Abstract
Basal cell carcinoma (BCC) is the most common human cancer. Patients with basal cell nevus syndrome (Gorlin syndrome) are highly susceptible to developing many BCCs as a result of a constitutive inactivating mutation in one allele of PATCHED 1, which encodes a tumor suppressor that is a major inhibitor of Hedgehog signaling. Dysregulated Hedgehog signaling is a common feature of both hereditary and sporadic BCCs. Recently, we showed remarkable anti-BCC chemopreventive efficacy of tazarotene, a retinoid with retinoic acid receptor (RAR) β/γ specificity, in Ptch1 +/− mice when treatment was commenced before carcinogenic insults. In this study, we assessed whether the effect of tazarotene against BCC carcinogenesis is sustained after its withdrawal and whether tazarotene is effective against preexisting microscopic BCC lesions. We found that BCCs did not reappear for at least 5 months after topical drug treatment was stopped and that already developed, microscopic BCCs were susceptible to tazarotene inhibition. In vitro, tazarotene inhibited a murine BCC keratinocyte cell line, ASZ001, suggesting that its effect in vivo is by direct action on the actual tumor cells. Down-regulation of Gli1, a target gene of Hedgehog signaling and up-regulation of CRABPII, a target gene of retinoid signaling, were observed with tazarotene treatment. Finally, we investigated the effects of topical applications of other retinoid-related compounds on BCC tumorigenesis in vivo. Tazarotene was the most effective of the preparations studied, and its effect most likely was mediated by RARγ activation. Furthermore, inhibition of basal RAR signaling in the skin promoted BCC carcinogenesis, suggesting that endogenous RAR signaling restrains BCC growth.
Introduction
Cutaneous basal cell carcinoma (BCC) affects ~1 million Americans every year. Approximately 1 in 50,000 individuals has the autosomal dominant disorder basal cell nevus syndrome (BCNS; Gorlin syndrome MIM 10900) and consequently develops many BCCs (1). BCNS results from an inactivating mutation of one PATCHED 1 (PTCH1) allele (2, 3). PTCH1 functions as a negative regulator of the Hedgehog pathway by inhibiting Smoothened, a positive effector of Hedgehog signaling. Binding of Hedgehog to PTCH1 abrogates the repression of Smoothened, allowing the transcriptional activation of Hedgehog target genes (including PTCH1 and GLI1 themselves) by GLI transcription factors (4). To identify potential chemopreventive treatments for BCC tumorigenesis, we are using the Ptch1 +/− mouse in which a nonfunctional Ptch1 allele was generated by disruption of the Ptch1 exons 1 and 2 with the lacZ gene (5). Exposure of these mice to ultraviolet (UV) radiation or ionizing radiation (IR) produces high BCC incidence (6).
Retinoids are vitamin A derivatives that are ligands for the retinoic acid receptor (RAR) and the retinoid × receptor (RXR) transcription factors and have been widely used to treat various cancers, such as leukemia and nonmelanoma skin cancers (7). RAR and RXR each have three main subtypes, α, β, and γ, of which further isoforms are generated by alternative splicing using different gene promoters (8). Endogenous retinoid signaling mediated by the bioactive retinoid, all-trans retinoic acid (ATRA), is essential for many cellular processes including cell specification, differentiation, and apoptosis (9). Although systemic retinoic acids have been used with some success to treat cancers including leukemia and nonmelanoma skin cancers, they cause significant toxicities and are often discontinued (10, 11). Long-term topical treatment with the synthetic retinoid “prodrug” tazarotene (Tazorac; Allergan) can cure one-fourth to one-half of sporadic visible human BCCs without significant toxicity (12, 13). Tazarotene is an acetylenic retinoid whose free acid metabolite, tazarotenic acid, specifically activates RARβ and RARγ and only weakly activates RARα (14). By contrast, ATRA activates all RARs and most likely all RXRs through conversion of ATRA to 9-cis retinoic acid, the RXR-specific ligand (15).
Because of the impressive, albeit limited, efficacy of tazarotene against established BCCs, we have studied its chemopreventive effect in the hope that such efficacy against smaller, preclinically detectable BCCs would be greater. Indeed, we found that tazarotene was highly effective when applications were started before the mutagenic insults and were continued “indefinitely” (16). In this study, we investigated the efficacy of tazarotene against murine BCCs under the clinically more relevant conditions of (a) starting applications “after” microscopic BCCs have developed because BCNS patients may have widespread microscopic BCC-like “budding” from the epidermis and (b) discontinuing the chemopreventive treatment to assess whether BCCs recur. In addition, the cellular and molecular effects of tazarotene treatment on BCC tumor cells were assessed in vitro. Finally, we investigated the effects of other retinoid agonists on BCC carcinogenesis in vivo as well as the consequence of inhibiting endogenous retinoic acid signaling in vivo.
Materials and Methods
Mice
Ptch1 +/− mice were maintained on an ~50:50 mixed C57BL/6 and DBA/2J background (5) under previously described husbandry conditions (16).
Murine In vivo Study Design
Tazarotene Withdrawal Study
Ptch1 +/− mice, randomized by sex and litter, were treated topically five times weekly, beginning at age ~1.5 months, with 0.1% tazarotene (AGN190168; ethyl 6-[2-(4,4-dimethylthiochroman-6-yl)-ethynyl] nicotinate) cream (pharmaceutical grade; Fig. 1A). Mice at age 2 months were exposed to 5 Gy Cs137 IR and skin biopsies were taken at age 7 months to confirm the presence of microscopic BCC lesions. Mice were then randomized into two groups by sex and litter, and each group was treated either with 0.1% tazarotene cream as before or with vehicle cream. A second skin biopsy was taken from the opposite side of the mid-back at age 12 months.
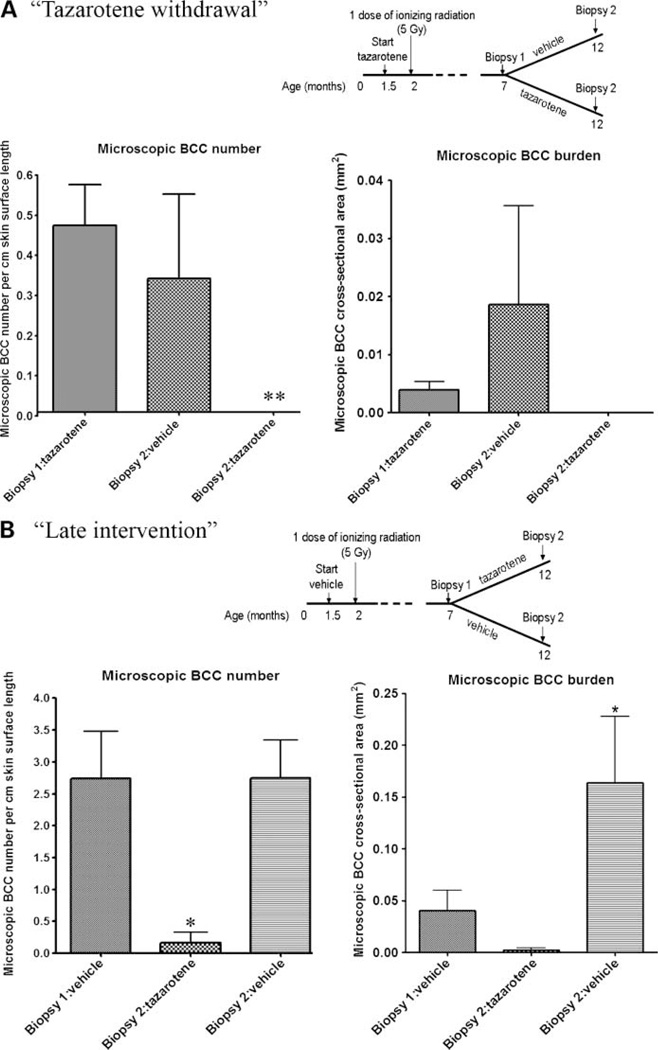
Figure 1.
Tazarotene withdrawal did not significantly increase the number and size of microscopic BCC lesions. Also, tazarotene inhibited the number and size of preexisting microscopic BCC lesions. A, tazarotene withdrawal: microscopic BCC number and size. As reported previously, tazarotene application reduced the number of microscopic BCC lesions after 5 months of treatment at age 7 months (biopsy 1: tazarotene; n = 10) compared with vehicle treatment. Mice switched to vehicle for a further 5 months had no increase in the average number and size of microscopic BCC lesions at age 12 months (biopsy 2: vehicle; n = 5), whereas mice continuously treated with tazarotene had no BCC lesions at age 12 months (biopsy 2: tazarotene; n = 5). Note: One mouse had a greater BCC tumor burden compared with the other mice in the group. B, tazarotene late intervention: at age 7 months, vehicle-treated mice (n = 13) had on average ~3 microscopic BCC lesions per centimeter skin surface length with a tumor burden of 0.04 mm2 (biopsy 1: vehicle). The group that was switched to tazarotene (n = 5) had a significant reduction in the number of microscopic BCC lesions at age 12 months (biopsy 1: control versus biopsy 2: tazarotene), whereas there was no significant difference in microscopic BCC number in 12-month-old mice continuously treated with control vehicle (biopsy 1: vehicle versus biopsy 2: vehicle; n = 8). There was no significant increase in microscopic BCC tumor burden in the 12-month-old mice that were switched to tazarotene. In mice continued on control vehicle, there appeared to be an increase in tumor burden. *, P < 0.05; **, P < 0.01.
Tazarotene Late Intervention Study
Ptch1 +/− mice, randomized by sex and litter, were treated topically five times weekly, beginning at age ~1.5 months, with vehicle cream (Fig. 1B). Cs137 IR (5 Gy) was given at age 2 months, and skin biopsies were taken at age 7 months. Mice were then randomized into two groups and treated with either 0.1% tazarotene cream or vehicle cream until rebiopsy at age 12 months.
Chemoprevention Study with Other Retinoid-Related Agents
Ptch1 +/− mice were randomized by sex and litter and treated topically either with pharmaceutical grade retinoid receptor–selective agonists, tazarotene (0.1% AGN190168; RARβ/γ agonist), 0.1% ATRA (pan-RAR agonist), 1.0% AGN195813 (RARα agonist), and 0.3% AGN194204 (pan-RXR agonist) or 0.01% AGN194310 (RAR antagonist) from age 1.5 months and exposed to 515 mJ/cm2 UVB radiation from age 2 months until age 9 months, when a single biopsy was taken. Mice were monitored for macroscopic tumors thereafter until age 16 months.
HistologicMicroscopic BCC Analysis
lacZ-encoded bacterial β-galactosidase activity and BCC number and size were assessed as described previously (6, 16).
Macroscopic Tumor Analysis
Mice were monitored for the development of visible BCCs, squamous cell carcinomas (SCC), and spindle-cell tumors (SpCT; fibrosarcomas) until age 16 months. Mice with tumors exceeding the Children’s Hospital Oakland Research Institute’s animal welfare guidelines, that is, when a tumor reached 2 cm in diameter or were ulcerated at 1 cm in diameter, were euthanized, and macroscopic tumors were recorded and examined histologically. Also, animals that appeared ill or lost >10% of their body weight after age 10 months were euthanized in accordance with the animal welfare guidelines, and tumor number and histology were assessed. Sick younger animals were not included in the assessment because we saw no visible skin tumors before age 10 months.
Cell Culture Studies
The ASZ001 cell line was maintained in 154CF medium (Cascade Biologics; ref. 17) supplemented with 2% calcium-free fetal bovine serum, 0.05 mmol/L calcium chloride, and antibiotics. C5N, an immortalized non-tumorigenic keratinocyte cell line (a generous gift from Dr. Allan Balmain; ref. 18), and Med1, a cell line established from a medulloblastoma tumor that developed in a Ptch1 +/− mouse in our laboratory, were maintained in DMEM (high-glucose) supplemented with 10% fetal bovine serum and antibiotics (University of California-San Francisco Cell Culture Facility). Tazarotene powder was dissolved in 100% DMSO at a stock concentration of 10 mmol/L and stored in darkness at −20°C until required. Cells at 70% confluency were serum starved for 1 h and treated with 1, 5, or 10 µmol/L tazarotene or 0.1% DMSO in the medium described above, except without serum, for 10, 24, and 48 h. To assess cell proliferation, cells were assayed after 48 h of treatment using the WST1 Cell Proliferation Assay (Roche Applied Science). Experiments were carried out in triplicate and done three times. For gene expression assays, cells were similarly treated, and cells were harvested at 10 and 24 h post-treatment.
Total RNA Preparation
Tumors were dissected away from hair and overlying epidermis and cut into small pieces in Trizol solution (Invitrogen). Total RNA was isolated, DNase I treated (Promega), and purified using the RNeasy mini kit (Qiagen) according to the manufacturers’ instructions.
Quantitative PCR
Total RNA was reverse transcribed into cDNA using the first-strand synthesis kit and quantitative PCR was carried out using the TaqMan Real-time PCR system (Applied Biosystems). Primer and probe sets for Gli1, Bcl2, CRABPII, and RARβ were purchased from Applied Biosystems or Qiagen. 18S rRNA expression was used as the internal standard. Each TaqMan PCR was carried out in duplicate, and the experiments were done at least twice.
Western Blotting
Back skin and macroscopic BCC tumors were obtained from IR- or UV-treated Ptch1 +/− mice. SCC tumors were obtained from DMBA/TPA-treated Ptch1 wild-type NIH/Swiss mice (generous gifts from Dr. Allan Balmain; ref. 18). Western blotting was carried out according to the antibody manufacturer’s instructions. Antibodies against RARα, RXRα (sc-551 and sc-553; Santa Cruz Biotechnology), and RARγ1(PA3-813; Affinity BioReagents) were used at dilutions of 1:200; 1:200, and 1:1,000, respectively. Equal loading of protein was monitored by incubating membranes with an antibody directed against β-actin (ab8227; Abcam). Signals were detected by use of an ECL-kit (GE Healthcare).
Immunohistochemistry
BCCs were fixed in 4% paraformaldehyde in PBS overnight at 4°C, washed in PBS, and stored in 70% ethanol. Samples were embedded in paraffin wax, sectioned at 10 µm, and stained according to the antibody manufacturer’s instructions. The antibodies used were those used for Western blotting, except at different dilutions: anti-RARα and RXRα (1:400) and anti-RARγ antibody (1:500). Antibody staining was carried out using the horseradish peroxidase VECTASTAIN ABC kit (Vector Laboratories).
Statistics
Graphs and statistical analyses were plotted and calculated using Microsoft Excel and Prism software. Statistical significance was calculated using the unpaired Student’s t test using the Prism software analysis.
Results
Tazarotene Withdrawal In vivo Study
We first investigated whether tazarotene had a curative rather than merely a growth-suppressive effect, that is, do BCCs recur when tazarotene treatment is stopped? Similar to our previous observations (16), we found that tazarotene given as a chemopreventive agent inhibited IR-induced microscopic BCCs at age 7 months (Fig. 1A; microscopic BCC number; biopsy 1: tazarotene) compared with vehicle-treated mice at age 7 months (Fig. 1B; microscopic BCC number; biopsy 1: vehicle). Tazarotene-treated mice that were switched to vehicle after age 7 months had no significant increase in microscopic BCCs once topical tazarotene was discontinued (Fig. 1A; microscopic BCC number; biopsy 1: tazarotene versus biopsy 2: vehicle, 0.47 versus 0.33; P > 0.05) and remained free of visible tumors until the study was ended at age 16 months (data not shown). Mice treated continuously with tazarotene had no detectable microscopic BCC at age 12 months (Fig. 1A; microscopic BCC number; biopsy 1: tazarotene versus biopsy 2: tazarotene, 0.47 versus 0; P < 0.01). The tumor burden at age 7 months in mice treated topically with tazarotene for 5 months from age 1.5 months was similar to our previous observations (Fig. 1A; microscopic BCC burden; ref. 16). At age 12 months, mice switched to vehicle had a slight increase in tumor burden (Fig. 1A; microscopic BCC burden; biopsy 2: vehicle). However, this increase was not statistically significant (biopsy 1: tazarotene versus biopsy 2: vehicle, 0.004 versus 0.019 mm2; P > 0.05), and the burden was ~8 times lower than that of Ptch1 +/− murine skin treated with vehicle from age 1.5 months to age 12 months (Fig. 1B; microscopic BCC burden; biopsy 2: vehicle).
Tazarotene Late Intervention In vivo Study
To test whether tazarotene is effective against microscopic BCC lesions, Ptch1 +/− mice were treated with vehicle cream five times weekly from age 1.5 months and exposed to a single dose of IR at age 2 months (Fig. 1B). Dorsal back skin biopsies taken at age 7 months (5 months after IR) from mice treated topically with vehicle had a number of microscopic BCC lesions similar to what we observed in our previously published studies (Fig. 1B; microscopic BCC number; biopsy 1: vehicle; ref. 16). Mice switched to tazarotene had significantly fewer microscopic BCCs at age 12 months than at age 7 months (Fig. 1B; microscopic BCC number; biopsy 1: vehicle versus biopsy 2: tazarotene, 2.74 versus 0.17; P < 0.05), and compared to 12 month old mice treated with vehicle continuously (Fig. 1B, microscopic BCC number; biopsy 2: vehicle versus biopsy 2: tazarotene, 2.75 versus 0.17; P < 0.01). There was no significant difference in microscopic BCC number in samples from vehicle-treated skin of mice age 7 and 12 months (Fig. 1B; microscopic BCC number; biopsy 1 vehicle versus biopsy 2: vehicle, 2.74 versus 2.75; P > 0.05).
The tumor burden in vehicle-treated 7-month-old skin was similar to our previous reports (Fig. 1B; ref. 16). Mice switched to tazarotene treatment had a decrease in tumor burden at age 12 months. However, this was not statistically significant (Fig. 1B; BCC burden; biopsy 1: vehicle versus biopsy 2: tazarotene, 0.04 versus 0.0023 mm2; P > 0.05). These data suggest that tazarotene has a growth-inhibitory effect on preexisting microscopic BCC lesions. By contrast, mice treated continuously with vehicle had a significant increase in microscopic BCC burden from age 7 to 12 months (Fig. 1B; BCC burden; biopsy 1: vehicle versus biopsy 2: vehicle, 0.04 versus 0.16 mm2; P < 0.05).
Tazarotene Inhibits Proliferation of BCC Keratinocytes In vitro
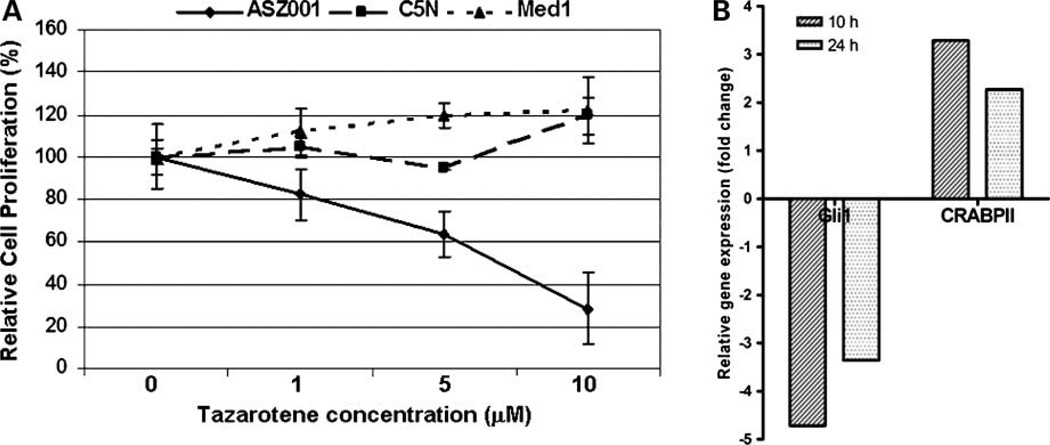
We tested whether tazarotene affects BCC epithelial cells directly by treating the murine BCC cell line ASZ001 (6, 17). Like BCC tumors in vivo (19), this cell line is growth inhibited by the Smoothened inhibitor, cyclopamine (17). To assess the specificity of the effects of tazarotene, we also treated an immortal, nontumorigenic murine epidermal keratinocyte cell line C5N (18) and a medulloblastoma cell line Med1 that was established from a Ptch1 +/− murine medulloblastoma tumor. Tazarotene treatment caused a dose-dependent reduction in cell proliferation in ASZ001 cells (the strongest effect on cell proliferation was at 10 µmol/L) but did not decrease proliferation of the C5Nor Med1 cell lines at 10 µmol/L (Fig. 2A), indicating that inhibition of ASZ001 cell proliferation observed with 10 µmol/L tazarotene was specific to BCC cells and not due to general cytotoxicity. We did not detect any apoptosis with tazarotene at 48 or 72 h as assayed by Western blotting for cleavage of caspase-3 and fluorescence-activated cell sorting analysis of DNA fragmentation of propidium iodide–stained nuclei (data not shown). Two other murine BCC epithelial cell lines generated in our laboratory, BSZ2 and CSZ001 (17), were also growth inhibited by 10 µmol/L tazarotene (data not shown).
Figure 2.
Tazarotene inhibited BCC tumor cell growth and affected Hedgehog and retinoic acid pathway target gene expression in vitro. A, tazarotene treatment of ASZ001 cells resulted in a dose-dependent decrease in cell proliferation but did not reduce cell numbers of C5N, a murine immortalized, nontumorigenic keratinocyte cell line, or Med1, a cell line established from a murine medulloblastoma tumor from a Ptch1 +/− mouse at any concentration tested. B, inhibition and activation of Hedgehog and retinoic acid target genes, respectively: tazarotene treatment of ASZ001 cells reduces Gli1 mRNA and increases CRABPII mRNA at 10 and 24 h post-treatment. Samples were normalized to 18S rRNA expression.
Tazarotene Affects the Expression of Hedgehog and Retinoid Target Genes In vitro
To investigate if tazarotene treatment affects Hedgehog target gene expression, we assessed mRNA expression of Gli1 in ASZ001 cells treated with 10 µmol/L tazarotene. We observed ~5- and 3.5-fold reductions in Gli1 mRNA levels at 10 and 24 h, respectively (Fig. 2B). Tazarotene treatment at 0.1 and 1.0 µmol/L for 24 h also decreased Gli1 mRNA levels in ASZ001 cells but to a lesser extent (data not shown). For Med1, 10 µmol/L tazarotene treatment for 24 h in serum-free conditions also reduced Gli1 transcripts, although no change in cell proliferation was observed at 48 h (data not shown). Tazarotene at 10 µmol/L in ASZ001 cells failed to affect mRNA levels of another Hedgehog target gene, Bcl2 (data not shown).
To test whether retinoid receptor signaling is intact in these cells, ASZ001 cells treated with 10 µmol/L tazarotene had a 2- to 3-fold induction of the retinoic acid target gene CRABPII, which is normally expressed in the epidermis (Fig. 2B). By contrast, RARβ, another direct retinoic acid target gene and tumor suppressor that is not normally expressed in the epidermis or in ASZ001 cells, was not induced by tazarotene (data not shown). In Med1 cells, RAR b expression was up-regulated by 10 µmol/L tazarotene, although there was no reduction in cell proliferation (data not shown).
Retinoid Receptor Expression in Skin Tumors In vivo
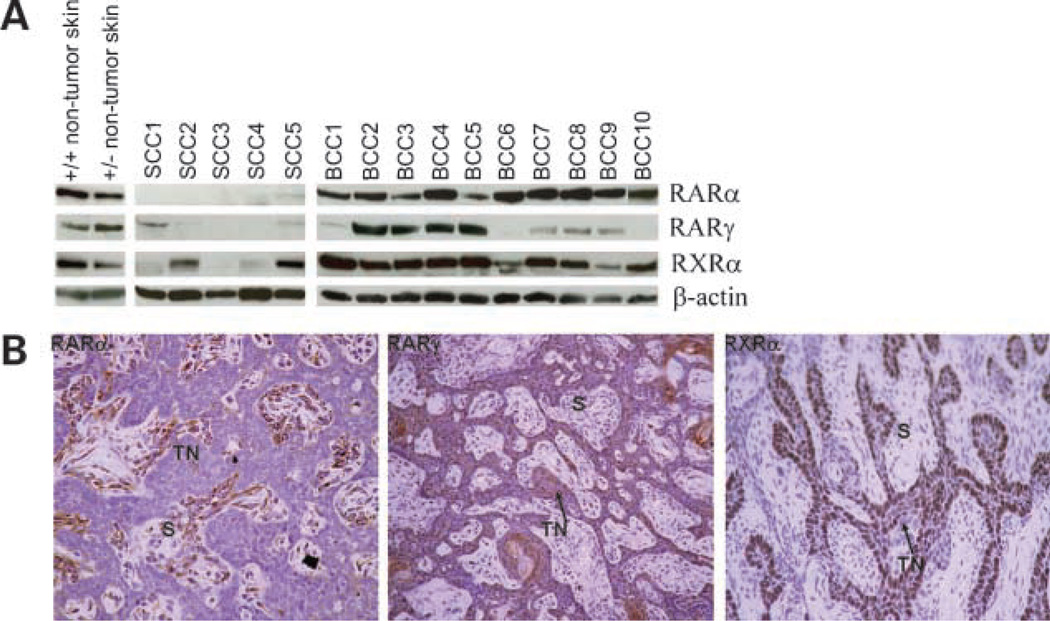
In human epidermis and BCC, RARγ and RXRα are the predominant retinoid receptors expressed, whereas RARα is present at lower levels. RXRβ is expressed at even lower levels (if at all) in human skin, whereas RARβ and RXRγ are not expressed (20, 21). To confirm that the same receptors are expressed in the skin and BCC tumors in our Ptch1 +/− mice, we carried out Western blotting on total protein lysates of 10 macroscopic BCCs and adjacent skin using antibodies against RARα, RARγ1, the predominant RARγ isoform in human epidermis (21, 22), and RXRα. Similar to human and mouse wild-type epidermis, RARα, RARγ1, and RXRα protein were expressed in murine Ptch1 +/− skin (Fig. 3A). Immunohistochemistry of murine Ptch1 +/− skin confirmed that the localization of the receptors in the epidermis was similar to published reports (data not shown). RARα and RXRα were also expressed in all 10 macroscopic BCCs analyzed, whereas 8 of these tumors expressed RARγ1 at different levels. By contrast, the five tested SCC tumors induced by topical DMBA and TPA treatment had either barely detectable or complete absence of RARα and RARγ1 proteins, and only two of five had moderate levels of RXRα protein. This loss of RAR expression in murine SCC tumors agrees with published data for murine and human SCCs (23–25). Of seven visible BCCs analyzed by immunohistochemistry, six tumors were positive for RARα protein, which was localized to the stromal component of the tumor. This observation contrasts with previous reports of RARα protein expression in epithelial cells of human BCC tumors (26). The majority of visible BCC tumors analyzed showed RARγ1 protein localized to the BCC keratinocytes (five of seven BCC) and, in six of seven tumors analyzed, RXRα protein localized to BCC keratinocytes at the edges of the tumor nests (Fig. 3B). These data suggest that, unlike SCCs, BCCs do not generally lose their retinoid receptor expression and can respond to tazarotene treatment.
Figure 3.
RARα, RXRγ, and RXRα proteins were expressed in visible murine BCC tumors that developed on Ptch1 +/− mice. A, RARα, RXRγ, and RXRα is expressed in Ptch1 wild-type and heterozygous skin and in the majority of murine macroscopic BCC tumors analyzed. SCC tumors generated by the DMBA/TPA two-step model of carcinogenesis murine expressed few, if any, of the skin-expressing retinoid receptors (RARα, RARγ, and RXRα). B, RARγ (middle) and RXRα (right) protein were localized to the tumor keratinocyte nests (TN) of visible murine BCC tumors, whereas RARα (left) protein was localized to the stromal component of the tumor (S).
Chemoprevention of BCCs with Other Retinoids In vivo: Microscopic BCC Lesions
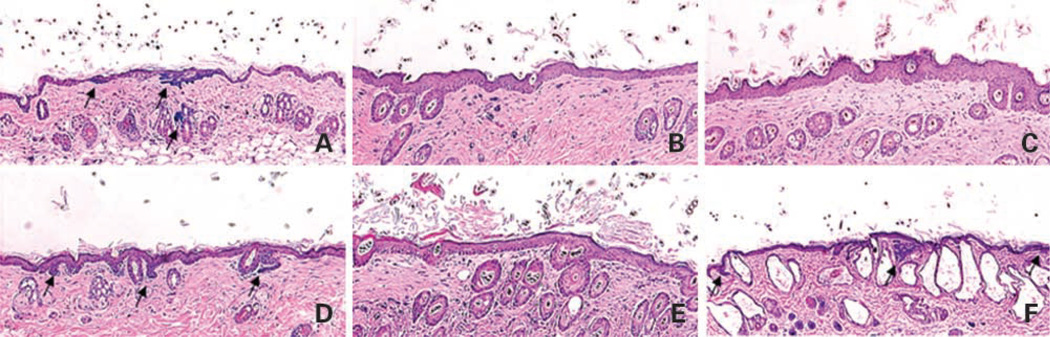
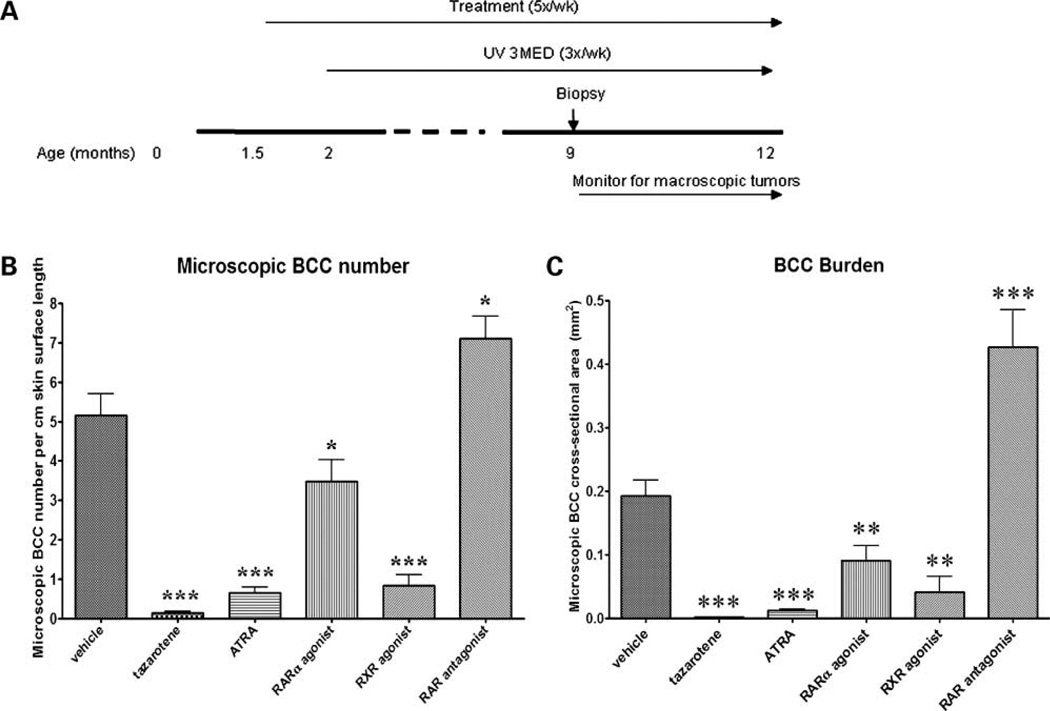
To test whether the anti-BCC effect is unique to tazarotene among retinoids, we treated Ptch1 +/− mice topically with either retinoid receptor–selective agonists or with a RAR antagonist from age 6 weeks and UV-irradiated mice from age 8 weeks until age 9 months. The major endogenous retinoid, ATRA, strongly activates all three RARs and potentially activates RXRs; AGN195183 specifically activates RARα; AGN194204 activates all RXRs, whereas AGN194310, a pan-RAR antagonist, strongly inhibits RAR signaling (27). Dorsal skin biopsies taken at age 9 months were assessed for microscopic BCC number and size. Compared with vehicle-treated skin (Fig. 4A), the previously reported tazarotene-induced epidermal thickening (Fig. 4B; ref. 16) was also observed in murine skin treated with ATRA (Fig. 4C), the RARα agonist (Fig. 4D), or the RXR agonist (Fig. 4E), which is consistent with previous reports (28). As expected, no epidermal hyperplasia was observed in skin taken from mice treated with vehicle or the RAR antagonist (Fig. 4F). However, in the latter, we observed large epidermal and dermal cysts, which are similar to those observed in mice with epidermal “knockout” of RXRα or vitamin D receptor (29, 30). These changes also indicated that at the concentrations used the retinoid-related agents effectively penetrated into the skin.
Figure 4.
Representive histology of the effects of vehicle (A), tazarotene (B), ATRA (C), RARα agonist (D), RXR agonist (E), or RAR antagonist (F) on UV-irradiated Ptch1 +/− dorsal back skin at age 9 months. Microscopic BCC lesions were stained blue for β-galactosidase activity (arrows).
At age 9 months, the tazarotene-treated mice had the fewest microscopic BCCs (Fig. 5B; tazarotene versus vehicle, 0.15 versus 5.16; P < 0.0001) and the lowest tumor burden (Fig. 5C; tazarotene versus vehicle, 0.0015 versus 0.19 mm2; P < 0.0001). ATRA and the RXR agonist were almost as effective as tazarotene at inhibiting microscopic BCC number (Fig. 5B; ATRA, 0.69 versus 5.16; P < 0.0001; RXR agonist, 0.82 versus 5.16; P < 0.0001) and size (Fig. 5C; ATRA, 0.012 versus 0.19 mm2; P < 0.0001; RXR agonist, 0.04 versus 0.19 mm2; P < 0.05). The RARα agonist moderately reduced microscopic BCC number (Fig. 5B; 3.48 versus 5.16; P < 0.05) and size (Fig. 5C; 0.09 versus 0.19 mm2; P < 0.001). Mice treated with the pan-RAR antagonist had an increased microscopic BCC number compared with vehicle-treated mice (Fig. 5B; 7.11 versus 5.16; P < 0.05). RAR antagonist-treated mice also had a significant increase in microscopic BCC size compared with the vehicle-treated group (Fig. 5C; 0.44 versus 0.19 mm2; P < 0.0001). When we tested a higher concentration of the pan-RAR antagonist (0.1%), all mice died within 2 months of initial treatment (data not shown). This was in contrast to using the 0.01% concentration, which did not cause any obvious physiologic changes in the treated mice.
Figure 5.
Effects of retinoid-related agents on microscopic BCC tumorigenesis. A, timeline of retinoid chemoprevention study. B, mice were topically treated with retinoid agonists, vehicle, or a RAR antagonist from age 1.5 months and from age 2 months, exposed to UV continuously until age 12 months. Biopsies taken at age 9 months indicated that mice treated with tazarotene had the lowest number of microscopic BCCs (n = 31) followed by those mice treated with ATRA (n = 33) or the RXR agonist (n = 11). Mice treated with the RARα agonist (n = 20) had slightly fewer BCC lesions than the vehicle control mice (n = 26), whereas mice treated with the RAR antagonist appeared to have a slight increase in microscopic BCC number (n = 22); compared with vehicle-treated mice. C, topical treatment with tazarotene, ATRA, RARα, and the pan-RXR agonist significantly reduced the microscopic BCC tumor burden compared with vehicle treatment. RAR antagonist-treated mice developed more microscopic BCC lesions than vehicle-treated mice. Note: One microscopic BCC lesion that arose in the pan-RXR agonist-treated mice was an “extreme” outlier (0.13 mm2) and was not included in the graphical and statistical analyses because this lesion size appeared to be an anomaly. *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
Chemoprevention of BCCs with Other Retinoids In vivo: Macroscopic Tumors
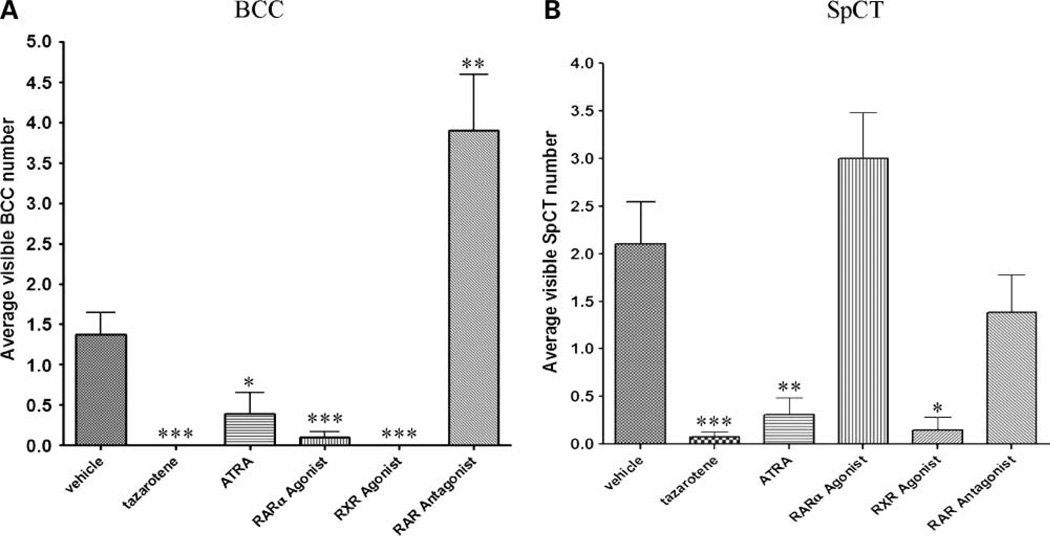
No visible BCCs were observed in any of the mice treated topically with tazarotene or the pan-RXR agonist (Fig. 6A; vehicle versus tazarotene/RXR agonist, 1.37 versus 0; P < 0.0001). On average, ATRA or RARα agonist-treated mice developed at least one macroscopic BCC. However, the macroscopic BCC number was significantly lower compared with vehicle-treated mice (Fig. 6A; vehicle versus ATRA, 1.37 versus 0.39; P < 0.05; vehicle versus RARα agonist, 1.37 versus 0.1; P < 0.0001). By contrast, mice treated with the pan-RAR antagonist had more visible BCCs than did vehicle-treated mice (Fig. 6A; 3.91 versus 1.37; P < 0.01), and those mice also had many small papular BCCs of ~1 to 3 mm in diameter in their skin at age 16 months (data not shown). Very few SpCTs were observed in mice treated topically with tazarotene, ATRA, or the pan-RXR agonist compared with the vehicle-treated group (vehicle versus tazarotene, P < 0.0001; ATRA, P < 0.01; RXR agonist, P < 0.05; Fig. 6B). Mice treated topically with the RARα agonist had a slight, nonsignificant increase in SpCT number compared with vehicle-treated mice (P > 0.05; Fig. 6B).
Figure 6.
Development of macroscopic BCCs and SpCTs. A, tazarotene (n = 26) and the RXR agonist (n = 7) were the most effective at inhibiting macroscopic BCC development followed by the RARα agonist (n = 20) and ATRA (n = 13). The RAR antagonist significantly increased the number of macroscopic BCC tumors (n = 19) compared with vehicle. B, tazarotene and the RXR agonist were the most effective at inhibiting macroscopic SpCT development followed by ATRA. Treatment with the RARα agonist appeared to increase the number of macroscopic SpCTs. The RAR antagonist did not significantly affect the number SpCT compared with vehicle. *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
Discussion
In this study, we have investigated the efficacy of tazarotene under more clinically relevant conditions. Withdrawing tazarotene application at age 7 months did not result in a significant increase in microscopic BCC number or size, suggesting that the chemopreventive efficacy of tazarotene is sustained and perhaps even curative. In the parallel study, tazarotene treatment of preexisting microscopic BCCs for 5 months significantly reduced their number and inhibited their growth. No macroscopic BCCs were observed in any of these mice. The lack of macroscopic BCCs in our tazarotene-treated mice in the “withdrawal” and “late intervention” studies suggests that it is not just microscopic BCCs of low growth potential that were inhibited. These results contrast to human studies in which new BCCs promptly appear after systemic retinoids are stopped in BCNS and XP patients (10, 11). Also, long-term therapy with low doses of systemic retinoids such as isotretinoin/ATRA and 13-cis retinoic acid, was not effective in reducing the occurrence of BCCs at new sites in patients with previously reported small numbers (1, 2) of BCCs (31). The differences between our observations and those observed for skin cancer patients treated with systemic retinoids may be (a) mode of delivery: topical tazarotene application may result in more retinoid reaching the tumor cells; (b) type of retinoid: the human studies were carried out using other pan-RAR agonists (that is, 13-cis retinoic acid and ATRA); from our observations, the outcome of activating different RARs in the skin may be quite different; (c) there may be a species difference in the response to retinoids; and/or (d) the sustained and curative effects of tazarotene applied topically may be specific to microscopic BCCs; previous observations showed that systemic retinoids prevented the occurrence of new BCCs in BCNS patients during therapy but had no effect against the majority of existing BCCs in these patients (10). However, reports that topical tazarotene application cures 30% to 50% of visible human BCCs (13) and our data suggest that topical tazarotene effectively cures both microscopic and macroscopic BCCs in contrast to the above-mentioned systemically used retinoids.
Mice treated topically with tazarotene for 5 months when microscopic BCC lesions were present (that is, from age 7 months) had a significant decrease in microscopic BCC number at age 12 months, suggesting that, rather than altering the “pre-BCC” skin environment into one that is nonpermissive for BCC development, tazarotene probably acts on the BCC tumor cells themselves. Indeed, in our model, (a) immunohistochemistry indicates that the BCC tumor cells express high levels of RARγ, (b) tazarotene inhibits ASZ001 cell proliferation, and (c) microscopic BCCs have very little detectable specialized stroma. Also, any stromal effect of tazarotene on BCCs is likely to be indirect because, in the macroscopic BCC tumors we analyzed, RARγ protein was localized to the BCC epithelial cells and not to the stromal cells.
Tazarotene at 10 µmol/L failed to reduce proliferation of C5N(the immortalized nontumorigenic keratinocyte cell line) and Med1 (the medulloblastoma cell line). Tazarotene has been reported previously to cause tumor regression in a xenograft model of medulloblastoma (32) unlike our in vitro findings for our medulloblastoma cell line, Med1. However, in their system, tazarotene and other retinoids exerted their tumor-inhibitory effects via a paracrine effect and not directly on the medulloblastoma epithelial cells themselves.
At a molecular level, in the ASZ001 cells, tazarotene down-regulated expression of Gli1, a Hedgehog target gene and key effector of Hedgehog signaling, before any morphologic or metabolic changes were observed. Down-regulation of Gli1 has been reported previously in ATRA-treated immortalized, nonmalignant murine keratinocytes (33). These data are consistent with the idea that tazarotene exerts its BCC growth-inhibitory effects by repressing Hedgehog signaling. However, Med1 was not growth inhibited by tazarotene, although Gli1 expression was down-regulated, suggesting that the down-regulation of Gli1 may not be sufficient for tazarotene-mediated growth inhibition at least in vitro. In ASZ001 cells, tazarotene upregulated expression of the retinoid target gene CRABPII, which is normally up-regulated in skin with ATRA treatment (34). This finding is consistent with the idea that RAR signaling is intact in these cells. Most of the murine visible BCC tumors analyzed in this study expressed at least one RAR and RXR: this may explain why tazarotene is efficacious against BCCs and not against SCCs, which lose expression of their retinoid receptors (23–25). However, not all visible murine BCCs from our Ptch1 +/− mice showed strong expression of RARγ. Because topical tazarotene treatment cures only 30% to 50% of sporadic human BCCs (12, 13), it is tempting to speculate that loss of RARγ expression might underlie the resistance of some macroscopic human BCCs.
In our comparison of the anti-BCC chemopreventive efficacy of various retinoids, tazarotene was the most effective followed by ATRA and the pan-RXR agonist. The RARα agonist reduced microscopic BCC number slightly, whereas the pan-RAR antagonist enhanced their development. The differences in efficacy between tazarotene and ATRA may be due to differences in tissue penetration rather than functional differences between the active agents, because the vehicle “carrying” ATRA in these pharmacologic preparations was different from that in which tazarotene was dissolved. However, our data suggest that it is the specificity of tazarotene for RARγ that makes it a more effective retinoid than ATRA (a pan- RAR agonist), because activating RARα did not have a dramatic effect against BCC carcinogenesis. The slight reduction in microscopic BCC number observed with the RARα agonist may be due to nonspecific activation of RARγ. RARγ has been suggested to be an important suppressor of murine SCC carcinogenesis because loss of RARγ, and not RARα, predisposes keratinocytes to υ -Ha-Ras -induced SCC tumorigenesis (35). Our data suggest that the tumor-suppressive ability of RARγ is not limited to SCC carcinogenesis but also extends to BCC carcinogenesis. Also, murine SCC tumor xenografts lacking both RARγ and RARα were significantly larger than were those with only RARγ loss (35), suggesting that RARα may act at later stages of carcinogenesis to prevent visible tumor growth. In support of this, we observed fewer visible BCC tumors in the RARα agonist-treated and ATRA-treated mice compared with vehicle-treated mice. The few macroscopic BCCs that arose in the RARα agonist-treated and ATRA-treated mice may have arisen because RARγ activation “alone” may be required to prevent early skin carcinogenesis. However, later in tumor development, RARα activation may prevent the progression of microscopic BCC lesions into macroscopic tumors. Indeed, a role for RARα in inhibiting macroscopic BCC tumorigenesis correlates with the observation that, in the untreated macroscopic BCC tumors in our study, RARα protein was localized to the tumor stroma, which is known to play an important role in the growth of established tumors (36).
Inhibition of RAR signaling by treatment with the pan- RAR antagonist enhanced BCC tumorigenesis, suggesting that basal levels of RAR signaling inhibit BCC carcinogenesis. Reports in which SCC carcinogenesis is promoted by the loss of RAR signaling (23–25) suggest that physiologic activation of RAR signaling is a general mechanism that prevents nonmelanoma skin carcinogenesis. Basal RAR signaling is also required to maintain skin homeostasis because inhibiting RARs results in the development of many epidermal and dermal cysts. Epidermal RXRα signaling also prevents epidermal and dermal cyst formation and inhibits murine SCC and melanocytic tumor growth (29, 37, 38). These published reports and our data suggest that both RAR and RXR signaling are important for normal skin homeostasis and skin tumor suppression.
In summary, we find that, in our murine model of BCC tumorigenesis, topical application of tazarotene is highly efficacious against microscopic BCC lesions as a chemopreventive and therapeutic agent. Drug withdrawal does not result in recurrence of BCC tumors, unlike that observed in human studies with systemic retinoids but consistent with the reports that topical application of tazarotene cures 30% to 50% of human BCCs. Our data have good implications for the clinical use of topical tazarotene as a chemopreventive agent against BCC tumorigenesis in BCNS patients, and such use would circumvent the toxicity problems of oral retinoids. Mechanistically, our data also suggest that it is the specific activation of RARγ that is required for early and late BCC inhibition and that activation of RARα may be required “later” to inhibit clinically significant BCC tumor development.
Acknowledgments
We thank Allergan, especially Rosh Chandraratna, Patricia Walker, and Richard Beard for providing tazarotene, AGN195183, AGN194204, AGN194310, and vehicle gel; Allan Balmain for providing the C5N cell line and the SCC tumors; Nancy Daniallania for cell culture assistance; and the members of the Epstein laboratory for animal husbandry assistance and article discussions.
Grant support: NIH grants CA81888 and CA109584 (E.H. Epstein, Jr.).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Gorlin RJ. Nevoid basal cell carcinoma (Gorlin) syndrome. Genet Med. 2004;6:530–539. doi: 10.1097/01.gim.0000144188.15902.c4. [DOI] [PubMed] [Google Scholar]
- 2.Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 4.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 5.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 6.Aszterbaum M, Epstein J, Oro A, et al. Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5:1285–1291. doi: 10.1038/15242. [DOI] [PubMed] [Google Scholar]
- 7.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 8.Chambon P. The retinoid signaling pathway: molecular and genetic analyses. Semin Cell Biol. 1994;5:115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- 9.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 10.Peck GL. Long-term retinoid therapy is needed for maintenance of cancer chemopreventive effect. Dermatologica. 1987;175(Suppl 1):138–144. doi: 10.1159/000248870. [DOI] [PubMed] [Google Scholar]
- 11.Peck GL, DiGiovanna JJ, Sarnoff DS, et al. Treatment and prevention of basal cell carcinoma with oral isotretinoin. J Am Acad Dermatol. 1988;19:176–185. doi: 10.1016/s0190-9622(88)70162-0. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi L, Orlandi A, Campione E, et al. Topical treatment of basal cell carcinoma with tazarotene: a clinicopathological study on a large series of cases. Br J Dermatol. 2004;151:148–156. doi: 10.1111/j.1365-2133.2004.06044.x. [DOI] [PubMed] [Google Scholar]
- 13.Orlandi A, Bianchi L, Costanzo A, Campione E, Giusto Spagnoli L, Chimenti S. Evidence of increased apoptosis and reduced proliferation in basal cell carcinomas treated with tazarotene. J Invest Dermatol. 2004;122:1037–1041. doi: 10.1111/j.0022-202X.2004.22414.x. [DOI] [PubMed] [Google Scholar]
- 14.Chandraratna RA. Tazarotene-first of a new generation of receptor-selective retinoids. Br J Dermatol. 1996;135(Suppl 49):18–25. doi: 10.1111/j.1365-2133.1996.tb15662.x. [DOI] [PubMed] [Google Scholar]
- 15.McGrane MM. Vitamin A regulation of gene expression: molecular mechanism of a prototype gene. J Nutr Biochem. 2007;18:497–508. doi: 10.1016/j.jnutbio.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 16.So PL, Lee K, Hebert J, et al. Topical tazarotene chemoprevention reduces Basal cell carcinoma number and size in Ptch1 +/− mice exposed to ultraviolet or ionizing radiation. Cancer Res. 2004;64:4385–4389. doi: 10.1158/0008-5472.CAN-03-1927. [DOI] [PubMed] [Google Scholar]
- 17.So PL, Langston AW, Daniallinia N, et al. Long-term establishment, characterization and manipulation of cell lines from mouse basal cell carcinoma tumors. Exp Dermatol. 2006;15:742–750. doi: 10.1111/j.1600-0625.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 18.Oft M, Akhurst RJ, Balmain A. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat Cell Biol. 2002;4:487–494. doi: 10.1038/ncb807. [DOI] [PubMed] [Google Scholar]
- 19.Athar M, Li C, Tang X, et al. Inhibition of smoothened signaling prevents ultraviolet B-induced basal cell carcinomas through regulation of Fas expression and apoptosis. Cancer Res. 2004;64:7545–7552. doi: 10.1158/0008-5472.CAN-04-1393. [DOI] [PubMed] [Google Scholar]
- 20.Fisher GJ, Datta SC, Voorhees JJ. Retinoic acid receptor-γ in human epidermis preferentially traps all-trans retinoic acid as its ligand rather than 9-cis retinoic acid. J Invest Dermatol. 1998;110:297–300. doi: 10.1046/j.1523-1747.1998.00112.x. [DOI] [PubMed] [Google Scholar]
- 21.Finzi E, Blake MJ, Celano P, Skouge J, Diwan R. Cellular localization of retinoic acid receptor-γ expression in normal and neoplastic skin. Am J Pathol. 1992;140:1463–1471. [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996;10:1002–1013. doi: 10.1096/fasebj.10.9.8801161. [DOI] [PubMed] [Google Scholar]
- 23.Darwiche N, Celli G, Tennenbaum T, Glick AB, Yuspa SH, De Luca LM. Mouse skin tumor progression results in differential expression of retinoic acid and retinoid X receptors. Cancer Res. 1995;55:2774–2782. [PubMed] [Google Scholar]
- 24.Darwiche N, Scita G, Jones C, et al. Loss of retinoic acid receptors in mouse skin and skin tumors is associated with activation of the ras(Ha) oncogene and high risk for premalignant progression. Cancer Res. 1996;56:4942–4649. [PubMed] [Google Scholar]
- 25.Xu XC, Wong WY, Goldberg L, et al. Progressive decreases in nuclear retinoid receptors during skin squamous carcinogenesis. Cancer Res. 2001;61:4306–4310. [PubMed] [Google Scholar]
- 26.Kamradt J, Reichrath J. Expression of retinoic acid receptor proteins in basal cell carcinomas: an immunohistochemical analysis. J Histochem Cytochem. 1996;44:1415–1420. doi: 10.1177/44.12.8985133. [DOI] [PubMed] [Google Scholar]
- 27.Johnson A, Chandraratna RA. Novel retinoids with receptor selectivity and functional selectivity. Br J Dermatol. 1999;140(Suppl 54):12–17. doi: 10.1046/j.1365-2133.1999.140s54012.x. [DOI] [PubMed] [Google Scholar]
- 28.Thacher SM, Standeven AM, Athanikar J, et al. Receptor specificity of retinoid-induced epidermal hyperplasia: effect of RXR-selective agonists and correlation with topical irritation. J Pharmacol Exp Ther. 1997;282:528–534. [PubMed] [Google Scholar]
- 29.Li M, Chiba H, Warot X, et al. RXR-α ablation in skin keratinocytes results in alopecia and epidermal alterations. Development. 2001;128:675–688. doi: 10.1242/dev.128.5.675. [DOI] [PubMed] [Google Scholar]
- 30.Xie Z, Komuves L, Yu QC, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11–16. doi: 10.1046/j.1523-1747.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- 31.Tangrea JA, Edwards BK, Taylor PR, et al. Long-term therapy with low-dose isotretinoin for prevention of basal cell carcinoma: a multicenter clinical trial. Isotretinoin-Basal Cell Carcinoma Study Group. J Natl Cancer Inst. 1992;84:328–332. doi: 10.1093/jnci/84.5.328. [DOI] [PubMed] [Google Scholar]
- 32.Hallahan AR, Pritchard JI, Chandraratna RA, et al. BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nat Med. 2003;9:1033–1038. doi: 10.1038/nm904. [DOI] [PubMed] [Google Scholar]
- 33.Goyette P, Allan D, Peschard P, Chen CF, Wang W, Lohnes D. Regulation of Gli activity by all-trans retinoic acid in mouse keratinocytes. Cancer Res. 2000;60:5386–5389. [PubMed] [Google Scholar]
- 34.Gendimenico GJ, Mallon JP, Cromie MA, Mezick JA, Astrom A, Elder JT. Regulation of cellular retinoic acid binding protein expression in rhino mouse skin by all-trans retinoic acid. Skin Pharmacol. 1995;8:167–172. doi: 10.1159/000211342. [DOI] [PubMed] [Google Scholar]
- 35.Chen CF, Goyette P, Lohnes D. RARγ acts as a tumor suppressor in mouse keratinocytes. Oncogene. 2004;23:5350–5359. doi: 10.1038/sj.onc.1207682. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101:805–815. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 37.Li M, Indra AK, Warot X, et al. Skin abnormalities generated by temporally controlled RXRα mutations in mouse epidermis. Nature. 2000;407:633–636. doi: 10.1038/35036595. [DOI] [PubMed] [Google Scholar]
- 38.Indra AK, Castaneda E, Antal MC, et al. Malignant transformation of DMBA/TPA-induced papillomas and nevi in the skin of mice selectively lacking retinoid-X-receptor α in epidermal keratinocytes. J Invest Dermatol. 2007;127:1250–1260. doi: 10.1038/sj.jid.5700672. [DOI] [PubMed] [Google Scholar]