, Trowsdale J. Post-translational modification of the NKG2D ligand RAET1G leads to cell surface expression of a glycosylphosphatidylinositol-linked isoform. J Biol Chem. 2010;285:16408–15. doi: 10.1074/jbc.M109.077636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–7. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 31.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1:119–26. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 32.Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM. Cutting Edge: Murine UL16-Binding Protein-Like Transcript 1: A Newly Described Transcript Encoding a High-Affinity Ligand for Murine NKG2D. J Immunol. 2002;169:4079–83. doi: 10.4049/jimmunol.169.8.4079. [DOI] [PubMed] [Google Scholar]
- 33.Takada A, Yoshida S, Kajikawa M, Miyatake Y, Tomaru U, Sakai M, et al. Two novel NKG2D ligands of the mouse H60 family with differential expression patterns and binding affinities to NKG2D. J Immunol. 2008;180:1678–85. doi: 10.4049/jimmunol.180.3.1678. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Mariuzza RA. Structural basis for recognition of cellular and viral ligands by NK cell receptors. Front Immunol. 2014;5:123. doi: 10.3389/fimmu.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31:413–41. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou Z, Nomura M, Takihara Y, Yasunaga T, Shimada K. Isolation and characterization of retinoic acid-inducible cDNA clones in F9 cells: a novel cDNA family encodes cell surface proteins sharing partial homology with MHC class I molecules. J Biochem (Tokyo) 1996;119:319–28. doi: 10.1093/oxfordjournals.jbchem.a021242. [DOI] [PubMed] [Google Scholar]
- 37.Ogasawara K, Benjamin J, Takaki R, Phillips JH, Lanier LL. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat Immunol. 2005;6:938–45. doi: 10.1038/ni1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung H, Hsiung B, Pestal K, Procyk E, Raulet DH. RAE-1 ligands for the NKG2D receptor are regulated by E2F transcription factors, which control cell cycle entry. J Exp Med. 2012;209:2409–22. doi: 10.1084/jem.20120565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guerra N, Pestal K, Juarez T, Beck J, Tkach K, Wang L, et al. A selective role of NKG2D in inflammatory and autoimmune diseases. Clin Immunol. 2013;149:432–9. doi: 10.1016/j.clim.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 41.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–66. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehrlich LI, Ogasawara K, Hamerman JA, Takaki R, Zingoni A, Allison JP, et al. Engagement of NKG2D by cognate ligand or antibody alone is insufficient to mediate costimulation of human and mouse CD8+ T cells. J Immunol. 2005;174:1922–31. doi: 10.4049/jimmunol.174.4.1922. [DOI] [PubMed] [Google Scholar]
- 43.Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103:3065–72. doi: 10.1182/blood-2003-06-2125. [DOI] [PubMed] [Google Scholar]
- 44.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–60. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 45.Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, et al. Coordinated Induction by IL15 of a TCR-Independent NKG2D Signaling Pathway Converts CTL into Lymphokine-Activated Killer Cells in Celiac Disease. Immunity. 2004;21:357–66. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Tang F, Chen Z, Ciszewski C, Setty M, Solus J, Tretiakova M, et al. Cytosolic PLA2 is required for CTL-mediated immunopathology of celiac disease via NKG2D and IL-15. J Exp Med. 2009;206:707–19. doi: 10.1084/jem.20071887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–33. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 48.Fielding CA, Aicheler R, Stanton RJ, Wang EC, Han S, Seirafian S, et al. Two novel human cytomegalovirus NK cell evasion functions target MICA for lysosomal degradation. PLoS Pathog. 2014;10:e1004058. doi: 10.1371/journal.ppat.1004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashiru O, Bennett NJ, Boyle LH, Thomas M, Trowsdale J, Wills MR. NKG2D ligand MICA is retained in the cis-Golgi apparatus by human cytomegalovirus protein UL142. J Virol. 2009;83:12345–54. doi: 10.1128/JVI.01175-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett NJ, Ashiru O, Morgan FJ, Pang Y, Okecha G, Eagle RA, et al. Intracellular sequestration of the NKG2D ligand ULBP3 by human cytomegalovirus. J Immunol. 2010;185:1093–102. doi: 10.4049/jimmunol.1000789. [DOI] [PubMed] [Google Scholar]
- 51.Stern-Ginossar N, Elefant N, Zimmermann A, Wolf DG, Saleh N, Biton M, et al. Host immune system gene targeting by a viral miRNA. Science. 2007;317:376–81. doi: 10.1126/science.1140956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lodoen M, Ogasawara K, Hamerman JA, Arase H, Houchins JP, Mocarski ES, et al. NKG2D-mediated Natural Killer Cell Protection Against Cytomegalovirus Is Impaired by Viral gp40 Modulation of Retinoic Acid Early Inducible 1 Gene Molecules. J Exp Med. 2003;197:1245–53. doi: 10.1084/jem.20021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arapovic J, Lenac T, Antulov R, Polic B, Ruzsics Z, Carayannopoulos LN, et al. Differential susceptibility of RAE-1 isoforms to mouse cytomegalovirus. J Virol. 2009;83:8198–207. doi: 10.1128/JVI.02549-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lodoen M, Abenes G, Umamoto S, Houchins JP, Liu F, Lanier LL. The Cytomegalovirus m155 Gene Product Subverts NK cell Antiviral Protection by Disruption of H60-NKG2D Interactions. J Exp Med. 2004;200:1075–81. doi: 10.1084/jem.20040583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krmpotic A, Hasan M, Loewendorf A, Saulig T, Halenius A, Lenac T, et al. NK cell activation through the NKG2D ligand MULT-1 is selectively prevented by the glycoprotein encoded by mouse cytomegalovirus gene m145. J Exp Med. 2005;201:211–20. doi: 10.1084/jem.20041617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lenac T, Budt M, Arapovic J, Hasan M, Zimmermann A, Simic H, et al. The herpesviral Fc receptor fcr-1 down-regulates the NKG2D ligands MULT-1 and H60. J Exp Med. 2006;203:1843–50. doi: 10.1084/jem.20060514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cerboni C, Neri F, Casartelli N, Zingoni A, Cosman D, Rossi P, et al. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol. 2007;88:242–50. doi: 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- 58.McSharry BP, Burgert HG, Owen DP, Stanton RJ, Prod'homme V, Sester M, et al. Adenovirus E3/19K promotes evasion of NK cell recognition by intracellular sequestration of the NKG2D ligands major histocompatibility complex class I chain-related proteins A and B. J Virol. 2008;82:4585–94. doi: 10.1128/JVI.02251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider CL, Hudson AW. The human herpesvirus-7 (HHV-7) U21 immunoevasin subverts NK-mediated cytoxicity through modulation of MICA and MICB. PLoS Pathog. 2011;7:e1002362. doi: 10.1371/journal.ppat.1002362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas M, Boname JM, Field S, Nejentsev S, Salio M, Cerundolo V, et al. Down-regulation of NKG2D and NKp80 ligands by Kaposi's sarcoma-associated herpesvirus K5 protects against NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2008;105:1656–61. doi: 10.1073/pnas.0707883105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campbell JA, Trossman DS, Yokoyama WM, Carayannopoulos LN. Zoonotic orthopoxviruses encode a high-affinity antagonist of NKG2D. J Exp Med. 2007;204:1311–7. doi: 10.1084/jem.20062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spear P, Wu MR, Sentman ML, Sentman CL. NKG2D ligands as therapeutic targets. Cancer immunity. 2013;13:8. [PMC free article] [PubMed] [Google Scholar]
- 63.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–71. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci U S A. 2001;98:11521–6. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 68.Kaiser BK, Yim D, Chow IT, Gonzalez S, Dai Z, Mann HH, et al. Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature. 2007;447:482–6. doi: 10.1038/nature05768. [DOI] [PubMed] [Google Scholar]
- 69.Salih HR, Rammensee HG, Steinle A. Cutting Edge: Down-Regulation of MICA on Human Tumors by Proteolytic Shedding. J Immunol. 2002;169:4098–102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 70.Wu JD, Higgins LM, Steinle A, Cosman D, Haugk K, Plymate SR. Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J Clin Invest. 2004;114:560–8. doi: 10.1172/JCI22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernandez-Messina L, Ashiru O, Boutet P, Aguera-Gonzalez S, Skepper JN, Reyburn HT, et al. Differential mechanisms of shedding of the glycosyl-phosphatidylinositol (GPI)-anchored NKG2D ligands. J Biol Chem. 2010;285:8543–51. doi: 10.1074/jbc.M109.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baragano Raneros A, Suarez-Alvarez B, Lopez-Larrea C. Secretory pathways generating immunosuppressive NKG2D ligands: New targets for therapeutic intervention. Oncoimmunology. 2014;3:e28497. doi: 10.4161/onci.28497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ullrich E, Koch J, Cerwenka A, Steinle A. New prospects on the NKG2D/NKG2DL system for oncology. Oncoimmunology. 2013;2:e26097. doi: 10.4161/onci.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crane CA, Austgen K, Haberthur K, Hofmann C, Moyes KW, Avanesyan L, et al. Immune evasion mediated by tumor-derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc Natl Acad Sci U S A. 2014;111:12823–8. doi: 10.1073/pnas.1413933111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deng W, Gowen BG, Zhang L, Wang L, Lau S, Iannello A, et al. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science. 2015;348:136–9. doi: 10.1126/science.1258867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived "suppressor" cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080–9. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sentman CL, Meehan KR. NKG2D CARs as cell therapy for cancer. Cancer J. 2014;20:156–9. doi: 10.1097/PPO.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trsan T, Busche A, Abram M, Wensveen FM, Lemmermann NA, Arapovic M, et al. Superior induction and maintenance of protective CD8 T cells in mice infected with mouse cytomegalovirus vector expressing RAE-1gamma. Proc Natl Acad Sci U S A. 2013;110:16550–5. doi: 10.1073/pnas.1310215110. [DOI] [PMC free article] [PubMed] [Google Scholar]