Abstract
Following up on the characterization of a new (heme)FeIII-superoxide species formed from the cryogenic oxygenation of a ferrous-heme (PPy)FeII (1) (PPy = a tetraarylporphyrinate with a covalently tethered pyridine group as a potential axial base), giving (PPy)FeIII-O2•- (2) (Li Y et al., Polyhedron 2013; 58: 60–64), we report here on (i) its use in forming a cytochrome c oxidase (CcO) model compound, or (ii) in a reaction with nitrogen monoxide (•NO; nitric oxide) to mimic nitric oxide dioxygenase (NOD) chemistry. Reaction of (2) with the cuprous chelate [CuI(AN)][B(C6F5)4] (AN = bis[3-(dimethylamino) propyl]amine) gives a meta-stable product [(PPy)FeIII-()-CuII(AN)][B(C6F5)4] (3a), possessing a high-spin iron(III) and Cu(II) side-on bridged peroxo moiety with a μ-η2:η2-binding motif. This complex thermally decays to a corresponding μ-oxo complex [(PPy)FeIII-(O2-)-CuII(AN)][B(C6F5)4] (3). Both (3) and (3a) have been characterized by UV-vis, 2H NMR and EPR spectroscopies. When (2) is exposed to •NO(g), a ferric heme nitrato compound forms; if 2,4-di-tert-butylphenol is added prior to •NO(g) exposure, phenol ortho-nitration occurs with the iron product being the ferric hydroxide complex (PPy) FeIII(OH) (5). The latter reactions mimic the action of NOD’s.
Keywords: heme-superoxo, high spin heme-copper peroxo, heme-copper-μ-oxo, peroxynitrite, nitrate
INTRODUCTION
Metalloenzymes form an essential component of the various biological and physiological functions that are essential for life [1]. Hemoproteins are perhaps the best-known class of metalloenzymes, and their reactions with dioxygen are foundational to aerobic life. These proteins are critical to dioxygen storage and transport (myoglobin and hemoglobin) [2], substrate oxygenation (cytochrome P-450 family), as well as dioxygenation [3] and peroxidation [4]. Heme-copper proteins are critical to cellular respiration (e.g. in cytochrome c oxidase). Nitric oxide is biosynthesized and interacts with hemoproteins as part for this molecule’s involvement in inflammatory responses, cellular signaling, and vasodilation. Nitric oxide dioxygenase (NOD) [5] and nitric oxide reductase (NOR) [6] enable cellular NO regulation/removal when it is present in excess.
It has been shown that CcO functionality is inhibited in the presence of NO [7, 8] via competition with O2 for binding at the binuclear center. Under certain physiological conditions the NO concentration in vivo can reach levels that significantly affect the reaction rate of CcO [9]. To compensate, myoglobin (Mb) and/ or hemoglobin (Hb) play a major role in scavenging •NO, helping to keep respiratory homeostasis. This maintains the proton gradient over the mitochondrial inner membrane, driving ATP synthesis. When •NO is overproduced in vivo as a component of inflammatory response, reactive nitrogen species (RNS) can be formed by the reaction of NO with reactive oxygen species (ROS) such as superoxide, to generate a peroxynitrite (O=NOO-). Peroxynitrite [10, 11] is a strong oxidant and nitrating agent, and reacts with a number of biological substrates such as thiols [12], tyrosine residues [13], lipids, CO2, DNA [14–16] and metalloproteins [17]. Hence, •NO and peroxynitrite scavenging by Hb/Mb, is critical not only to respiration, but also for the mitigation of oxidative damage via NOD activity [18, 19].
Our own research group is particularly interested in providing basic coordination chemistry insights into the possible reactive intermediates formed during CcO turnover by using synthetic functional models [20–24]. CcO is responsible for the reduction of O2 to water as a terminal step of the respiratory chain of mitochondria and many aerobic bacteria [25–27]. A ferric superoxo species is the most well studied dioxygen intermediate generated upon initial O2-reaction with the fully reduced activesite heme Fe(II)–Cu(I) center [28]. This species forms prior to O–O bond cleavage and as such has attracted considerable interest [29–31]. Here, we describe the reactivity of an iron(III)-superoxo species (2) towards (i) a cuprous-chelated complex [CuI(AN)][B(C6F5)4] and (ii) •NO, these reactions representing synthetic functional models for CcO and NOD, respectively.
RESULTS AND DISCUSSION
Reactivity of the iron(III)-superoxo complex (2) towards [CuI(AN)][B(C6F5)4]
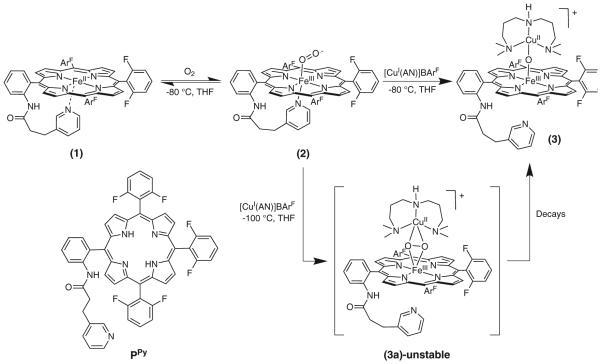
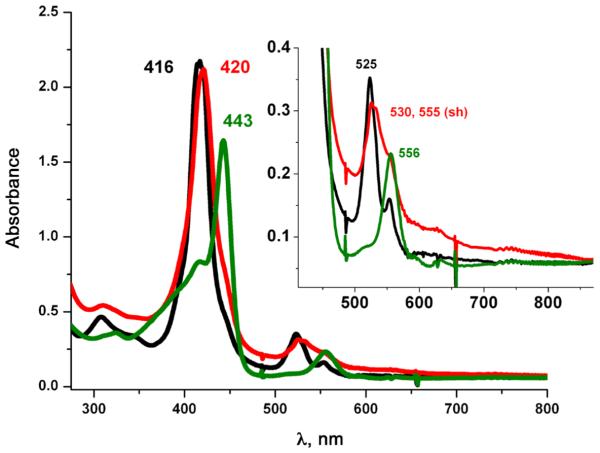
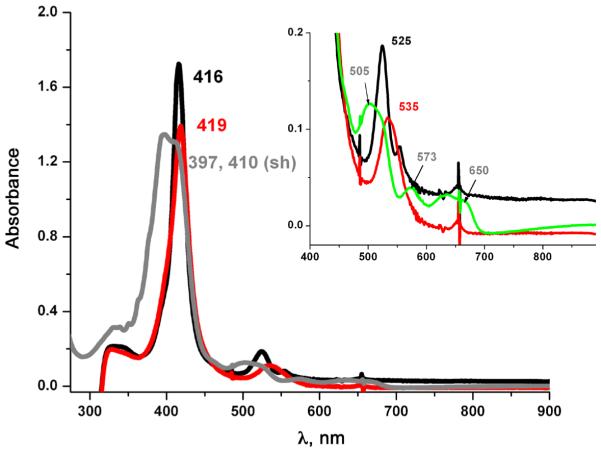
Earlier work in our group [31] described the synthesis and characterization of (PPy)FeII (1) [(PPy) = pyridyl tailed porphyrinate (2-)] (λmax = 416, 525, 554 (sh) nm) which reacts reversibly with dioxygen to give a diamagnetic iron(III)-superoxo species (PPy)FeIII-(O2•-) (2) (UV-vis, λmax= 419, 535 nm; EPR, silent). This is stable in solution below −30 °C in coordinating solvents such as tetrahydrofuran (THF), acetone, or acetonitrile as well as in non-coordinating solvents like dichloromethane (DCM). The use of copper ion complexes with tridentate alkylamino ligand AN has previously been useful [23, 32–34] and as such this copper chelate was employed here. Addition of one equivalent of [CuI(AN)][B(C6F5)4] (Scheme 1) to the superoxo compound (2) in THF at −100 °C, monitored by UV-vis spectroscopy, leads to the immediate formation of a heme-copper-O2 adduct (3a) [λmax = 420, 530, 555 (sh) nm], see Fig. 1. The UV-vis spectrum of (3a) is very similar to our previously described high-spin [(heme)FeIII-()-CuII(L)]+ species where L is a tri- or tetradentate alkylamino or pyridylalkylamino ligand; these possess a side-on binding of peroxide to both metal ions, as depicted in Scheme 1 [35–37]. Thermal decomposition of (3a) leads to the formation of complex (3) with a red shifted Soret band at 443 nm and Q-band at 556 nm (Fig. 1). These are characteristic features for μ-oxo FeIII-O-CuII like species, such as the previously structurally and spectroscopically characterized complex [(F8)FeIII-(O2-)-CuII(TMPA)]+ [38] (TMPA = tris(2-pyridylmethyl)amine). Complex (3) can also be obtained by direct bubbling of dioxygen to a 1:1 mixture of (PPy) FeII (1) and [CuI(AN)][B(C6F5)4] at −80 °C in THF. The μ-oxo complex [(PPy)FeIII-(O2-)-CuII(AN)]+ (3) (or rather a protonated μ-hydroxo conjugate acid form), are of interest since such species derive from dioxygen reactivity, thus perhaps related to CcO reaction chemistry [39, 40].
Scheme 1.
Formation of (PPy)FeIII-(O2•-) (2) via oxygenation of (PPy)FeII (1) and subsequent reaction with [CuI(AN)]+ to give the meta-stable intermediate, the high-spin heme-peroxo-copper complex (3a), which decays to give the m-oxo complex [(PPy)FeIII-(O2-)- CuII(AN)]+ (3)
Fig. 1.
UV-vis spectra of (1, black) a reduced (PPy)FeII + [CuI(AN)]+ 1:1 mixture; (3a, red) high spin peroxo complex [(PPy)FeIII-() CuII(AN)]+; (3, green) μ-oxo complex [(PPy)FeIII-(O2-)-CuII(AN)]+
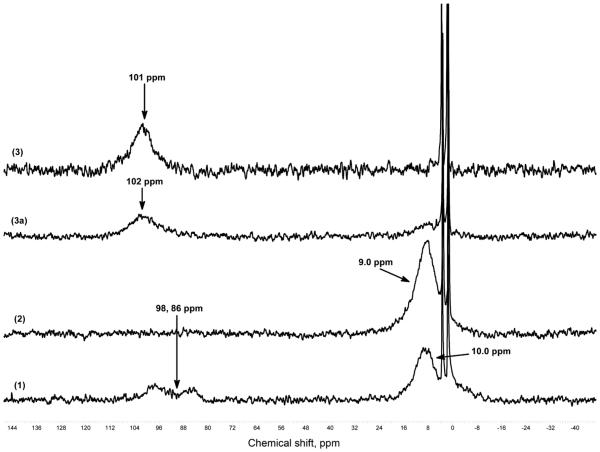
Further characterization of μ-oxo complex (3) was provided by low temperature 2H NMR spectroscopy of the pyrrole deuterated analogue of (PPy)FeII (1), (d8-PPy) FeII. Figure 2 shows 2H NMR spectra of the oxygenation reaction of a (PPy)FeII-THF (1) exhibits a pyrrole resonance at † 10 ppm, indicative of a low-spin (S = 0) six-coordinate ferrous heme at low temperature, but we also observe pyrrole resonances at † 98 and 86 ppm, which are characteristic of a penta-coordinated high spin heme. We interpret this observed NMR spectroscopic data as indicating that complex (1) is a mixture of 6-coordinate (pyridyl + THF) low-spin iron(II) and 5 or 6-coordinate high-spin iron(II) (e.g. pyridyl arm off, THF bound, or vice versa, and also possibly a bis-THF ligated ferrous heme). Direct bubbling of dioxygen to (1) gives superoxo complex (2) where a pyrrole resonance occurs in the diamagnetic region, at † 9.0 ppm. Subsequent addition of one equiv. of [CuI(AN)]+ to the cold solution of superoxo complex (2) in an NMR tube leads to the formation of [(PPy)FeIII-(O2-)-CuII(AN)]+ (3), with a downfield shifting of the pyrrole resonance to † 102 ppm (Fig. 2), indicative of a high-spin ferric heme (also, see below). Complex (3) is stable at room temperature. We have previously reported this characteristic pattern of a downfield shifted pyrrole resonance for (P)FeIII-X-CuII (X = or O2-) systems having overall S = 2 spin states [37, 41–43], which arise from the antiferromagnetic coupling of the S = 5/2 high-spin heme-Fe(III) center to an S = ½ copper(II) moiety, through the bridging X ligand in (3) or (3a). When monitoring peroxo complex (3a) by 2H NMR spectroscopy, a clean thermal transformation to (3) is observed. EPR spectroscopic interrogation of (3) and (3a) revealed that both are EPR inactive, consistent with their formulations.
Fig. 2.
2H NMR spectra at −80 °C in THF (1) (d8-PPy)FeII-THF, (2) (d8-PPy)FeIII-() (3a) [(d8-PPy)FeIII-(O2)-CuII(AN)]+ and (3) [(d8-PPy)FeIII-(O2-)-CuII(AN)]+
Reactivity of iron(III)-superoxo complex (2) towards •NO(g)
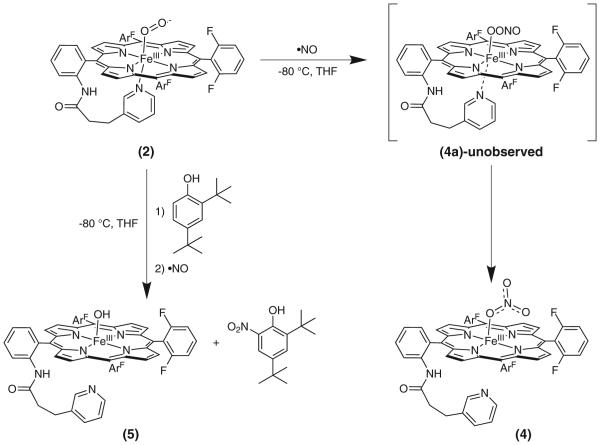
Using a gas-tight three way syringe, addition of •NO(g) to superoxo species (PPy)FeIII-(O2•-) (2) at −80 °C in THF, as monitored by UV-vis spectroscopy, led to the immediate formation of a five-coordinate nitrato compound (PPy) FeIII-(ONO2) (4) [UV-vis, λmax = 397, 410 (sh), 505, 573, 650 nm; EPR, g = 6, 14 K, high-spin iron(III)], as indicated in Scheme 2 and with spectra shown in Fig. 3. Product (4) yields a positive test for nitrate ion, as determined using semiquantitative QUANTOFIX nitrate (NO3-)/nitrite (NO2-) test paper; no NO2- ion was detected and the yield of nitrate ion was estimated to be > 75% (see Experimental). These results are very similar to what we observed in a previous study of (F8) FeII (F8 = tetrakis(2,6-difluorophenyl)porphyrinate(2-)) where addition of •NO(g) to the superoxo complex (F8) FeIII-(O2•-), yields a five-coordinate nitrato heme complex [44]. While no transient species were detected following addition of •NO(g) to (2) and isolation of (4), the formation of a nitrite complex supports the intermediacy of a peroxynitrite -OON=O species (4a) which formed during the reaction (Scheme 2), indicating an NOD type of reaction mechanism (see below).
Scheme 2.
Reaction sequence where •NO(g) is added to superoxo complex (2) to give nitrato complex (4). In the presence of a phenolic substrate, the same reaction gives (5) as a final product along with the ortho-nitrated phenol
Fig. 3.
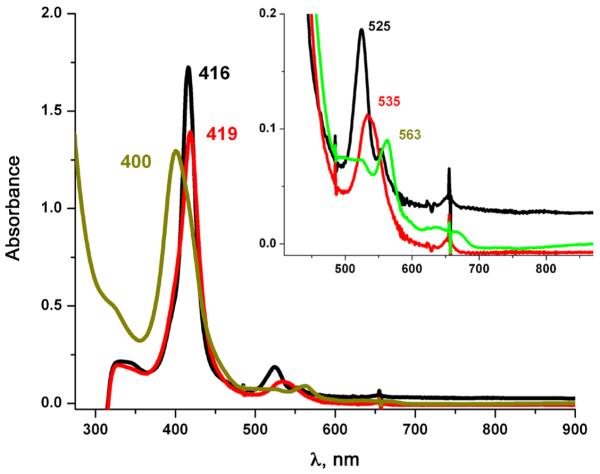
UV-vis spectra showing superoxo (2, red) formed from reduced (PPy)FeII (1, black) by bubbling O2(g) at −80 °C; nitrato complex (PPy)FeIII-ONO2 (4, grey) generated immediately after addition of •NO(g)
Thus, we sought chemical evidence which might support our supposition involving the formation of a peroxynitrite species during the reaction of (2) with •NO(g). Here, a tyrosine analog, 2,4-di-tert-butylphenol (DTBP), was added into the solution of superoxo complex (PPy)FeIII-(O2•-) (2). When •NO(g) was subsequently added, we observed the immediate formation of (PPy) FeIII(OH) (5) [λmax= 400, 563 nm] as indicated by the Q-band feature in the UV-vis spectrum (Fig. 4). We then isolated the pure (PPy)FeIII(OH) (5) (λmax= 410, 563) from this mixture (see Experimental). An authentic sample of (PPy)FeIII(OH) (5) (λmax= 412, 563) was prepared (see Experimental), and has nearly identical UV-vis features as those observed for the reaction product. The UV-vis and EPR spectroscopic features observed here also closely match those known for (F8)FeIII(OH) (λmax = 408, 572 nm high-spin, EPR, g = 6.0) [44] (Fig. 4). Workup of the reaction solution revealed that the product (5) forms along with high yields (>85%) of 2,4-di-tert-butyl-6-nitrophenol (NO2-DTBP), as confirmed via gaschromatography. This reaction mixture was also tested for the presence of any NO3-/NO2- ions and yielded a negative result for both.
Fig. 4.
UV-vis spectroscopy in THF at −80 °C. The black spectrum is reduced (PPy)FeII (1); red is (2 + DTBP), and green is (5)
These studies indicate the involvement of a hemeperoxynitrite like intermediate [(PPy)FeIII-OONO] (4a), which we could not detect, but that formed when superoxo complex (PPy)FeIII-(O2•-) (2) was reacted with •NO(g) (Scheme 2). There were previous reports that suggested the detection of heme-peroxynitrite species in the reaction of oxy-heme (i.e. ferric superoxo) with •NO(g), either via UV-vis or EPR spectroscopy [18, 45]. However these results were refuted by Moënne-Loccoz and co-workers [46] using rapid freeze quench resonance Raman spectroscopy with Mb, which revealed that such intermediates were in fact iron-bound nitrate species formed prior to their decay to metMb. Still, the generally accepted mechanism of •NO dioxygenase involves direct reaction of the FeIII-(O2•-) oxy complex with •NO, giving a peroxynitrite intermediate. Subsequent homolytic O–O bond cleavage produces an oxo-ferryl (FeIV=O) species and the free radical nitrogen dioxide (•NO2); the latter attacks the ferryl O-atom to produce a N–O bond, yielding nitrate [47–50]. Recent work with oxy-coboglobin models [51, 52] exhibiting NODlike activity has led to the detection of peroxynitrite intermediates using low temperature FTIR, this work has helped to shed light onto favorable conditions for generating peroxynitrite intermediates. Also, recent work by Nam and Karlin [53] has shown an alternative method for mimicking NOD activity that is isoelectronic to the methods discussed above. In this case, NOD activity was exhibited using a nitrosonium ion added to a non-heme iron peroxo species. Following these literature precedents, we can hypothesize that (4a) undergoes homolysis to give a ferryl + •NO2 radical, which can be captured by phenol present in solution. The ferryl would oxidize the phenol to a phenoxyl radical which will further react with •NO2 to give ortho-nitration of the phenol and form a very stable ferric hydroxo complex (4), as we observed (Scheme 2).
EXPERIMENTAL
General
All reagents and solvents used were of commercially available analytical quality except as noted. Dioxygen was dried by passing through a short column of supported P4O10 (Aquasorb, Mallinkrodt). Nitrogen monoxide (•NO) gas was obtained from Matheson Gases (High Purity Grade, Full cylinder ~500 psig @ 70 °F) and purified as follows: it was first passed through multiple columns containing Ascarite II (Thomas Scientific) to remove higher nitrogen oxide impurities. Further purification by distillation was completed by warming frozen •NO (as crystalline N2O2) from 78 K in a liquid N2 cooled vacuum trap to 193 K through use of an acetone/dry-ice (−78 °C) bath, and collection in a second liquid N2 cooled evacuated vacuum trap. This secondary flask was again warmed to 193 K and the purified •NO was collected in an evacuated Schlenk flask (typically 50 mL) closed with a rubber septum secured tightly by copper wire. The •NO in the Schlenk flask is collected and kept at higher pressures (<1 atm). Addition of •NO and O2 to the metal complex solutions was accomplished using a three-way long needle syringe connected to a Schlenk line.
THF and Pentane were distilled over Na/benzophenone ketyl or calcium hydride. 2,4-di-tert-butylphenol (DTBP) was purchased from Sigma-Aldrich and purified by multiple recrystallizations in toluene under Ar. All other reagents were used as received.
Preparation and handling of air-sensitive compounds were performed under an argon atmosphere using standard Schlenk techniques or in an MBraun Labmaster 130 inert atmosphere (<1 ppm O2, <1 ppm H2O) drybox filled with nitrogen gas. Solvents were purged with Ar prior to use. All UV-vis measurements were carried out by using a Hewlett Packard 8453 diode array spectrophotometer with a 10 mm path quartz cell. The spectrometer was equipped with HP Chemstation software and a Unisoku thermostated cell holder for low temperature experiments. All NMR spectra were recorded in 7 inch, 5 mm o.d. NMR tubes. Low-temperature 2H NMR (Bruker 300 MHz spectrometer equipped with a tunable deuterium probe to enhance deuterium detection) measurements were performed at −80 °C under a N2 atmosphere. The 2H chemical shifts are calibrated to natural abundance deuterium solvent peaks. EPR measurements of the frozen solutions were carried out at 14K on an X-Band Bruker EMX CW EPR spectrometer controlled with a Bruker ER 041 XG microwave bridge operating at the X-band (~9 GHz). Gas chromatography (GC) was performed on an Agilent 6890 gas chromatograph fitted with a HP-5 (5%-phenyl)-methylpolysiloxane capillary column (30 m * 0.32 mm * 0.25 mm) and equipped with a flame-ionization detector.
Synthesis
(PPy)FeII/(d8-PPy)FeII (1) and [CuI(AN)]BArF were synthesized as previously described [31, 32].
Preparation of 2H NMR and EPR samples In-situ generation of complexes (3a) and (3).
In a typical experiment, 0.57 mL of a (d8-PPy)FeII (5 mM) solution in THF was placed in a 5 mm rubber septum capped NMR tube. After cooling down the NMR tube to −80 °C (acetone/N2(liq) bath), dioxygen was bubbled through the solution mixture to form the (d8-PPy) FeIII(O2•-) complex (2). The NMR tube was transferred rapidly into the NMR instrument which was precooled to −85 °C. Similar to our UV-vis experiments, complex (3a) was prepared by removing any excess of dioxygen by vacuum/Ar cycles from (2) and careful addition of 1 equiv. of [CuI(AN)]BArF complex. Finally, Complex (3a) was warmed to RT to obtain decomposed product (3). EPR samples were prepared in a similar way by using 9 mm EPR tube.
In-situ generation of complex (4)
In a typical experiment, 0.650 mL of (PPy)FeII (1 mM) in THF was placed in a 9 mm rubber septum capped EPR tube. After cooling down the EPR tube to −80 °C (acetone/N2(liq) bath), 3 mL dioxygen was bubbled through the solution mixture to form the (PPy)FeIII(O2•-) complex (2). Similar to our UV-vis experiments, complex (4) was prepared by removing excess of dioxygen by vacuum/Ar cycles from (2) and careful addition of 2 mL •NO(g) via 3-way gas tight syringe. Excess gas was removed by vacuum/ Ar cyles. After generation of all complexes, the tubes were frozen in N2(liq) and brought to the spectrometer for measurement.
Synthesis of (PPy)FeIII-OH (5)
(PPy)FeIII-(OH) (5) was prepared using a modified procedure for the synthesis of its previously published [31] chloride analog, (PPy)FeIII-(Cl). In this case (PPy)FeIII-(Cl) was dissolved in ~250 mL DCM, and this DCM layer was then stirred vigorously with ~250 mL of 3.0 M NaOH in a 1000 mL round-bottom flask for 3 h. Separation of the organic layer, followed by drying with magnesium sulfate, and solvent removal yielded the (PPy)FeIII-(OH) (5) Yield 600 mg (57.8%). This compound was then characterized by UV-vis, and 1H NMR spectroscopies as well as ESI-MS. UV-vis (THF): λmax, nm 412, 563, room temperature. 1H NMR (300 MHz; CD2Cl2): dpyrrole 80.63 ppm. MS (ESI): m/z 924. EPR g = 6.
Procedure for nitrate/nitrite test
In a 25 mL-Schlenk flask compound (1) was prepared in 10 mL THF (0.1 mM solution, inside dry box). In a typical bench top reaction, the flask was cooled to −80 °C (Acetone/dry ice bath) and dioxygen was bubbled through complex 1, resulting in the formation of the superoxo species, (2). Excess O2(g) was removed by several cycles of Ar bubble followed by vacuum. Addition of •NO(g) to (2) resulted in formation of (4), immediately. The reaction mixture was for 10 min at −80 °C. After 10 mins, solvent was removed and solid product was isolated in 5 mL of DCM and extracted with 10 mL of aqueous NaCl solution (6 mM). The presence of a significant amount of nitrate ion in the aquous layer was confirmed by semiquantitative QUANTOFIX nitrate/nitrite test strips.
Nitration of the 2,4-di-tert-butylphenol (DTBP)
The formation of the superoxo complex (2) in THF at −80 °C was carried out as described above for (1) (0.1 mM) in a schlenk cuvette, and the reaction was monitored by UV-vis. Excess O2(g) was removed by bubbling the solution with Ar and vacuum purge cycles as before. Two equivalents of 2,4-di-tert butylphenol (DTBP) (0.1 mmol) were added. Upon addition of the DTBP no change in the UV-vis spectrum was observed. •NO(g) was added by using a three-way gas tight syringe, leading to the formation of (5). The resulting solution was concentrated in vacuo and pentane was added to precipitate the Fe product. The pentane solution was collected by decanting. The Fe product was washed several times with pentane, and the pentane solution was removed and collected by decanting after each wash. The solid Fe product (PPy)FeIII-(S) (S = solvent, H2O, -OH) was dried in vacuo, redissolved in THF and its UV-vis spectrum was recorded [λmax= 410, 563 nm; a small change in the Soret position compared to the reaction mixture is observed, possibly due to the different solution conditions and different temperature for the spectra recorded]; these spectral parameters matched those of authentically synthesized (PPy)FeIII(OH) (see above). The pentane solution containing the phenolic products was filtered to remove any trace of Fe product and the solvent was removed in vacuo. The resulting solid was re-dissolved in MeOH and dodecane used as internal standard and injected into a GC. This showed 2,4-di-tbutyl-6-nitrophenol (NO2-DTBP) (82% yield) and unreacted DTBP as the only products of the reaction. These were identified by comparison to the spectra obtained from commercial 2,4-di-t-butyl-6-nitrophenol, and 2,4-di-t-butylphenol respectively.
CONCLUSION
From this investigation we can conclude that our present ferric superoxo complex (2) can show cytochrome c oxidase reactivity (CcO) in the presence of a cuprous complex, generating a peroxo-bridged heme-Cu intermediate which can thermally transform to a heme-(O2-)-Cu(L) μ-oxo product, which is derived from dioxygen reactivity. Alternatively, (2) can also function as a •NO scavenger by oxidizing it to the biologically benign nitrate ion, complex (4). Further work is going on in our laboratory using this system.
Acknowledgements
We are grateful to the US National Institutes of Health for support of this research (GM60353).
REFERENCES
- 1.Holm RH, Kennepohl P, Solomon EI. Chem. Rev. 1996;96:2239–2314. doi: 10.1021/cr9500390. [DOI] [PubMed] [Google Scholar]
- 2.Perutz MF. Annu. Rev. Physiol. 1990;52:1–25. doi: 10.1146/annurev.ph.52.030190.000245. [DOI] [PubMed] [Google Scholar]
- 3.Sono M, Roach MP, Coulter ED, Dawson JH. Chem. Rev. 1996;96:2841–2888. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]
- 4.English AM, Tsaprailis G. In: Advances in Inorganic Chemistry. Sykes AG, editor. Academic Press; 1995. pp. 79–125. [Google Scholar]
- 5.Gardner PR, Gardner AM, Martin LA, Salzman AL. Proc. Natl. Acad. Sci. USA. 1998;95:10378–10383. doi: 10.1073/pnas.95.18.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wasser IM, De Vries S, Moenne–Loccoz P, Schroder I, Karlin KD. Chem. Rev. 2002;102:1201–1234. doi: 10.1021/cr0006627. [DOI] [PubMed] [Google Scholar]
- 7.Cleeter MWJ, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AHV. FEBS Letters. 345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 8.Brown GC, Cooper CE. FEBS Letters. 356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 9.Brown GC. FEBS Letters. 369:136–139. doi: 10.1016/0014-5793(95)00763-y. [DOI] [PubMed] [Google Scholar]
- 10.Radi R. Proc. Natl. Acad. Sci. USA. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szabo C, Ischiropoulos H, Radi R. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 12.Radi R, Beckman JS, Bush KM, Freeman BA. J. Biol. Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 13.Ischiropoulos H. Arch. Biochem. Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 14.Radi R, Beckman JS, Bush KM, Freeman BA. Arch. Biochem. Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 15.Denicola A, Freeman BA, Trujillo M, Radi R. Arch. Biochem. Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- 16.King PA, Anderson VE, Edwards JO, Gustafson G, Plumb RC, Suggs JW. J. Am. Chem. Soc. 1992;114:5430–5432. [Google Scholar]
- 17.Radi R. Chem. Res. Toxicol. 1996;9:828–835. doi: 10.1021/tx950176s. [DOI] [PubMed] [Google Scholar]
- 18.Herold S, Exner M, Nauser T. Biochemistry. 2001;40:3385–3395. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 19.Herold S, Shivashankar K, Mehl M. Biochemistry. 2002;41:13460–13472. doi: 10.1021/bi026046h. [DOI] [PubMed] [Google Scholar]
- 20.Kim E, Helton ME, Wasser IM, Karlin KD, Lu S, Huang HW, Moenne-Loccoz P, Incarvito CD, Rheingold AL, Honecker M, Kaderli S, Zuberbuhler AD. Proc. Natl. Acad. Sci. USA. 2003;100:3623–3628. doi: 10.1073/pnas.0737180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim E, Chufan EE, Kamaraj K, Karlin KD. Chem. Rev. 2004;104:1077–1133. doi: 10.1021/cr0206162. [DOI] [PubMed] [Google Scholar]
- 22.Chufan EE, Puiu SC, Karlin KD. Acc. Chem. Res. 2007;40:563–572. doi: 10.1021/ar700031t. [DOI] [PubMed] [Google Scholar]
- 23.Halime Z, Kieber-Emmons MT, Qayyum MF, Mondal B, Gandhi T, Puiu SC, Chufan EE, Sarjeant AA, Hodgson KO, Hedman B, Solomon EI, Karlin KD. Inorg. Chem. 2010;49:3629–3645. doi: 10.1021/ic9020993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halime Z, Kotani H, Li Y, Fukuzumi S, Karlin KD. Proc. Natl. Acad. Sci. USA. 2011;108:3990–3994. doi: 10.1073/pnas.1104698108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson-Miller S, Babcock GT. Chem. Rev. 1996;96:2889–2908. doi: 10.1021/cr950051s. [DOI] [PubMed] [Google Scholar]
- 26.Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. Science. 1995;269:1069–1074. doi: 10.1126/science.7652554. [DOI] [PubMed] [Google Scholar]
- 27.Iwata S, Ostermeier C, Ludwig B, Michel H. Nature. 1995;376:660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- 28.Varotsis C, Zhang Y, Appelman EH, Babcock GT. Proc. Natl. Acad. Sci. USA. 1993;90:237–241. doi: 10.1073/pnas.90.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collman JP, Sunderland CJ, Berg KE, Vance MA, Solomon EI. J. Am. Chem. Soc. 2003;125:6648–6649. doi: 10.1021/ja034382v. [DOI] [PubMed] [Google Scholar]
- 30.Momenteau M, Reed CA. Chem. Rev. 1994;94:659–698. [Google Scholar]
- 31.Li Y, Sharma SK, Karlin KD. Polyhedron. 2013;58:190–196. doi: 10.1016/j.poly.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang HC, Zhang CX, Henson MJ, Sommer RD, Hatwell KR, Kaderli S, Zuberbuhler AD, Rheingold AL, Solomon EI, Karlin KD. J. Am. Chem. Soc. 2002;124:4170–4171. doi: 10.1021/ja0125265. [DOI] [PubMed] [Google Scholar]
- 33.Kieber-Emmons MT, Qayyum MF, Li Y, Halime Z, Hodgson KO, Hedman B, Karlin KD, Solomon EI. Angew. Chem. Int. Ed. Engl. 2012;51:168–172. doi: 10.1002/anie.201104080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kieber-Emmons MT, Li Y, Halime Z, Karlin KD, Solomon EI. Inorg. Chem. 2011;50:11777–11786. doi: 10.1021/ic2018727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chufan EE, Mondal B, Gandhi T, Kim E, Rubie ND, Moenne-Loccoz P, Karlin KD. Inorg. Chem. 2007;46:6382–6394. doi: 10.1021/ic700363k. [DOI] [PubMed] [Google Scholar]
- 36.Del Rio D, Sarangi R, Chufan EE, Karlin KD, Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 2005;127:11969–11978. doi: 10.1021/ja043374r. [DOI] [PubMed] [Google Scholar]
- 37.Kim E, Helton ME, Lu S, Moenne-Loccoz P, Incarvito CD, Rheingold AL, Kaderli S, Zuberbuhler AD, Karlin KD. Inorg. Chem. 2005;44:7014–7029. doi: 10.1021/ic050446m. [DOI] [PubMed] [Google Scholar]
- 38.Karlin KD, Nanthakumar A, Fox S, Murthy NN, Ravi N, Huynh BH, Orosz RD, Day EP. J. Am. Chem. Soc. 1994;116:4753–4763. [Google Scholar]
- 39.Sharma V, Karlin KD, Wikström M. Proc. Natl. Acad. Sci. USA. 2013;110:16844–16849. doi: 10.1073/pnas.1220379110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox S, Nanthankumar A, Wikstrom M, Karlin KD, Blackburn NJ, et al. J. Am. Chem. Soc. 1996;118:24–34. [Google Scholar]
- 41.Ghiladi RA, Karlin KD. Inorg. Chem. 2002;41:2400–2407. doi: 10.1021/ic0103547. [DOI] [PubMed] [Google Scholar]
- 42.Ghiladi RA, Huang H-W, Moenne-Loccoz P, Stasser J, Blackburn NJ, Woods AS, Cotter RJ, Incarvito CD, Rheingold AL, Karlin KD. J. Biol. Inorg. Chem. 2005;10:63–77. doi: 10.1007/s00775-004-0609-1. [DOI] [PubMed] [Google Scholar]
- 43.Ghiladi RA, Chufan EE, Rio DD, Solomon EI, Kerbs C, Huynh BH, Huang H-W, Moenne-Loccoz P, Kaderli S, Honecker M, Zuberbuhler AD, Marzilli L, Cotter RJ, Karlin KD. Inorg. Chem. 2007;46:3889–3902. doi: 10.1021/ic061726k. [DOI] [PubMed] [Google Scholar]
- 44.Schopfer MP, Mondal B, Lee DH, Sarjeant AA, Karlin KD. J. Am. Chem. Soc. 2009;131:11304–11305. doi: 10.1021/ja904832j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. Free Radic. Biol. Med. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 46.Yukl ET, De Vries S, Moenne-Loccoz P. J. Am. Chem. Soc. 2009;131:7234–7235. doi: 10.1021/ja9026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herold S, Koppenol WH. Coord. Chem. Rev. 2005;249:499–506. [Google Scholar]
- 48.Gunaydin H, Houk KN. J. Am. Chem. Soc. 2008;130:10036–10037. doi: 10.1021/ja711365e. [DOI] [PubMed] [Google Scholar]
- 49.Pacher P, Beckman JS, Liaudet L. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boccini F, Herold S. Biochemistry. 2004;43:16393–16404. doi: 10.1021/bi0482250. [DOI] [PubMed] [Google Scholar]
- 51.Kurtikyan TS, Eksuzyan SR, Goodwin JA, Hovhannisyan GS. Inorg. Chem. 2013;52:12046–12056. doi: 10.1021/ic4018689. [DOI] [PubMed] [Google Scholar]