Abstract
Background
Constipation and L-dopa-induced gastric dysmotility are common gastrointestinal (GI) symptoms in Parkinson’s disease (PD). We investigate the novel ghrelin agonist, HM01 influence on GI motor dysfunctions in 6-hydroxydopamine (6-OHDA) rats.
Methods
HM01 pharmacological profiles were determined in vitro and in vivo in rats. We assessed changes in fecal output and water content, and gastric emptying (GE) in 6-OHDA rats treated or not with orogastric (og) HM01 and L-dopa/carbidopa (LD/CD, 20/2 mg kg−1). Fos immunoreactivity (ir) cells in specific brain and lumbosacral spinal cord were quantified.
Key results
HM01 displayed a high binding affinity to ghrelin receptor (Ki: 1.42 ± 0.36 nM), 4.3±1.0 h half-life and high brain/plasma ratio. 6-OHDA rats had reduced daily fecal output (22%) and water intake (23%) compared to controls. HM01 (3 and 10 mg kg−1) similarly reversed the decreased 4-h fecal weight and water content in 6-OHDA rats. Basal GE was not modified in 6-OHDA rats, however, LD/CD (once or daily for 8 days) delayed GE in 6-OHDA and control rats that was prevented by HM01 (3 mg kg−1 acute or daily before LD/CD). HM01 increased Fos-ir cell number in the area postrema, arcuate nucleus, nucleus tractus solitarius and lumbosacral intermediolateral column of 6-OHDA rats where 6-OHDA had a lowering effect compared to controls.
Conclusions & Inferences
6-OHDA rats display constipation- and adipsia-like features of PD and L-dopa-inhibited GE. The new orally active ghrelin agonist, HM01 crosses the blood brain barrier and alleviates these alterations suggesting a potential benefit for PD with GI disorders.
Keywords: Constipation, gastric emptying, ghrelin agonist, 6-hydroxydopamine, L-dopa, Parkinson’s disease
Introduction
Parkinson’s disease (PD) patients suffer from a variety of gastrointestinal (GI) motor symptoms in addition to the cardinal motor disorders (1, 2). Constipation is the most common GI dysfunction occurring with 60% to 80% prevalence in PD patients (1). Furthermore, L-dopa, a standard treatment in PD (3), delays gastric emptying in PD patients and healthy humans (4, 5).
Development of therapies for GI symptoms in PD patients is still an unmet need (6). Ghrelin is a gut peptide mainly secreted from the gastric A/X-like endocrine cells (7). Ghrelin binds to growth hormone-secretagogue receptor (GHS-R)1a (8) to exert potent prokinetic effects on GI. Namely, it is well documented that peripheral administration of ghrelin enhances basal gastric emptying in rodents (9, 10), healthy volunteers (11) and patients with gastroparesis (12, 13). We previously reported that intravenous ghrelin prevented the oral L-dopa-induced delayed gastric emptying in naïve rats (14). Interestingly, peripheral administration of ghrelin has no effect on colonic motor activity. However, peripheral administration of ghrelin agonists that cross the blood-brain barrier (BBB) can elicit propulsive colonic contractions and defecation in rats (15–17). Several compounds targeting the GHS-R1a are under clinical trials for GI disorders such as diabetic gastroparesis, postoperative ileus, and idiopathic chronic constipation (12). Whether ghrelin or ghrelin agonists improve GI dysfunctions in PD is yet to be explored experimentally.
Rat PD models in which the neurotoxin, 6-hydroxydopamine (6-OHDA) is microinjected at target sites of the nigrostriatal pathway (18) have been widely used in preclinical PD research to test new compounds alleviating PD symptomatology and largely contributed to drug development (19, 20). In these PD models, rats also display features of altered GI motor function (21–23). Namely, unilateral microinjection of 6-OHDA into the medial forebrain bundle (mfb, containing the dopaminergic axons of nigrostriatal dopaminergic neurons) decreased daily fecal output after treatment and more prominently at 3–4 weeks (23). When 6-OHDA is injected bilaterally or unilaterally into the substantia nigra, rats showed delayed gastric emptying of solid meal (21, 22).
In the present study, we seek to establish in rats whether oral treatment with a novel ghrelin agonist can alleviate GI motor alteration in the 6-OHDA PD model generated by unilateral microinjection of 6-OHDA into the mfb (18, 23). First, we assessed symptomatic features of rats 3–4 weeks after the 6-OHDA injection, including daily defecation, food and water intake, body weight, gastric emptying and circulating ghrelin levels compared to vehicle (control). We also determined relevant characteristics of the newly developed ghrelin agonist, HM01, namely the binding affinity and intracellular calcium signaling in HEK293 cells expressing recombinant human GHS-R1a and pharmacokinetic properties including plasma half-life and brain penetration after peripheral administration in rats. Then, we tested the influence of acute or repeated orogastric (og) treatment with HM01 on GI alterations in 6-OHDA rats treated with or without L-dopa combined with carbidopa, one of the L-amino acid decarboxylase inhibitors to reduce L-dopa conversion to dopamine in the periphery (24). Lastly, we used c-Fos as a neuronal marker to get insight as to whether hypothalamus, medullary and spinal cord sites which are known to be involved in the regulation of GI functions and food intake were altered in 6-OHDA without or with HM01 treatment.
Materials and Methods
Animals
Adult male Sprague-Dawley (SD) rats (230–250 g, Harlan Laboratories, San Diego, CA, USA and in pharmacokinetic studies, 200–240 g, Shanghai SIPPR-BK Laboratory Animal CO. LTD, Shanghai, China) were kept under controlled illumination (12:12 h light/dark cycle, lights on/off: 6:00 AM/6:00 PM) and temperature (22 ± 2°C). Animals were fed standard rodent diet (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO and Shanghai SLAC laboratory animal CO. LTD.) and tap water ad libitum. Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the federal authority for animal research conduct. All procedures were approved by the Animal Research Committee at Veterans Affairs Greater Los Angeles Healthcare System (protocol #01002-12), and Shanghai Laboratory Animal Center (protocol #2013-DMPK-009).
Reagents
Small molecule ghrelin agonist, HM01 (Helsinn SA Lugano, Lugano, Switzerland; patent) was suspended in vehicle (0.5% carboxymethylcellulose), except otherwise stated. L-dopa and carbidopa were dissolved in vehicle [saline:dimethylsulfoxide (DMSO):Tween-80 as 85:10:5, containing 1% ascorbic acid]. Desipramine hydrochloride was dissolved in saline and 6-OHDA hydrochloride was dissolved in saline with 0.2% ascorbic acid. Acetaminophen was suspended in 1.5% methylcellulose (4000 cp). All reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO).
6-OHDA rat model of PD
The procedures were based on previous studies (23, 25) with modifications. Rats were anesthetized with an intramuscular injection of ketamine hydrochloride (75 mg kg−1, Ketanest; Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (5 mg kg−1, Rompun; Mobay Corporation, Shawnee, KS), and placed on stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Then, 6-OHDA (12 μg in 3 μL) or vehicle (3 μL saline with 0.2% ascorbic acid) was microinjected unilaterally into the mfb using the following coordinates (mm) from the bregma (antero-posterior: −4.4; medio-lateral: +1.6; and dorso-ventral: −8.0) according to Paxinos and Watson’s atlas (26). Desipramine (25 mg kg−1) was injected intraperitoneally 30 min before the microinjection to prevent the uptake of 6-OHDA into noradrenergic neurons (27). Thereafter, rats were housed individually and experiments started after a 4-week recovery period.
Measurements
Calcium signaling assay and binding assay
Binding assay and calcium signaling assay were performed using cultured cells that express human GHS-R1a according to standard methods detailed in supporting information.
Pharmacokinetics of HM01
HM01 (3 mg kg−1 in 3 mL kg−1) was given og as a suspension in vehicle (2% DMSO and 98% of 0.5% carboxymethylcellulose). Repeated blood samplings (0.2–0.3 mL) were performed at 0.25, 0.5, 1, 2, 4, 8, and 24 h after the oral administration by puncturing the jugular vein and collecting blood into polypropylene tubes containing ethylenediaminetetraacetic acid (EDTA)-K2. Plasma samples were separated by centrifugation and stored at −20°C until analysis. In other groups of rats, brains were harvested after euthanasia by CO2 inhalation at 2, 4, and 8 h following oral administration, homogenized with five-fold volume of phosphate buffer saline (PBS) (pH 7.4), and stored at −20°C until analysis. Plasma and brain HM01 concentrations were determined with liquid chromatography tandem mass spectrometry (LC-MS/MS) (supporting information).
Twenty-four hours basal food and water intake, and fecal output
Rats housed individually were given pre-weighed food and water bottle with a ballpoint sipper tube to avoid spillage. Twenty-four hours later, food and water bottle were weighed and the amount consumed was calculated. At the same time, 24 h basal fecal pellets were counted and weighed.
Four-hour fecal output and fecal water content without access to food and water
Each rat was placed in new individual cage with a wire grid at the cage bottom without food and at 1, 2, and 4 h later, fecal pellets were collected and weighed. Feces were dried in an oven for 24 h and weighed. Water content of the feces was calculated by following equation: .
Gastric emptying
Gastric emptying of a non-nutrient viscous liquid was measured using the acetaminophen (N-acetyl-p-aminophenol) method as described in the supportive information.
Plasma total- and acyl-ghrelin levels
Blood samples were collected from the tail vein using EDTA-treated capillaries and immediately transferred to ice-cold tube containing serine proteinase inhibitor (Pefabloc SC, 1 mg mL−1 blood) and centrifuged. Then, plasma was acidified with HCl (0.05 M, final concentration) and stored in −80°C until assay. Plasma total- and acyl-ghrelin levels were determined using specific ELISA kits (EZRGRT-91K and EZRGRA-90K, respectively, Millipore, Billerica, MA).
Immunohistochemistry
Brain and spinal cord tissue preparation, processing of free-floating sections for Fos immunohistochemistry and quantitative assessment of positive cells were as detailed in our previous report (28) and supportive information.
Experimental protocols
Basal body weight, and 24-h food and water intake, and fecal output
Twenty-four hour food and water intake, fecal output and body weight were monitored in rats 3–4 weeks after the microinjection of 6-OHDA or vehicle (control) into the mfb.
Effects of og HM01 on fecal output and water content in 6-OHDA rats
Experiments were performed in rats microinjected with 6-OHDA or vehicle 5–8 weeks before. Non-fasted rats received an og administration of vehicle or HM01 (1, 3, and 10 mg kg−1) and fecal pellets were collected at 1, 2 and 4 h thereafter. Rats had no access to water and food during the 4-h experimental period. Fecal output was expressed as 4-h cumulative wet weight and water content. HM01 at the 3 mg kg−1 was found to be the maximal effective dose and selected for all subsequent studies.
Effects of acute administration of HM01 on delayed gastric emptying induced by LD/CD in 6-OHDA rats
Experiments were performed at 5–8 weeks after microinjection of 6-OHDA or vehicle in rats. First, basal gastric emptying was determined in 6-OHDA and control rats fasted overnight, gavaged with acetaminophen and blood collected over 2.5 h to determine plasma acetaminophen levels. One week after, the 6-OHDA and control rats were fasted overnight and pretreated with either vehicle or HM01 (3 mg kg−1, og) and 30 min later with LD/CD (20/2 mg kg−1, og). Ten minutes after LD/CD treatment, rats were gavaged with acetaminophen and gastric emptying was measured over 1 h based on the first study showing maximal plasma levels of acetaminophen at that time. The selected LD/CD doses were based on our previous experiment (14) and a pilot study.
Effects of one-week daily administration of HM01 and LD/CD on food and water intake, body weight and gastric emptying in 6-OHDA rats
Rats were randomly assigned for different treatment groups. Experiments were performed with crossover design with 1-week washout period at 2–3 and 5–6 months after 6-OHDA or vehicle microinjection. 6-OHDA and control rats received oral gavage of either vehicle or HM01 (3 mg kg−1) and 30 min later that of LD/CD (20/2 mg kg−1) every day for one week. Food/water intake and body weight were monitored every day. Then on day 7, all rats were fasted overnight. On day 8, vehicle or HM01 (3 mg kg−1) was gavaged to rats 30 min before gavage of LD/CD (20/2 mg kg−1), and 10 min later viscous liquid containing acetaminophen was gavaged to measure gastric emptying.
Plasma total- and acyl-ghrelin levels in 6-OHDA rats
Blood (150 μL) was collected from the tail vein before and after an overnight fasting in 6-OHDA and control rats at 7 weeks after microinjection into the mfb. Samples were processed for the measurement of plasma total and acyl-ghrelin levels.
Effects of HM01 on c-Fos expression in the brain and spinal cord in 6-OHDA rats
At 4 months after the microinjection of 6-OHDA or vehicle, non-fasted rats were treated with vehicle or HM01 (3 mg kg−1, og) and perfused 2 h later for brain and L6–S1 collection and processed for Fos immunoreactivity. Food and water were removed after the treatment to avoid influence of differences in food intake on Fos expression.
Statistical analysis
Data are shown as mean ± SEM. AUC for plasma acetaminophen levels was calculated using trapezoid rule. Comparison between two groups was done by Student’s or Welch’s t-test, following the F-test. Comparison between multiple groups was performed by two-way or three-way ANOVA followed by Tukey post hoc multiple comparisons. A P values < 0.05 was considered significant.
Results
6-OHDA rats show constipation- and adipsia-like symptoms
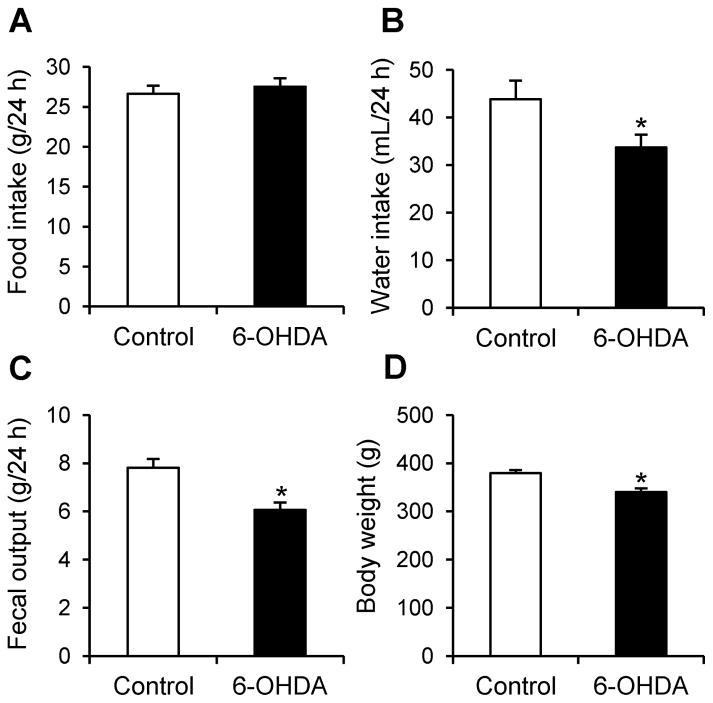
Four weeks after microinjection of 6-OHDA unilaterally in the mfb, rats showed no change in 24-h food intake compared to vehicle microinjected rats (27.5 ± 1.0 g vs. 26.6 ± 1.0 g, P = 0.57; Fig. 1A) while there were significant reductions in the 24-h water intake (33.7 ± 2.7 mL vs. 43.8 ± 3.9 mL, P < 0.05, Fig. 1B) and 24-h fecal output weight (6.1 ± 0.3 g vs. 7.8 ± 0.4 g, P < 0.01; Fig. 1C). However, the number of daily fecal pellets did not show difference between 6-OHDA and control rats (42.2 ± 1.5 vs. 42.5 ± 1.6 pellets). Body weight of 6-OHDA rats was lower than that of control group (340.4 ± 7.5 g vs. 379.4 ± 6.2 g, P < 0.01, Fig. 1D). There was a correlation between water intake and body weight (r2=0.29, P < 0.05) or fecal output weight (r2=0.27, P < 0.05), and the absence of correlation between body weight and food intake (r2=0.08, P > 0.05) or fecal output weight (r2=0.17, P > 0.05), and food intake and fecal output weight (r2=0.06, P > 0.05) or water intake (r2=0.00, P > 0.05).
Figure 1.
Body weight, daily water intake and fecal weight were reduced in 6-OHDA rats 3-4 weeks after microinjection. Basal 24-h food intake (A), water intake (B), fecal weight (C) and body weight (D) were measured in a home cage. Data are mean ± SEM of 7 and 10 rats for control and 6-OHDA rats, respectively. *P < 0.05 vs. control.
Pharmacological profile of HM01
HM01 showed high binding affinity to human GHS-R1a (Ki, 1.42 ± 0.36 nM) and induced potent activation of intracellular calcium signaling (EC50, 1.25 ± 0.15 nM; Table 1). Bioavailability and half-life of HM01 after oral gavage (3 mg kg−1) in rats was 70.6% and 4.3 h, respectively. The ratio of brain to plasma concentration of HM01 at 2, 4, and 8 h after oral gavage of HM01 (3 mg kg−1,) was 0.73, 0.80 and 0.67, respectively (Table 1), indicative of the BBB penetration with potential actions in the central nervous system.
Table 1.
Pharmacological profile of HM01
| Determination | Values |
|---|---|
| hGHS-R1a binding, Ki | 1.42 ± 0.36 nM |
| hGHS-R1a calcium signaling, EC50 | 1.25 ± 0.15 nM |
| Bioavailability (rat) | 70.6 ± 26.9% |
| Half-life in plasma (rat) | 4.3** ± 1.0 h |
| CNS permeability, Kp brain/plasma (rat) | 0.73 ± 0.14 ( at 2 h) 0.80 ± 0.23 (at 4 h) 0.67* (at 8 h) |
: n = 1
: Half-life calculated after HM01 oral administration
Kp: ratio of brain and plasma concentrations
Acute orogastric administration of HM01 reverses reduced fecal output and water content in 6-OHDA rats
To avoid the potential influence of HM01 in food and water intake, the fecal output and water content were assessed for 4 h in 6-OHDA and control rats without access to food and water. In control rats, HM01 (1, 3 and 10 mg kg−1, og) induced a similar increase in the fecal output weight during the first 1 h post treatment (1.09 ± 0.31 g, 1.32 ± 0.22 g, and 1.12 ± 0.15 g, respectively vs. 0.16 ± 0.11 g vehicle, n=5–7 per group, all P < 0.05), while there was no effect on 2- and 4-h cumulative weight (Fig. 2A). In 6-OHDA rats treated with og vehicle, the cumulative weight of fecal output was significantly smaller than that of control rats at 2 and 4 h (0.18 ± 0.10 g vs. 0.78 ± 0.24 g and 0.53 ± 0.20 g vs. 1.49 ± 0.2 g, respectively, both P < 0.05; Fig. 2A). In 6-OHDA rats, HM01 (1, 3, and 10 mg kg−1, og) significantly increased the fecal output compared to og vehicle reaching its maximal effect at 3 mg kg−1 (1.38 ± 0.39 g vs. 0.13 ± 0.09 g, 1.48 ± 0.38 g vs. 0.18 ± 0.10 g, and 1.60 ± 0.35 g vs. 0.53 ± 0.20 g, respectively at 1, 2, and 4 h after treatment, all P < 0.05). The highest dose (10 mg kg−1) showed similar stimulatory effect as that of 3 mg kg−1 while 1 mg kg−1 had no significant effect (Fig. 2A). Two-way ANOVA on fecal weight at each time point showed a significant influence of HM01 at 1 h (F3,44 = 13.9, P < 0.001) and at 2 h (F3,44 = 8.7, P < 0.001), and a significant interaction of 6-OHDA × HM01 (F3,44 = 3.8, P < 0.05) at 4 h.
Figure 2.
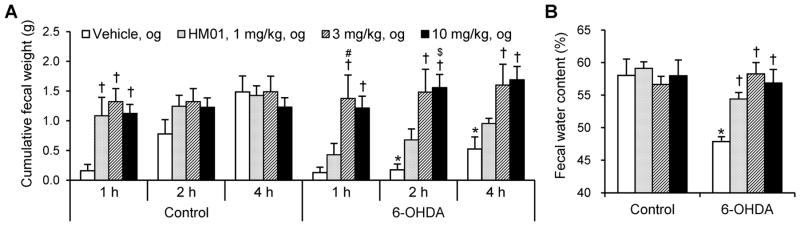
HM01 increased fecal weight and water content 4 h after the treatment. Non-fasted rats were gavaged with vehicle or HM01 (1, 3, and 10 mg kg−1) and defecation were monitored for 4 h without food and water. (A) Fecal weight. (B) Fecal water content. Data are mean + SEM of 5–7 rats per each group. *P < 0.05 6-OHDA + og vehicle vs. control + og vehicle at each time point. †P < 0.05 vs. each vehicle at each time point. #P < 0.05 HM01, 3 mg kg−1 vs. HM01, 1 mg kg−1. $P < 0.05 HM01 10 mg kg−1 vs. HM01 1 mg kg−1.
The 6-OHDA rats treated with og vehicle had significantly lower water content in the 4-h fecal output compared to control rats (48 ± 1% vs. 58 ± 2%, P < 0.01, Fig. 2B). HM01 (1, 3, and 10 mg kg−1, og) which had no effect on fecal water content in control rats, reversed the reduction observed in 6-OHDA rats bringing water content values to those of control (54 ± 2%, 58 ± 2%, and 57 ± 2% respectively, P < 0.05, 0.01, and 0.01, respectively; Fig. 2B). Two-way ANOVA showed a significant influence of 6-OHDA (F1,42 = 6.8, P < 0.05) and 6-OHDA × HM01 (F3,42 = 3.7, P < 0.05) on fecal water content.
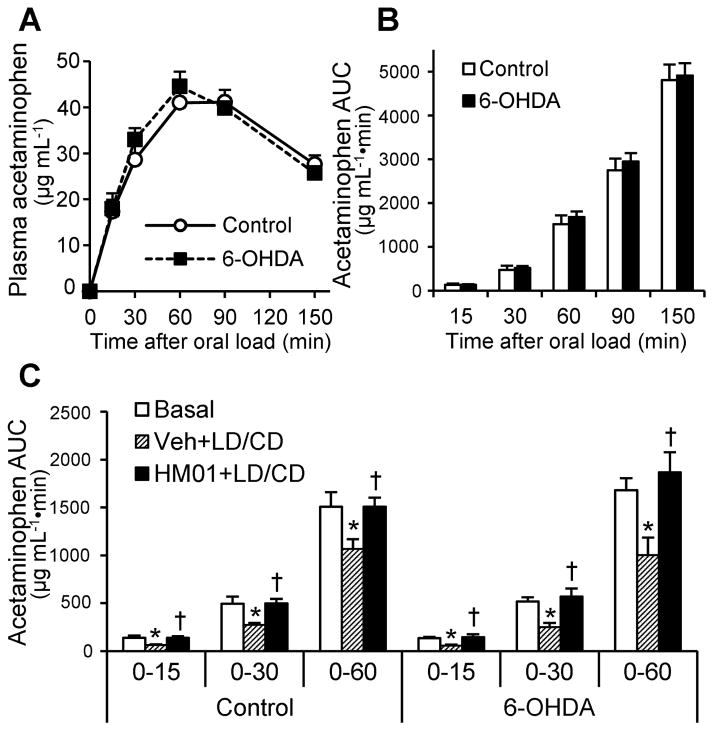
HM01 prevents the reduction of gastric emptying acutely induced by LD/CD both in 6-OHDA and control rats
Basal gastric emptying assessed by the acetaminophen method was similar between 6-OHDA and control rats (135 ± 14 vs. 130 ± 30, 517 ± 46 vs. 474 ± 94, 1680 ± 125 vs. 1518 ± 200, 2944 ± 196 vs. 2749 ± 263, and 4909 ± 284 vs. 4810 ± 351 μg mL−1•min, assessed as AUC at 15, 30, 60, 90, and 150 min respectively; Fig. 3A, B). Single treatment of LD/CD (20/2 mg kg−1, og) reduced gastric emptying significantly and similarly in 6-OHDA to 43%, 49%, and 60% and control rats to 46%, 55%, and 71% of basal level at 0–15, 0–30, and 0–60 min respectively (all P < 0.05, Fig. 3C) assessed as AUC of plasma acetaminophen levels. Pretreatment with HM01 (3 mg kg−1, og) 30 min prior to LD/CD (20/2 mg/kg, og) prevented LD/CD-induced reduction of gastric emptying with similar values reaching 109%, 110%, 111% of basal level in 6-OHDA (all P < 0.05) and 98%, 100%, and 100% of basal level in control rats (all P < 0.05) at the 0–15, 0–30 or 0–60 min time periods respectively. Two-way ANOVA showed a significant influence of HM01 (F2,48 = 11.0, P < 0.001) not 6-OHDA (F1,48 = 1.6, P = 0.21) on gastric emptying assessed as AUC of acetaminophen up to 60 min.
Figure 3.
HM01 prevented acute LD/CD-induced delayed gastric emptying. First, basal gastric emptying in 6-OHDA and control rats was examined without drug treatment. Viscous liquid (1.0 mL) containing acetaminophen (100 mg kg−1) was gavaged to overnight-fasted rats and plasma acetaminophen levels were monitored (A). Gastric emptying assessed as AUCs of plasma acetaminophen levels was comparable between two groups (B). Data are mean ± SEM of 7 and 9 rats for control and 6-OHDA rats, respectively. Next, vehicle or HM01 (3 mg kg−1) was gavaged to overnight-fasted rats 30 min before gavage of LD/CD (20/2 mg kg−1), and 10 min later viscous liquid containing acetaminophen was gavaged to measure gastric emptying (C). Data are mean ± SEM of 7 and 9 rats for control and 6-OHDA rats, respectively. *P < 0.05 Veh+LD/CD vs. basal; †P < 0.05 HM01+LD/CD vs. Veh+LD/CD at each time point of each group.
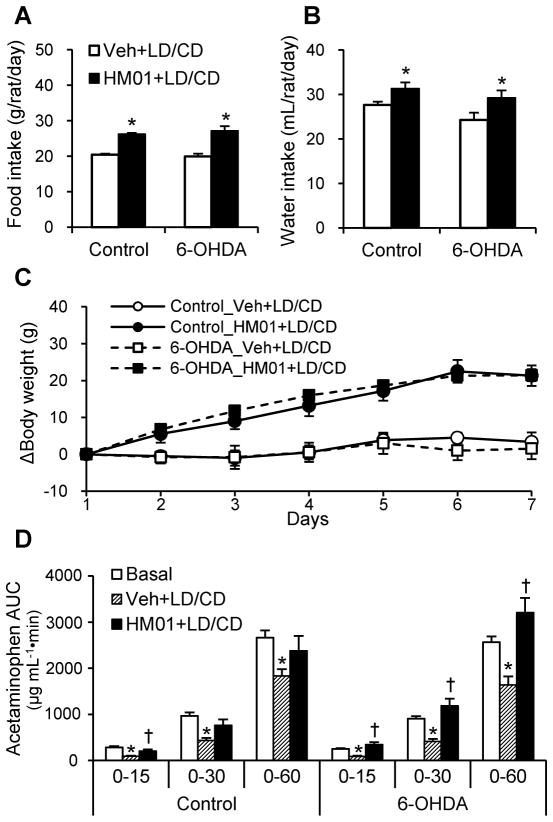
HM01 promotes food and water intake, gastric emptying and body weight gain after the 1-week daily administration in 6-OHDA rats treated with LD/CD
Both 6-OHDA and control rats receiving og administration of vehicle followed by that of LD/CD (20/2 mg kg−1) 30 min later daily for one week had similar daily food consumption, water intake and change in body weight (Fig. 4A–C). HM01 (3 mg/kg) administered og for one week increased similarly the daily food intake in 6-OHDA and control rats compared to daily og vehicle plus LD/CD (27.1 ± 1.3 g vs. 20.0 ± 0.7 g and 26.2 ± 0.3 g vs. 20.5 ± 0.3 g, respectively, both P < 0.01; Fig. 4A and B), as well as the body weight gain (21.4 ± 1.6 vs. 1.6 ± 2.8 g and 21.3 ± 2.8 vs. 3.3 ± 2.6 g; Fig. 4C). HM01 daily og treatment for one week also increased the daily water intake compared with og vehicle in 6-OHDA and control rats (29.2 ± 1.6 mL vs. 24.3 ± 1.6 and 31.3 ± 1.4 vs. 27.6 ± 0.7 mL, both P < 0.05 vs. vehicle, respectively, Fig. 4B).
Figure 4.
Effects of 1-week treatment of HM01 combined with LD/CD on food and water intake, body weight and gastric emptying. Rats received 1-week repeated og administration of vehicle or HM01 (3 mg kg−1) combined with LD/CD (20/2 mg kg−1). (A) Average food intake and (B) water intake during 1-week repeated administration. (C) Body weight changes during 1-week treatment. Data are mean ± SEM of n = 6 in control and n = 9 in 6-OHDA groups. *P < 0.05 vs. each Veh+LD/CD. On Day 8, vehicle or HM01 (3 mg kg−1) was gavaged to overnight-fasted rats 30 min before gavage of LD/CD (20/2 mg kg−1), and 10 min later viscous liquid containing acetaminophen was gavaged to measure gastric emptying (D). Data was analyzed comparing with basal gastric emptying measured earlier in the same animals. *P < 0.05 Veh+LD/CD vs. basal, †P < 0.05 HM01+LD/CD vs. Veh+LD/CD at each time point of each group.
After 1-week daily og vehicle plus LD/CD administration, gastric emptying assessed as AUC at 15, 30, and 60 min was significantly reduced to 35%, 45%, and 64% of basal level in 6-OHDA rats and similarly to 31%, 45%, and 69% of basal level in control rats respectively (Fig. 4D). HM01 after one week treatment still significantly restored gastric emptying values to those of basal at each time point in both 6-OHDA and control rats treated with LD/CD (Fig. 4D). Two-way ANOVA showed a significant influence of HM01 (F2,39 = 11.7, P < 0.001) and no effect of 6-OHDA (F1,39 = 0.9, P = 0.35) and HM01 × 6-OHDA (F2,39 = 2.9, P = 0.07) compared to control group on gastric emptying assessed as AUC of acetaminophen up to 60 min.
6-OHDA rats showed no change in plasma ghrelin levels
To examine the possible alterations in endogenous ghrelin levels in 6-OHDA rats, total- and acyl-ghrelin plasma levels were compared to control rats in non-fasted and overnight-fasted conditions. There was no statistically significant difference between the two groups in both conditions, while overnight fasting increased significantly plasma total- and acyl-ghrelin levels respectively by 91.4% and 64.9% in 6-OHDA and similarly by 91.4% and 81.8% in control rats (Table 2).
Table 2.
Total- and acyl-ghrelin plasma levels
| Treatment | Total ghrelin (ng mL−1)
|
Acyl-ghrelin (ng mL−1)
|
||
|---|---|---|---|---|
| Non-fasted | Fasted | Non-fasted | Fasted | |
| Control (n = 7) | 1.51 ± 0.11 | 2.89 ± 0.34* | 0.55 ± 0.04 | 1.00 ± 0.08* |
| 6-OHDA (n = 8–9) | 1.39 ± 0.11 | 2.66 ± 0.16* | 0.57 ± 0.06 | 0.94 ± 0.05* |
: P < 0.05 vs. respective non-fasted groups. No statistically significant difference between 6-OHDA and control groups.
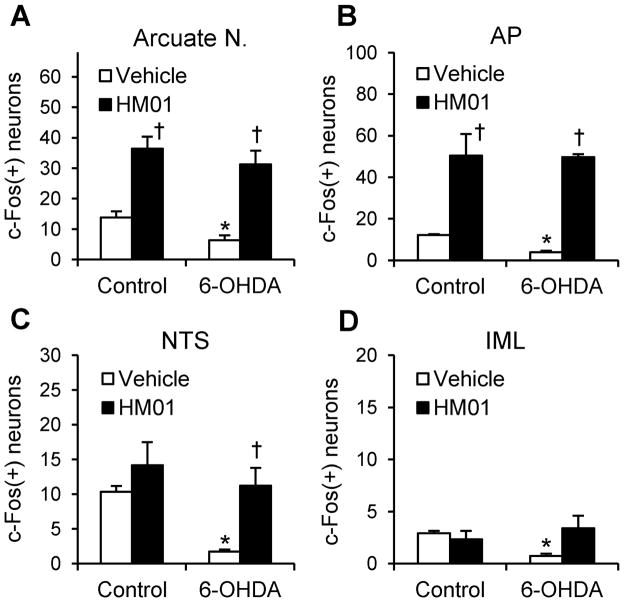
HM01 increases Fos immunoreactivity in selective brain areas of 6-OHDA and control rats
In og vehicle treated control rats, the numbers of c-Fos-immunoreactive (ir) neurons were low in numbers. However, Fos-ir neurons were significantly fewer in 6-OHDA rats (n = 4) than in controls (n = 3) namely in the arcuate nucleus (6.3 ± 1.7 vs. 13.9 ± 2.0 cells per section, P < 0.05), area postrema (AP) (3.9 ± 0.7 vs. 12.2 ± 0.4 cells per section, P < 0.05), nucleus tractus solitarius (NTS) (1.7 ± 0.3 vs. 10.4 ± 0.8 cells per section, P < 0.05) and intermediolateral column (IML) of the lumbosacral spinal cord (0.7 ± 0.2 vs. 2.9 ± 0.2 cells per section, P < 0.05; Figs. 5A–D and S1–4). HM01 (3 mg kg−1, og) increased similarly the number of c-Fos-ir (cells/section) in both 6-OHDA (n = 4) and control (n = 4) rats in the arcuate nucleus (31.2 ± 4.5 and 36.4 ± 4.0, P < 0.05 vs. og vehicle; Figs. 5A and S1), and AP (49.7 ± 1.4 and 50.4 ± 10.4; P < 0.01 and 0.05 vs. og vehicle; Figs. 5B and S2). In the NTS, HM01 increased Fos-ir in the 6-OHDA rats compared to the basal that was very low (11.3 ± 2.6 vs. 1.7 ± 0.3 cells per section, P < 0.05), but not in control rats (Figs. 5C and S2). In the IML of lumbosacral spinal cord L6-S1, HM01 showed a trend, not significant, to restore the decreased Fos positive neurons in 6-OHDA rats (3.4 ± 1.2 vs. 0.7 ± 0.2 cells/section by vehicle, P = 0.053, n = 4/group), while no difference was observed in control rats (2.3 ± 0.8 vs. 2.9 ± 0.2 cells/section; Figs. 5D and S3). In 6-OHDA rats, the c-Fos expression induced by og HM01 in the arcuate nucleus, AP, NTS and IML were 4.9, 12.9, 6.6 and 4.9 folds higher respectively than those in og vehicle. There was no increase in Fos immunoreactivity in the paraventricular nucleus of the hypothalamus after og HM01 (Fig. S4). The TH immunostaining showed that all rats injected unilaterally with 6-OHDA into the mfb lost most of dopaminergic neurons in the striatum and substantia nigra in the injected side (Supporting information, Fig. S5).
Figure 5.
Cells counts of HM01 induced c-Fos expression in the selective brain and spinal cord areas of 6-OHDA and control rats. Non-fasted rats treated with og administration of vehicle or HM01 (3 mg kg−1) were sacrificed 2 h later for immunohistochemical analysis. Positive neurons for c-Fos were counted in the arcuate nucleus (A), AP (B), NTS (C), and IML of lumbrosacral the spinal cord (D). Data are mean ± SEM of n = 3–4 rats per group. *P < 0.05 6-OHDA_Vehicle vs. Control_Vehicle; †P < 0.05 HM01 vs. each Vehicle. AP, area postrema; IML, intermediolateral column; NTS: nucleus of tractus solitarius.
Discussion
In the present study, the microinjection of 6-OHDA unilaterally in the mfb reduced significantly the 24-h fecal weight and water intake by 22% and 23% respectively while not altering food intake, and body weight was lowered by 10% compared to control rats as monitored 3–4 weeks after the lesion. These results are consistent with previous report showing a reduction of daily fecal output with unchanged food intake in rats at 3–4 weeks after 6-OHDA unilateral injection into the mfb (23). The reduction was in fecal weight without change in numbers of fecal pellets. When 6-OHDA rats had no access to food and water for a 4-h experimental period, we observed a 64% reduction in the weight of fecal output and the fecal water content was reduced by 17% compared to controls. Another studies also showed that unilateral injection of 6-OHDA into the substantia nigra reduced both the weight and water content of feces monitored for 1 h (22). Collectively, these data indicate that 6-OHDA models display PD constipation-like feature not secondary to decreased gut content linked to differences in food intake.
The reduced defecation is likely to represent a PD-associated alteration of colonic motor function. Supportively, we observed that Fos expression was significantly lower in the lumbosacral IML of 6-OHDA than control rats. This may indicate a decreased sacral parasympathetic outflow regulating colonic motor function. In addition, supraspinal mechanisms related to decreased brain DA neuronal activity may also contribute. There is pharmacological evidence that icv injection of D1 or D2 agonists in rats increased colonic contractions (29). At the enteric level, neuronal and functional alterations have been reported in 6-OHDA rats. Those include increased vasoactive intestinal peptide immunoreactivity and decreased neuronal nitric oxide synthase in the distal ileum and proximal colon associated with a reduced propulsive efficiency, uncoordinated peristalsis, smaller magnitude of contractions of the colonic longitudinal muscles monitored ex vivo (23, 30), and decreased cecorectal transit displayed in vivo by radiological imaging (31) in rats. The dryer fecal content in 6-OHDA rats is likely related to slower intestinal transit allowing enhanced water absorption of fecal content and/or to reduced water intake.
Indeed, we also observed a reduction of 24-h water intake in rats 3–4 weeks after unilateral microinjection of 6-OHDA into the mfb which is not correlated to food intake, as the 6-OHDA rats ate similarly as control rats. One previous study showed also that water intake was reduced at different time points after unilateral 6-OHDA lesion in rats (23). Although little studied, one clinical report showed that PD patients have reduced water intake that is accompanied with occurrence of constipation (32). Supportively, D2 receptor activation in the striatum increased water intake in rats (33). Based on these observations, the 6-OHDA lesioned rats by unilateral injection in the mfb provide a relevant experimental model to investigate brain signaling circuits regulating water intake linked with the dopaminergic system, which might be involved in mechanisms in constipation.
Delayed gastric emptying is a common upper GI dysfunction in PD patients (1, 34). However, only a few animal models of PD show delayed gastric emptying, namely rats microinjected bilaterally or unilaterally with 6-OHDA into the substantia nigra (21, 22) and rats chronically treated with rotenone via a subcutaneous minipump (35). A newly published study in unilaterally injected 6-OHDA rats into the mfb reported no change in gastric emptying at 4 weeks, while at 8 weeks a delay was observed (31). The PD model used in this study with unilateral injection of 6-OHDA in the mfb did not reduce gastric emptying in 4–8 weeks after 6-OHDA injection. The reason for the difference is not clear and could involve different methods and our use of desipramine before the microinjection to prevent the uptake of 6-OHDA into noradrenergic neurons (27). We further demonstrated that L-dopa combined with carbidopa inhibited gastric emptying similarly in 6-OHDA and control rats, consistent with clinical studies in PD patients (36), healthy human volunteers (4) and naïve rats (37).
To test the influence of ghrelin in this model, we used the newly developed ghrelin agonist, HM01 that displays high affinity to the human GHS-R1a (Ki, 1.42 ± 0.36 nM), long half-life (4.3 h), oral bioavailability and brain penetrance. There are a few other ghrelin agonists crossing the BBB (16, 38). HM01 has similar brain/blood ratio as GSK894281 (about 0.7), but longer half-life (4.3 vs. 3.4 h) (16). Ulimorelin (TZP-101) has a very long half-life in humans (13 h) (39, 40), while being short in rats (1.5 h) (41). The anti-constipation effect of ulimorelin suggests that it may cross the BBB (13, 17). However, it was reported to have low brain penetration in rats (41), and it could not be administered orally (17, 39, 40, 42). Some BBB-crossing ghrelin agonists desensitize readily, such as GSK894281 and CP464709 (17, 38, 43, 44), while in the present study, HM01 is still effective in vivo after one week of daily treatment.
We found that oral administration of HM01 either acutely or daily for one week prevented L-dopa-induced delayed gastric transit in both control and 6-OHDA rats. Similarly in our previous report, a bolus iv injection of ghrelin normalized gastric emptying delayed by oral L-dopa in rats (14). These data are indicative that activation of GHS-R1a counteracts the alterations of gastric transit induced by L-dopa and that there is no drug tolerance to repeated oral administrations of HM01. Previous in vivo and in vitro studies delineated that ghrelin’s gastroprokinetic effect is neurally mediated through GHS-R1a expressed on the nodose ganglion neurons (45) and/or enteric neurons (46). Based on its high affinity to ghrelin receptor, HM01 may act via the vagal afferent to signal the brain. However, in the NTS known to receive afferent terminals of the gastric vagal innervation, we found only a small-number of Fos-ir cells in 6-OHDA rats treated with HM01 compared to almost none by vehicle. In contrast, there was a 12.7 fold increase in the number of Fos positive cells in the AP where GHS-R1a are strongly expressed (47). This points to HM01 possible action through brainstem circuits regulating gastric function via the AP. The lack of Fos expression in dorsal motor nucleus neurons known to express GHS-R1a (47) also ruled out a direct action at this site. Further studies are needed to delineate whether HM01 action is primarily mediated through a direct activation of enteric and/or brainstem neurons.
We also showed that oral administration of HM01 stimulated defecation in controls and 6-OHDA PD model with a constipation-like feature. Previous studies demonstrated that peripherally administered ghrelin did not influence colonic transit (48, 49). By contrast, intrathecal administration of ghrelin, or ghrelin agonists, capromorelin, CP464709 and GSK894281 that cross the BBB, or ulimorelin that acts on the pelvic nerves increased colorectal propulsive activity and expulsion of colonic contents in naïve rats and stimulated defecation in spinal cord injured rats (15, 16, 50). The pharmacokinetic studies showed that 2 h after oral administration of HM01, the brain/plasma ratio was 0.73, suggesting that the ghrelin agonist may penetrate the blood-spinal cord barrier and activates GHS-R1a located in the spinal cord (51) to stimulate colonic propulsive motor function. This is supported also by our data that HM01 increased fecal output and showed a trend to enhance Fos expression in the lumbosacral spinal cord which is lower in 6-OHDA than control rats. Furthermore, HM01 may act on supraspinal areas that regulate colonic functions, such as the arcuate nucleus (52). Previous studies showed that activation of arcuate nucleus neurons by microinjection of kainic acid at this site resulted in the stimulation of colonic motility in rats (52). It is also well established that ghrelin or ghrelin agonists administrated peripherally activates neuropeptide (NPY) neurons in the arcuate nucleus in rodents (53, 54). Other study showed that NPY contributes to ghrelin injected into the PVN-induced stimulatory action on colonic motility (55). Since we did not observe activation of PVN neurons (Fig. S5) while Fos positive cells increased by 5.0 fold in the arcuate nucleus, these data suggest that hypothalamic site of HM01 action to stimulate colonic motility may be primarily initiated in the arcuate nucleus that bears NPY neurons projecting to the PVN. So far, there is a paucity of studies on Fos expression in the brain or spinal cord induced by recently developed stable ghrelin agonists compared to the native peptide ghrelin. However, earlier studies using one of GHSs – growth hormone-releasing hexapeptide also showed Fos induction in the arcuate nucleus of rats (56, 57).
HM01 also normalized the fecal water content reduced in 6-OHDA lesioned rats. This is unlikely to reflect a pro-secretory effect of HM01. First, to the best of our knowledge, there is no report on that ghrelin or agonists increase intestinal secretion. Second, in control rats, the percentage of fecal output fluid content was similar in HM01 and vehicle treated groups, indicative that the ghrelin agonist did not increase intestinal secretion. Lastly, the decreased fecal water content in 6-OHDA rats was normalized by HM01 treatment to the similar percentage as in controls, which is likely to reflect lesser fluid absorption resulted from decreased intestinal transit time.
Beside its GI prokinetic actions, HM01 given orally for one week exerts anabolic effect by increasing similarly food and water intake in vehicle and 6-OHDA rats and preventing the body weight lost occurring in the PD rat model. Unintended weight loss is common in PD patients and is considered as a risk factor for worsening the prognosis of the disease (58). HM01 effects may be mediated by the activation of NPY neurons in the arcuate nucleus as reported with ghrelin (53, 59). Those neurons are well established to integrate signals for food intake and body weight gain (60). In addition, drinking and feeding are closely related behaviors (61, 62), and it is likely that oral HM01 induced increased water intake in 6-OHDA rats is secondary to the stimulation of food intake rather than an independent action. Indeed, ghrelin inhibits water intake in dehydrated rats monitored in the absence of food (63).
In conclusion, our study established that the novel orally active and brain penetrant ghrelin agonist, HM01, ameliorates the GI dysfunctions in PD model induced by unilateral 6-OHDA injection into the mfb in rats treated with LD/CD. HM01 reversed the constipation-like alterations by stimulating fecal output and normalizing the fecal water content. HM01 under conditions of acute or repeated treatment prevented the delay of gastric emptying induced by acute or chronic LD/CD. These data suggest the potential use of HM01 to treat both upper and lower GI symptoms that developed with PD and L-dopa treatment. In addition to improve the GI dysfunction, we showed that HM01 might have benefits to keep an adequate nutritional state and body weight. To our knowledge, this is the first preclinical report in a PD rat model showing the therapeutic potential of a ghrelin agonist.
Supplementary Material
Key massages.
The new orally active ghrelin agonist, HM01 that crosses the blood-brain barrier may have potential therapeutic benefit to alleviate alterations of gastrointestinal motor function associated with Parkinson’s disease (PD) when tested in a rat chemical model of PD.
The study aimed to investigate in a rat PD model whether orally administered HM01 influenced constipation and the delayed gastric emptying induced by the standard PD medication, L-dopa/carbidopa.
The rat PD model was induced by microinjection of 6-hydroxydopamine (6-OHDA) unilaterally into the rat medial forebrain bundle and HM01 was administered by orogastric gavage.
HM01 counteracted the reduced fecal output and water content, and L-dopa/carbidopa-inhibited gastric emptying in 6-OHDA rats.
HM01 increased Fos expression in selective brain and spinal cord areas regulating gastrointestinal function that were reduced in 6-OHDA rats.
Acknowledgments
This work was supported by NIHDDK-41303 (Animal core, YT, LW), Fox Foundation Target Validation Grant (LW, YT), and Veterans Administration Research Career Scientist Award (YT). We are grateful to Mrs. Honghui Liang for excellent technical support.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- ABC
avidin-biotin-peroxidase complex
- AP
area postrema
- AUC
area under the curve
- BBB
blood-brain barrier
- DMSO
dimethylsulfoxide
- EDTA
ethylenediaminetetraacetic acid
- ir
immunoreactivity or immunoreactive
- iv
intravenous or intravenously
- ED
effective dose
- GE
gastric emptying
- GHS-R
growth hormone secretagogue receptor
- GI
gastrointestinal
- IML
intermediolateral column
- LD/CD
L-dopa/carbidopa
- mfb
medial forebrain bundle
- NPY
neuropeptide Y
- NTS
nucleus tractus solitarius
- og
orogastric or orogastrically
- PD
Parkinson’s disease
- PVN
paraventricular nucleus of the hypothalamus
- TH
tyrosine hydroxylase
Footnotes
Authorship contributions
Conceived and designed the experiments: YT, LW. Performed the experiments: HK, XX, CG, SY, LW. Analyzed the data: HK, CP, XX, YT, LW. Contributed reagents/materials/analysis tools: CP, CG, SGR. Wrote the paper: HK, YT, LW. Reviewed the paper: CP, CG, SGR, SY.
References
- 1.Jost WH. Gastrointestinal dysfunction in Parkinson’s Disease. J Neurol Sci. 2010;289:69–73. doi: 10.1016/j.jns.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Sakakibara R, Kishi M, Ogawa E, et al. Bladder, bowel, and sexual dysfunction in Parkinson’s disease. Parkinsons Dis. 2011;2011:924605. doi: 10.4061/2011/924605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poewe W, Antonini A, Zijlmans JC, Burkhard PR, Vingerhoets F. Levodopa in the treatment of Parkinson’s disease: an old drug still going strong. Clin Interv Aging. 2010;5:229–238. doi: 10.2147/cia.s6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson DR, Renwick AG, Macklin B, et al. The influence of levodopa on gastric emptying in healthy elderly volunteers. Eur J Clin Pharmacol. 1992;42:409–412. doi: 10.1007/BF00280127. [DOI] [PubMed] [Google Scholar]
- 5.Kurlan R, Rothfield KP, Woodward WR, et al. Erratic gastric emptying of levodopa may cause “random” fluctuations of parkinsonian mobility. Neurology. 1988;38:419–421. doi: 10.1212/wnl.38.3.419. [DOI] [PubMed] [Google Scholar]
- 6.Meissner WG, Frasier M, Gasser T, et al. Priorities in Parkinson’s disease research. Nat Rev Drug Discov. 2011;10:377–393. doi: 10.1038/nrd3430. [DOI] [PubMed] [Google Scholar]
- 7.Mizutani M, Atsuchi K, Asakawa A, et al. Localization of acyl ghrelin- and des-acyl ghrelin-immunoreactive cells in the rat stomach and their responses to intragastric pH. Am J Physiol Gastrointest Liver Physiol. 2009;297:G974–G980. doi: 10.1152/ajpgi.00147.2009. [DOI] [PubMed] [Google Scholar]
- 8.Gahete MD, Rincon-Fernandez D, Villa-Osaba A, et al. Ghrelin gene products, receptors, and GOAT enzyme: biological and pathophysiological insight. J Endocrinol. 2014;220:R1–24. doi: 10.1530/JOE-13-0391. [DOI] [PubMed] [Google Scholar]
- 9.Trudel L, Tomasetto C, Rio MC, et al. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G948–G952. doi: 10.1152/ajpgi.00339.2001. [DOI] [PubMed] [Google Scholar]
- 10.Kitazawa T, De Smet B, Verbeke K, Depoortere I, Peeters TL. Gastric motor effects of peptide and non-peptide ghrelin agonists in mice in vivo and in vitro. Gut. 2005;54:1078–1084. doi: 10.1136/gut.2005.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin F, Edholm T, Schmidt PT, et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab. 2006;91:3296–3302. doi: 10.1210/jc.2005-2638. [DOI] [PubMed] [Google Scholar]
- 12.Avau B, Carbone F, Tack J, Depoortere I. Ghrelin signaling in the gut, its physiological properties, and therapeutic potential. Neurogastroenterol Motil. 2013;25:720–732. doi: 10.1111/nmo.12193. [DOI] [PubMed] [Google Scholar]
- 13.Ejskjaer N, Vestergaard ET, Hellstrom PM, et al. Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis. Aliment Pharmacol Ther. 2009;29:1179–1187. doi: 10.1111/j.1365-2036.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Murphy NP, Stengel A, et al. Ghrelin prevents levodopa-induced inhibition of gastric emptying and increases circulating levodopa in fasted rats. Neurogastroenterol Motil. 2012;24:e235–e245. doi: 10.1111/j.1365-2982.2012.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu Y, Chang EC, Shafton AD, et al. Evidence that stimulation of ghrelin receptors in the spinal cord initiates propulsive activity in the colon of the rat. J Physiol. 2006;576:329–338. doi: 10.1113/jphysiol.2006.116160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shafton AD, Sanger GJ, Witherington J, et al. Oral administration of a centrally acting ghrelin receptor agonist to conscious rats triggers defecation. Neurogastroenterol Motil. 2009;21:71–77. doi: 10.1111/j.1365-2982.2008.01176.x. [DOI] [PubMed] [Google Scholar]
- 17.Pustovit RV, Callaghan B, Kosari S, et al. The mechanism of enhanced defecation caused by the ghrelin receptor agonist, ulimorelin. Neurogastroenterol Motil. 2014;26:264–271. doi: 10.1111/nmo.12259. [DOI] [PubMed] [Google Scholar]
- 18.Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- 19.Duty S, Jenner P. Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011;164:1357–1391. doi: 10.1111/j.1476-5381.2011.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bove J, Perier C. Neurotoxin-based models of Parkinson’s disease. Neuroscience. 2012;211:51–76. doi: 10.1016/j.neuroscience.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 21.Zheng LF, Wang ZY, Li XF, et al. Reduced expression of choline acetyltransferase in vagal motoneurons and gastric motor dysfunction in a 6-OHDA rat model of Parkinson’s disease. Brain Res. 2011;1420:59–67. doi: 10.1016/j.brainres.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Zhu HC, Zhao J, Luo CY, Li QQ. Gastrointestinal dysfunction in a Parkinson’s disease rat model and the changes of dopaminergic, nitric oxidergic, and cholinergic neurotransmitters in myenteric plexus. J Mol Neurosci. 2012;47:15–25. doi: 10.1007/s12031-011-9560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blandini F, Balestra B, Levandis G, et al. Functional and neurochemical changes of the gastrointestinal tract in a rodent model of Parkinson’s disease. Neurosci Lett. 2009;467:203–207. doi: 10.1016/j.neulet.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 24.Deleu D, Ebinger G, Michotte Y. Clinical and pharmacokinetic comparison of oral and duodenal delivery of levodopa/carbidopa in patients with Parkinson’s disease with a fluctuating response to levodopa. Eur J Clin Pharmacol. 1991;41:453–458. doi: 10.1007/BF00626368. [DOI] [PubMed] [Google Scholar]
- 25.Decressac M, Mattsson B, Bjorklund A. Comparison of the behavioural and histological characteristics of the 6-OHDA and alpha-synuclein rat models of Parkinson’s disease. Exp Neurol. 2012;235:306–315. doi: 10.1016/j.expneurol.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Orlando: Academic Press; 2007. [Google Scholar]
- 27.Carvalho MM, Campos FL, Coimbra B, et al. Behavioral characterization of the 6-hydroxidopamine model of Parkinson’s disease and pharmacological rescuing of non-motor deficits. Mol Neurodegener. 2013;8:14. doi: 10.1186/1750-1326-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Goebel-Stengel M, Stengel A, Wu SV, Ohning G, Tache Y. Comparison of CRF-immunoreactive neurons distribution in mouse and rat brains and selective induction of Fos in rat hypothalamic CRF neurons by abdominal surgery. Brain Res. 2011;1415:34–46. doi: 10.1016/j.brainres.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gue M, Gleizes-Escala C, Del Rio-Lacheze C, Junien JL, Bueno L. Reversal of CRF- and dopamine-induced stimulation of colonic motility by CCK and igmesine (JO 1784) in the rat. Br J Pharmacol. 1994;111:930–934. doi: 10.1111/j.1476-5381.1994.tb14828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colucci M, Cervio M, Faniglione M, et al. Intestinal dysmotility and enteric neurochemical changes in a Parkinson’s disease rat model. Auton Neurosci. 2012;169:77–86. doi: 10.1016/j.autneu.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Vegezzi G, Al HZ, Levandis G, et al. Radiological analysis of gastrointestinal dysmotility in a model of central nervous dopaminergic degeneration: Comparative study with conventional in vivo techniques in the rat. J Pharmacol Toxicol Methods. 2014;70:163–169. doi: 10.1016/j.vascn.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Ueki A, Otsuka M. Life style risks of Parkinson’s disease: association between decreased water intake and constipation. J Neurol. 2004;251(Suppl 7):vII18–vII23. doi: 10.1007/s00415-004-1706-3. [DOI] [PubMed] [Google Scholar]
- 33.Amato D, Muller CP, Badiani A. Increased drinking after intra-striatal injection of the dopamine D2/D3 receptor agonist quinpirole in the rat. Psychopharmacology (Berl) 2012;223:457–463. doi: 10.1007/s00213-012-2735-8. [DOI] [PubMed] [Google Scholar]
- 34.Heetun ZS, Quigley EM. Gastroparesis and Parkinson’s disease: A systematic review. Parkinsonism Relat Disord. 2012;18:433–440. doi: 10.1016/j.parkreldis.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Greene JG, Noorian AR, Srinivasan S. Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson’s disease. Exp Neurol. 2009;218:154–161. doi: 10.1016/j.expneurol.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardoff R, Sula M, Tamir A, et al. Gastric emptying time and gastric motility in patients with Parkinson’s disease. Mov Disord. 2001;16:1041–1047. doi: 10.1002/mds.1203. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Mogami S, Karasawa H, et al. Preventive effect of rikkunshito on gastric motor function inhibited by l-dopa in rats. Peptides. 2014;55:136–144. doi: 10.1016/j.peptides.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanger GJ. Ghrelin and motilin receptor agonists: time to introduce bias into drug design. Neurogastroenterol Motil. 2014;26:149–155. doi: 10.1111/nmo.12300. [DOI] [PubMed] [Google Scholar]
- 39.Lasseter KC, Shaughnessy L, Cummings D, et al. Ghrelin agonist (TZP-101): safety, pharmacokinetics and pharmacodynamic evaluation in healthy volunteers: a phase I, first-in-human study. J Clin Pharmacol. 2008;48:193–202. doi: 10.1177/0091270007310380. [DOI] [PubMed] [Google Scholar]
- 40.Wargin W, Thomas H, Clohs L, et al. Contribution of protein binding to the pharmacokinetics of the ghrelin receptor agonist TZP-101 in healthy volunteers and adults with symptomatic gastroparesis: two randomized, double-blind studies and a binding profile study. Clin Drug Investig. 2009;29:409–418. doi: 10.2165/00044011-200929060-00004. [DOI] [PubMed] [Google Scholar]
- 41.Fraser GL, Hoveyda HR, Tannenbaum GS. Pharmacological demarcation of the growth hormone, gut motility and feeding effects of ghrelin using a novel ghrelin receptor agonist. Endocrinology. 2008;149:6280–6288. doi: 10.1210/en.2008-0804. [DOI] [PubMed] [Google Scholar]
- 42.Fraser GL, Venkova K, Hoveyda HR, Thomas H, Greenwood-Van MB. Effect of the ghrelin receptor agonist TZP-101 on colonic transit in a rat model of postoperative ileus. Eur J Pharmacol. 2009;604:132–137. doi: 10.1016/j.ejphar.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Tolle V, Zizzari P, Tomasetto C, Rio MC, Epelbaum J, Bluet-Pajot MT. In vivo and in vitro effects of ghrelin/motilin-related peptide on growth hormone secretion in the rat. Neuroendocrinology. 2001;73:54–61. doi: 10.1159/000054620. [DOI] [PubMed] [Google Scholar]
- 44.Camina JP, Carreira MC, El Messari S, Llorens-Cortes C, Smith RG, Casanueva FF. Desensitization and endocytosis mechanisms of ghrelin-activated growth hormone secretagogue receptor 1a. Endocrinology. 2004;145:930–940. doi: 10.1210/en.2003-0974. [DOI] [PubMed] [Google Scholar]
- 45.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 46.Dass NB, Munonyara M, Bassil AK, et al. Growth hormone secretagogue receptors in rat and human gastrointestinal tract and the effects of ghrelin. Neuroscience. 2003;120:443–453. doi: 10.1016/s0306-4522(03)00327-0. [DOI] [PubMed] [Google Scholar]
- 47.Bron R, Yin L, Russo D, Furness JB. Expression of the ghrelin receptor gene in neurons of the medulla oblongata of the rat. J Comp Neurol. 2013;521:2680–2702. doi: 10.1002/cne.23309. [DOI] [PubMed] [Google Scholar]
- 48.Poitras P, Tomasetto C. The potential of ghrelin as a prokinetic. Regul Pept. 2009;155:24–27. doi: 10.1016/j.regpep.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Hirayama H, Shiina T, Shima T, et al. Contrasting effects of ghrelin and des-acyl ghrelin on the lumbo-sacral defecation center and regulation of colorectal motility in rats. Neurogastroenterol Motil. 2010;22:1124–1131. doi: 10.1111/j.1365-2982.2010.01553.x. [DOI] [PubMed] [Google Scholar]
- 50.Ferens DM, Habgood MD, Saunders NR, et al. Stimulation of defecation in spinal cord-injured rats by a centrally acting ghrelin receptor agonist. Spinal Cord. 2011;49:1036–1041. doi: 10.1038/sc.2011.60. [DOI] [PubMed] [Google Scholar]
- 51.Furness JB, Cho HJ, Hunne B, et al. Identification of neurons that express ghrelin receptors in autonomic pathways originating from the spinal cord. Cell Tissue Res. 2012;348:397–405. doi: 10.1007/s00441-012-1405-9. [DOI] [PubMed] [Google Scholar]
- 52.Tebbe JJ, Pasat IR, Monnikes H, Ritter M, Kobelt P, Schafer MK. Excitatory stimulation of neurons in the arcuate nucleus initiates central CRF-dependent stimulation of colonic propulsion in rats. Brain Res. 2005;1036:130–138. doi: 10.1016/j.brainres.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Saint-Pierre DH, Tache Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y - synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- 54.Kohno D, Yada T. Arcuate NPY neurons sense and integrate peripheral metabolic signals to control feeding. Neuropeptides. 2012;46:315–319. doi: 10.1016/j.npep.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Tebbe JJ, Mronga S, Tebbe CG, Ortmann E, Arnold R, Schafer MK. Ghrelin-induced stimulation of colonic propulsion is dependent on hypothalamic neuropeptide Y1- and corticotrophin-releasing factor 1 receptor activation. J Neuroendocrinol. 2005;17:570–576. doi: 10.1111/j.1365-2826.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- 56.Dickson SL, Luckman SM. Induction of c-fos messenger ribonucleic acid in neuropeptide Y and growth hormone (GH)-releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH-releasing peptide-6. Endocrinology. 1997;138:771–777. doi: 10.1210/endo.138.2.4907. [DOI] [PubMed] [Google Scholar]
- 57.Hewson AK, Tung LY, Connell DW, Tookman L, Dickson SL. The rat arcuate nucleus integrates peripheral signals provided by leptin, insulin, and a ghrelin mimetic. Diabetes. 2002;51:3412–3419. doi: 10.2337/diabetes.51.12.3412. [DOI] [PubMed] [Google Scholar]
- 58.Cloud LJ, Greene JG. Gastrointestinal features of Parkinson’s disease. Curr Neurol Neurosci Rep. 2011;11:379–384. doi: 10.1007/s11910-011-0204-0. [DOI] [PubMed] [Google Scholar]
- 59.Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol. 2000;12:1047–1049. doi: 10.1046/j.1365-2826.2000.00584.x. [DOI] [PubMed] [Google Scholar]
- 60.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 61.Johnson RF, Johnson AK. Meal-related and rhythmic drinking: effects of abolition of rat’s eating rhythm. Am J Physiol. 1991;261:R14–R19. doi: 10.1152/ajpregu.1991.261.1.R14. [DOI] [PubMed] [Google Scholar]
- 62.Fitzsimons TJ, Le MJ. Eating as a regulatory control of drinking in the rat. J Comp Physiol Psychol. 1969;67:273–283. doi: 10.1037/h0026772. [DOI] [PubMed] [Google Scholar]
- 63.Hashimoto H, Fujihara H, Kawasaki M, et al. Centrally and peripherally administered ghrelin potently inhibits water intake in rats. Endocrinology. 2007;148:1638–1647. doi: 10.1210/en.2006-0993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.