Abstract
Background
Crohn’s disease (CD) is considered a contraindication to ileal pouch—anal anastomosis (IPAA). In this study, we compare outcomes of CD and ulcerative colitis (UC) patients undergoing IPAA.
Methods
Patients were considered to have CD before surgery based on a history of small bowel disease, perianal disease, noncrypt-associated granuloma, or pretreatment skip colonic lesions. Patients were prospectively assessed for pouchitis or CD. Postoperative CD (pouch inflammation into the afferent limb or pouch fistula) or pouch failure (need for permanent diversion) were assessed. Preoperative serum was assayed for IBD-associated antibodies using enzyme-linked immunosorbent assay (ELISA).
Results
Seventeen patients with preoperative CD were identified. Seven (41%) patients developed postoperative recurrent CD in the afferent limb (n = 3) or pouch fistulizing disease (n = 4). One patient (6%) required pouch excision. The incidence of postoperative CD was higher (P = 0.002) in preoperative CD patients (41%) than UC patients (11%). There was no significant difference in pouchitis or pouch failure. There was also no significant difference in any preoperative clinical feature between patients with or without postoperative CD. Afferent limb inflammation developed in three (50%) of the six patients with pANCA+/OmpC− expression compared to none of the 11 patients without this serologic profile (P = 0.03).
Conclusions
Although the intentional use of IPAA in CD has a higher incidence of postoperative disease vs. UC patients, there was no significant difference in pouch failure. Demographics, clinical features, and serologic factors do not predict outcome of CD patients undergoing IPAA. IBD serology may identify the phenotype manifestation of postoperative recurrent CD.
Keywords: ileal pouch-anal anastomosis, Crohn’s disease, surgical complications, pouch failure
Ileal pouch-anal anastomosis (IPAA) is the operative approach for patients requiring colectomy because of familial polyposis, ulcerative colitis (UC), or in selected patients with inflammatory bowel disease unclassified (IBDU).1,2 A preoperative diagnosis of Crohn’s disease (CD) is generally considered a contraindication to IPAA as it is generally believed that: 1) disease recurrence within or above the ileal reservoir is high; 2) there is a high potential for fistulas, stricture, suture line failure, and abscess formation; and 3) pouch excision will ultimately be necessary. In addition, extensive small-bowel resection to correct the condition could result in impaired nutrient and water absorption.3,4
This viewpoint, however, has been challenged by some authors, citing data showing low pouch loss and favorable functional results in patients with CD confined to the large bowel without associated perianal disease.5,6 Little is known, however, regarding the outcome of IPAA in colorectal CD patients with associated disease in the small bowel and/or perianal disease. One recent small study, however, demonstrated that although postoperative CD was common in this unique patient subgroup, it was not an inevitable outcome.7 Clinical or subclinical risk factors for the development of recurrent CD, however, were not reported. In addition, the study was limited by referral center bias at a specialized “Pouchitis Clinic.”
Serologic immune markers have been associated with IBD. Perinuclear antineutrophil cytoplasmic antibodies (pANCA) are found in 60%–80% of UC patients.8,9 pANCA is also present in 15%–25% of patients with CD, where it is associated with a “UC-like” clinical picture.10 Anti-Saccharomyces cerevisiae antibodies (ASCA) are found in about 60% of CD patients.11,12 While serum pANCA and ASCA are the best-studied serologic markers for IBD, antibodies against the outer membrane porin C (anti-OmpC) of Escherichia coli and anti-CBir1 are also found in about 50% of patients with CD and to a lesser degree in UC patients.9,13 The value of these disease markers in predicting the outcome of intentional IPAA in CD patients has not been defined.
In an effort to understand this problem, we compared surgical outcomes of well-characterized UC and colorectal CD patients, some of whom also had small bowel and/or perianal disease. In addition, we attempted to identify associations between clinical and IBD-associated seromarker expression and surgical outcomes in this highly selected group of CD patients.
MATERIALS AND METHODS
Study Population
Consecutive UC and CD patients requiring colectomy for medically refractory disease or dysplasia from 1994 to 2010 were studied in a prospectively maintained database. Mucosectomy and hand-sewn anastomosis was performed in all patients by one surgeon (P.F.). Patients were seen for follow-up examination every 3 months for the first year after stoma closure and yearly afterwards. All research related activities were approved by the Cedars-Sinai Medical Center Institutional Review Board (IRB no. 3358).
Assessment of Clinical Characteristics
Detailed clinical profiles were prospectively generated by one investigator (P.F.) using chart and patient interview. Demographic information assessed included patients’ gender, age at time of surgery, smoking history, and family history of IBD. Patients smoking at the time of surgery and/or after surgery were considered smokers. Disease characteristics examined included disease duration, extent of diseased colon, extra-intestinal manifestations, and length of follow-up after surgery. Disease duration refers to the time interval between IBD diagnosis and the date of colectomy. Disease extent was classified as either pancolitis or left-sided colitis.
Treatment characteristics tabulated included the nature of medical therapy before colectomy (steroids, immunomodulators, biologics) and indications for surgery (medically refractory disease vs. dysplasia/cancer). Patients treated with multiple medications were categorized by the highest level of immunosuppression (biologics > immunomodulators > steroids). Medication used to treat postoperative CD was assessed, including oral antibiotics, steroids, immunomodulators, and/or biologic agents. The effectiveness of medical therapy was assessed as either pouch salvage or pouch failure.
Diagnosis of UC and CD
Clinical, endoscopic, and pathologic criteria were reviewed in all patients to determine the diagnosis of UC or CD. Clinically, UC patients had no perianal disease, and endoscopic features included continuous macroscopic disease extending varying distances from the dentate line. Radiologic evaluation revealed the distinct absence of either a colonic stricture or small-bowel disease, and histologic patterns of continuous microscopic inflammation were found.
Patients were considered to have CD before surgery based on the presence of small bowel disease, perianal disease, noncrypt-associated granuloma, or pretreatment skip lesions within the colon. Small-bowel inflammation was determined with small-bowel imaging, direct visualization on wireless capsule endoscopy, or histopathologic evaluation of resected small bowel. Perianal disease was defined as the presence of perianal/rectal abscesses or fistulas. Since granulomas may be present in the mucosal layer of patients with UC in response to mucin release from a ruptured crypt,14 the diagnosis of CD was only made if histologic evaluation showed noncrypt-associated granuloma. Patients with clinical features of UC with some features suggestive but not diagnostic of CD, such as those with posttreatment skip lesions, were considered to have IBDU and were excluded from the study.
Surgical Treatment
Although a two-stage total proctocolectomy with IPAA was the intended surgical approach, an initial subtotal colectomy (STC) was necessary in some patients. Reasons for STC included toxic megacolon and/or signs of perforation, those in whom an IPAA was not technically feasible, or in patients where the tissues were thought to be too “fragile” to safely stretch the ileal pouch into the pelvis. While somewhat subjective, these criteria reflect current “standard of practice” as they are used widely by surgeons commonly operating on IBD patients. Postoperative morbidity and mortality was recorded during the 30-day period from surgery. Only complications arising from the initial colectomy (i.e., IPAA or STC) were recorded. These complications were classified as either medical or surgical, and were further characterized as being either major or minor in nature. The complication descriptions and divisions were based on definitions established from a prior IBD surgical study.15 In essence, patients requiring postoperative ICU transfer, reoperation, and/or hospital readmission were considered to have a major complication. All other complications were classified as minor in nature. If a patient had more than one complication, then the most severe complication or the complication that was the most likely source for the others was included.
Dignosis of Pouchitis and CD After Surgery
Pouchitis was defined as a clinical syndrome characterized by the onset of increased stool frequency often with bloody diarrhea, pelvic discomfort, urgency, malaise, and fever. The diagnosis of pouchitis was confirmed in all cases by endoscopy with afferent ileal limb intubation. Acute pouchitis was defined as antibiotic responsive flares occurring at least 4 months apart, during which time the patient was completely asymptomatic and had returned to his/her usual bowel pattern. Chronic pouchitis (CP) required continuous antibiotic treatment for symptom relief and also included patients refractory to antibiotic treatment. Stool studies were obtained when conventional antibiotic therapy was unsuccessful or in patients with CP. However, pouchoscopy with biopsies was performed in all CP patients looking for granulomatous inflammation or cytomegalovirus inclusion bodies.16 For patients with persistent symptoms, studies were done to exclude mechanical complications of surgery such as an anal stricture or partial small bowel obstruction. Patients not consenting to have blood drawn for research purposes and those using nonsteroidal anti-inflammatory drugs17 were excluded from analysis.
Postoperative CD was diagnosed either when mucosal inflammation (five or more ulcers) involved the afferent ileal limb (small bowel mucosa proximal to the ileal pouch) any time after surgery and/or when a pouch fistula or anal complication developed more than 3 months after ileostomy closure. The diagnosis of CD was confirmed in all cases using a number of criteria, including examination under anesthesia and/or contrast pouchography. All patients also underwent pouchoscopy, including specific evaluation and biopsy of the afferent limb and pouch. Histologic evidence of granulomatous inflammation in pouch or small bowel biopsies was not necessary for the diagnosis of postoperative CD. Time to diagnosis of pouchitis or postoperative CD was defined as the time period from ileostomy closure.
Serologic Analysis
Serum was drawn immediately before colectomy, coded, and stored for future analysis. All sera were analyzed in a blinded fashion by Prometheus Laboratories (San Diego, CA) or performed at Cedars-Sinai Medical Center. Stored serum was analyzed for expression of IBD-associated antibodies, including ASCA, anti-OmpC, anti-CBir1, anti-I2, and pANCA in a blinded fashion by enzyme-linked immunosorbent assay (ELISA). All assays for anti-I2 were performed at Cedars-Sinai Medical Center. Qualitative positivity to any antibody was defined as being greater than cutoff values greater than 2 standard deviations above mean control titers for each assay. All assays were performed blinded without knowledge of patient clinical characteristics. Similarly, clinical course after IPAA was assessed without knowledge of the patient’s serologic profile. Patients lacking complete preoperative serologic analysis were excluded from the analysis.
Statistical Analysis
All data were entered into a standardized database computer program (Microsoft Excel, Seattle, WA). For continuous covariates, medians were compared with Wilcoxon’s nonparametric tests. Categorical variables were compared with the use of the chi-squared method or Fisher’s; exact test (if expected cell counts were less than 5). P < 0.05 was considered statistically significant.
RESULTS
Patient Demographics and Clinical Characteristics
Patient demographic and clinical characteristics of the preoperative CD (n = 17) and UC (n = 261) study patients are shown in Table 1. The diagnosis of CD before surgery was based on involvement of the small bowel (n = 5), noncrypt-associated granulomas (n = 5), perianal disease (n = 4), or discontinuous inflammation (n = 3). All patients with small bowel disease had previously undergone surgical resection, and none had evidence of active disease at the time of IPAA. CD patients with perianal disease included anal fistula (n = 3) and stenosis (n = 1). At IPAA, all fistulas had been treated with seton drainage and were clinically uninfected. There was a majority of males in both patient groups. Most patients had surgery for medically refractory disease. There was a significantly higher use of biologic therapy and three-stage IPAA in the preoperative CD patient group compared to the preoperative UC patient group.
TABLE 1.
Clinical Features of the Study Groups
| Preoperative Crohn’s Disease (n=17) |
Preoperative Ulcerative Colitis (n=261) |
|
|---|---|---|
| Sex (M/F) | 11/6 | 156/105 |
| Median age in years | 45 (13–64) | 38 (7–81) |
| Median disease duration in months |
101 (24–636) | 72 (1–600) |
| Disease extent (%) | ||
| Pancolitisa | 12 (71) | 200 (76) |
| Left-sided | 5 (29) | 61 (24) |
| Preoperative medication (%) | ||
| Steroids alone | 2 (12) | 34 (13) |
| Immunomodulators | 4 (23) | 176 (67) |
| Biologics | 11 (65) | 51 (20)b |
| Extraintestinal disease (%) | ||
| Arthritis | 2 (12) | 42 (16) |
| PSC | 0 | 8 (3) |
| Other | 0 | 13 (5) |
| Family history of IBD (%) | 3 (18) | 63 (24) |
| Smoking history (%) | 4 (24) | 81 (31) |
| Indication for IPAA (%) | ||
| Medically unresponsive | 15 (88) | 209 (80) |
| Cancer/dysplasia | 2 (12) | 52 (20) |
| Surgical Treatment | ||
| 2-stage IPAA | 7 (41) | 196 (75) |
| 3-stage IPAA | 10 (59) | 65 (25)c |
| Preoperative features of CD (%) | ||
| Small bowel disease | 5 (29) | — |
| Noncaseating granuloma | 5 (29) | — |
| Discontinuous inflammation | 3 (18) | — |
| Perianal disease | 4 (24) | — |
Pancolitis included skip lesions in CD patient group.
P = 0.0001.
P = 0.004.
Values in parentheses except range denote percentage.
PSC, primary sclerosing cholangitis; IBD, inflammatory bowel disease; IPAA, ileal pouch-anal anastomosis; CD, Crohn’s disease.
Serologic Expression
Initially, we determined the serum reactivity to each of these antigens in our study cohorts (Fig. 1). Interestingly, none of preoperative CD patients expressed ASCA IgA or IgG. In the UC group and preoperative CD group, serum pANCA, anti-I2, anti-OmpC, and anti-CBir1 were detected in 146 patients (58%) vs. 10 patients (59%), 57 patients (22%) vs. 2 patients (12%), 40 patients (15%) vs. 4 patients (24%), and 30 patients (11%) vs. 5 patients (29%), respectively. None of these trends, however, were statistically significant. Quantitative antibody levels were also comparable between patient groups (Table 2).
FIGURE 1.
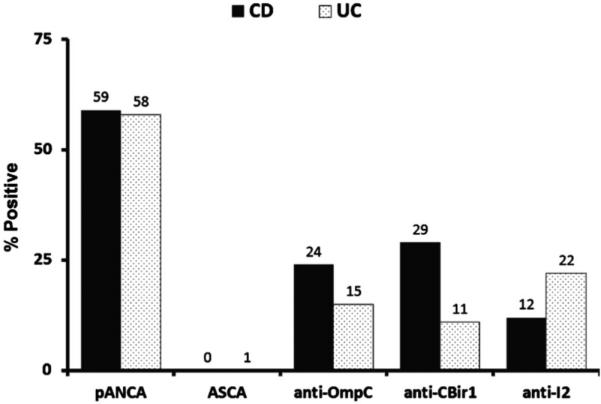
Seromarker expression in the preoperative Crohn’s dis ease (CD) and ulcerative colitis (UC) patient groups.
TABLE 2.
IBD Serologic Expression
| Preoperative Crohn’s Disease (n=17) |
Preoperative Ulcerative Colitis (n=261) |
|
|---|---|---|
| pANCA | ||
| Positive | 10 (59) | 146 (58) |
| Median level | 35 | 30 |
| High-level (>100 EU/ml) | 2 (12) | 23 (9) |
| ASCA | ||
| IgA+ | 0 | 4 (1.5) |
| IgG+ | 0 | 1 (0.4) |
| Either IgA+ or IgG+ | 0 | 3 (1.1) |
| Both IgA+ and IgG+ | 0 | 1 (0.4) |
| Median level IgA+ | 0 | 5 |
| Median level IgG+ anti-OmpC |
0 | 6 |
| Positive | 4 (24) | 40 (15) |
| Median level anti-CBirl |
11 | 13 |
| Positive | 5 (29) | 30 (11) |
| Median level anti-I2 |
18 | 16 |
| Positive | 2 (12) | 57 (22) |
| Median level | 9 | 11 |
All levels expressed as ELISA units/mL.
Short-term Surgical Outcomes
There was no postoperative mortality in this study (Table 3). Postoperative complications were noted in 99 patients, representing an overall rate of 35%. The most common complications were superficial surgical site infection (n = 21) and ileus (n = 30). There was no statistically significant difference in surgical morbidity between patient groups.
TABLE 3.
Surgical Outcomes After Ileal Pouch-Anal Anastomosis
| Preoperative Crohn’s Disease (n=17) |
Preoperative Ulcerative Colitis (n=261) |
|
|---|---|---|
| Postoperative complications (%) | 4 (24) | 95 (36) |
| Major medical | 2 (12) | 13 (13) |
| Minor medical | 0 | 10 (4) |
| Major surgical | 1 (6) | 38 (15) |
| Minor surgical | 1 (6) | 34 (13) |
| Pouchitis (%) | 6 (35) | 75 (29) |
| Acute pouchitis | 3 (18) | 40 (15) |
| Chronic pouchitis | 3 (18) | 35 (13) |
| Postoperative CD | 7 (41) | 27 (11)a |
| Pouch failure (%) | 1 (6) | 5 (2) |
| Length of follow up (months) | 60 (17–159) | 36 (1–194) |
P = 0.002. CD, Crohn’s disease.
Long-term Surgical Outcomes
The median follow-up time after ileostomy closure for the entire study cohort was 38 months (range, 1–194 months) and was not significantly different between patient groups. There was also no significant difference in the incidence of acute or chronic pouchitis between patient groups. Median time to acute pouchitis was 4 months (range, 4–45 months) in the preoperative CD group vs. 9 months (range, 2–57 months) in the preoperative UC group. Median time to chronic pouchitis was 8 months (range, 7–45 months) in the preoperative CD group vs. 7 months (range, 4–57 months) in the preoperative UC group. None of these differences were statistically significant.
Seven patients (41%) in the preoperative CD group developed postoperative CD vs. 27 patients (11%) of the UC group (P = 0.002). Median time to postoperative CD diagnosis was similar for the preoperative CD group (10 months; range, 3–48) and the preoperative UC group (9 months; range, 4–184). In the preoperative CD group, recurrent CD was diagnosed on the basis of perianal disease in four (57%) patients and afferent ileal limb disease in three (43%) patients. As for the preoperative UC group, 23 patients had afferent limb inflammation and four patients developed perianal disease. Of the seven CD patients with recurrent inflammation, three patients were maintained on immunosuppressive therapy and another three patients were controlled with antibiotics alone. Only one patient (6%) of the preoperative CD patient cohort with severe pouch inflammation and perianal disease required pouch excision and permanent ileostomy after failing aggressive medical therapy. The incidence of pouch failure was not statistically significant between patient groups.
Factors Associated with Development of Postoperative CD
There was no clinical factor associated with the development of recurrent inflammation or pattern of inflammation in CD patients undergoing IPAA (Table 4). We could also not demonstrate any significant correlation between individual or combination seromarker expression and recurrent inflammation or pattern of inflammation (data not shown). However, afferent limb inflammation developed in three (50%) of the six patients with pANCA+/OmpC− expression compared to none of the 11 patients without this serologic profile (P = 0.03) (Fig. 2).
TABLE 4.
Clinical Characteristics in Patients with and Without Recurrent CD
| Recurrent Crohn’s Disease (n=17) |
Afferent Limb Inflammation (n=3) |
Pouch Fistula (n=4) |
||
|---|---|---|---|---|
| No (n=10) | Yes (n=7) | |||
| Sex (M/F) | 6/4 | 5/2 | 3/0 | 2/2 |
| Median age in years | 44 (13–64) | 48 (21–57) | 50 (45–68) | 48 (21–57) |
| Median disease duration in months | 66 (26–576) | 106 (72–636) | 180 (120–336) | 106 (72–636) |
| Disease extent (%) | ||||
| Pancolitis | 6 (60) | 6 (86) | 3 (100) | 3 (75) |
| Left-sided | 4 (40) | 1 (14) | 0 | 1 (25) |
| Preoperative medication (%) | ||||
| Steroids alone | 1 (10) | 1 (14) | 0 | 1 (25) |
| Immunomodulators | 4 (40) | 0 | 0 | 0 |
| Biologics | 5 (50) | 6 (86) | 3 (100) | 3 (75) |
| Extraintestinal disease (%) | ||||
| Arthritis | 2 (20) | 0 | 0 | 0 |
| PSC | 0 | 0 | 0 | 0 |
| Other | 0 | 0 | 0 | 0 |
| Family history of IBD (%) | 2 (20) | 1 (14) | 1 (33) | 0 |
| Smoking history (%) | 4 (40) | 0 | 0 | 0 |
| Indication for IPAA (%) | ||||
| Medically unresponsive | 10 (100) | 5 (71) | 2 (67) | 3 (75) |
| Cancer/dysplasia | 0 | 2 (28) | 1 (33) | 1 (25) |
| Surgical Treatment | ||||
| 2-stage IPAA | 5 (50) | 5 (71) | 3 (100) | 2 (50) |
| 3-stage IPAA | 5 (50) | 2 (28) | 0 | 2 (50) |
| Preoperative features of CD (%) | ||||
| Small bowel disease | 4 (40) | 2 (28) | 2 (67) | 0 |
| Noncaseating granuloma | 3 (30) | 0 | 0 | 0 |
| Discontinuous inflammation | 2 (20) | 2 (28) | 1 (33) | 1 (25) |
| Perianal disease | 1 (10) | 3 (42) | 0 | 3 (75) |
Values in parentheses except range denote percentage.
PSC, primary sclerosing cholangitis; IBD, inflammatory bowel disease; IPAA, ileal pouch-anal anastomosis; CD, Crohn’s disease.
FIGURE 2.
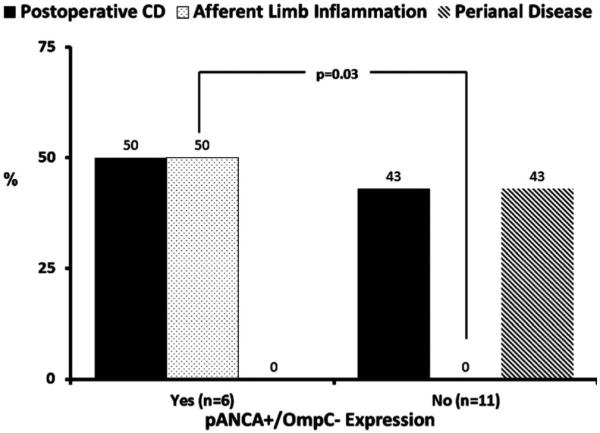
Association between pANCA+/OmpC− expression and CD phenotype after ileal pouch-anal anastomosis.
DISCUSSION
The main finding of this study is that the intentional use of IPAA in patients with preoperative CD is associated with a higher incidence of postoperative CD compared to UC patients. However, there was no significant difference in pouch failure. Although CD patients with disease isolated to the colon can be satisfactorily managed with a total colectomy and ileorectal anastomosis, surgical decision-making becomes more difficult when the rectum is too diseased for a safe surgery, particularly when there is associated small bowel and/or perianal disease. While most colorectal surgeons would not recommend an IPAA in this circumstance, some motivated CD patients, particularly young of age, would rather ignore the potential for disease recurrence, reoperation, pouch failure, and permanent stoma and submit to an IPAA. Based on the data from this study, this surgical approach may be a viable option in these highly selected CD patients, particularly if they do not express the seromarker profile pANCA+/OmpC−.
There was a much higher incidence of using biologic therapy in the preoperative CD patient group vs. the preoperative UC group. This observation may simply reflect that infliximab was FDA-approved for use in CD 7 years before its approval in UC. However, the widely held belief that medically refractory CD patients cannot be safely treated with an IPAA may have led the patients’ physicians to prolong medical therapy at all costs. However, as many surgeons are also reluctant to offer IPAA to patients with atypical features of UC due to the presumed increased risk of complications, pouch failure, and evolution into CD, the use of staged surgical procedures was also higher in the preoperative CD patient group. Perhaps data from this study will begin to alter these practice patterns.
A unique feature of this study is that we included CD patients with inflammation of the colorectum also having small bowel and/or perianal disease. Despite there being a reasonable body of literature examining the association between IPAA and CD, most studies have not answered the vexing question of whether we should intentionally be performing IPAA in these CD patients (Table 5). Many of the prior reports also included patients diagnosed with CD in the immediate postoperative period, based primarily on histopathologic evaluation of the resected colon. As we have previously demonstrated that no single atypical histopathologic feature of CD, or combination of features, appears to be associated with any adverse pouch outcome after IPAA,18 it is clear that patients diagnosed with “CD” after surgery based on histopathologic criteria behave differently than well-characterized CD patients diagnosed before surgery.
TABLE 5.
Studies of Ileal Pouch-Anal Anastomosis for Crohn’s Disease
| Diagnostic Criteria for Crohn’s Disease |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study (yr) | N | Skip Lesion |
Granuloma | Perianal Disease |
Stricture | Transmural Inflammation |
Small Bowel Disease |
Postop CD (%) |
Pouch Failure (%) |
| Preop CD Diagnosis | |||||||||
| Hyman (91) | 9 | + | − | + | + | − | + | NS | 89 |
| Regimbeau (01) | 26 | + | All | − | − | − | − | 15 | NS |
| Melton (08) | 20 | − | All | − | − | + | − | NS | 15 |
| Shen (10) | 11 | Pre-rx | All | NCG | + | + | + | 64 | 9 |
| Grucela (11) | 13 | − | All | − | + | + | − | NS | 15 |
| Current study | 17 | Pre-rx | True | + | − | − | + | 41 | 6 |
| Postop CD Diagnosis | |||||||||
| Grobler (93) | 10 | NS | All | NS | NS | NS | NS | NS | 30 |
| Morpurgo (03) | 13 | − | All | + | − | + | − | 62 | 23 |
| De Oca (03) | 12 | + | All | − | − | + | − | 16 | 16 |
| Brown (05) | 36 | + | All | + | − | + | + | NS | 56 |
CD, Crohn’s disease; Pre-rx, pretreatment; NCG, noncryptoglandular; NS, not stated.
Studies specifically examining the role of intentional IPAA for CD are also shown in Table 5. The incidence of CD development after IPAA varies widely (15%–64%). The wide discrepancy in pouch outcomes among published studies may be attributable to multiple factors, including study inclusion and exclusion criteria, diagnostic criteria used for CD of the pouch, and possibly issues regarding differences in surgical expertise. It is clear, however, that not all CD patients undergoing IPAA develop recurrent disease of the pouch and/or afferent limb.
IBD patients with distinct serologic patterns have different forms of pouch inflammation following IPAA.9 While not the primary finding of this study, we did find that fully one-half of CD patients undergoing IPAA who expressed the IBD seromarker combination pANCA+/OmpC− developed CD after IPAA. It is possible that the hypothesis-generating results of this study may reflect a type 1 error, and this observation must be independently validated in future studies. Despite this limitation, the present study has begun to uncover factors that may predispose some CD patients to continued inflammation after IPAA while at the same time define CD patients successfully treated with an IPAA.
Another interesting finding was the relatively low incidence (6%) of pouch loss, even in patients developing inflammation after IPAA. Older studies suggested that almost one-half of patients with CD in their pouch required fecal diversion for symptom control.19-21 More recent studies have reported a much lower incidence of pouch failure, ranging from 16%–28%. In the current study, only one of seven (14%) CD patients with recurrent inflammation after IPAA required a permanent diverting ileostomy. It is more than likely that these results can only be expected to improve with the increasing use of potent therapies such as biologic agents. Interestingly, 14 of the 17 (82%) study patients were treated with biologics after surgery.
A major limitation of this study was that the follow-up of the CD patients was relatively short (60 months). It is well known that the development of CD after IPAA in patients with UC can occur many years after surgery.22 Before definitive conclusions regarding the outcomes of IPAA intentionally performed in CD can be made, follow-up and reanalysis of the study cohort in few years will be necessary. Another study limitation is the small number of patients. Despite being one of the largest reported series of CD patients intentionally undergoing IPAA, small patient numbers precluded robust analysis of the association between clinical and serologic data and postoperative outcome.
In conclusion, despite the presence of perianal and/or small bowel disease before colectomy, CD of the pouch does not always develop after IPAA. These preliminary observations suggest that highly motivated, young patients with colorectal CD also involving the more proximal and/or distal gastrointestinal tract may wish to undergo IPAA. Furthermore, the data provided herein will enable information for fully informed consent and risk–benefit analyses as patients consider their surgical options in this setting.
REFERENCES
- 1.Meagher AP, Farouk R, Dozois RR, et al. J ileal pouch-anal anastomosis for chronic ulcerative colitis: complications and long-term outcome in 1310 patients. Br J Surg. 1998;85:800–803. doi: 10.1046/j.1365-2168.1998.00689.x. [DOI] [PubMed] [Google Scholar]
- 2.Murrell ZA, Melmed GY, Ippoliti A, et al. A prospective evaluation of the long-term outcome of ileal pouch-anal anastomosis in patients with inflammatory bowel disease-unclassified and indeterminate colitis. Dis Colon Rectum. 2009;52:872–878. doi: 10.1007/DCR.0b013e31819f5d4c. [DOI] [PubMed] [Google Scholar]
- 3.Brown CJ, Maclean AR, Cohen Z, et al. Crohn’s disease and indeterminate colitis and the ileal pouch-anal anastomosis: outcomes and patterns of failure. Dis Colon Rectum. 2005;48:1542–1549. doi: 10.1007/s10350-005-0059-z. [DOI] [PubMed] [Google Scholar]
- 4.Reese GE, Lovegrove RE, Tilney HS, et al. The effect of Crohn’s disease on outcomes after restorative proctocolectomy. Dis Colon Rectum. 2007;50:239–250. doi: 10.1007/s10350-006-0777-x. [DOI] [PubMed] [Google Scholar]
- 5.Regimbeau JM, Panis Y, Pocard M, et al. Long-term results of ileal pouch-anal anastomosis for colorectal Crohn’s disease. Dis Colon Rectum. 2001;44:769–778. doi: 10.1007/BF02234693. [DOI] [PubMed] [Google Scholar]
- 6.Melton GB, Fazio VW, Kiran RP, et al. Long-term outcomes with ileal pouch-anal anastomosis and Crohn’s disease: pouch retention and implications of delayed diagnosis. Ann Surg. 2008;248:608–616. doi: 10.1097/SLA.0b013e318187ed64. [DOI] [PubMed] [Google Scholar]
- 7.Shen B, Patel S, Lian L. Natural history of Crohn’s disease in patients who underwent intentional restorative proctocolectomy with ileal pouch-anal anastomosis. Aliment Pharmacol Ther. 2010;31:745–753. doi: 10.1111/j.1365-2036.2009.04227.x. [DOI] [PubMed] [Google Scholar]
- 8.Saxon A, Shanahan F, Landers C, et al. A distinct subset of antineutrophil cytoplasmic antibodies is associated with inflammatory bowel disease. J Allergy ClinImmunol. 1990;86:202–210. doi: 10.1016/s0091-6749(05)80067-3. [DOI] [PubMed] [Google Scholar]
- 9.Fleshner PR, Vasiliauskas EA, Dubinsky M, et al. Both preoperative pANCA and anti-CBir1 expression in ulcerative colitis patients influence pouchitis development after ileal pouch-anal anastomosis. ClinGastroenterol Hepatol. 2008;6:561–568. doi: 10.1016/j.cgh.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasiliauskas EA, Plevy SE, Landers CJ, et al. Perinuclear antineutrophil cytoplasmic antibodies in patients with Crohn’s disease define a clinical subgroup. Gastroenterology. 1996;110:1810–1819. doi: 10.1053/gast.1996.v110.pm8964407. [DOI] [PubMed] [Google Scholar]
- 11.Quinton JF, Sendid B, Reumaux D, et al. Anti-Saccharomyces cerevisiaemannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–791. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutton CL, Yang H, Li Z, et al. Familial expression of anti-Saccharomyces cerevisiaemannan antibodies in affected and unaffected relatives of patients with Crohn’s disease. Gut. 2000;46:58–63. doi: 10.1136/gut.46.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Targan SR, Landers CJ, Yang H, et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128:2020–2028. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 14.Mahadeva U, Martin JP, Patel NK, et al. Granulomatous ulcerative colitis: a re-appraisal of the mucosal granuloma in the distinction of Crohn’s disease from ulcerative colitis. Histopathology. 2002;41:166–168. doi: 10.1046/j.1365-2559.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 15.Hyde GM, Jewell DP, Kettlewell MGW, et al. Cyclosporin for severe ulcerative colitis does not increase the rate of perioperative complications. Dis Colon Rectum. 2001;44:1436–1440. doi: 10.1007/BF02234594. [DOI] [PubMed] [Google Scholar]
- 16.Munoz-Juarez M, Pemberton JH, Sandborn WJ, et al. Misdiagnosis of specific cytomegalovirus infection of the ileoanal pouch as refractory idiopathic chronic pouchitis. Dis Colon Rectum. 1999;42:117–120. doi: 10.1007/BF02235196. [DOI] [PubMed] [Google Scholar]
- 17.Shen B, Fazio VW, Remzi FH, et al. Comprehensive evaluation of inflammatory and noninflammatory sequelae of ileal pouch-anal anastomosis. Am J Gastroenterol. 2005;100:93–101. doi: 10.1111/j.1572-0241.2005.40778.x. [DOI] [PubMed] [Google Scholar]
- 18.Nasseri Y, Melmed GY, Wang HL, et al. Rigorous histopathologic assessment of the colectomy specimen in patients with inflammatory bowel disease-unclassified does not predict outcome after ileal pouch-anal anastomosis. Am J Gastroenterol. 2010;105:155–161. doi: 10.1038/ajg.2009.510. [DOI] [PubMed] [Google Scholar]
- 19.Peyregne V, Francois Y, Gilly FN, et al. Outcome of ileal pouch after secondary diagnosis of Crohn’s disease. Int J Colorectal Dis. 2000;15:49–53. doi: 10.1007/s003840050007. [DOI] [PubMed] [Google Scholar]
- 20.Melmed G, Fleshner PR, Bardakcioglu O, et al. Family history and serology predict Crohn’s disease after ileal pouch-anal anastomosis for ulcerative colitis. Dis Colon Rectum. 2008;51:100–108. doi: 10.1007/s10350-007-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen B, Remzi FH, Brzezinski A, et al. Risk factors for pouch failure in patients with different phenotypes of Crohn’s disease of the pouch. Inflamm Bowel Dis. 2008;14:942–948. doi: 10.1002/ibd.20409. [DOI] [PubMed] [Google Scholar]
- 22.Melmed G, Fleshner PR, Bardakcioglu O, et al. Family history and serology predict Crohn’s disease after ileal pouch-anal anastomosis for ulcerative colitis. Dis Colon Rectum. 2008;51:100–108. doi: 10.1007/s10350-007-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


