Abstract
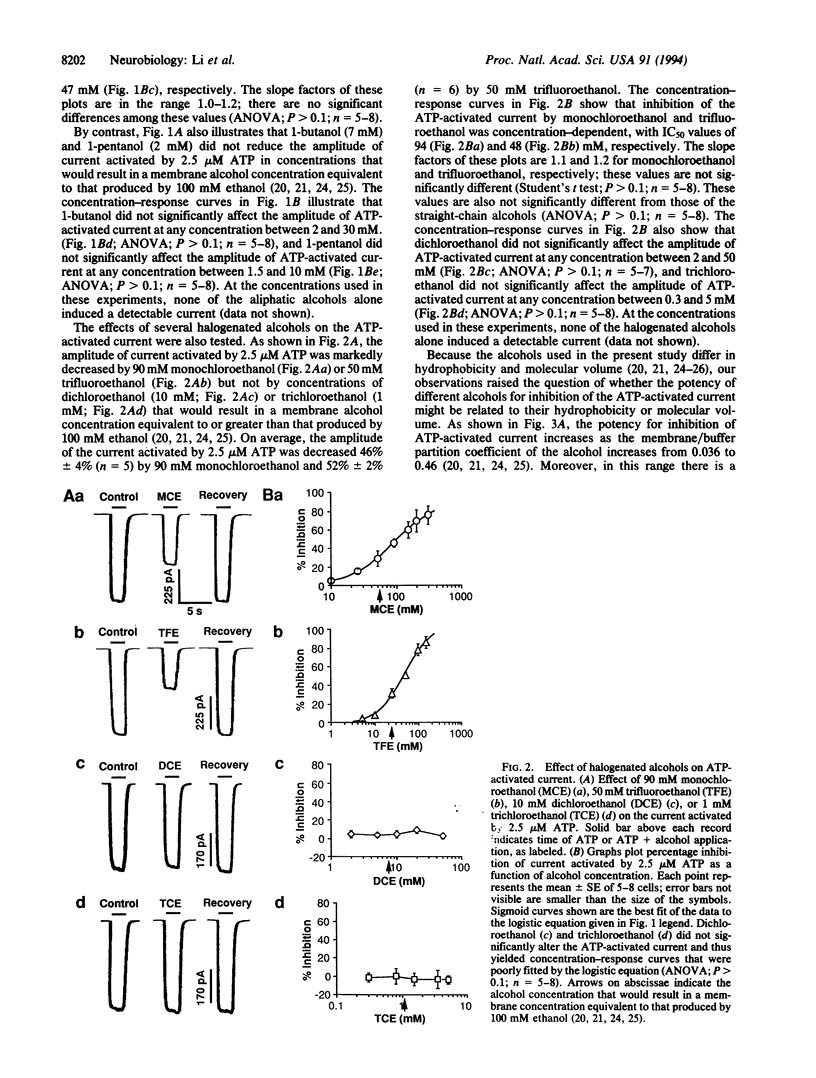
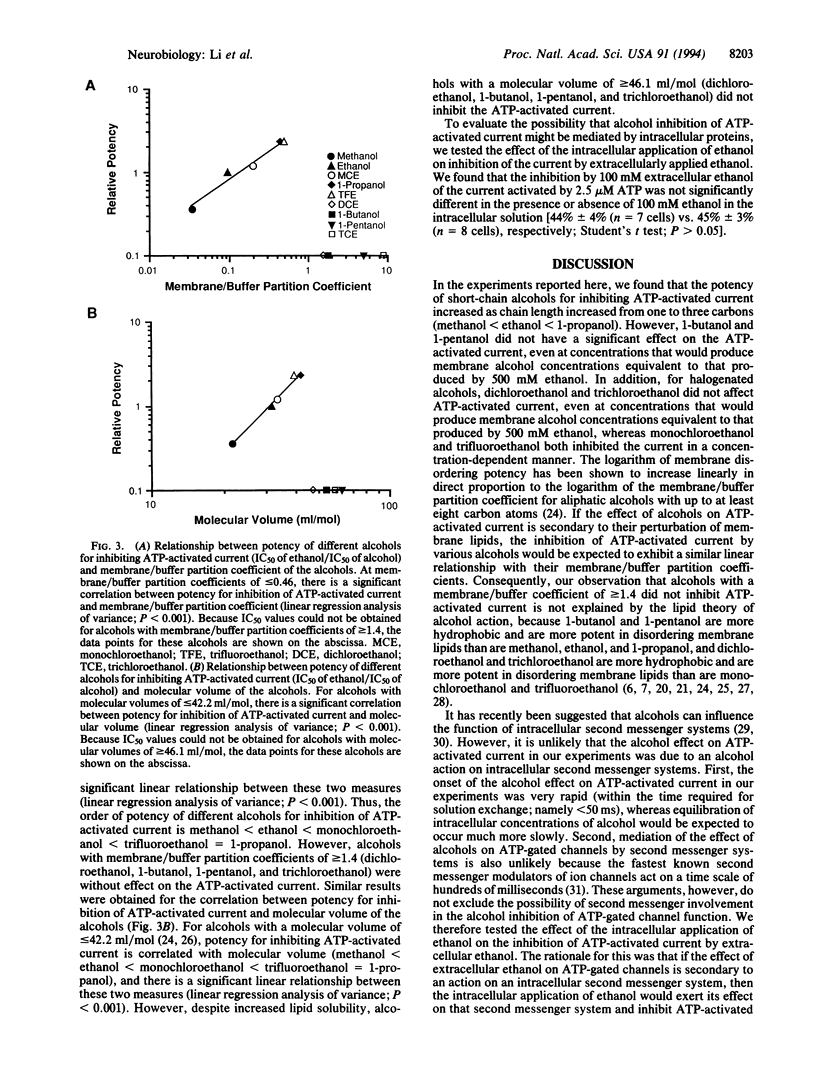
For almost a century, alcohols have been thought to produce their effects by actions on the membrane lipids of central nervous system neurons--the well known "lipid theory" of alcohol action. The rationale for this theory is the correlation of potency with oil/water or membrane/buffer partition coefficient. Although a number of recent studies have shown that alcohols can affect the function of certain neuronal neurotransmitter receptors, there is no evidence that the alcohols interact directly with these membrane proteins. In the present study, we report that inhibition of a neuronal neurotransmitter receptor, an ATP-gated ion channel, by a series of alcohols exhibits a distinct cutoff effect. For alcohols with a molecular volume of < or = 42.2 ml/mol, potency for inhibiting ATP-activated current was correlated with lipid solubility (order of potency: 1-propanol = trifluoroethanol > monochloroethanol > ethanol > methanol). However, despite increased lipid solubility, alcohols with a molecular volume of > or = 46.1 ml/mol (1-butanol, 1-pentanol, trichloroethanol, and dichloroethanol) were without effect on the ATP-activated current. The results suggest that alcohols inhibit the function of this neurotransmitter receptor by interacting with a small hydrophobic pocket on the receptor protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J Neurosci. 1990 Jan;10(1):1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992 Mar;13(3):87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Williams C. A., Ceelen P. W. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990 Jan;10(1):11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Do general anaesthetics act by competitive binding to specific receptors? Nature. 1984 Aug 16;310(5978):599–601. doi: 10.1038/310599a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Mapping of general anaesthetic target sites provides a molecular basis for cutoff effects. Nature. 1985 Jul 25;316(6026):349–351. doi: 10.1038/316349a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Partitioning of long-chain alcohols into lipid bilayers: implications for mechanisms of general anesthesia. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5116–5120. doi: 10.1073/pnas.83.14.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. B. The effects of drugs on membrane fluidity. Annu Rev Pharmacol Toxicol. 1984;24:43–64. doi: 10.1146/annurev.pa.24.040184.000355. [DOI] [PubMed] [Google Scholar]
- Illes P., Nörenberg W. Neuronal ATP receptors and their mechanism of action. Trends Pharmacol Sci. 1993 Feb;14(2):50–54. doi: 10.1016/0165-6147(93)90030-n. [DOI] [PubMed] [Google Scholar]
- Li C., Aguayo L., Peoples R. W., Weight F. F. Ethanol inhibits a neuronal ATP-gated ion channel. Mol Pharmacol. 1993 Oct;44(4):871–875. [PubMed] [Google Scholar]
- Lyon R. C., McComb J. A., Schreurs J., Goldstein D. B. A relationship between alcohol intoxication and the disordering of brain membranes by a series of short-chain alcohols. J Pharmacol Exp Ther. 1981 Sep;218(3):669–675. [PubMed] [Google Scholar]
- McCreery M. J., Hunt W. A. Physico-chemical correlates of alcohol intoxication. Neuropharmacology. 1978 Jul;17(7):451–461. doi: 10.1016/0028-3908(78)90050-3. [DOI] [PubMed] [Google Scholar]
- Pringle M. J., Brown K. B., Miller K. W. Can the lipid theories of anesthesia account for the cutoff in anesthetic potency in homologous series of alcohols? Mol Pharmacol. 1981 Jan;19(1):49–55. [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Silinsky E. M., Gerzanich V. On the excitatory effects of ATP and its role as a neurotransmitter in coeliac neurons of the guinea-pig. J Physiol. 1993 May;464:197–212. doi: 10.1113/jphysiol.1993.sp019630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater S. J., Cox K. J., Lombardi J. V., Ho C., Kelly M. B., Rubin E., Stubbs C. D. Inhibition of protein kinase C by alcohols and anaesthetics. Nature. 1993 Jul 1;364(6432):82–84. doi: 10.1038/364082a0. [DOI] [PubMed] [Google Scholar]
- Snell L. D., Tabakoff B., Hoffman P. L. Involvement of protein kinase C in ethanol-induced inhibition of NMDA receptor function in cerebellar granule cells. Alcohol Clin Exp Res. 1994 Feb;18(1):81–85. doi: 10.1111/j.1530-0277.1994.tb00884.x. [DOI] [PubMed] [Google Scholar]
- Weight F. F. Cellular and molecular physiology of alcohol actions in the nervous system. Int Rev Neurobiol. 1992;33:289–348. doi: 10.1016/s0074-7742(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Yatani A., Brown A. M. Rapid beta-adrenergic modulation of cardiac calcium channel currents by a fast G protein pathway. Science. 1989 Jul 7;245(4913):71–74. doi: 10.1126/science.2544999. [DOI] [PubMed] [Google Scholar]



