Abstract
Objective
To identify the cause of childhood onset involuntary paroxysmal choreiform and dystonic movements in 2 unrelated sporadic cases and to investigate the functional effect of missense mutations in adenylyl cyclase 5 (ADCY5) in sporadic and inherited cases of autosomal dominant familial dyskinesia with facial myokymia (FDFM).
Methods
Whole exome sequencing was performed on 2 parent–child trios. The effect of mutations in ADCY5 was studied by measurement of cyclic adenosine monophosphate (cAMP) accumulation under stimulatory and inhibitory conditions.
Results
The same de novo mutation (c.1252C>T, p.R418W) in ADCY5 was found in both studied cases. An inherited missense mutation (c.2176G>A, p.A726T) in ADCY5 was previously reported in a family with FDFM. The significant phenotypic overlap with FDFM was recognized in both cases only after discovery of the molecular link. The inherited mutation in the FDFM family and the recurrent de novo mutation affect residues in different protein domains, the first cytoplasmic domain and the first membrane-spanning domain, respectively. Functional studies revealed a statistically significant increase in β-receptor agonist-stimulated intracellular cAMP consistent with an increase in adenylyl cyclase activity for both mutants relative to wild-type protein, indicative of a gain-of-function effect.
Interpretation
FDFM is likely caused by gain-of-function mutations in different domains of ADCY5—the first definitive link between adenylyl cyclase mutation and human disease. We have illustrated the power of hypothesis-free exome sequencing in establishing diagnoses in rare disorders with complex and variable phenotype. Mutations in ADCY5 should be considered in patients with undiagnosed complex movement disorders even in the absence of a family history.
We recently reported a missense mutation in adenylyl cyclase 5 (ADCY5; Gene ID 111; Online Mendelian Inheritance in Man database [OMIM] #600293) as the cause of familial dyskinesia and facial myokymia (FDFM; OMIM #606703), an autosomal dominant disorder characterized by paroxysmal chorea, dystonia, and facial myokymia.1 Linkage analysis in a single family with 10 affected individuals in 3 generations had confined the locus to a 72.5Mb region on chromosome 3p21–3q21. Only 1 of the 5 variants in this region in the exome of 1 affected individual (c.2176G>A, p.A726T in ADCY5) was highly conserved, predicted to be pathogenic, and cosegregated perfectly with disease status. ADCY5 is 1 of 9 membrane-bound adenylyl cyclases that convert adenosine triphosphate (ATP) to pyrophosphate and cyclic adenosine-3′,5′-monophosphate (cAMP), the second messenger in a broad range of cellular activities.2,3 ADCY5 expression is particularly high in striatum and myocardium.4 We hypothesized that the adventitious movements in FDFM resulted from a gain-of-function effect of the mutation in ADCY5.1 This hypothesis was based on the phenotype of mice deficient in Adcy5 that develop parkinsonianlike motor dysfunction5—in some ways the opposite of what is observed in the family with FDFM. Despite 2 earlier publications that provided detailed phenotypic descriptions,6,7 at that time no other families with a diagnosis of FDFM were identified in which to substantiate the role of ADCY5. This vexing problem in the study of rare diseases is further complicated by phenotypic variability that characterizes many genetic disorders, as the appropriateness of a candidate gene may not be obvious.
Herein, by identification of identical de novo ADCY5 missense mutation in 2 subjects with a paroxysmal choreic/dystonic movement disorder and by functional characterization of this mutation and the previously reported inherited mutation, we confirm that FDFM is caused by mutations in ADCY5 that likely confer a gain-of-function effect. Alterations in gene sequence can have more than one effect, and such effects might be tissue specific. Therefore, it will be necessary to study additional variants in ADCY5 found in patients with undiagnosed movement disorders to further evaluate the pathogenesis of FDFM. As other variants in ADCY5 are found, we plan to evaluate their functional effects in neuronal cell cultures and perhaps in model organisms. FDFM represents the first direct link between adenylyl cyclase mutations and a human disease.
Patients and Methods
Subjects
Subjects were evaluated in the Neurology Clinic at Rady Children’s Hospital, University of California at San Diego and the Scripps Translational Science Institute (ID1 and her parents) or the Genetic Medicine Clinic at the University of Washington, Seattle (UW1 and her parents) under protocols approved by the relevant institution.
Whole Exome, Whole Genome Sequencing, Variant Calling, and Filtration
Genomic DNA was extracted from whole blood using the QIAamp system (Qiagen, Valencia, CA). Enriched exome libraries were prepared using the Agilent Sureselect XT kit (Agilent, Santa Clara, CA). Whole exome sequences (WES) were obtained at the Scripps Translational Science Institute on the HiSeq2000 (Illumina, San Diego, CA) platform with a 100bp, indexed, paired-end sequencing run (TruSeq SBS Kit v3 200 cycle; Illumina). Mean coverage of 79 to 119× per individual was achieved with 93 to 95% of the target exome covered by >10 reads. Sequence alignment and variant calling were performed against the reference human genome (NCBI 37/hg 19). For single nucleotide polymorphisms and indels, variant calling and genotyping was done with the GATK Unified Genotyper. Filtered variant annotation was performed using the SG-ADVISER and Cypher Genomics systems with a combination of population-, inheritance-, annotation-, and functional impact–based filters. These included removal of variants that (1) are present at >1% allele frequency in the HapMap8 or 1,000 Genomes databases, or 385 genomes from the Scripps Wellderly population (individuals aged >80 years, without common chronic conditions, sequenced on the Complete Genomics [Mountain View, CA] platform); (2) are in segmental duplication regions that are prone to produce false-positive variant calls due to mapping errors10; (3) are not nonsynonymous, frameshift, or nonsense, or do not affect canonical splice-site donor/acceptor sites; and (4) are not present within the trio in a manner consistent with affectation status.
Whole genome sequencing was performed on ID1 and her parents by Complete Genomics, utilizing their standard sequencing and variant calling service. A mean coverage of 49 to 50× per individual was achieved with ∼98.5% of the genome covered by >10 reads.
Clinical WES for UW1 and her parents was performed at GeneDx (Gaithersburg, MD). Mean depth of coverage was 114×, with a quality threshold of 98.2%.
Generation of an Expression Plasmid Vector for Human ADCY5
We obtained a full-length human ADCY5 cDNA (Genbank accession #BC156217.1; Source Bioscience, Nottingham, UK) and introduced SalI and KpnI restriction enzyme sites by polymerase chain reaction amplification using forward primer ADCY5_F: 5′-ctgcaGTCGACaaaatgtccggctccaaaag-3′ and reverse primer ADCY5_R: 5′-cccgcGGTACCactgagcggggg ccctccatt-3′ (restriction sites are uppercase; the underlined nucleotides were added to enhance the start codon recognition and expression). This fragment was topo-cloned into the entry vector pCR-XL-TOPO (Life Technologies, Carlsbad, CA). ADCY5 was then excised using SalI and KpnI and cloned into the SalI and KpnI sites of the pEGFP-C1 expression vector (Clontech Laboratories, Mountain View, CA). The resulting constructs were verified for fidelity and orientation of the gene by restriction enzyme digestion–gel analysis and Sanger sequencing.
Site-Directed Mutagenesis
Mutations were introduced by site-directed mutagenesis of pEGFP-C1-ADCY5 with primers containing the specific nucleotide substitutions (QuikChange II XL Mutagenesis Kit; Agilent Technologies, Santa Clara, CA) and verified by Sanger sequencing. The primer pairs for p.A726T and p.R418W were F 5′-ccgtgccattgacaccaggagcattg-3′/R 5′-caatgctcctggtgtcaatggca cgg-3′ and F 5′-gcatccaggcggggctccactcg-3′/R 5′-cgagtggagccccgc ctggatgc-3′, respectively. Resulting constructs for transfection were: vector alone (Vector), wild-type ADCY5 (ADCY5-WT), ADCY5 with the mutation p.A726T (ADCY5–726T), and ADCY5 with the mutation p.R418W (ADCY5–418W).
Transfection of ADCY5 Constructs into HEK293 Cells
EGFP-ADCY5 constructs were transfected into HEK293 cells using Lipofectamine 2000 (Life Technologies). Slides were stained with Hoechst dye, and EGFP expression in approximately 200 cells in 4 fields was assessed to estimate transfection efficiency.
Western Blot Analysis
HEK293 cells were harvested 24-hours post-transfection. Cell lysis and protein isolation, fractionation on gels, and transfer to polyvinylidene difluoride membranes were performed as described previously.11 After treatment for 30 minutes in Super-Block Blocking Buffers (Thermo Scientific, Waltham, MA), membranes were probed overnight at 4°C with 1:500 rabbit anti-ADCY5 (Abcam, Cambridge, MA) in SuperBlock Blocking Buffer. Rabbit anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Sigma-Aldrich, St Louis, MO) at 1:5,000 was used as an internal control. Membranes then were incubated with antirabbit horseradish peroxidase–conjugated secondary antibodies (Life Technologies) at 1:3,000 against ADCY5 and at 1:6,000 against GAPDH. Protein bands were visualized by enhanced chemiluminescence (Thermo Scientific) on a Bio-Chemi digital imaging system (UVP, Upland, CA), quantified by densitometry, and analyzed with ImageJ software (NIH). Transfected EGFP-tagged ADCY5 was distinguished from endogenous protein by its larger size. ADCY5 expression was normalized with GAPDH level. The data were averaged from triplicate experiments and analyzed by Student t test.
Intracellular cAMP Assays
Two methods, plated in triplicate, were used to assess ADCY5 activity, the GE Healthcare cAMP Biotrak competitive enzyme immunoassay (EIA; GE Healthcare Life Sciences, Pittsburgh, PA) and incorporation of [2, 8-3H]-adenine. For the EIA method, 24 hours after transfection of the EGFP-ADCY5 constructs, medium was replaced with serum-free medium containing 1mM isobutylmethylxanthine (IBMX; Sigma-Aldrich) and incubated for 45 minutes to inhibit cAMP phosphodiesterase activities. Transfected cells were then treated for 15 minutes with 50µM forskolin or 10µM isoproterenol for the stimulant conditions or 10µM propranolol for 15 minutes followed by 10µM isoproterenol for 15 minutes for the blocking condition (reagents all from Sigma-Aldrich). The reaction was terminated by removal of the incubation solution and addition of diluted lysis reagent. Intracellular cAMP was assessed using the nonacetylation EIA procedure according to the manufacturer’s instructions. Optical density determination was carried out in a plate reader at 450nm. A standard curve was generated by a series of dilutions, giving standard levels of cAMP from 25 to 3,200 fmol/well. The data were averaged from 3 independent experiments and analyzed by Student t test.
Assessment of intracellular cAMP accumulation by incorporation of [2, 8-3H]-adenine was done as previously described12,13 with minor modifications. Twenty-four hours after transfection, 2µCi of [3H]adenine (PerkinElmer, Boston, MA) was added to each well for overnight incubation. Medium was then replaced with serum-free media containing 1mM IBMX and forskolin or isoproterenol as described above. Nor-epinephrine (10µM), an α2 antagonist, was used as an irrelevant/control reagent. After stopping the reaction,12 acid-soluble nucleotides were separated by Dowex AG 50W–X4 (Bio-Rad Laboratories, Hercules, CA) and neutral alumina (Sigma-Aldrich) chromatography. Effluents were analyzed on a Beck-man liquid scintillation counter. cAMP accumulation was determined by the ratio of [3H]cAMP to the total ATP, adenosine diphosphate, and AMP pool as published.12
Results
Clinical Presentations
ID1, a 15-year-old girl of European ancestry, presented with hypotonia, weakness, and abnormal involuntary movements. Developmental milestones were delayed with unsupported sitting at 15 months. Independent ambulation occurred at 14 months but remained minimal until assisted with a walker at 30 months. Paroxysmal choreic movements of the limbs were first noted at 19 months. Daytime movements included intermittent resting tremors and myoclonus affecting all limbs as well as kinesigenic dyskinesia with standing. Nighttime movements associated with frequent awakenings included severe generalized myoclonic jerks and at times ballistic movements arising from stages N2 and N3 non–rapid eye movement sleep, without associated epileptiform discharges. Recently, the paroxysmal movements are milder during the day, but at night they continue to cause significant sleep disruption. Episodes are worsened by anxiety. Her ability to ambulate has fluctuated, with an overall very slow deterioration. Currently, ambulation is impossible at times and at maximum can be sustained for 30 feet. The most recent examination shows axial hypotonia with limb hypertonia, hyperreflexia, generalized weakness, intermittent tremors and paroxysmal dyskinesia, dystonia, and myoclonus both at rest and with activity. There is poor head support due to marked neck muscle weakness. When she is able to ambulate, there is marked camptocormia. Speech is hypophonic and severely dysarthric. Perioral and periorbital dyskinesias were previously noted but not considered distinguishing until the molecular diagnosis was made. In retrospect, these muscle movements could represent myokymia, but the subject would not allow electromyography (EMG) of this area.
UW1 is an 18-year-old woman of European ancestry with onset of limb and body choreiform movements at approximately 5 years. Choreoathetosis and dystonia fluctuate, are most prominent at rest, and are alleviated by physical activity. Initially abnormal movements involved only the limbs, but progressed to include facial twitches and mild dysarthria. More prolonged bouts may persist for days to weeks. Despite the movement disorder, she has been very active, participating in multiple sports through the Special Olympics. A few facial twitches were noted, but nothing that would have been described as myokymia. At the most recent examination, there were nearly constant involuntary choreic and dystonic movements, although these were somewhat diminished while walking. Her strength and muscle tone were normal.
Both probands underwent extensive investigations at multiple major academic medical centers, with combinations of metabolic screens, electroencephalography, brain magnetic resonance imaging, single photon emission computed tomography brain scan, EMG/nerve conduction studies, and muscle and nerve biopsy, but these workups failed to provide a diagnosis, and numerous therapeutic interventions were tried without lasting benefit.
Sequencing and Candidate Variant Identification
WES was performed on both trios (ID1, UW1, and their unaffected parents) under the assumption that disease in ID1 and UW1 was Mendelian—resulting from a de novo dominant mutation or inherited in recessive manner. For ID1, 5 variants in 3 genes survived the filtration process and were corroborated by whole genome sequencing: a de novo missense variant in ADCY5 (c.1252C>T, p.R418W), a de novo missense variant (c.5645A>G, p.N1882S) and a maternally inherited mis-sense variant in DOCK3 (c.2557C>T, p.R853C), and 2 inherited heterozygous missense variants in FAT4 (paternal: c.524G>T, p.R175L; maternal: c.4000G>A, p.V1334M). We recently reported a mutation in ADCY5 as likely causative in a family with FDFM, an autosomal dominant movement disorder1 (Supplementary Video). Although hypotonia was not seen in patients with FDFM and the facial movements were not initially recognized as significant in ID1, the ADCY5 variant was the strongest candidate given the clinical overlap. This mutation is a nonconservative amino acid substitution of a positively charged, polar arginine with a neutral, non-polar tryptophan at a residue that is conserved across species. Moreover, ADCY5 is in the top 1% of genes intolerant to rare variants.14 FAT4 (OMIM #612411) is a protocadherin active in the Hippo signaling pathway that controls organ size. Loss of FAT4 in mice leads to a constellation of morphological anomalies of the kidneys, ears, and spinal cord,15 defects not consistent with ID1’s condition. DOCK3 (OMIM #603123) is a guanine nucleotide exchange factor, primarily expressed in neurons and testis, which is involved in axonal transport by promoting cytoskeletal reorganization.16,17 Dock3 null mice present with central axonal dystrophy, a concomitant loss of sensorimotor function, and impaired axoplasmic flow, but no loss in motor learning abilities or loss or disorganization of central nervous system structures, raising the possibility that the DOCK3 mutations contribute to ID1’s severe phenotype.
For UW1, the same heterozygous de novo R418W (c.1252C>T) ADCY5 mutation was the only variant deemed pathogenic. Because the mutant allele was under-represented in comparison to the normal allele, the possibility of somatic mosaicism was raised. Uneven amplification of the DNA related to primer placement was ruled out by reamplification and sequencing using alternative primers, but the level of mosaicism could not be precisely determined as Sanger sequencing is not quantitative.
Functional Studies of ADCY5 Mutations
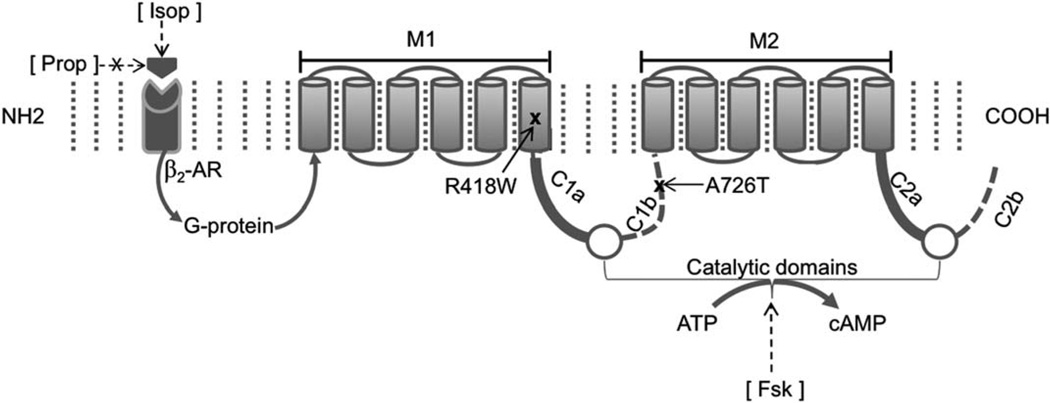
A schematic of the domain structure of ADCY5 protein with the locations of the mutations is shown in Figure 1. The p.A726T mutation lies in the second portion of the first cytoplasmic domain (C1b), and the p.R418W mutation lies in the 6th helical segment of the first transmembrane domain (M1). To obtain further evidence for the pathogenic role of both ADCY5 mutations, we studied their effect on protein expression and enzyme activity in HEK293 cells transfected with ADCY5 constructs.
FIGURE 1.
Schematic of ADCY5 showing the protein domains and locations of the mutations. The protein contains two 6-helical section membrane-spanning domains, M1 and M2, and 2 bipartite cytoplasmic domains, C1 and C2, which when brought together form a catalytic pocket for conversion of adenosine triphosphate (ATP) to cyclic adenosine-3′,5′-monophosphate (cAMP). Brackets denote synthetic reagents used. Fsk=forskolin, a receptor-independent stimulator of most adenylyl cyclases; Isop=isoproterenol, a β-adrenergic receptor (AR) agonist; Prop=propranolol, a β-AR antagonist.
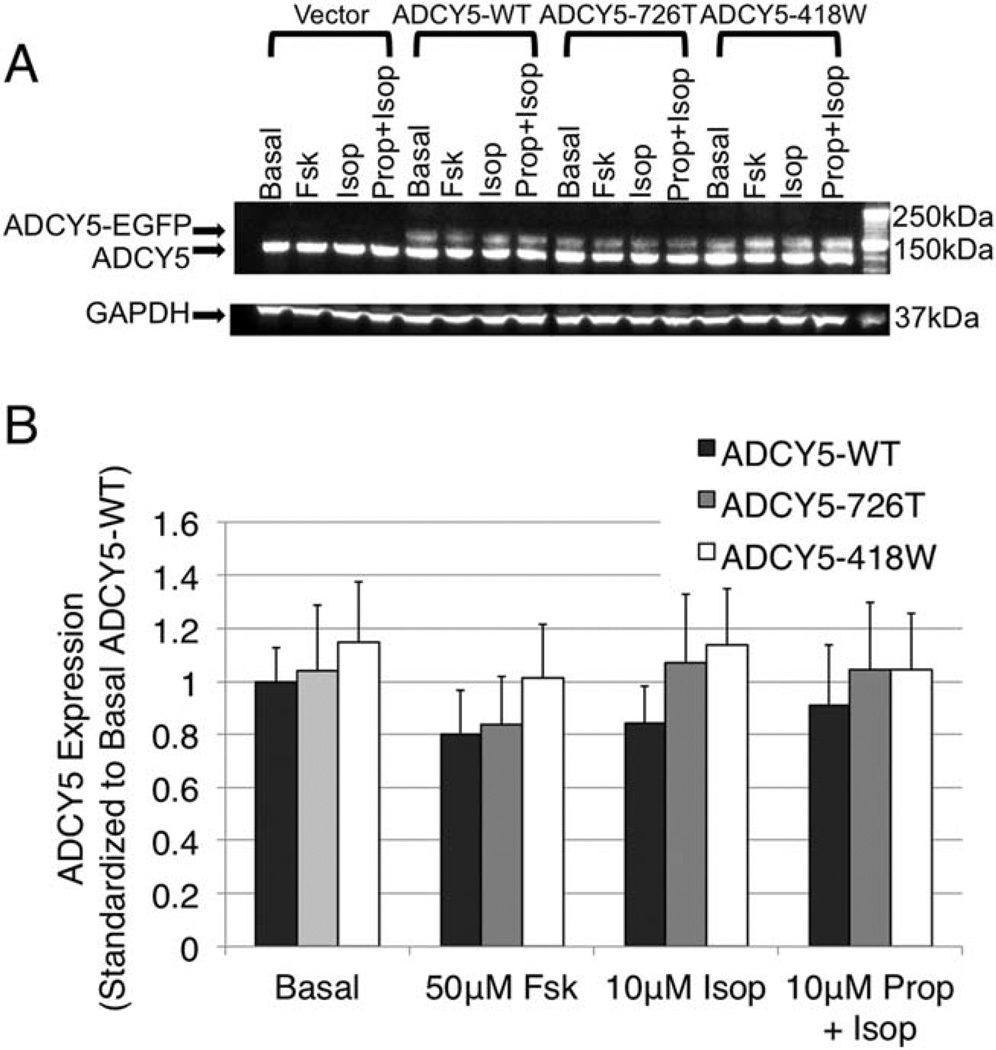
Cells were transfected with expression constructs in p-EGFP vector containing no insert (Vector), ADCY5-WT, or mutant ADCY5 (ADCY5–418W or ADCY5–726T). The viability of transfected cultures was not reduced compared to untransfected HEK293 cells. Trans-fection efficiency reached about 65% and was identical in each experiment. Immunoblot using anti-ADCY5 revealed 2 bands at ∼130kDa and ∼160kDa (Fig 2A), which correspond to endogenous ADCY5 and transfected ADCY5 fused with the EGFP tag, respectively. In general, endogenous ADCY5 was more highly expressed than transfected ADCY5. Expression levels of both mutant transfected ADCY5 forms were not significantly different from that of transfected ADCY5-WT (p > 0.05), suggesting that the mutations have no effect on expression of the protein (see Fig 2B).
FIGURE 2.
Assessment of effect of ADCY5 mutations on protein expression in HEK293 cells transfected with ADCY5 expression constructs. HEK293 cells were transfected with plasmid DNA of enhanced green fluorescence protein (EGFP) vector alone, EGFP-tagged wild-type (ADCY5-WT), mutant ADCY5–726T, or mutant ADCY5–418W. Twenty-four hours later, cells were treated with carrier alone, forskolin (Fsk), isoproterenol (Isop), or propranolol (Prop) plus isoproterenol for 15 minutes. Cells were then harvested, and protein expression was measured by immunoblotting with anti-ADCY5. (A) ADCY5 immunoblot. Two bands at ∼130kDa and ∼160kDa were detected, corresponding to endogenous ADCY5 and transfected ADCY5 fused with the EGFP tag (ADCY5-EGFP), respectively. (B) Analysis of ADCY5 expression levels. Expression levels of ADCY5 were quantified and normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels and standardized to expression in cells transfected with ADCY5-WT with basal carrier treatment. Error bars are standard errors from triplicate determinations. Student t test showed that there was no significant difference (p>0.05) between wild-type and mutant ADCY5 constructs for any of the conditions.
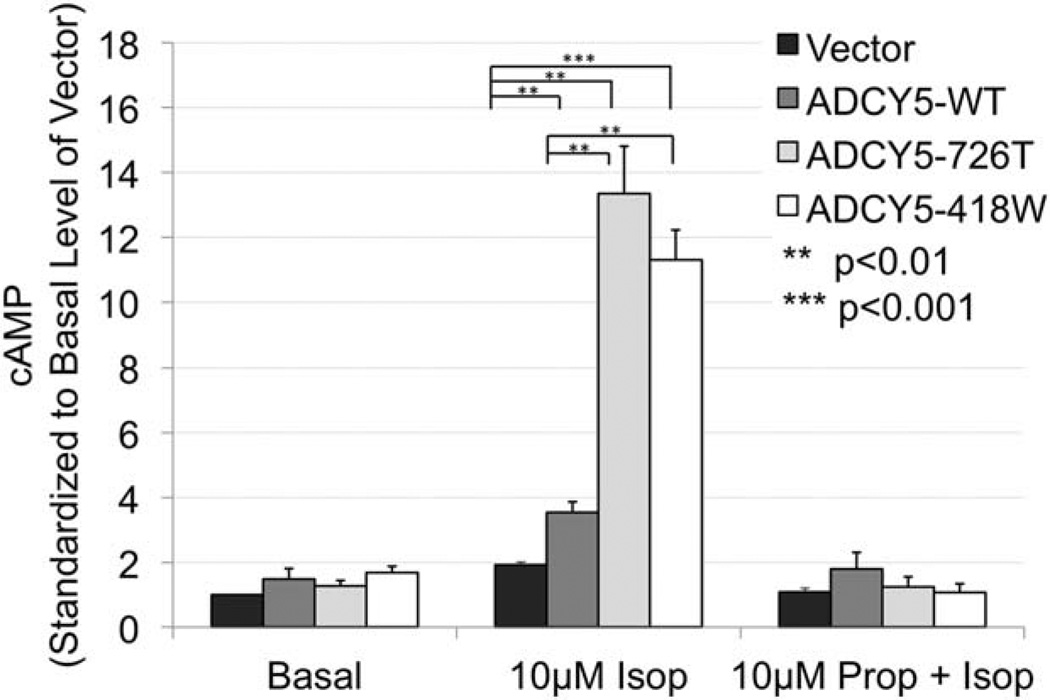
ADCYs convert ATP to cAMP. Enzyme immunoassay was used to detect intracellular cAMP under forskolin or isoproterenol stimulation or with isoproterenol in the presence of propranolol or with only the carrier (basal state). To assess the magnitude of cAMP increases, for each construct and treatment, the level of cAMP was standardized to that of the basal cAMP level of the empty vector. Without stimulation, there was no significant difference in basal activity level in cells transfected with the empty vector or any of the ADCY5 constructs (Fig 3). Forskolin, which directly binds and stimulates most ADCYs, bypassing other cellular signaling mechanisms, serves as a positive control. Treatment of ADCY5-transfected cells with forskolin markedly increased cAMP levels from basal for all constructs (data not shown). Treatment with isoproterenol, a β-adrenergic agonist, resulted in dramatically increased cAMP production in cells transfected with either mutant ADCY5 compared to cells transfected with ADCY5-WT (p < 0.001). There was no statistical difference between the 2 mutations. As expected, addition of the β-adrenergic receptor antagonist propranolol blocked the response to isoproterenol such that cells treated with this combination had similar levels of cAMP as the carrier-treated cells, demonstrating the specificity of the assay and the treatment conditions. Similar results were obtained for ADCY5–726T assessed by an isotope-based method (data not shown). These results are consistent with a gain-of-function effect of both mutations, specifically in response to receptor stimulating activation, and strongly support their causative role in all 3 families.
FIGURE 3.
Intracellular cyclic adenosine-3′,5′-monophosphate (cAMP) assessed by enzyme immunoassay. HEK293 cells were transfected with empty enhanced green fluorescence protein (EGFP) vector (Vector) or expression constructs of EGFP tagged wild type (WT)-, 418W- or 726T–ADCY5 for 24 hours. Cells were treated under different adenylyl cyclase enzyme activating conditions—carrier alone, isoproterenol (Isop), or propranolol (Prop) plus Isop for 15 minutes—and cAMP level was assessed using an enzyme immunoassay. Treatment with Isop resulted in markedly increased cAMP from the carrier-treated basal levels. In comparison to ADCY5-WT, both mutant constructs demonstrated significantly increased levels of intracellular cAMP. Pretreatment with Prop abrogated the stimulatory effect of Isop for all constructs. Data shown are averages of 3 independent replicates standardized to activity of the empty vector under carrier-treated basal conditions. This value is designated as 1 unit on the y-axis. Statistical analyses were performed using Student t test; significant p values are indicated in the figure. Error bars indicate standard error of the mean.
Discussion
A pervasive problem in the study of rare diseases is the difficulty in ascertaining additional cases to verify gene–disease associations and to establish that mutations have similar detrimental effects on gene function. Family-based exome sequencing is a powerful approach to molecular genetic diagnosis of rare and undiagnosed disease, especially for simplex cases where linkage studies are not possible. In both cases, exome sequencing, coupled with previous findings from a single multigenerational family, enabled molecular diagnosis and confirmed the association of ADCY5 mutation with FDFM. It is important to note that neither case we present could have been definitively diagnosed without the prior publication of ADCY5 as a candidate gene for FDFM, highlighting the value of index case publications. ID1 and UW1 were evaluated at different institutions, and neither had a family history of a movement disorder, precluding linkage analysis as supportive evidence. Variant classification poses an increasingly common challenge as we tackle rare or clinically heterogeneous disorders where only one or a few cases have been reported and the full spectrum of manifestations is not known. A stringent genetic approach has been proposed to classify variants in 1 of 4 categories: pathogenic, likely pathogenic variant of uncertain significance (VUS), VUS, or likely benign VUS18 based upon allele frequency and segregation data. Using these criteria, the variant in these 2 cases meets criteria for pathogenicity based upon an allele frequency less than the frequency of the disease in the population, segregation in our previously reported large family, and now 2 proven de novo events in trios. Even with extensive clinical evaluation, it can be difficult to recognize features that suggest a specific diagnostic category. In ID1 and UW1, the perioral and periorbital movements, a prominent feature of FDFM,1,6 had not been recognized as significant and other features differed from the one reported family. By themselves, the more prominent chorea and dystonia do not define a single disorder. Myokymia can be a subtle finding that may be missed unless specifically sought and may require EMG corroboration; in the original report of the FDFM family their disease was described as essential chorea19,20 until myokymia of the face and hand were confirmed by EMG.6 The scheme of whole exome sequencing followed by a step- wise series of filters provided a manageable list of candidate variants to consider in an agnostic approach to disease gene discovery. Once the de novo mutations in ADCY5 in ID1 and UW1 were found, the significant overlap with FDFM was recognized, including onset in early childhood, paroxysmal dystonic and choreiform movements, likely facial myokymia, and worsening with anxiety.
ADCYs catalyze the formation of cAMP and pyrophosphate from ATP when stimulated through G-protein–coupled receptors.21,22 ADCY5 is 1 of 9 membrane-associated ADCYs (see Kamenetsky et al for review23). These ADCYs have 2 transmembrane domains, M1 and M2, each comprised of 6 helices of hydrophobic amino acids, and 2 major cytoplasmic domains, C1 and C2. There is experimental evidence suggesting that C1 and C2 are brought together to form an ATP substrate-binding site within a catalytic pocket for hydrolysis of ATP.24 As residue 726 lies in the C1b domain, substitution of polar threonine for the smaller nonpolar alanine could affect strength of substrate binding or interaction between C1 and C2. The second mutation, p.R418W, lies in M1 and replaces a branched chain amino acid with the most complex amino acid. This alteration might affect response to or transmission of the β-adrenergic receptor stimulation of the enzyme via trimeric G-protein. Additionally, these mutations might affect the enzyme’s interactions with other proteins upstream or downstream in signaling events.
cAMP changes have been associated with a few human diseases.25–27 In prior reports, neither the connection between ADCY and the disorder nor the nature of the cAMP changes were clearly defined. Our findings, that ADCY5 mutations in FDFM in different protein domains increase intracellular cAMP in mutant ADCY5-transfected cells in response to isoproterenol, an agonist of the β-adrenergic system through its receptor, are to our knowledge the first demonstration directly linking mutations in an ADCY and a human disease.
ID1 and UW1 are both more severely affected than affected members of the initially reported FDFM family, possibly due to the different locations of the respective mutations in the gene. Delayed motor milestones, axial hypotonia, and progressive inability to ambulate present in ID1 but not in UW1 may reflect an additional contribution of the compound heterozygous DOCK3 mutations. Alternatively, if UW1 has somatic mosaicism for the ADCY5 mutation, as is suggested by its reduced sequence reads compared to the wild-type allele, this might have mitigated her phenotype. With future ascertainment of additional cases, we can delineate the spectrum of mutations, the effects of genotypic differences on phenotypic variability, and the relative frequencies of de novo and inherited mutations, as well as explore the possibility of decreased penetrance. Finally, these findings lay the groundwork for rational investigation into therapeutic options for FDFM.
Supplementary Material
Acknowledgment
This work is supported by Scripps Genomic Medicine, an NIH National Center for Advancing Translational Sciences Clinical and Translational Science Award (5 UL1 RR025774) to Scripps Translational Science Institute, as well as funding from the Shaffer Family Foundation and the Anne and Henry Zarrow Foundation. Further support is from NIH/NHGRI U01 HG006476 (A.T.), NIH/NINDS R01 NS069719 (W.H.R.), and NIH/NINDS NS20498 (D.S.), the Department of Veterans Affairs (T.D.B., W.H.R.), the American Association for Cancer Research (N.J.S.), and a research service contract with Johnson & Johnson (N.J.S.).
We thank the members of our review panel for their dedication and support: K. Bethel, B. Darst, J. Diamant, S. Haaser, N. Hywnn, E. Kavalerchik, B. Patay, J. Sheard, R. Simon, and G. Williams. Dr N. Zheng provided helpful information and advice about adenylyl cyclase biochemistry. V. Li provided expert technical support for the ADCY5 functional studies.
Footnotes
Potential Conflicts of Interest
J.R.F.: husband is biotech investor with investments in the sequence analysis and therapeutics space. P.H.P.: employment, stock, Cypher Genomics. A.A.S.-V.Z.: cofounder, employee, stock, Cypher Genomics. E.J.T.: cofounder, stock, Cypher Genomics; advisor, Illumina. T.D.B.: patent licensing royalties from Athena Diagnostics. N.J.S.: cofounder, consultant, patent, stock, Cypher Genomics. A.T.: cofounder, consultant, patent, stock, Cypher Genomics.
References
- 1.Chen YZ, Matsushita MM, Robertson P, et al. Autosomal dominant familial dyskinesia and facial myokymia: single exome sequencing identifies a mutation in adenylyl cyclase 5. Arch Neurol. 2012;69:630–635. doi: 10.1001/archneurol.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linder JU. Class III adenylyl cyclases: molecular mechanisms of catalysis and regulation. Cell Mol Life Sci. 2006;63:1736–1751. doi: 10.1007/s00018-006-6072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck J, Sinclair ML, Schapal L, et al. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci U S A. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuoka I, Suzuki Y, Defer N, et al. Differential expression of type I, II, and V adenylyl cyclase gene in the postnatal developing rat brain. J Neurochem. 1997;68:498–506. doi: 10.1046/j.1471-4159.1997.68020498.x. [DOI] [PubMed] [Google Scholar]
- 5.Iwamoto T, Okumura S, Iwatsubo K, et al. Motor dysfunction in type 5 adenylyl cyclase-null mice. J Biol Chem. 2003;278:16936–16940. doi: 10.1074/jbc.C300075200. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez M, Raskind W, Wolff J, et al. Familial dyskinesia and facial myokymia (FDFM): a novel movement disorder. Ann Neurol. 2001;49:486–492. [PubMed] [Google Scholar]
- 7.Raskind WH, Matsushita M, Peter B, et al. Familial dyskinesia and facial myokymia (FDFM): follow-up of a large family and linkage to chromosome 3p21-3q21. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:570–574. doi: 10.1002/ajmg.b.30879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frazer KA, Ballinger DG, Cox DR, et al. International HapMap Consortium. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abecasis GR, Auton A, Brooks LD, et al. 1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey JA, Gu Z, Clark RA, et al. Recent segmental duplications in the human genome. Science. 2002;297:1003–1007. doi: 10.1126/science.1072047. [DOI] [PubMed] [Google Scholar]
- 11.Chen DH, Naydenov A, Blankman JL, et al. Two novel mutations in ABHD12: expansion of the mutation spectrum in PHARC and assessment of their functional effects. Hum Mutat. 2013;34:1672–1678. doi: 10.1002/humu.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan GC, Tonegawa S, Storm DR. Hippocampal neurons express a calcineurin-activated adenylyl cyclase. J Neurosci. 2005;25:9913–9918. doi: 10.1523/JNEUROSCI.2376-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salomon Y. Cellular responsiveness to hormones and neurotrans-mitters: conversion of [3H]adenine to [3H]cAMP in cell mono-layers, cell suspensions, and tissue slices. Methods Enzymol. 1991;195:22–28. doi: 10.1016/0076-6879(91)95151-9. [DOI] [PubMed] [Google Scholar]
- 14.Petrovski S, Wang Q, Heinzen EL, et al. Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet. 2013;9:e1003709. doi: 10.1371/journal.pgen.1003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saburi S, Hester I, Goodrich L, McNeill H. Functional interactions between Fat family cadherins in tissue morphogenesis and planar polarity. Development. 2012;139:1806–1820. doi: 10.1242/dev.077461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namekata K, Enokido Y, Iwasawa K, Kimura H. MOCA induces membrane spreading by activating Rac1. J Biol Chem. 2004;279:14331–14337. doi: 10.1074/jbc.M311275200. [DOI] [PubMed] [Google Scholar]
- 17.Chen Q, Peto CA, Shelton GD, et al. Loss of modifier of cell adhesion reveals a pathway leading to axonal degeneration. J Neurosci. 2009;29:118–130. doi: 10.1523/JNEUROSCI.3985-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorschner MO, Amendola LM, Turner EH, et al. Actionable, pathogenic incidental findings in 1,000 participants’ exomes. Am J Hum Genet. 2013;93:631–640. doi: 10.1016/j.ajhg.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bird TD, Carlson CB, Hall JG. Familial essential (“benign”) chorea. J Med Genet. 1976;13:357–362. doi: 10.1136/jmg.13.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bird TD, Hall JG. Additional information on familial essential (benign) chorea. Clin Genet. 1978;14:271–272. doi: 10.1111/j.1399-0004.1978.tb02146.x. [DOI] [PubMed] [Google Scholar]
- 21.Onda T, Hashimoto Y, Nagai M, et al. Type-specific regulation of adenylyl cyclase. Selective pharmacological stimulation and inhibition of adenylyl cyclase isoforms. J Biol Chem. 2001;276:47785–47793. doi: 10.1074/jbc.M107233200. [DOI] [PubMed] [Google Scholar]
- 22.Cooper EC, Jan LY. M-channels: neurological diseases, neuromo-dulation, and drug development. Arch Neurol. 2003;60:496–500. doi: 10.1001/archneur.60.4.496. [DOI] [PubMed] [Google Scholar]
- 23.Kamenetsky M, Middelhaufe S, Bank EM, et al. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol. 2006;362:623–639. doi: 10.1016/j.jmb.2006.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whisnant RE, Gilman AG, Dessauer CW. Interaction of the two cytosolic domains of mammalian adenylyl cyclase. Proc Natl Acad Sci U S A. 1996;93:6621–6625. doi: 10.1073/pnas.93.13.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardin M, Zielinski J, Wan ES, et al. CHRNA3/5, IREB2, and ADCY2 are associated with severe chronic obstructive pulmonary disease in Poland. Am J Respir Cell Mol Biol. 2012;47:203–208. doi: 10.1165/rcmb.2012-0011OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nordman S, Abulaiti A, Hilding A, et al. Genetic variation of the adenylyl cyclase 3 (AC3) locus and its influence on type 2 diabetes and obesity susceptibility in Swedish men. Int J Obes (Lond) 2008;32:407–412. doi: 10.1038/sj.ijo.0803742. [DOI] [PubMed] [Google Scholar]
- 27.Pierre S, Eschenhagen T, Geisslinger G, Scholich K. Capturing adenylyl cyclases as potential drug targets. Nat Rev Drug Discov. 2009;8:321–335. doi: 10.1038/nrd2827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.