Abstract
Conjoined twins (CT) are a very rare developmental accident of uncertain etiology. Prevalence has been previously estimated to be 1 in 50,000 to 1 in 100,000 births. The process by which monozygotic twins do not fully separate but form CT is not well understood. The purpose of the present study was to analyze diverse epidemiological aspects of CT, including the different variables listed in the Introduction Section of this issue of the Journal. The study was made possible using the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR) structure. This multicenter worldwide research includes the largest sample of CT ever studied. A total of 383 carefully reviewed sets of CT obtained from 26,138,837 births reported by 21 Clearinghouse Surveillance Programs (SP) were included in the analysis. Total prevalence was 1.47 per 100,000 births (95% CI: 1.32–1.62). Salient findings including an evident variation in prevalence among SPs: a marked variation in the type of pregnancy outcome, a similarity in the proportion of CT types among programs: a significant female predominance in CT: particularly of the thoracopagus type and a significant male predominance in parapagus and parasitic types: significant differences in prevalence by ethnicity and an apparent increasing prevalence trend in South American countries. No genetic, environmental or demographic significant associated factors were identified. Further work in epidemiology and molecular research is necessary to understand the etiology and pathogenesis involved in the development of this fascinating phenomenon of nature.
Keywords: conjoined twins, epidemiology, multicentric study, ICBDSR
INTRODUCTION
Conjoined twins (CT) are a rare embryologic developmental accident of uncertain etiology. Prevalence, although variable, has been estimated to be 1 in 50,000 to 1 in 100,000 births [Hanson, 1975; Källén and Rybo, 1978; Edmonds and Layde, 1982; Viljoen et al., 1983; Castilla et al., 1988; ICBDMS, 1991; Rees et al., 1993; Martínez-Frías et al., 2009]. CT is not restricted to humans; it has been reported in fish, reptiles, birds, primates, and other mammals [Levin et al., 1996; Canfield et al., 2000]. The first aspect to consider is as stated by Weber and Sebire [2010] that “CT is itself a malformation and is associated with secondary changes related to abnormal conjoined organs and superimposed effects of abnormal hemodynamics.” Proposed mechanisms of the defect cannot explain the alterations in the normal developmental process, by which a pair of monozygotic (MZ) twins do not fully separate from each other and continue their normal embryologic development.
Historical Background
Ancient citations of CT exist from quotations in different cultures such as in early pre-Colombian ceramics of the Moche Peruvian civilization [Berrin and Larco, 1997], to more formally scientific documented cases. Probably one of the first cases documented was a pair of rachipagus CT born in the Isle-Brewers, England, joined at the back from the middle chest to near the lumbar region [Bondeson, 1993]. Another very interesting pair of CT and one of the earliest and well-documented cases were the girls known as Mary and Eliza Chulkhurst who were joined at the hip (pygopagus). They were born in year 1100 in the town of Biddenden, County of Kent, England, and died in 1134 [Ballantyne, 1895]. Although controversy existed regarding the true existence of these CT, because of their generosity to the local church, every Easter Sunday small cakes with the twins’ images were distributed to the poor in their honor for centuries [Bondeson, 1992]. Other cases of pygopagus CT described were the Hungarian Helena and Judith sisters (1701–1723) and the Rosa and Josepha Blazek twins (1879–1922) that were born in Skreychov, Bohemia, now the Czech Republic [Guttmacher, 1967]. Rosa supposedly gave birth to a male child in 1910. To our knowledge this is the only example of a female CTwho had a healthy child. One of the most famous sets of CT was Eng and Chang Bunker, who were born in the Kingdom of Siam (now Thailand) in 1811 and died in 1874 in North Carolina, USA. The term “Siamese twins” was coined as a reference to them. They became famous while working in an international circus. They were considered xiphopagus (thoraco-pagus) as they were joined at the lower thorax by soft tissue and shared a common liver. They married sisters, fathered 21 children, and were one of the longest living CT at 63 years [Bondeson, 1992]. One of the first cases of CT reported in the Spanish medical literature was an asymmetric type born in the city of Durango, México in 1868 [Rodríguez, 1870].
A fascinating book published at the end of the 19th century, Anomalies and Curiosities of Medicine, contained an encyclopedic collection of rare and extraordinary cases. In chapter V under the title of Major Terata, a large number of rare birth defects are quoted as monstrosities, including a collection of CT. In the same chapter, the authors made reference to Ambroise Paré (1510–1590), a famous barber-surgeon who described the several types of CTas they are currently classified [Gould and Pyle, 1896].
The Uncertain Embryology of Conjoined Twins
MZ twins originate from the division and separation of a single early embryo. Depending on the completeness of the inner cell mass division of the blastocyst in the early stages of human development, MZ twins can be dichor-ionic diamniotic (30–40%), monochor-ionic diamniotic (60–70%), and very much less common, monochorionic monoamniotic MZ twins. It is assumed that the last type evolve from the partition of the embryonic axis into two parallel ones, giving origin to the monoammiotic monochorionic type of MZ twins [Kaufman, 2004; Sadler, 2010]. This type of placentation is characteristic of CT. Currently, it is accepted that CToriginate from a failure in the development of primitive structures at later stages of development, that is, Carnegie stage 6 (days 12–15), or the primitive streak stage of human development [Levin et al., 1996; Kaufman, 2004; Sadler, 2010]. However, the exact mechanisms of CT remain obscure.
Two opposing theories have been suggested to explain the sequence of events of CT. Those supporting a “fusion” process, postulate that with the exception of the parapagus type, all other types of CT can be explained by the fusion of two separated embryos (Box II) [Spencer, 1992, 2000a,b; Logroño et al., 1997; Machin, 1998]. However, cases described by Logroño et al. [1997] and Machin [1998] were exceptional ones. According to Machin and Sperber [1991], the origin of para-pagus could be explained by the bifurcation of a single notochord. Spencer [2000a] also stated that “No theoretical fission of the vertebrate embryo at any stage of development, in any plane, in any direction can explain the selection of the observed sites of fusion, the details of the union, or the limitation to the specific areas in which the twins are found to be joined.”
BOX 2. Classification of Conjoined Twins.
| Types | Definitions |
|---|---|
| Cephalopagus | There are two faces and are joined from the top of the head to the umbilicus |
| Thoracopagus | Are joined face-to-face from the upper thorax to the upper part of the abdomen and always involve the heart |
| Omphalopagus | The fusion includes the umbilicus region frequently at the lower thorax, but never the heart |
| Ischiopagus | The union usually includes the lower abdomen and duplicated fused pelvic bones, and external genitalia and anus are always involved |
| Parapagus | Are laterally joined, regularly share the pelvis. Varieties of parapagus conjoined twins are parapagus dithoracic (separated thoraces), parapagus dicephalus (one trunk two separate heads), and parapagus diprosopus (one trunk, one head, and two faces) |
| Craniopagus | Joined by the skull, share meninges but rarely the brain surface and do not include the face and trunk |
| Pygopagus | Are dorsally fused sharing the perineal and sacrococcygeal areas, has only one anus but two rectums |
| Rachipagus | Dorsally fused, the defect may involve the dorsolumbar vertebral column and rarely the cervical vertebrae and the occipital bone |
| Other symmetrical | Includes CT that some authors classify differently and also a variety of rare types of symmetrical CT |
| Asymmetric | Parasitic CT and fetus in fetus |
In contrast, supporters of the fission theory mention that CTare the result of an incomplete split of the embryonic axis [Simpson, 1869; Aird, 1959; Machin and Sperber, 1987; ICBDMS, 1991; Kaufman, 2004; Spitz, 2005; Weber and Sebire, 2010]. Kaufman [2004] states that with the exception of parasitic twins, all CT are symmetrical and “the same parts are always united to the same parts.” The same author mentioned that “if fusion, rather than fission, accounted for all cases of conjoined twins, the incidence of mirror-imaging should be the same in all monoamniotic twins, whether they are conjoined or not” and “if the incidence of mirror-image is higher in conjoined twins than in separate twins, the fusion hypothesis cannot be correct.”
GENETICS AND OTHER RISK FACTORS ASSOCIATED TO CONJOINED TWINS
There is no record in the literature of familial aggregation of CT, nor for preferential associations with other unrelated anomalies. As for the former, an example is the large multigenerational kindred, descendants from the famous Eng and Chang Bunker CT. Among 1,500 descendants of both of them, several pairs of twins including MZ twins were born, but no other CTwere recorded [Newman, 2006]. In the Online Mendelian Inheritance in Man (OMIM 164750) only one instance of CT concordant for omphalocele is reported, which constitutes a related defect to the omphalopagus type of CT [Bugge, 2010].
A report by Rosa et al. [1987] mentioned the exposure during pregnancy to griseofulvin, an antifungal medication, was noted in two sets of CT in humans, but this was not further confirmed by Knudsen [1987] and Métneki and Czeizel [1987]. Griseofulvin crosses the placental barrier and is recognized as a human teratogen. In a population-based study on 22,843 pregnancy outcomes with birth defects and 38,151 controls, the authors reported that a 0.03% and 0.06% of cases and controls mothers were treated during pregnancy with this drug. CT was observed in 55 pregnancies; however none of the mothers of the CT were exposed to griseofulvin [Czeizel et al., 2004]. Some proteins such as activin, nodal, and Sonic hedgehog have been associated with laterality defects in chicken CT, but not in humans [Levin et al., 1996]. Recently, it has been reported [Wertelecki, 2010] that chronic low-dose radiation exposure could favor the occurrence of twinning and the prevalence of CT. The analysis of approximately 100,000 births born between 2000 and 2006 in the area of Rivne, close to Chernobyl, Ukraine, showed an apparent cluster of CT (5 in 96,438 births). However, numbers were too small to reach conclusions.
EPIDEMIOLOGY OF CONJOINED TWINS
As mentioned above, worldwide prevalence of CT, although variable, has been estimated to be 1 in 50,000 pregnancies, but approximately 1 in 200,000 live-births (LB) [Spitz, 2005]. However, some studies reported prevalences as high as 1 in 2,800 LB in India [Mudaliar, 1930], to as low as 1 in 200,000 LBs in the USA [Bender, 1967].
Prevalence of conjoined twins observed in diverse populations studied: 1930–2010
Prevalence of symmetrical CT can be assessed in four categories: (i) higher than 1:20,000 births; (ii) between 1:20,000 and 1:50,000 births; (iii) between 1:50,000 and 1:100,000 births; and (iiii) between 1:100,000 and 1:200,000 births (Box I). The marked differences reported could be attributed to the population size monitored, and inclusion or not of stillbirths (SB), spontaneous abortions, and elective termination of pregnancy for fetal anomaly (ETOPFA). Significant under-registration of non-liveborn prenatal or perinatal cases in any of the four categories may explain, in part, the differences observed among populations studied [Hanson, 1975; Källén and Rybo, 1978; Liang et al., 1999; Tang et al., 2007] (Box I).
BOX I. Prevalence of Conjoined Twins Observed in Diverse Populations Studied: 1930-2010.
| Prevalence | Population studied |
|---|---|
| Higher than 1:20,000 births | 1:2,800 India [Mudaliar, 1930] |
| 1:4,242 Uganda [Bland and Hammar, 1962] | |
| 1:6,500 Taiwan [Emanuel et al., 1972] | |
| 1:14,000 Rhodesia-Africa [Zake, 1984] | |
|
| |
| Between 1:20,000 and 1:50,000 births | 1:20,000 Sweden [Ryden, 1934] |
| 1:20,100 CDC-USA (CDC-Atlanta, 1973) | |
| 1:22,284 Brazil [Berezowski et al., 2010] | |
| 1:25,000 Maltese Islands [Savona-Ventura et al., 2009] | |
| 1:30,600 China [Liang et al., 1999] | |
| 1:35,100 China [Tang et al., 2007] | |
|
| |
| Between 1:50,000 and 1:100,000 births | 1:50,000 USA-Los Angeles [Robertson, 1953] |
| 1:50,000 USA-Chicago [Potter, 1961] | |
| 1:55,865 24 countries WHO project [Stevenson et al., 1966] | |
| 1:68,500 Hungary [Métneki and Czeizel, 1989] | |
| 1:74,626 South America-ECLAMC [Castilla et al., 1988] | |
| 1:75,000 Sweden [Källén and Rybo, 1978] | |
| 1:91,131 ICBDMS [ICBDMS, 1991] | |
| 1:97,560 USA-Atlanta [Edmonds and Layde, 1982] | |
| Between 1:100,000 and 1:200,000 births | 1:100,000 Japan [Imaizumi, 1988] |
| 1:151,500 Spain [Martínez-Frías et al., 2009] | |
| 1:166,000 New York-USA [Milham, 1966] | |
| 1:200,000 USA [Bender, 1967] | |
Recent studies on the epidemiology of CT are relatively scarce, but the prevalence does not seem to differ significantly (1.02–1.34 per 100,000 births) in Western populations [Källén and Rybo, 1978; Edmonds and Layde, 1982; Castilla et al., 1988; Métneki and Czeizel, 1989; ICBDMS, 1991]. However, increased prevalences of 3.27:100,000 births [Liang et al., 1999] and 2.85:100,000 births [Tang et al., 2007] have been reported in two studies in Chinese populations from the same Surveillance Program (SP) at different times.
It is difficult to know the real prevalences of CT in SB because practically all epidemiological studies that consider total prevalences includes LB, SB, and ETOPFA as total births. Proportion of SB among CT vary from close to 40% to approximately 60% [Edmonds and Layde, 1982; ICBDMS, 1991; Tang et al., 2007]. Variation among studies depends on the methodological approach and the legal access to ETOPFA [Métneki and Czeizel, 1989; ICBDMS, 1991; Pajkrt and Jauniaux, 2005]. Regarding the prevalence of CT in spontaneous abortions, the only reference found was a study performed on 661 consecutive spontaneous abortions reporting 15 pairs of twins, among which 2 were conjoined. These data allow an estimate of the prevalence of CT of 3.03 per 1,000 (95% CI: 0.40–10.89) in spontaneous abortions [Uchida et al., 1983].
Although in one epidemiologic study [Castilla et al., 1988] predominance of females was not observed, many other studies have shown a 1.5–2.5 predominance of female sex over male sex [Edmonds and Layde, 1982; Imaizumi, 1988; Métneki and Czeizel, 1989; ICBDMS, 1991; Tang et al., 2007; Martínez-Frías et al., 2009].
Asymmetric or parasitic CT is another fairly rare atypical presentation of MZ twins, where one of them is significantly underdeveloped and considered parasitic from the other, often unaffected. Parasitic twins occur when one embryo of a pair of MZ twins starts to develop, but the pair does not fully separate, and one embryo’s development prevails over the other. Rather than conjoined, it is considered parasitic because it is incompletely formed or wholly dependent on the body functions of the complete fetus. Prevalence has been estimated to be approximately 20 times less frequent than the prevalence of the symmetrical types [Edmonds and Layde, 1982; ICBDMS, 1991]. Another type of twins considered by some authors as parasitic is the fetus in fetus. However, this developmental anomaly is considered by others as a different parasitic twin fetus growing within its host twin very early in a MZ pregnancy, where one fetus grows around the other. The internal twin survival depends on the survival of its host twin [Aquino et al., 1997; Arlikar et al., 2009; Sharma et al., 2010].
Survival of CT is precarious, most dying during the very early perinatal period or as the result of surgical separation. Survival mainly depends on the type of CT, the sharing of organs, and timely and appropriate surgical or non-surgical treatment. Options for therapy include emergency or planned separation if appropriate [Bland and Hammar, 1962; Hoyle, 1990; Kingston et al., 2001; Spitz, 2003].
The purpose of the present study was to identify the main epidemiological characteristics associated to this very rare defect. Variables considered in the analysis are described in detail in introductory article of this issue [Castilla and Mastroiacovo, 2011].
MATERIALS AND METHODS
Population Studied
The sample of CT was obtained from 21 worldwide SPs who are all members of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR). Each had agreed to participate in the analysis of the epidemiology of this very rare defect. Programs were asked to provide re-identified case records following a common protocol, with information on phenotype, genetic testing and selected demographic and prenatal information. Data were submitted according to a designed Excel database to obtain more uniform information from each participating SP. The time in years covered and information sent by each SP was variable, although all covered a minimum of 5 years period of time of epidemiological surveillance of birth defects. Some covered more than 30 years. Information regarding the individual characteristics of each SP is described in introductory article of this issue [Castilla and Mastroiacovo, 2011]. Submitted data were reviewed by two of the authors (J.A.V. and L.L.M.) and the principal investigator (O.M.M.) to identify the cases, confirm the diagnosis, classify the CT, and decide upon the inclusion or exclusion in the sample. From a total of 402 CT pairs reported, 15 were excluded because they were included twice and 4 had a wrong diagnosis. This resulted in a total of 383 sets of CT born among a total of 26,138,837 births. Statistical analysis included the chi-squared test and Fisher’s exact test to compare proportions, the chi-squared test for trends for the analysis of time trends, the Poisson test to estimate exact 95% confidence intervals (CI) and the cumulative Poisson P-values for comparisons of total prevalence between programs. More detailed information is provided in the introductory article of this issue [Castilla and Mastroiacovo, 2011].
Classification of Conjoined Twins
According to the site of union, symmetrical CT are classified in different manners, including diverse wide-ranging classifications [ICBDMS, 1991; Phelan and Hall, 2006] and simplified commonly used ones [Edmonds and Layde, 1982; Métneki and Czeizel, 1989; Spencer, 1996, 2000a,b; Kingston et al., 2001; Kaufman, 2004]. We decided to adopt the classification exhibited in Box II. Eight well-defined types are listed for symmetrical CT, one for very rare types of CT, and an extra category for asymmetrical types. Some classifications includes more types resulting from the extension of the junction, although not all authors accept combined types such as cephalo-thoracopagus or thoracoomphalopagus, arguing that practically cephalopagus always includes part of the thorax and thoracopagus includes part of the abdomen [Spencer, 1996, 2000a,b; Kingston et al., 2001; Kaufman, 2004]. The classification chosen for our analyses (Box II) is the one that fits our data well and permits comparisons with previously reported data.
Classification of Unrelated Congenital Anomalies
Only those major congenital anomalies not related to the site of union of the CT and those cases in which the defects were clearly described were included in the analysis, independent of the occurrence of the anomalies in one or both twins. Malformations were grouped by developing system. If the same malformation occurred in each twin of a CT pair, it was counted only once. Proportion of each type of congenital anomaly was estimated among the total number of malformations.
RESULTS
Total Prevalence and Prevalence by Surveillance Program
Total prevalence of CT was 1.47 (95% CI: 1.32–1.62) per 100,000 births (Table I). Prevalences show a marked variation among SPs, from as high as 3.22 (95% CI: 2.04–4.84) per 100,000 births in the Finland SP to as low as less than 0.08 per 100,000 births in the Italy-North East program. Besides Finland, three other SPs have prevalence over 2 per 100,000 births: South America-ECLAMC, México-RYVEMCE, and Germany Saxony-Anhalt, in decreasing order. Nine other SPs, USA-Atlanta, Wales, Australia-Victoria, USA-Utah France-Central East, China-Beijing, Northern Netherlands, Hungary, and Canada-Alberta showed a prevalence of more than 1 but less than 2 per 100,000 births; and the remaining eight programs, Spain-ECEMC, Italy-Emilia Romagna, Israel, USA-Texas, Italy-Tuscany, Italy-Campania, Slovak Republic, and Italy-North East, reported a prevalence lower than 1 per 100,000 births. As shown in Table I, the prevalence reported by only 7 of the 21 participating SPs differed significantly from the total prevalence; Finland, South America-ECLAMC, and Mexico-RYVEMCE SPs had a statistically significant high prevalence, and Italy-North East, Italy-Campania, Spain-ECEMC, and USA-Texas SPs had a statistically significant low prevalence. Prevalence per 100,000 births and 95% CI for each of the 21 participating SPs is presented in Figure 1 by decreasing prevalence.
TABLE I.
Total Prevalence of Conjoined Twins in 21 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR)
| Surveillance Program | Period | Births | Total cases | Prevalence (per 100,000 births) | 95% CI | P-value* |
|---|---|---|---|---|---|---|
| Canada-Alberta | 1980–2005 | 1,062,483 | 11 | 1.04 | 0.52–1.85 | 0.150 |
| USA-Utah | 1997–2004 | 380,706 | 6 | 1.58 | 0.58–3.43 | 0.673 |
| USA-Atlanta | 1968–2004 | 1,283,999 | 25 | 1.95 | 1.26–2.87 | 0.099 |
| USA-Texas | 1996–2002 | 2,054,788 | 13 | 0.63 | 0.34–1.08 | 0.0004 |
| Mexico-RYVEMCE | 1978–2005 | 1,058,885 | 24 | 2.27 | 1.45–3.37 | 0.027 |
| South America-ECLAMC | 1982–2006 | 4,556,173 | 108 | 2.37 | 1.94–2.86 | <0.0001 |
| Finland | 1993–2004 | 713,494 | 23 | 3.22 | 2.04–4.84 | 0.0005 |
| Wales | 1998–2004 | 222,309 | 4 | 1.80 | 0.49–4.61 | 0.411 |
| Northern Netherlands | 1981–2003 | 369,658 | 5 | 1.35 | 0.44–3.16 | 0.543 |
| Germany Saxony-Anhalt | 1980–2004 | 355,184 | 8 | 2.25 | 0.97–4.44 | 0.155 |
| Slovak Republic | 2000–2005 | 318,257 | 1 | 0.31 | 0.01–1.75 | 0.054 |
| Hungary | 1980–2005 | 3,022,194 | 40 | 1.32 | 0.95–1.80 | 0.291 |
| France-Central East | 1979–2004 | 2,500,214 | 37 | 1.48 | 1.04–2.04 | 0.498 |
| Italy-North East | 1981–2004 | 1,186,497 | 1 | 0.08 | 0.00–0.47 | <0.0001 |
| Italy Emilia Romagna | 1982–2004 | 558,176 | 4 | 0.72 | 0.20–1.83 | 0.090 |
| Italy-Tuscany | 1992–2004 | 336,744 | 2 | 0.59 | 0.07–2.15 | 0.131 |
| Italy-Campania | 1992–2004 | 643,962 | 3 | 0.47 | 0.10–1.36 | 0.016 |
| Spain-ECEMC | 1980–2004 | 2,045,751 | 16 | 0.78 | 0.45–1.27 | 0.004 |
| Israel | 1975–2005 | 151,562 | 1 | 0.66 | 0.02–3.68 | 0.348 |
| China-Beijing | 1992–2005 | 1,927,622 | 28 | 1.45 | 0.97–2.10 | 0.582 |
| Australia-Victoria | 1983–2004 | 1,390,179 | 23 | 1.65 | 1.05–2.48 | 0.308 |
| Total | 26,138,837 | 383 | 1.47 | 1.32–1.62 |
ECEMC, Estudio Colaborativo Español de malformaciones Congénitas; ECLAMC, Estudio Colaborativo Latino Americano de Malformaciones Congénitas; RYVEMCE, Registro y Vigilancia Epidemiológica de Malformaciones Congénitas.
P: exact cumulative Poisson P-value. Bold values denote statistically significant high or low CT prevalence.
Figure 1.
Total prevalence per 100,000 births (bar) and 95% confidence interval (line) by Surveillance Programs of conjoined twins in 21 Surveillance Programs of the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR).
Pregnancy Outcome of Conjoined Twins by Surveillance Program
Table II shows the proportion of cases delivered as LB, SB, or ETOPFA. The largest proportion corresponded to LB sets of CT (45.6%), contrary to the literature reporting a higher proportion of SB among CT [Edmonds and Layde, 1982; ICBDMS, 1991]. The total proportion of SB cases and ETOPFA was identical, at 27.2% each. However, when considering only the 16 programs where termination for fetal anomaly is available the proportion of ETOPFA is 50.7% (103/203). There are some SPs with a very high prevalence of ETOPFA for CT such as France, Finland, and Germany Saxony-Anhalt. There are other programs like Mexico-RYVEMCE and South American-ECLAMC in countries in which ETOPFA is not permitted and do not offer termination of pregnancy for fetal anomaly. However, in these SPs as well as in programs where termination is permitted (China-Beijing, USA-Atlanta, and Spain-ECEMC), the prevalence of CT is also higher in live born infants.
TABLE II.
Proportion of Pregnancy Outcomes by Surveillance Program
| Livebirth
|
Stillbirth
|
ETOPFA
|
Total
|
|||||
|---|---|---|---|---|---|---|---|---|
| Surveillance Program | n | % | n | % | n | % | n | % |
| Canada-Alberta | 6 | 54.5 | 3 | 27.3 | 2 | 18.2 | 11 | 100.0 |
| USA-Utah | 2 | 33.3 | 0 | 0.0 | 4 | 66.7 | 6 | 100.0 |
| USA-Atlanta | 13 | 56.5 | 4 | 17.4 | 6 | 26.1 | 23 | 100.0 |
| USA-Texas | 4 | 30.8 | 6 | 46.2 | 3 | 23.1 | 13 | 100.0 |
| Mexico-RYVEMCE | 17 | 70.8 | 7 | 29.2 | NP | — | 24 | 100.0 |
| South America-ECLAMC | 86 | 79.6 | 22 | 20.4 | NP | — | 108 | 100.0 |
| Finland | 5 | 22.7 | 1 | 4.5 | 16 | 72.7 | 22 | 100.0 |
| Wales | 0 | 0.0 | 1 | 25.0 | 3 | 75.0 | 4 | 100.0 |
| Northern Netherlands | 3 | 60.0 | 1 | 20.0 | 1 | 20.0 | 5 | 100.0 |
| Germany Saxony-Anhalt | 1 | 12.5 | 1 | 12.5 | 6 | 75.0 | 8 | 100.0 |
| Slovak Republic | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 | 1 | 100.0 |
| Hungary | 10 | 25.0 | 14 | 35.0 | 16 | 40.0 | 40 | 100.0 |
| France-Central East | 4 | 10.8 | 3 | 8.1 | 30 | 81.1 | 37 | 100.0 |
| Italy-North East | 0 | 0.0 | 0 | 0.0 | 1 | 100.0 | 1 | 100.0 |
| Italy-Emilia Romagna | 1 | 25.0 | 2 | 50.0 | 1 | 25.0 | 4 | 100.0 |
| Italy-Tuscany | 0 | 0.0 | 0 | 0.0 | 2 | 100.0 | 2 | 100.0 |
| Italy-Campania | 1 | 33.3 | 0 | 0.0 | 2 | 66.7 | 3 | 100.0 |
| Spain-ECEMC | 10 | 62.5 | 6 | 37.5 | NR | — | 16 | 100.0 |
| China-Beijing | 6 | 21.4 | 22 | 78.6 | NR | — | 28 | 100.0 |
| Australia-Victoria | 3 | 13.0 | 10 | 43.5 | 10 | 43.5 | 23 | 100.0 |
| Total | 173 | 45.6 | 103 | 27.2 | 103 | 27.2 | 379 | 100.0 |
Surveillance Programs are ordered by geography North-South and West-East. Israel Surveillance Program was excluded since their single reported case hand an unknown birth outcome. Three cases, 1 from Finland and 2 from USA Atlanta were also excluded.
ETOPFA, elective termination of pregnancy for fetal anomaly; NP, not permitted; NR, not reported; ECEMC, Estudio Colaborativo Español de Malformaciones Congénitas; ECLAMC, Estudio Colaborativo Latino Americano de Malformaciones Congénitas; RYVEMCE, Registro y Vigilancia Epidemiológica de Malformaciones Congénitas.
Proportion of the Different Types of Conjoined Twins by Surveillance Program
The proportion of the different types of CT by SP (Table III) is presented according to the classification scheme described in Box II. Proportions of the total number of cases include 82 cases (21.4%) in which the type of CTwas not specified. The different types of CT are displayed by decreasing prevalence. Thoracopagus CT, that also includes thoracoomphalopagus CT, represent the largest number of cases reported (42.0%). The second most common CT type was parapagus dicephalus (11.5%). The remaining most common types were craniopagus and omphalopagus with 5.5% each. Other CT types such as parapagus diprosopus, ischiopagus, rachipagus, and pygopagus were observed in less than 3% of the cases, with the last two being the rarest types (1.0%). Parasitic CT were observed in 3.9% of all specified CT reported.
TABLE III.
Number of Types of Conjoined Twins Reported by Each Surveillance Program and Total Prevalence Proportions
| Surveillance Programa | Thoracopagus | Parapagus dicephalus | Cephalopagus | Omphalopagus | Parasitic | Craniopagus | Parapagus diprosopus | Ischiopagus | Rachipagus | Pygopagus | Not specified | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canada-Alberta | 5 | 1 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 2 | 11 |
| USA-Atlanta | 11 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 9 | 25 |
| USA-Texas | 3 | 3 | 3 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 13 |
| Mexico-RYVEMCE | 5 | 5 | 4 | 0 | 2 | 0 | 3 | 2 | 1 | 0 | 2 | 24 |
| South America-ECLAMC | 51 | 10 | 4 | 15 | 8 | 5 | 6 | 0 | 3 | 0 | 6 | 108 |
| Finland | 11 | 1 | 5 | 0 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 23 |
| Hungary | 28 | 7 | 1 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 40 |
| France-Central East | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 37 | 37 |
| Spain-ECEMC | 12 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 16 |
| China-Beijing | 14 | 7 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 3 | 28 |
| Australia-Victoria | 5 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 16 | 23 |
| Others | 16 | 5 | 0 | 1 | 0 | 4 | 0 | 2 | 0 | 2 | 5 | 35 |
| Total (n) | 161 | 44 | 21 | 21 | 15 | 13 | 11 | 7 | 4 | 4 | 82 | 383 |
| Total (%) | 42.0 | 11.6 | 5.5 | 5.5 | 3.9 | 3.4 | 2.9 | 1.8 | 1.0 | 1.0 | 21.4 | 100.0 |
ECEMC, Estudio Colaborativo Español de Malformaciones Congénitas; ECLAMC, Estudio Colaborativo Latino Americano de Malformaciones Congénitas; RYVEMCE, Registro y Vigilancia Epidemiológica de Malformaciones Congénitas.
Only those Surveillance Programs that reported 10 or more sets of CTwere specified. The remaining 10 reporting less than 10 cases are grouped together as “Others” (USA-Utah, Wales, Northern Netherlands, Germany Saxony-Anhalt, Slovak Republic, Italy-North East, Italy-Emilia Romagna, Italy-Tuscany, Italy-Campania, Israel).
Interestingly, a similar pattern of proportions of cases was observed in all participating programs, except for the omphalopagus type. For this type, 15 (71.4%) of the 21 cases were reported by the South America-ECLAMC SP, the rest by the Hungarian, USA-Texas, and USA-Utah registries.
Sex Ratio by Type of Conjoined Twins
Findings on the sex ratio were similar to previous studies, which reported a predominance of female cases. However, in the present sample the number of female cases was more than twice as frequent as that of males (218 females and 108 males; 20 CT cases were of indeterminate sex and 37 of sex not specified). Prevalence at birth of CTwas 56.9% for females and 28.2% for males (Table IV). Using an estimate of the number of males and females from the total of 26,138,837 births reported, a significant statistical difference was observed (P <0.01; OR =2.23; 95% CI: 1.76–2.83). Although, omphalopagus CT was four times more frequent in females than in males, the difference was not statistically significant. Female predominance was significantly higher for the thoracopagus CT type (P <0.01; OR =3.27; 95% CI: 1.83–5.99). Para-pagus as a whole (P <0.01; OR =2.39; 95% CI: 1.21–4.71), parapagus dice-phalus alone (P <0.05; OR =2.23; 95% CI: 1.06–4.68), and parasitic (P <0.01; OR =5.32; 95% CI: 1.42–24.22) CT types showed a significantly higher prevalence in males. No significant differences were observed for the rest of the CT types analyzed. Indeterminate sex was found in a small proportion (5.2%) of cases (Table IV).
TABLE IV.
Distribution of Sex by Type of Conjoined Twins
| Male
|
Female
|
Indeterminate sex
|
NS
|
Total
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | n | % | n | % | n | % | n | % | n | % |
| Thoracopagus | 28 | 17.4 | 110 | 68.3 | 5 | 3.1 | 18 | 11.2 | 161 | 100.0 |
| Parapagus dicephalus | 19 | 43.2 | 21 | 47.7 | 2 | 4.5 | 2 | 4.5 | 44 | 100.0 |
| Cephalopagus | 6 | 28.6 | 13 | 61.9 | 1 | 4.8 | 1 | 4.8 | 21 | 100.0 |
| Omphalopagus | 4 | 19.0 | 16 | 76.2 | 1 | 4.8 | 0 | 0.0 | 21 | 100.0 |
| Parasitic | 9 | 60.0 | 4 | 26.7 | 2 | 13.3 | 0 | 0.0 | 15 | 100.0 |
| Craniopagus | 6 | 46.2 | 4 | 30.8 | 0 | 0.0 | 3 | 23.1 | 13 | 100.0 |
| Parapagus diprosopus | 5 | 45.5 | 5 | 45.5 | 1 | 9.1 | 0 | 0.0 | 11 | 100.0 |
| Ischiopagus | 4 | 57.1 | 2 | 28.6 | 1 | 14.3 | 0 | 0.0 | 7 | 100.0 |
| Rachipagus | 2 | 50.0 | 2 | 50.0 | 0 | 0.0 | 0 | 0.0 | 4 | 100.0 |
| Pygopagus | 0 | 0.0 | 2 | 50.0 | 0 | 0.0 | 2 | 50.0 | 4 | 100.0 |
| Not specified | 25 | 29.4 | 39 | 49.4 | 7 | 8.2 | 11 | 12.9 | 82 | 100.0 |
| Total | 108 | 28.2 | 218 | 56.9 | 20 | 5.2 | 37 | 9.7 | 383 | 100.0 |
NS, not specified.
Unrelated Congenital Anomalies Associated by Type of Conjoined Twin
Cases were carefully reviewed for unrelated associated anomalies (Table V), although in some cases and types of CT it was difficult to determine with certainty whether other defects present were unrelated malformations. Associated congenital anomalies were observed in 115 pairs of 182 CT sets in which the information was available (63.2%). However, the malformation was adequately described in only 73 cases. In these 73 cases, 111 malformations were described, but detailed information of whether just one or both twins had the malformations was not noted. The malformations more frequently reported were those affecting the genitourinary tract (19.8%), the central nervous system (18.9%), comprising neural tube defects (9.9%), hydrocephalus (3.6%), microphthalmia (0.9%), and other central nervous system defects (4.5%), and the musculoskeletal system (12.6%). Combining musculoskeletal anomalies with limb deficiency defects and polydactyly, the proportion of limb anomalies increases to 20.7%. Other frequent malformations reported were gastrointestinal atresias (9.9%) and facial clefts (9.9%).
TABLE V.
Number and Proportion of Major Congenital Malformations Unrelated to the Site of Union Observed in 73 Sets of Conjoined Twins
| Malformation | Thoracopagus | Parapagus Dicephalus | Omphalopagus | Parasitic | Craniopagus | Parapagus Diprosopus | Cephalopagus | Ischiopagus | Pygopagus | Total | % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neural tube defects | 4 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 11 | 9.91 |
| Hydrocephaly | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 3.60 |
| Other central nervous system defects | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 4.50 |
| Micro-ophthalmia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.90 |
| Facial clefts | 5 | 1 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 11 | 9.91 |
| Congenital heart defects | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 2 | 7 | 6.31 |
| Gastrointestinal atresias | 1 | 3 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 11 | 9.91 |
| Abdominal wall defects | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 4 | 3.60 |
| Genitourinary defects | 6 | 1 | 5 | 1 | 1 | 1 | 1 | 2 | 4 | 22 | 19.82 |
| Musculoskeletal anomalies | 6 | 3 | 2 | 0 | 1 | 0 | 0 | 2 | 0 | 14 | 12.61 |
| Limb reduction anomalies | 5 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 7 | 6.31 |
| Polydactyly/syndactyly | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1.80 |
| Other | 5 | 2 | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 12 | 10.81 |
| Total | 37 | 18 | 17 | 7 | 5 | 8 | 3 | 7 | 9 | 111 | 100.00 |
Ethnicity and Conjoined Twins
Conjoined twins were also stratified by ethnicity in four categories: Anglo-Saxon/Caucasian, Chinese, Latin American, and Latin European. Prevalence was higher in the Latin American ethnic group. Statistical differences were observed when compared to the Anglo-Saxon/Caucasian (P <0.01; OR =1.51, 95% CI: 1.20–1.91), Chinese (P =0.02: OR =1.65, 95% CI: 1.06–2.49), and Latin European (P <0.01; OR =2.50, 95% CI: 1.84–3.40) ethnic groups. Other comparisons were not statistically significant.
Total Prevalence Time Trends
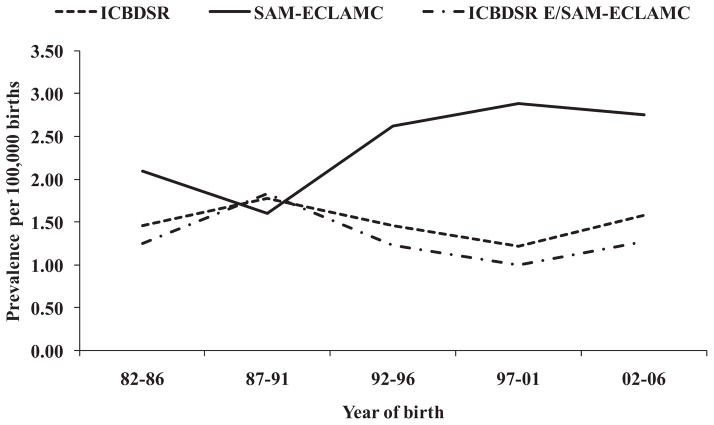
Time trends are presented in Figure 2, with the data analyzed in three different ways: (i) all participating SPs of the ICBDSR; (ii) South America-ECLAMC alone; and (iii) ICBDSR SPs excluding South America-ECLAMC. As shown in Figure 2, a slight steadily increasing but statistically non-significant trend in the prevalence of CT is present in the South America-ECLAMC SP for years 1987–2006, that differ from the decreasing prevalence trend observed in the ICBDSR excluding South America-ECLAMC.
Figure 2.
Prevalence of conjoined twins by 5-year period, 1982–2006 (ICBDSR: Clearinghouse; SAM-ECLAMC: South America-ECLAMC; ICBDSR E/SAM-ECLAMC: Clearinghouse excluding South America-ECLAMC).
Genetic, Demographic, and Environmental Risk Factors
Analyses were performed to evaluate for associations with genetic factors such as consanguinity, familial aggregation, and cytogenetic studies; demographic characteristics such as maternal age and maternal education; reproductive data, including gestational age, birth order, birth weight, spontaneous previous abortions; and environmental factors such as maternal exposures and diseases during pregnancy. No associations were observed between the mentioned genetic factors and CT. No familial cases were observed, consanguinity was reported in 5 of 209 CT cases (2.38%; 95% CI: 0.78–5.49) and all chromosome studies performed (12.1%) were normal.
Maternal age analysis is shown in Figure 3. The small differences in prevalence between maternal age groups were not statistically significant. Maternal education was known for 165 women. Those mothers of a LB and SB CT had an average of 9.3 years, and those women having ETOPFA had an average of 11.8 years of education. Although it is difficult to evaluate birth weight and gestational age in CT, an analysis was attempted in 159 LB cases, in which the information was reported. The data were stratified by weight (<2,500 and ≥2,500 g) and gestational age (≤37 and ≥38 weeks). Low birth weight was observed in 63 CT pairs (39.0%). Weight was stratified by gestational age for 131 CT cases in which both data values were available. Preterm CT pairs were observed in a very high proportion (100 cases; 76.3%). Of these preterm CT pairs, 49% weighed less than 2,500 g. However, among CT pairs delivered at term, only 6.5% of them had a low birth weight.
Figure 3.

Conjoined twins prevalence ratios and 95% CI for maternal age groups relative to the reference group of 25–29 years.
Birth order showed an even distribution: 29.7% of CT cases born to primipara mothers, and similar proportions (34.9% and 35.3%) for CT born to mothers with parity =2 and ≥3, respectively. The number of CT born to primipara mothers was very low in the Mexico-RYVEMCE SP (1 case among the 24 reported cases) and in the Latin America-ECLAMC program (72 cases among the 108 reported cases). Expected and observed values differed significantly (cumulative Poisson P-values were P =0.001 and P <0.0001, respectively).
Among 187 cases in which prior pregnancy outcomes for multipara mothers were reported, previous spontaneous abortions were reported in 37 (19.79%; 95% CI: 14.33–26.23%). Of 164 women that answered questions on supplement usage, no one had supplemented their diet with folic acid and only 6 took multivitamins during pregnancy. Unspecified fertility problems were reported by 6.8% of women, preconceptional existing diabetes was reported twice, and epilepsy was reported only once. The medications taken by these women were not analyzed due to the very high proportion (82.2%) of missing data and poor quality of data reported.
DISCUSSION
This worldwide study includes the largest sample of CT ever studied before. A total of 383 carefully revised sets of CT obtained from 26,138,837 births reported by 21 Clearinghouse participating SPs were included in the analysis. Total prevalence (1.47; 95% CI: 1.32–1.62) per 100,000 births is within the range of the more frequently reported prevalence in large populations studied (Box I). Although, this is true for total prevalence, significant heterogeneity is observed when the prevalence of each SP is considered (Table I, Fig. 1).
The main sources of variation in this study are most likely attributable to the population types and sizes monitored, and inclusion or exclusion of SB and ETOPFA. The routine follow-up of every pregnancy and the feasibility of an early prenatal ultrasound diagnosis in some cases may have introduced an underascertainment due to unreported ETOPFA. As reported by Martínez-Frías et al. [2009] in Spain, termination for fetal anomaly has been offered by the public health system for many years, but it does not have for a system for including cases with ETOPFA in its registry program. As a result, current prevalence of CT in this SP decreased considerably to 0.68 per 100,000 births. This probably accounts for the differences observed within and between the four depicted categories when prevalence results were described (Table I). The underreporting is also a dilemma when present results are compared with previous reports [Hanson, 1975; Källén and Rybo, 1978; Edmonds and Layde, 1982; Viljoen et al., 1983; Castilla et al., 1988; ICBDMS, 1991; Liang et al., 1999; Tang et al., 2007] and other studies listed in Box II.
Pregnancy outcomes, LB (45.6%), SB (27.2%), and ETOPFA (27.2%) are shown in Table II. The proportion of CT described in each of these categories depends in part on the health services characteristics and registration systems in the communities served by the SP, as well as the availability of legal ETOPFA. This perhaps could account for the low proportion of SB reported in the present study in comparison with other surveys carried out when ETOPFA was not available [Edmonds and Layde, 1982; Métneki and Czeizel, 1989; ICBDMS, 1991]. The proportion of CT resulting in ETOPFA varies from zero in Mexico-RYVEMCE and South America-ECLAMC where ETOPFA is not legal to as much as 81.1% in France Central East where elective termination of pregnancy is a common practice (Table II).
The observed prevalence of types of CT (Table III) shows a predominance (42.0%) of the thoracopagus type as reported in previous epidemiological studies with similar proportions: 49.3% [Edmonds and Layde, 1982], 43.5% [Castilla et al., 1988], 43.6% [Métneki and Czeizel, 1989], and 39.1% [ICBDMS, 1991]. It is not clear whether the high proportion of omphalopagus cases (71.4%) reported by the South America-ECLAMC program is a real cluster or an anomaly. Perhaps one way to evaluate this would be to look at the hospitals of origin of the 15 reported cases, mindful of the fact that the South America-ECLAMC SP collects data from 10 different countries and from close to 100 hospitals. Except for an uncommon high proportion of para-pagus dicephalus and diprosopus CT in the Mexican SP (33.3%), other types of reported CT showed a similar distribution among the 21 participating Clearinghouse SPs (Table III).
The predominance of female sex observed in the present study has been previously reported [Edmonds and Layde, 1982; Imaizumi, 1988; Métneki and Czeizel, 1989; ICBDMS, 1991; Tang et al., 2007; Martínez-Frías et al., 2009]. Considering the entire CT sample, prevalence was more than twice (2.02) as frequent in females as in males. Changes in prevalence by sex may depend on sample size and chance. In a previous study of the South American SP, Castilla et al. [1988] reported a very similar number of male and female CT, but in the data reported by the South American SP for the present study an overt female predominance of 2.09 was observed (69 females and 33 males, data not shown). Another interesting finding was the contrasting and significant differences observed when prevalence by sex is analyzed by type of CT. Thoracopagus type is almost four times more frequent in females than in males (P <0.01; OR 3.13; 95% CI: 1.76–5.63). However, parapagus and parasitic types were significantly more frequent in males than in females, P =0.01 and P <0.01, respectively (Table IV). No significant differences were observed for the rest of the CT types analyzed. Detailed similar information of this type of data (Table IV) was reported previously [ICBDMS, 1991], although proportions reported were different from the ones reported herein, and no comments were made regarding statistical analysis. No explanation is proposed for the sex differences but future studies could look at these in the CT outcomes in the SB and ETOPFA groups. More research is needed to explore the relation and severity of types of CT with sex to identify a possible biased selection in utero of some CT types that might explain the observed differences.
The proportion of associated malformations unrelated to the site of union of the twins has been reported in other studies [Edmonds and Layde, 1982; Métneki and Czeizel, 1989; ICBDMS, 1991], and was similar to the 63% observed in the present study. However, only two studies [Métneki and Czeizel, 1989; ICBDMS, 1991] stratified the associated malformations according to the CT type. Neural tube defects were observed in more than 15% of parapagus dicephalus and diprosopus types. Genitourinary anomalies were recorded in more than 15% of thoracopagus, omphalopagus, ischiopagus, and pygo-pagus types, and musculoskeletal defects in more than 15% of thoracopagus, parapagus dicephalus, craniopagus, and ischiopagus types. Oral clefts and gastrointestinal atresias were evenly distributed (Table V). The reported proportion of associated anomalies does not differ significantly from those reported by Métneki and Czeizel [1989], and the ICBDMS [1991]. Although, some anomalies occurred more frequently with certain CT types, numbers are still too small to suggest specific associations and could merely represent spurious associations.
Ethnicity analysis showed that the prevalence of CT is significantly higher in Latin American SPs than in Anglo-Saxon/Caucasian, Chinese and Latin European programs (Table I). A possible explanation of these findings could be the under-registration of CT pregnancies undergoing ETOPFA in some SPs, but particularly in the Latin European SP. This observation agrees with the proportion of prenatal diagnosis before 20 weeks of gestation and the high proportion of ETOPFA in European SPs (Table II). This could also explain the significantly higher prevalence in Latin American SPs than in the one of China, where ETOPFA was not described in the 28 CT cases reported, although elective termination of pregnancy is permitted but not recorded.
Although several references to clusters of CT have been observed [Hanson, 1975; Källén and Rybo, 1978; Mabogunje and Lawrie, 1980; Viljoen et al., 1983; Zake, 1984; Rees et al., 1993; Savona-Ventura et al., 2009] a clear explanation has not been found. Different explanations bias in the results have been considered, particularly with respect to population types and sizes surveyed, and hospital records not contributing to the regional or national SPs or a multicentric hospital-based surveillance system. Retrospective versus prospective CT sample collection and the lack of a population reference of total births born during the same period of time of diagnosis could also be sources of bias.
When prevalence data were analyzed in a time trend, the South America-ECLAMC SP, exclusively for years 1987–2006, showed an increasing but statistically non-significant prevalence trend for CT (χ for trend: 2.83; P =0.09). Although not significant, it would be important to monitor the increasing prevalence trend in this SP.
Genetic and environmental risk factors such as consanguinity, familial aggregation, cytogenetic studies, and maternal exposures, and acute and chronic diseases during pregnancy did not reveal any association with the occurrence of CT, in general, or for any particular type of CT. No familial cases were reported and all karyotypes performed were normal. Regarding reproductive characteristic patterns, maternal age does not show any significant trend or association with CT (Fig. 3). However, other related variables, such as gestational age, birth weight, birth order, previous spontaneous abortions, and maternal education showed certain peculiar relationships. A high proportion of preterm deliveries (76.3%) occurred among CT cases; however, interestingly low birth weight varied by gestational age (49.0% in pregnancies ≤37 weeks and 6.5% in pregnancies of >37 weeks). The significant difference in birth order in the Mexico RYVEMCE and the Latin America ECLAMC SPs is an unexpected finding compared with other SPs that reported information on birth order in more than 20 CT pairs. Previous spontaneous abortions were reported in a high proportion (19.8%) of CT mothers. These figures are higher than that usually reported in most healthy populations studied.
Even though success in surgical separation of CT pairs has improved, surgical separation is still a major challenge. The procedure requires a multi-disciplinary team, accurate imaging to assess organ sharing, and a consideration of aspects related to survival and ethics in each case. Experiences of dedicated groups in surgical separation, clinical supervision, and support directives for parents and patients are available from the literature [Bland and Hammar, 1962; Hoyle, 1990; Spitz, 1996; Kingston et al., 2001; Pearn, 2001; Spitz and Kiely, 2002; Spitz, 2003, 2005; Arkinson, 2004; Pajkrt and Jauniaux, 2005; Votteler and Lipsky, 2005; Arlikar et al., 2009; Sharma et al., 2010; Weber and Sebire, 2010].
Review of variable total prevalence, variable prevalence by occurrence type, predominance in females, a higher prevalence in some ethnic groups such as Blacks [Edmonds and Layde, 1982] and Chinese [Liang et al., 1999; Tang et al., 2007], geographic variation, socio-demographic variables, genetic and environmental factors, has not helped to advance the understanding of the altered mechanisms that interrupt the normal separation of an inner cell mass leading to the occurrence of a set of CT. Considering the fission theory as currently accepted [ICBDMS, 1991; Kaufman, 2004; Spitz, 2005; Sadler, 2010; Weber and Sebire, 2010], a simple molecular disorder at a deep cellular level distorting cell adhesion or apoptosis in a very early stage of embryogenesis could be involved in a etiology of CT that involves incomplete split of the inner mass cell.
This is the largest international collaborative study of CT to date. Salient findings were a notable variation in prevalence among SPs; a marked variation in the type of pregnancy outcome; a significant female predominance in CT, particularly of the thoracopagus type, and a significant male predominance in parapagus and parasitic types; significant differences in prevalence by ethnicity; and apparent non-significant increasing prevalence trend in South American countries. Further work in epidemiology and molecular research is needed to elucidate the etiologic processes involved and associated risk factors for the development of this fascinating phenomenon from nature.
Acknowledgments
The authors are grateful to each Surveillance Program’s Staff and Members for their work in collecting case data and submission to the ICBDSR Centre. The work conducted at the ICBDSR Centre was supported by the Centers for Disease Control and Prevention (1U50DD000524-02). Grant sponsor for South America ECLAMC: MCT/ CNPq, Brazil; Grant numbers: 573993/ 2008-4, 476978/2008-4, 554755/ 2009-2; 306750/2009-0; 402045/ 2010-6. This work was in part supported by Instituto de Salud Carlos III (ISCIII, Ministry of Science and Innovation) of Spain, and the Fundación 1000 sobre Defectos Congénitos, of Spain. CIBERER is an initiative of ISCIII. Components of ECEMC’s Peripheral Group are gratefully acknowledged. The Tuscany Registry of Birth Defects is funded by the “Direzione Generale Diritti di cittadinanza e Coesione sociale—Regione Toscana.” Public Health Genetics, Murdoch Childrens Research Institute and Department of Paediatrics, University of Melbourne, Parkville, Victoria, Australia, formerly Victorian Birth Defects Register.
Grant sponsor: Centers for Disease Control and Prevention; Grant number: 1U50DD000524-02; Grant sponsor: South America ECLAMC: MCT/ CNPq, Brazil; Grant numbers: 573993/2008-4, 476978/2008-4, 554755/2009-2, 306750/2009-0, 402045/2010-6; Grant sponsor: Instituto de Salud Carlos III (ISCIII, Ministry of Science and Innovation) of Spain; Grant sponsor: Fundación 1000 sobre Defectos Congénitos, of Spain; Grant sponsor: The Tuscany Registry of Birth Defects is funded by the “Direzione Generale Diritti di cittadinanza e Coesione sociale—Regione Toscana”
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Aird I. Conjoined twins: Further observations. Br Med J. 1959;1(5133):1313–1315. doi: 10.1136/bmj.1.5133.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino DB, Timmons C, Burns D, Lowichik A. Craniopagus parasiticus: A case illustrating its relationship to craniopagus conjoined twinning. Pediatr Pathol Lab Med. 1997;17(6):939–944. [PubMed] [Google Scholar]
- Atkinson L. Ethics and conjoined twins. Childs Nerv Syst. 2004;20:504–507. doi: 10.1007/s00381-004-0983-6. [DOI] [PubMed] [Google Scholar]
- Arlikar J, Mane S, Dhende N, Sanghavi Y, Valand A, Butale P. Fetus in fetus: Two case reports and review of literature. Pediatr Surg Int. 2009;25(3):289–292. doi: 10.1007/s00383-009-2328-8. [DOI] [PubMed] [Google Scholar]
- Ballantyne JW. The Biddenden Maids: The medieval pygopagus. Teratologia. 1895;2:268–274. [Google Scholar]
- Bender C. Studies on symmetrically conjoined twins. J Pediatr. 1967;70(6):1010–1011. doi: 10.1016/s0022-3476(67)80282-8. [DOI] [PubMed] [Google Scholar]
- Berezowski AT, Duarte G, Rodrigues R, de Carvalho Cavalli R, de Olivera Cardoso dos Santos R, de Moraes Villela de Andrade Vicente YA, Galli Sorita Tazima M, de F. Conjoined twins: An experience of a tertiary hospital in Southeast Brazil. Rev Bras Ginecol Obstet. 2010;32(2):61–65. [PubMed] [Google Scholar]
- Berrin K, Larco M. The spirit of ancient Peru: treasures from the Museo Arqueoló-gico Rafael Larco Herrera. New York: Thames and Hudson; 1997. p. 216. [Google Scholar]
- Bland KG, Hammar B. Xiphopagus twins. Report of obstetric and surgical management of a case. Cent Afr J Med. 1962;8:371–375. [PubMed] [Google Scholar]
- Bondeson J. The Biddenden Maids: A curious chapter in the history of conjoined twins. J R Soc Med. 1992;85(4):217–221. doi: 10.1177/014107689208500413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondeson J. The Isle-Brewers conjoined twins of 1680. J R Soc Med. 1993;86(2):106–109. [PMC free article] [PubMed] [Google Scholar]
- Bugge M. Twins with omphalocele in Denmark (1970–1989) Am J Med Genet Part A. 2010;152A(8):2048–2052. doi: 10.1002/ajmg.a.33494. [DOI] [PubMed] [Google Scholar]
- Canfield D, Brignolo L, Peterson PE, Hendrickx AG. Conjoined twins in a rhesus monkey (Macaca mulatta) J Med Primatol. 2000;29(6):427–430. doi: 10.1111/j.1600-0684.2000.290608.x. [DOI] [PubMed] [Google Scholar]
- Castilla EE, Mastroiacovo P. Very rare defects: what can we learn? 2011 doi: 10.1002/ajmg.c.30315. This issue. [DOI] [PubMed] [Google Scholar]
- Castilla EE, López-Camelo JS, Orioli IM, Sánchez O, Paz JE. The epidemiology of conjoined twins in Latin America. Acta Genet Med Gemellol (Roma) 1988;37(2):111–118. doi: 10.1017/s0001566000004013. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Metneki J, Kazy Z, Puho E. A population-based case–control study of oral griseofulvin treatment during pregnancy. Acta Obstet Gynecol Scand. 2004;83(9):827–831. doi: 10.1111/j.0001-6349.2004.00598.x. [DOI] [PubMed] [Google Scholar]
- Edmonds LD, Layde PM. Conjoined twins in the United States, 1970–1977. Teratology. 1982;25(3):301–308. doi: 10.1002/tera.1420250306. [DOI] [PubMed] [Google Scholar]
- Emanuel I, Huang SW, Gutman LT, Yu FC, Lin CC. The incidence of congenital malformations in a Chinese population: The Taipei Collaborative Study. Teratology. 1972;5(2):159–169. doi: 10.1002/tera.1420050206. [DOI] [PubMed] [Google Scholar]
- Gould GM, Pyle WL. Major terata. In: Gould GM, Pyle WL, editors. Anomalies and curiosities of medicine. Philadelphia: W.B. Saunders; 1896. pp. 162–213. [Google Scholar]
- Guttmacher AF. In: Biographical notes on some famous conjoined twins. 1. Bergsma D, editor. III. New York: National Foundation-March of Dimes; 1967. pp. 10–17. Birth defects original Article series. [Google Scholar]
- Hanson JW. Letter: Incidence of conjoined twinning. Lancet. 1975;2(7947):1257. doi: 10.1016/s0140-6736(75)92092-9. [DOI] [PubMed] [Google Scholar]
- Hoyle RM. Surgical separation of conjoined twins. Surg Gynecol Obstet. 1990;170(6):549–562. [PubMed] [Google Scholar]
- ICBDMS. Conjoined twins—An epidemiological study based on 312 cases. The International Clearinghouse for Birth Defects Monitoring Systems. Acta Genet Med Gemellol (Roma) 1991;40(3–4):325–335. doi: 10.1017/s0001566000003512. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y. Conjoined twins in Japan, 1979–1985. Acta Genet Med Gemellol (Roma) 1988;37(3–4):339–345. doi: 10.1017/s0001566000003937. [DOI] [PubMed] [Google Scholar]
- Källén B, Rybo G. Conjoined twinning in Sweden. Acta Obstet Gynecol Scand. 1978;57(3):257–259. doi: 10.3109/00016347809154894. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The embryology of conjoined twins. Childs Nerv Syst. 2004;20(8–9):508–525. doi: 10.1007/s00381-004-0985-4. [DOI] [PubMed] [Google Scholar]
- Kingston CA, McHugh K, Kumaradevan J, Kiely EM, Spitz L. Imaging in the preoperative assessment of conjoined twins. Radiographics. 2001;21(5):1187–1208. doi: 10.1148/radiographics.21.5.g01se011187. [DOI] [PubMed] [Google Scholar]
- Knudsen LB. No association between griseofulvin and conjoined twinning. Lancet. 1987;2(8567):1097. doi: 10.1016/s0140-6736(87)91533-9. [DOI] [PubMed] [Google Scholar]
- Levin M, Roberts DJ, Holmes LB, Tabin C. Laterality defects in conjoined twins. Nature. 1996;384(6607):321. doi: 10.1038/384321a0. [DOI] [PubMed] [Google Scholar]
- Liang J, Xu CI, Wang Y. Epidemiological survey of conjoined twins in China. Hua Xi Yi Ke Da Xue Xue Bao. 1999;30(1):56–58. [PubMed] [Google Scholar]
- Logroño R, Garcia-Lithgow C, Harris C, Kent M, Meisner L. Heteropagus conjoined twins due to fusion of two embryos: Report and review. Am J Med Genet. 1997;73(3):239–243. [PubMed] [Google Scholar]
- Mabogunje OA, Lawrie JH. Conjoined twins in West Africa. Arch Dis Child. 1980;55(8):626–630. doi: 10.1136/adc.55.8.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin GA. Heteropagus conjoined twins due to fusion of two embryos. Am J Med Genet. 1998;78(4):388–389. doi: 10.1002/(sici)1096-8628(19980724)78:4<388::aid-ajmg19>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Machin GA, Sperber GH. Lessons from conjoined twins. Am J Med Genet. 1987;28(1):89–97. doi: 10.1002/ajmg.1320280113. [DOI] [PubMed] [Google Scholar]
- Machin GA, Sperber GH. Comments on “Unique anomalies in cephalothoracopagus janiceps conjoined twins with implications for multiple mechanisms in the abnormal embryogenesis. Teratology. 1991;44(5):481–483. doi: 10.1002/tera.1420440502. [DOI] [PubMed] [Google Scholar]
- Martínez-Frías ML, Bermejo E, Mendioroz J, Rodríguez-Pinilla E, Blanco M, Egüés J, Félix V, García A, Huertas H, Nieto C, López JA, López S, Paisán L, Rosa A, Vázquez MS. Epidemiological and clinical analysis of a consecutive series of conjoined twins in Spain. J Pediatr Surg. 2009;44(4):811–820. doi: 10.1016/j.jpedsurg.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Métneki J, Czeizel A. Griseofulvin teratology. Lancet. 1987;1(8540):1042. doi: 10.1016/s0140-6736(87)92318-x. [DOI] [PubMed] [Google Scholar]
- Métneki J, Czeizel A. Conjoined twins in Hungary, 1970–1986. Acta Genet Med Gemellol (Roma) 1989;38(3–4):285–299. doi: 10.1017/s0001566000002695. [DOI] [PubMed] [Google Scholar]
- Milham S. Symmetrical conjoined twins: An analysis of the birth records of twenty-two sets. J Pediatr. 1966;69(4):643–647. doi: 10.1016/s0022-3476(66)80054-9. [DOI] [PubMed] [Google Scholar]
- Mudaliar AL. Double monsters: A study of their circulatory system and some other anatomical abnormalities and the complications in labor. BJOG. 1930;37(4):753–768. [Google Scholar]
- Newman C. Together forever. Natl Geogr. 2006;209(6):148–152. [Google Scholar]
- Pajkrt E, Jauniaux E. First-trimester diagnosis of conjoined twins. Prenat Diagn. 2005;25(9):820–826. doi: 10.1002/pd.1267. [DOI] [PubMed] [Google Scholar]
- Pearn J. Bioethical issues in caring for conjoined twins and their parents. Lancet. 2001;357:1968–1971. doi: 10.1016/S0140-6736(00)05070-4. [DOI] [PubMed] [Google Scholar]
- Phelan MC, Hall JG. Twins. In: Stevenson RE, Hall JG, editors. Human malformations and related anomalies. 2. New York: Oxford University Press; 2006. pp. 1377–1495. [Google Scholar]
- Potter EL. Pathology of the fetus and infant. Chicago: Chicago Year Book Medical Publishers; 1961. p. 217. [Google Scholar]
- Rees AE, Vujanic GM, Williams WM. Epidemic of conjoined twins in Cardiff. Br J Obstet Gynaecol. 1993;100(4):388–391. doi: 10.1111/j.1471-0528.1993.tb12987.x. [DOI] [PubMed] [Google Scholar]
- Robertson EG. Craniopagus parietalia. Arch Neurol Psychiatry. 1953;70:189–205. doi: 10.1001/archneurpsyc.1953.02320320055005. [DOI] [PubMed] [Google Scholar]
- Rodríguez JM. Descripción de un monstruo humano cuádruple, nacido en Durango el año de 1868. Gac Med Mex V. 1870:18–31. [Google Scholar]
- Rosa FW, Hernandez C, Carlo WA. Griseofulvin teratology, including two thoracopagus conjoined twins. Lancet. 1987;1(8525):171. doi: 10.1016/s0140-6736(87)92015-0. [DOI] [PubMed] [Google Scholar]
- Ryden AL. Kasuistischur beitrog zur kenntris der gerburtron thoracapagen. Zbl Gynak. 1934;58:972–975. [Google Scholar]
- Sadler TW. Third month to birth: The fetus and placenta. In: Sadler TW, editor. Langman’s Medical Embryology. 11. Philadelphia: Lippincott Williams & Wilkins; 2010. pp. 91–111. [Google Scholar]
- Savona-Ventura C, Grima S, Buttigieg GG. Conjoint twinning in the Maltese Islands. J Obstet Gynaecol. 2009;29(7):599–604. doi: 10.1080/01443610903082500. [DOI] [PubMed] [Google Scholar]
- Sharma G, Mobin SS, Lypka M, Urata M. Heteropagus (parasitic) twins: A review. J Pediatr Surg. 2010;45(12):2454–2463. doi: 10.1016/j.jpedsurg.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Simpson JY. A lecture on the Siamese and other viable United twins. Br Med J. 1869;1(428):229–233. doi: 10.1136/bmj.1.428.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R. Conjoined twins: Theoretical embryologic basis. Teratology. 1992;45(6):591–602. doi: 10.1002/tera.1420450604. [DOI] [PubMed] [Google Scholar]
- Spencer R. Anatomic description of conjoined twins: A plea for standardized terminology. J Pediatr Surg. 1996;31(7):941–944. doi: 10.1016/s0022-3468(96)90417-0. [DOI] [PubMed] [Google Scholar]
- Spencer R. Theoretical and analytical embryology of conjoined twins: Part I: Embryogenesis. Clin Anat. 2000a;13(1):36–53. doi: 10.1002/(SICI)1098-2353(2000)13:1<36::AID-CA5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Spencer R. Theoretical and analytical embryology of conjoined twins: Part II: Adjustments to union. Clin Anat. 2000b;13(2):97–120. doi: 10.1002/(SICI)1098-2353(2000)13:2<97::AID-CA5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Spitz L. Conjoined twins. Br J Surg. 1996;83(8):1028–1030. doi: 10.1002/bjs.1800830803. [DOI] [PubMed] [Google Scholar]
- Spitz L. Surgery for conjoined twins. Ann R Coll Surg Engl. 2003;85(4):230–235. doi: 10.1308/003588403766274917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz L. Conjoined twins. Prenat Diagn. 2005;25(9):814–819. doi: 10.1002/pd.1268. [DOI] [PubMed] [Google Scholar]
- Spitz L, Kiely EM. Experience in the management of conjoined twins. Br J Surg. 2002;89(9):1188–1192. doi: 10.1046/j.1365-2168.2002.02193.x. [DOI] [PubMed] [Google Scholar]
- Stevenson AD, Johnston HA, Stewart MIP, et al. Congenital malformations. Bull World Health Org. 1966;34(Suppl):9–127. [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Zhu J, Zhou GX, Dai L, Wang YP, Liang J. An epidemiological study on conjoined twins in China, from 1996 to 2004. Zhonghua Yu Fang Yi Xue Za Zhi. 2007;41(Suppl):146–149. [PubMed] [Google Scholar]
- Uchida Ia, Freeman VCP, Gedeon M, Goldmaker J. Twining rate in spontaneous abortions. Am J Hum Genet. 1983;35:987–993. [PMC free article] [PubMed] [Google Scholar]
- Viljoen DL, Nelson MM, Beighton P. The epidemiology of conjoined twinning in Southern Africa. Clin Genet. 1983;24(1):15–21. doi: 10.1111/j.1399-0004.1983.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Votteler TP, Lipsky K. Long-term results of 10 conjoined twin separations. J Pediatr Surg. 2005;40(4):618–629. doi: 10.1016/j.jpedsurg.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Weber MA, Sebire NJ. Genetics and developmental pathology of twinning. Semin Fetal Neonatal Med. 2010;15(6):313–318. doi: 10.1016/j.siny.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Wertelecki W. Malformations in a Chornobil-impacted region. Pediatrics. 2010;125(4):e836–e843. doi: 10.1542/peds.2009-2219. [DOI] [PubMed] [Google Scholar]
- Zake EZ. Case reports of 16 sets of conjoined twins from a Uganda hospital. Acta Genet Med Gemellol (Roma) 1984;33(1):75–80. doi: 10.1017/s0001566000007534. [DOI] [PubMed] [Google Scholar]