Abstract
The Alexandrium tamarense species complex is one of the most studied marine dinoflagellate groups due to its ecological, toxicological and economic importance. Several members of this complex produce saxitoxin and its congeners – potent neurotoxins that cause paralytic shellfish poisoning. Isolates from this complex are assigned to A. tamarense, A. fundyense, or A. catenella based on two main morphological characters: the ability to form chains and the presence/absence of a ventral pore between Plates 1′ and 4′. However, studies have shown that these characters are not consistent and/or distinctive. Further, phylogenies based on multiple regions in the rDNA operon indicate that the sequences from morphologically indistinguishable isolates partition into five clades. These clades were initially named based on their presumed geographic distribution, but recently were renamed as Groups I–V following the discovery of sympatry among some groups. In this study we present data on morphology, ITS/5.8S genetic distances, ITS2 compensatory base changes, mating incompatibilities, toxicity, the sxtA toxin synthesis gene, and rDNA phylogenies. All results were consistent with each group representing a distinct cryptic species. Accordingly, the groups were assigned species names as follows: Group I, A. fundyense; Group II, A. mediterraneum; Group III, A. tamarense; Group IV, A. pacificum; Group V, A. australiense.
Keywords: Alexandrium fundyense, Alexandrium mediterraneum, Alexandrium tamarense, Alexandrium pacificum, Alexandrium australiense
Introduction
Dinoflagellates are among the most important primary producers in marine systems. A small minority of these species cause harmful algal blooms (HABs) that adversely impact ecosystem services and function, often through the production of phycotoxins that can accumulate within marine food webs (Sunda et al. 2006). Many of these toxins contaminate seafood and pose a significant public health threat (Anderson et al. 2012; Smayda 1997). Toxigenic dinoflagellates belonging to the Alexandrium tamarense (M. Lebour) Balech species complex are among the most widely distributed HAB-causing taxa globally. Currently, the A. tamarense species complex contains three morphologically defined species: A. tamarense (M. Lebour) Balech, A. fundyense Balech and A. catenella (Whedon & Kof.) Balech. Numerous strains of each species produce saxitoxin and its congeners (hereafter referred to collectively as ‘saxitoxins’), a group of phycotoxins that cause paralytic shellfish poisoning (PSP; Anderson et al. 1994, 2012; Balech 1985). Balech (1995) provided detailed information on the morphology of these species based on extensive sampling from different geographic regions. The three morphospecies share the same plate formula and can be distinguished by differences in their length to width ratios, the presence or absence of a ventral pore along the suture between 1′ and 4′ thecal plates, and differences in the shape of the sp and sa thecal plates. Other defining characteristics reported by Balech included (1) the ability of A. catenella, but not A. fundyense or A. tamarense to form long chains (more than 4 cells; Balech 1985, 1995; Balech and Tangen 1985), (2) the more flattened shape of A. catenella cells which exhibited lower length:width ratios than the other two species, and (3) the presence of a ventral pore only in A. tamarense (Balech 1985).
More recent field and culture studies have revealed cells exhibiting morphologies intermediate to those described as characteristic for A. catenella, A. fundyense, and A. tamarense. These morphological intermediates are often observed within the same field population as well as within cultures started from a single cell, casting serious doubt on the validity of the morphospecies concept of all three species (Anderson et al. 1994; Balech 1985; Cembella and Taylor 1986; Destombe et al. 1992; Gayoso and Fulco 2006; Kim et al. 2002; Orlova et al. 2007; Taylor 1984). In contrast, rDNA sequences from the three morphologically defined species have consistently fallen into five phylogenetically discrete, non-overlapping clades, two of which are comprised of strains having different morphospecies classifications (John et al. 2003a; Lilly et al. 2007; Miranda et al. 2012; Orr et al. 2011; Scholin et al. 1995; Wang et al. 2014). Thus, there has been a long-standing discordance between the morphology-based classifications in the A. tamarense species complex and the group’s molecular phylogeny. This has led many researchers to adopt a clade-based (Groups I–V) numbering scheme to identify the “species” being studied when reporting the results of ecological, toxicological, or other studies (e.g. Baggesen et al. 2012; Brosnahan et al. 2010; Collins et al. 2009; Genovesi et al. 2011; Ho et al. 2012; Jedlicki et al. 2012; Lilly et al. 2007; Murray et al. 2012; Orr et al. 2011; Toebe et al. 2013; Touzet et al. 2010).
The “Group” naming scheme was proposed as an ad interim revision to address the speciation apparent in their analysis of LSU sequences from globally dispersed Alexandrium tamarense species complex isolates (Lilly 2003; Lilly et al. 2007). The publication did not provide an authoritative taxonomic revision but recommended that the group designations be used until the taxonomy was reevaluated and new species were proposed. Wang et al. (2014) recently proposed species names for Groups I–V based on ITS rDNA phylogenies, following previous work that demonstrated the utility of ITS sequences as species-specific DNA barcodes for dinoflagellates (Adachi et al. 1994; Gottschling and Plötner 2004; Gottschling et al. 2005; Litaker et al. 2007). Their results were in agreement with those obtained previously using SSU and LSU sequences, again indicating that Groups I–V are distinct species (John et al. 2003a; Lilly et al. 2007; Miranda et al. 2012; Scholin et al. 1995). However, none of these workers has undertaken the precise and careful taxonomic revision required by the International Code of Nomenclature for algae, fungi, and plants (ICN, McNeill et al. 2012) to establish each of these groups as valid species.
The inability to reliably distinguish A. catenella, A. fundyense and A. tamarense can complicate cell-based HAB monitoring programs used to identify the onset of toxic blooms and provide public health officials and resource managers with sufficient lead-time to implement direct measurements of shellfish toxicity and post timely warnings to the public. In some coastal regions toxic and non-toxic A. tamarense species complex taxa co-occur, making it impossible to determine threat levels from cell counts alone. In these instances, rapid, quantitative species-specific or gene specific (sxtA) molecular assays may offer the best means of distinguishing the toxic potential of incipient blooms (Anderson et al. 1994, 2005; Dyhrman et al. 2006, 2010; Erdner et al. 2010; Godhe et al. 2007; John et al. 2003b, 2005; Scholin et al. 1995; Toebe et al. 2013). Development and validation of these assays, however, depends on accurate species definitions.
The subject of this paper is therefore to present a complete taxonomic revision of the A. tamarense species complex, which directly addresses the long-standing taxonomic difficulties within this group. This analysis includes a review of mating and cell toxicity studies conducted using globally distributed isolates, toxicity screening using the newly developed sxtA4 gene marker, and a thorough phylogenetic assessment of the rDNA operon including LSU, SSU, and ITS sequences, and secondary structure modeling of compensatory base changes (CBCs) within the ITS2 domain. The preponderance of evidence compiled here is consistent with the existence of at least five cryptic species within the A. tamarense species complex, some of which produce PSP toxins and others that do not. Type representatives for each of the cryptic species are presented, including complete morphological descriptions based on light and scanning electron microscopy. Epitypes are proposed for A. fundyense (Group I) and A. tamarense (Group III) and new species names are assigned to Group II, IV and V.
Results and Discussion
A detailed review of the historical and experimental data on rDNA phylogenetic relationships, ITS/5.8S species-specific genetic distances, ITS2 complementary base pair changes (CBCs), mating incompatibilities, and production patterns of saxitoxins, were uniformly consistent with the existence of five cryptic species within the A. tamarense species complex.
Evidence that the Three Morphologically Defined Species within the A. tamarense Species Complex are Insufficient.
The first name assigned to an A. tamarense species complex isolate was Gonyaulax tamarensis and was described by Lebour (1925) as follows: “This little species was found up the River Tamar, in estuarine water. Cell roundish, rather longer than broad. No apical horn; 2 very small antapical spines. Girdle hardly displaced one girdle width, with no overhang, with no lists. Longitudinal furrow much expanded posteriorly. Plate formula 4′, 0a, 6″, 6‴, 1p, 1″″. First apical rather broad. Theca smooth. Length 36 μm. Found only in the River Tamar estuary, near Plymouth”. Lebour’s description was accompanied by drawings showing the plate pattern in ventral, dorsal, apical (in interior view) and antapical view; the cell depicted in the drawings lacked a ventral pore. The species was subsequently studied by various authors who reassigned the species as follows; Gonyaulax tamarensis Lebour var. excavata (Braarud 1945), Gonyaulax excavata (Braarud) Balech (Balech 1971), Gessnerium tamarensis (Loeblich and Loeblich 1979) and Protogonyaulax excavata (Braarud) F.J.R. Taylor (Taylor 1979). In 1985 Balech transferred Gonyaulax tamarensis M. Lebour, G. excavata (Braarud) Balech and G. catenella Whedon & Kof. to the genus Alexan-drium. Alexandrium excavatum was retained as a distinct species from A. tamarense based on generally well-defined shoulders in the epitheca and a concave antapical portion in A. excavatum. The Balech (1985) publication also provided the original description of A. fundyense with a very short diagnosis: ‘Very close to A. excavatum but constantly lacking of ventral pore. Dimensions: L 27–46, A27–44 μm, Distribution in the Bay of Fundy’. Balech also added the disclaimer ‘Perhaps a subspecies’.
Based on additional morphological studies, Balech (1995) concluded that A. excavatum should be considered a synonym of A. tamarense. He further distinguished A. fundyense and A. tamarense as follows (Balech 1995, page 43), “The primary difference is the lack of a ventral pore, which hardly seems enough to separate them as species. However, I believe that the proven constancy of this character justifies separation of A. fundyense from the Lebour species”. Variability in cell shape (e.g. Balech 1971; Braarud 1945), cell size, and in the presence or absence of the ventral pore (e.g. Anderson et al. 1994; Loeblich and Loeblich III 1975; Loeblich et al. 1975), however, was frequently reported in A. tamarense. In his extensive study of natural samples of A. tamarense and A. fundyense obtained from different geographic areas, Balech (1995) specifically noted that in certain cases these characters were of limited value when identifying species. Similarly, other field and culture studies have recorded cells exhibiting morphologies intermediate between those described for the A. fundyense and A. tamarense morphotypes (Anderson et al. 1994; Cembella and Taylor 1986; Destombe et al. 1992; Gayoso and Fulco 2006; Kim et al. 2002; Orlova et al. 2007; Taylor 1984; Fig. 1; Table 1).
Figure 1.
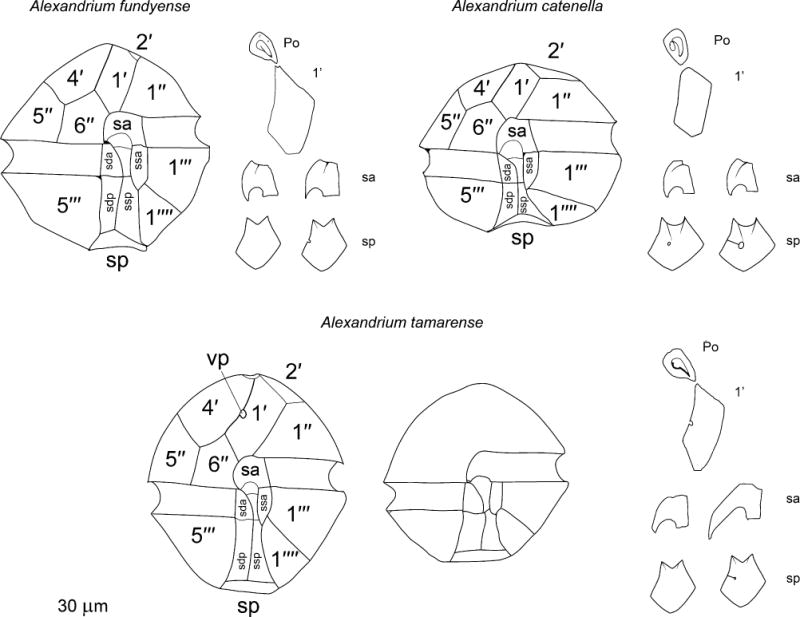
A synthetic representation of the morphological differences among A. catenella, A. fundyense and A. tamarense, as described by Balech (1985; 1990; 1995). The A. fundyense and A. catenella drawings represent the two extreme morphotypes observed in the A tamarense species complex. po = apical pore plate, sa = anterior sulcal plate, sda = right anterior lateral sulcal plate, sdp = right posterior lateral sulcal plate, sma = anterior median sulcal plate, smp = posterior median sulcal plate, sp = posterior sulcal plate, ssa = left anterior lateral sulcal plate, ssp = left posterior lateral sulcal plate, vp = ventral pore.
Table 1.
The morphological descriptions of A. catenella, A. fundyense, and A. tamarense provided by Balech (1995).
| Alexandrium catenella | Alexandrium fundyense | Alexandrium tamarense | |
|---|---|---|---|
| Current authority | Alexandrium catenella (Whedon & Kofoid) Balech 1985: p. 34, figure 2 | Alexandrium fundyense Balech 1985: p. 37, figure 18 | Alexandrium tamarense (Lebour) Balech 1985, p. 37, figure 20 |
| Cell Characteristics From Balech 1995 | Cell small- to medium-sized, somewhat flattened anterior-posteriorly. Usually forms curved chairs. Epitheca has more or less noticeable shoulder and a rather upraised apical region. Cigulum is very excavated, descending (one, sometimes a little more). It generally has an overlapping membrane or curtain fin that extends from the projecting flange on the epitheca to the corresponding flange on the hypotheca. Sulcus is rather deep, abruptly widened on the posterior. The 1′ lacks a ventral pore and directly contacts the Po. It is asymmetrically rhomboidal. Usually, the anterior right margin is clearly concave. Plates 2′, 3′ and 4′ have raised flanges and support the Po. 2′ is the largest apical plate and usually connected with 3′ by sinuous margin that is mostly concave. 3′ is hexagonal and clearly asymmetrical with the anterior left side up to twice as long as the anterior right side. 4′ is relatively short and wide. 6″ is medium wide; its internal margin border with the S.a. and has a barely pronounced concavity. In the hypotheca, the 5‴ is wide and has a somewhat reinforced internal margin that is slightly S-shaped and supports a narrow list. 1″″ is rather narrow, long and very oblique. Its sulcal list is moderately wide, wider anteriorly than posteriorly. 2″″ is transversely elongated. | “By its shape and size, it cannot be distinguished from A. tamarense. Therefore, instead of providing a detailed description, I will note only its variations and differences from the last species.” (Balech et al. 1995). Most specimens that are not obviously collapsed are as wide or wider than they are long. The L/W ratio is almost always less than one. “The primary difference from A. tamarense is the lack of the ventral pore, which hardly seems enough to separate them as species. However, I believe that the proven consistency of this character justifies separation of A. fundyense from the Lebour species. Some other small differences occur, but they are not consistent.” | Cell small- to relatively large-sized and is somewhat isodiametric. In lateral view, the shape is irregularly pentagonal and convex. The cell frequently has one or two shoulders that may or may not be very noticeable. The hypotheca is regularly trapezoidal with convex and sometime irregular sides. A concavity may be located on the left side of the hypothecae; sometimes a protuberance is above it. The concavity is not deep, but is noticeable. The posterior margin is very often asymmetric, sloped forward and to the right. The descending cingulum is excavated and has a very narrow list. The sulcus is variably deep and has moderately developed lists. The Po is often very wide and markedly angular. The 1′ has a relatively variable width. Usually, the anterior angle and, especially, the posterior angle are rather extensively truncated. The anterior right margin is often rather concave. Sometimes, the margin is abruptly angled at the location of the ventral pore. The ventral pore is small and always exists. Both its position on the margin and the degree of its indentation on the plate may vary. 3′ is always clearly asymmetrical, but the degree of asymmetry varies. 1″″ is somewhat narrow. The most variable feature is the development of the left sulcal list which is rather wide in some cases and rather narrow in others. The right sulcal list, supported by the 5‴ plate, is usually barely conspicuous. 2″″ is variable. It is transversely extended in some thecae or dorsoventrally in others, with some in transition. This species is widely distributed and therefore its characteristics are more variable. |
| Cellular Dimensions From (Balech 1995) | Length 20–39.5 μm, but generally 24 to 34 μm; width 22–44 μm. The length/width (L/W) ratio varies, but the species is generally wider than long and the anterior-posterior flattening is often rather conspicuous. However, length can equal the width in some specimens. Some specimens can be difficult to distinguish from A. tamarense. | Length 39–50 μm, but generally 27 to 37 μm; Width not given only the L/W ratio of 0.87–1, but generally 0.93 to 0.98. Interestingly, the larger cells usually have a rather different shape. They are somewhat elongated and the L/W ratio in almost all of them is equal to or greater than one (generally 1.01 to 1.09) averaging approximately 1.04. Additionally, their hypothecae are more regularly convex. | Length 22–51 μm, but generally 28 to 35 μm; Width 22–44 μm, but usually between 35 and 44 μm. [Width] is almost equal to L, but may be a little larger or smaller. |
Alexandrium catenella was originally described as Gonyaulax catenella by Whedon and Kofoid (1936) from natural samples collected off San Francisco (California) and along the Oregon coast, USA. The detailed description of cell shape, size and thecal plates was accompanied by drawings of cells in ventral, dorsal, apical and antapical view as well as by a sketch of a 4-cell chain and two drawings in which the location of the horseshoe-shaped nucleus was illustrated (Whedon and Kofoid 1936). Balech (1995) stated that the distinctive character between A. catenella and A. fundyense is the formation of chains in the former species, where connecting pores are present on both, the outer apical pore (po) and posterior sulcal (sp) plates. However, though long chains are frequently recorded in samples from the natural environment, this character is markedly reduced in culture, where cells may be single or in couplets (e.g. MacKenzie et al. 2004).
These observations indicate that variations in cell size and shape, the presence or absence of a small pore on the margin of the 1′ plate, and the degree of chain formation originally used to differentiate these species are both more plastic within a single strain and more variable within a species than was originally assumed (Fig. 1; Tables 1, 2). The presence or absence of the pore along the border between 1′ and 4′ plates, which was the primary characteristic separating A. fundyense and A. tamarense, was shown to be particularly unreliable, sometimes present and other times not. Measurement of all the other diagnostic morphological features also overlapped between species, indicating that all three species instead represent a single, morphologically plastic group (Tables 1, 2). Therefore, the original A. catenella, A. fundyense, and A. tamarense species descriptions are not useful, because the morphological characters do not allow an unequivocal species circumscription.
Table 2.
Comparison of cell size measurements (provided only for the strains selected as epitypes/holotypes of the different species), presence or absence of a vental pore in the 1′ plate, tendency to form chains (no species forms chains 100% of the time), PSP toxicity, presence of sxtA4, whether on not species overlap (occur sympatrically) in the same geographic ranges, and number of nucleotide differences in rRNA genes among Alexandrium fundyense, A. mediterraneum, A. pacificum, A. tamarense and A. australiense.
| Type strain |
A. fundyense (Group I) SPE10-03 |
A. mediterraneum (Group II) SZN01 |
A. tamarense (Group III) ATSW01-1 |
A. pacificum (Group IV) ACPP01 |
A. australiense (Group V) ATBB01 |
|---|---|---|---|---|---|
| Size (mean) | |||||
| length | 34.0 ±2.4 (n=20) | 36.8 (n=20) | 36.0 ±2.3 (n=20) | 35.7 ±3.4 (n=20) | 32.7 (n=20) |
| width | 32.7 ±2.4 (n=20) | 39.6 (n=20) | 33.9 ±2.1 (n=20) | 34.3 ±2.9 (n=20) | 31.6 (n=20) |
| L:W ratio | 1.04 | 0.93 | 1.06 | 1.04 | 1.04 |
| Ventral pore | |||||
| Presence/ absence | present/absent | present | present/absent | present/absent | present |
| Chain formation | |||||
| Single cells | yes | yes | yes | yes | yes |
| Occasionally 2–4 cell chain | yes | yes | yes | ||
| Long chains (> 4 cells) | yes | yes | |||
| Compensatory Base pair Changes in the ITS2 region (helix III) | present | present | absent | absent | present |
| PSP toxicity | |||||
| Presence/absence | present | absent | absent | present | present/absent |
| sxtA4 gene presence | present | absent | absent | present | present |
| Occurs sympatrically in combination with other Groups | Groups 2, 3, 4, 5 *references1,2,3,4,5 |
Groups 1, 4 *references4,5 |
Groups 1, 4 *references6,7 |
Groups 1, 3, 5 *references8,9 |
Groups 1, 4 *reference5 |
| Ribosomal sequence differences within species | |||||
| SSU (1710 bp) | 79 bp | 12 bp | 1 bp | 8 bp | 1 bp |
| ITS (519 bp) | 43 bp | 2 bp | 4 bp | 12 bp | 2 bp |
| LSU (599 bp) | 36 bp | 4 bp | 7 bp | 12 bp | 3 bp |
Phylogenetic Relationships within the A. tamarense Species Complex Support the Existence of Five Cryptic Species
Phylogenetic analyses of the rDNA complex genes, including SSU rDNA, ITS1/5.8S/ITS2 rDNA and LSU rDNA consistently recovered five distinct clades (Groups I–V) within the A. tamarense species complex that are inconsistent with the morphologically based species (Fig. 2 and Supplementary Material Figs S3–S6, S8–S10). Moreover, the genetic distances separating the Groups are as large as those observed between other Alexandrium species (Fig. 2 and Supplementary Material Figs S3–S6; Adachi et al. 1996; Anderson et al. 2012; Guillou et al. 2002; Ho et al. 2012; John et al. 2003a; Lilly et al. 2007; MacKenzie et al. 2004; Montresor et al. 2004; Murray et al. 2012; Penna et al. 2005, 2008; Ruiz Sebastián et al. 2005; Touzet et al. 2008; Wang et al. 2014). The greatest divergences within Group I were due to a deletion in some sequences (Fig. 2, Supplementary Material Figs S4, S5). Despite this deletion, all the sequences fell within Group I and not Groups II–V. This is consistent with the results of previous analyses, which found that even highly divergent pseudogenes in Group I genomes never segregate with those found in the other groups (Ho et al. 2012; Medlin et al. 1998; Miranda et al. 2012; Orr et al. 2011; Wang et al. 2014).
Figure 2.
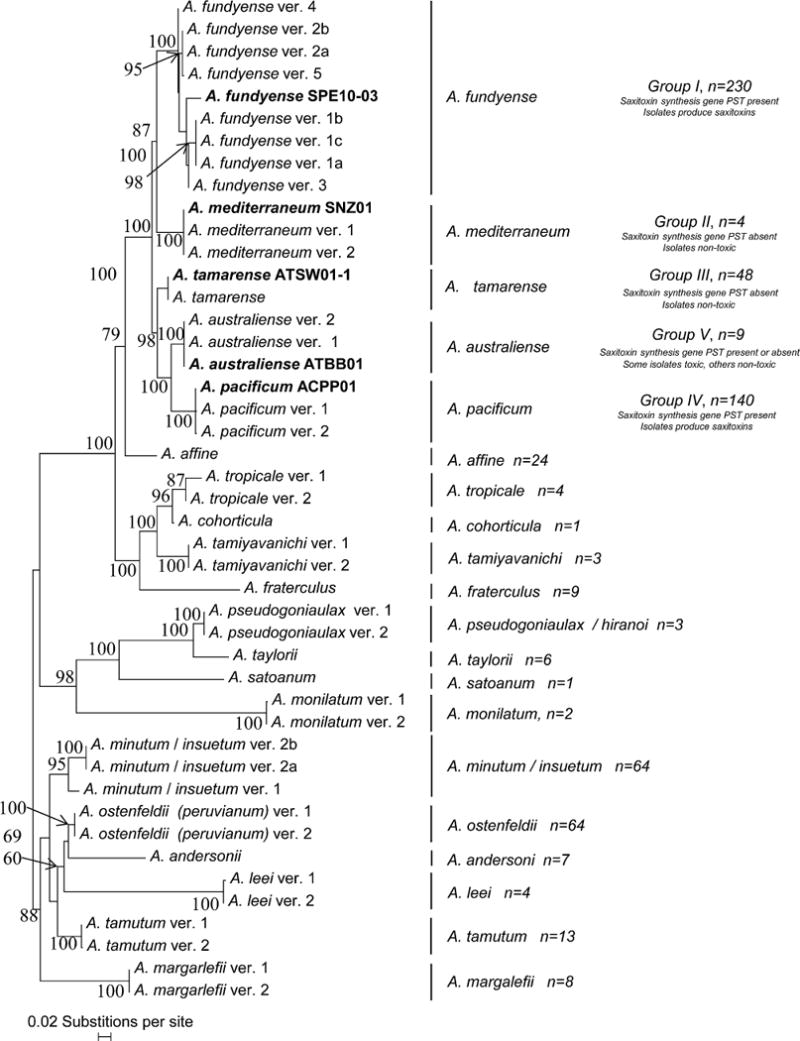
Phylogenetic tree of the D1–D2 LSU rDNA utilizing all available Alexandrium tamarense species complex sequences as well as those from other Alexandrium species sequences present in GenBank. RAxML CAT analyses with 1000× bootstrap support, which is shown at the nodes of the tree. To simplify the analysis, the sequence data were filtered to remove sequences with differences of ≤2bp (see Methods). Once these groups were sorted, one representative sequence from each was selected for inclusion in the phylogenetic analysis. N = the number of sequences of each species included in the analysis. Groups that possess the sxtA4 gene and produce saxitoxins are marked as well as those which are non toxic.
Divergence in the ITS/5.8S region has been proposed as a way to identify species boundaries in dinoflagellates, and as a DNA barcoding region (Litaker et al. 2007). Specifically, average genetic distances (p values) exceeding 0.04 substitutions per site have been found to be consistent with species level divergences in dinoflagellates (Litaker et al. 2007). The average ITS/5.8S genetic distances among sequences belonging to different Alexandrium tamarense species complex Groups in this study ranged from 0.155 to 0.217 substitutions per site (Table 3), far exceeding the p>0.04 threshold. In contrast, the within Group genetic distances varied from 0.001 to 0.027 substitutions per site, well below the p>0.04 species-level divergence threshold. Further, these estimates represent the maximum possible divergences because no effort was made to eliminate pseudogenes from the analysis (i.e., all sequences present in GenBank were included). If the pseudogene sequences had been omitted, the estimate of within species genetic distance variation would have been substantially lower. The fact that average genetic distances remained below p<0.03 substitutions per site supports the A. tamarense species complex groups I–V as representing separate species.
Table 3.
Within and between species genetic distances (p, substitutions per site) calculated using aligned ITS1/5.8S/ITS2 rDNA sequences. Pairwise p values were calculated for every possible sequence pair both within and between species. The n value indicated that total number of pairwise comparisions made for each analysis. The average p value, as well as the smallest and largestp valued observed (in parentheses) in each between or within species analysis are presented.
|
A. fundyense (Group I) |
A. mediteraneum (Group II) |
A. tamarense (Group III) |
A. pacificum (Group IV) |
A. australiense (Group V) |
|
|---|---|---|---|---|---|
|
A. fundyense (Group I) |
Average 0.027 | Average 0.192 | Average 0.196 | Average 0.218 | Average 0.217 |
| (0.000 – 0.087) n = 3,003 |
(0.179 – 0.232) n = 539 |
(0.187 – 0.226) n = 1,771 |
(0.202 – 0.265) n = 9,779 |
(0.206 – 0.253) n = 77 |
|
|
A. mediterraneum (Group II) |
Average 0.002 | Average 0.152 | Average 0.196 | Average 0.187 | |
| 0.000 – 0.004 n = 21 |
0.151 – 0.158 n = 161 |
(0.188 – 0.217) n = 910 |
0.186 – 0.189 n = 7 |
||
|
A. tamarense (Group III) |
Average 0.001 | Average 0.177 | Average 0.164 | ||
| (0.000 – 0.008) n = 253 |
(0.169 – 0.198) n = 2,921 |
(0.164 – 0.166) n = 23 |
|||
|
A. pacificum (Group IV) |
Average 0.009 | Average 0.155 | |||
| (0.000 – 0.038) n = 8,001 |
0.147 – 0.176 n = 127 |
||||
|
A. australiense (Group V) |
0.03 | ||||
| n = 2 |
Field studies have shown that representatives of the Alexandrium tamarense species complex have been found in different regions of the world, frequently in sympatry, but no hybridization signals have been detected in those areas (Bolch and de Salas 2007; Collins et al. 2009; Gu et al. 2013; Kamikawa et al. 2007; MacKenzie et al. 2004; Murray et al. 2012; Orlova et al. 2007; Penna et al. 2008; Toebe et al. 2013; Touzet et al. 2010; Table 2). A single exception is the report of a Group I-Group III hybrid resting cysts in Belfast Lough, Northern Ireland (Brosnahan et al. 2010). However, as described in more detail below, the same study also demonstrated that such hybrids are not capable of resuming mitotic division when produced in culture, affirming the classification of the parent cells as distinct species.
Evidence for Hybridization Barriers Between Alexandrium tamarense Species Complex Groups
For sexual organisms like species of Alexandrium, genetic recombination occurs via mating, a process that entails the sexual fusion of free-swimming haploid cells to form diploid zygotes, followed by meiosis and the production of new haploid vegetative cells (Pfiester and Anderson 1987). Barriers to the exchange of genetic material between individuals define the boundaries between different species and the characterization of these barriers typically must consider both pre-zygotic and post-zygotic mechanisms. Here, we review both direct evidence of pre- and postzygotic hybridization barriers and also consider the accumulation of CBCs, a genetic marker that has been empirically associated with speciation in a diverse array of eukaryotic species (Coleman 2009).
Within the A. tamarense species complex, the most thoroughly examined sexual life cycles are those from Groups I, III and IV. In these species, diploids mature via passage through a resting cyst or hypnozygote stage. The resting cyst stage has special adaptive value because it can survive long periods of poor growth conditions and therefore enables the persistence of these species in many areas (Anderson and Wall 1978). Though passage through the resting cyst stage may not be necessary for all species (several congenerics have been shown to exit the sexual cycle without encystment; Anderson et al. 2012), cyst production by two strains is evidence of a lack of pre-zygotic barriers to hybridization.
In an assessment of compatibility among strains from Groups I–V, Brosnahan et al. (2010) reported that 6 of 10 possible combinations form cysts in co-culture. However the same study also found higher rates of compatibility within ribosomally defined Groups than between them. Therefore a pre-zygotic barrier limits but does not completely prevent hybridization between the A. tamarense Groups. The same study also confirmed zygosis between Group I and Group III cells through a nested-PCR assay and demonstrated a complete failure of Group I-Group III hybrids to yield new, viable cultures. The latter result indicates a complete post-zygotic barrier to hybridization between the Group I and Group III lineages.
No similar effort has been made to characterize the viability of hybrid cysts produced through combinations of the other ribosomally defined A. tamarense Groups but the Group I–Group III results are in contrast to studies that have reported production of viable cultures from combinations of “A. tamarense” and “A. catenella” morphotype, Group IV ribotype clones (MacKenzie et al. 2004) and combinations of “A. tamarense” and “A. fundyense” morphotype, Group I ribotype clones (Anderson et al. 1994; Brosnahan et al. 2010). Successful hybridizations between different morphotypes demonstrate the failure of the morphological species criteria to predict barriers to genetic recombination between the A. tamarense complex species.
The existence of hybridization barriers between the A. tamarense ribosomal Groups is further supported by the presence of compensatory base pair changes (CBCs) by pairwise comparisons in the ITS2 region between Groups I, II, and V (Table 2; Fig. 3; Coleman 2003; Fabry et al. 1999). Though not mechanistically linked to the development of either pre- or post-zygotic incompatibility, when present, CBCs or hemi-CBCs consistently correlate with species-level divergences even among closely related lineages (Klöpper et al. 2013, Ruhl et al. 2009, and Vanormelingen et al. 2007). Not all speciation events, however, lead to the development of CBCs or hemi-CBCs. Consequently, the lack of CBCs between Groups II and IV should not be interpreted as evidence against their being separate species.
Figure 3.
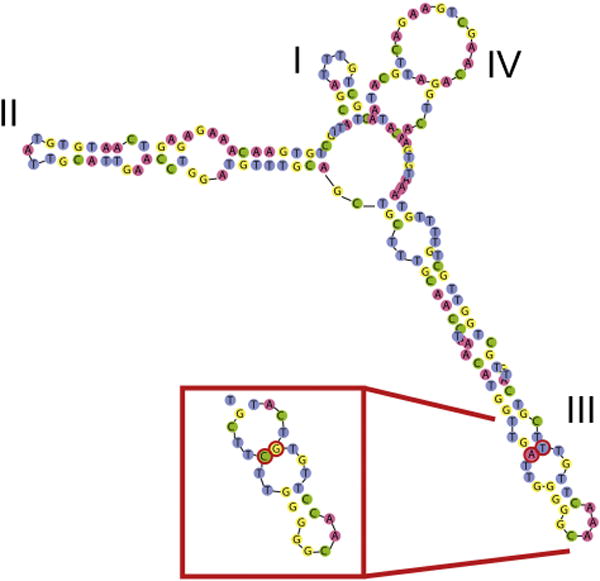
Secondary structure model of ITS2 region of rRNA for Alexandrium fundyense, based on type strain SPE10-03. The helices I–IV are shown. The boxed insert shows the compensatory base change detected in helix III for A. mediterraneum and A. australiense (also see Supplementary Material Fig. S11).
Broadly, these results are consistent with species inferences made through phylogenetic analyses of the ribosomal DNA operon. Given the close evolutionary relationships among the clades, it is not surprising that individual clones can sometimes initiate hybridization by proceeding through gamete conjugation and encystment. However, direct evidence of Group I-Group III cyst inviability and the accumulation of CBCs within three of the five A. tamarense Groups together provide strong evidence that hybridization barriers exist, affirming classification of the Groups as distinct species.
Group-specific Toxin Production
Analyses of toxicity have revealed that Group I and Group IV (Lilly et al. 2007; Orr et al. 2011) produce saxitoxins, but Group II (John et al. 2003a; Lilly et al. 2007; Orr et al. 2011; Penna et al. 2008) and III do not (Higman et al. 2001; John et al. 2003b; Lilly et al. 2007; Orr et al. 2011; Table 2). Group V isolates can produce saxitoxins or not, depending on the strain (Murray et al. 2012). In this study, the reference strain representing each of the groups was screened for the presence or absence of sxtA4 domain of the sxtA gene, a sequence that has been found in all saxitoxin-producing dinoflagellates species, including the A. tamarense species complex, as well as A. minutum, A. ostenfeldii, A. tamayavanichi, Pyrodinium bahamense, and Gymnodinium catenatum, but not from non-toxic species (Murray et al. 2011; Orr et al. 2013; Stüken et al. 2011). In this study, Group I, IV and V isolates tested positive for the presence of the sxtA4 domain, but Group II and III isolates were negative. These results are consistent with previous studies that have found sxtA4 to be present in all strains of Groups I, IV, and V that have been examined to date (Murray et al. 2011; Orr et al. 2013; Stüken et al. 2011; SM, unpublished data), but contrast with a report from Stüken et al. (2011) that sxtA4 was present in a single Group III strain (CCMP1771). Given that only two Group III strains have been tested for the presence of sxtA4, the question of sxtA4 presence/absence in this species is one that requires further examination.
More broadly with respect to the species complex, our own sxtA4 screening results were consistent with the measurements of the propensity of strains to produce saxitoxins. Further, the amplification of sxtA4 from Group V suggests that the apparent nontoxicity among some of Group V isolates is due to variability in other genes of the saxitoxin biosynthetic pathway, post-transcriptional mechanisms, or other genetic or physiological factors. Overall, the pattern of toxicity and non-toxicity within four of the five groups demonstrates that the five species are genetically and physiologically different from one another in important ways. Therefore the results of the analyses of toxin production data support the classification of these five groups as separate species.
Taxonomic Revision of the A. tamarense Species Complex
Based on genetic distances between their LSU, ITS/5.8 and SSU sequences, compensatory base changes within the ITS2 sequences, toxicity data, and apparent mating incompatibilities, we conclude that the five phylogenetic Groups observed within the Alexandrium tamarense species complex represent distinct species. Moreover, these species are not distinguished reliably using morphological criteria, so that species boundaries are here defined primarily on the basis of molecular criteria. Revision of the species names is therefore needed. Such a revision is long overdue, especially because some of these species pose a significant threat to human health through the production of saxitoxins. Current monitoring programs often rely on a combination of approaches, including estimates of the abundance of the different species complex taxa, an approach that is particularly problematic in areas where both toxic and non-toxic species occur in sympatry. Examples include sympatic blooms of toxic Group I and non-toxic Group III species that co-occur in the North and Irish Seas (Brosnahan et al. 2010; Medlin et al. 1998; Toebe et al. 2013; Touzet et al. 2010), non-toxic Group II and toxic Group IV that co-occur in the Mediterranean Sea (Lilly et al. 2002; Masseret et al. 2009; Vila et al. 2001); blooms of Groups I, III, and IV in Japanese waters (Adachi et al. 1996); and both toxic and non-toxic Group V with toxic Group IV in southern Australian waters (Murray et al. 2012).
Even though significant sympatries exist, the geographic ranges originally ascribed to A. fundyense and A. tamarense correspond well with the locations where the majority of Group I and III isolates have been collected. Consequently, most studies involving Group I isolates identify the species involved as A. fundyense and those where Group III isolates were present identify them as A. tamarense. Hence, the species designations for A. fundyense (Group I) and A. tamarense (Group III) can be retained with minimal confusion in the literature.
Such is not the case with A. catenella. The identity of the type material on which this species was based is unclear. Alexandrium catenella was first described from material collected off the California and Oregon coasts (eastern Pacific coast; Balech 1995; Whedon and Kofoid 1936); however, species ‘morphologically’ identified as Alexandrium catenella from this region fall within Group I (e.g. Garneau et al. 2011; Jester et al. 2009; Ruiz Sebastián et al. 2005). One could make an argument that A. catenella should supplant A. fundyense and be applied to all Group I strains, because its original description predates that of A. fundyense by 50 years. However this ignores the large number of Group I studies published using the name A. fundyense that would remain valid under our revision, and also the similarly large number of published Group IV studies whose naming convention would be made incorrect. For this reason, we have submitted the proposal to reject the name Alexandrium catenella under Art. 56 of ICN (John et al. 2014) and rename Group IV as A. pacificum. The remaining two Groups (II and V) have also been assigned new species names. Each of the species descriptions includes molecular and toxicological data that could be used for unambiguous identification in future biogeographic, ecological, physiological or toxicological studies.
Species Descriptions
Validity of the previously described species
The rules for a valid description of taxa are specified in nomenclatural codes, and dinoflagellate taxa have been described following the rules of both the International Code of Zoological Nomenclature (ICZN, Ride et al. 1999) and what is now the International Code of Nomenclature for algae, fungi, and plants (ICN, McNeill et al. 2012; previously the International Code of Botanical Nomenclature). The genus Alexandrium was initially described following the ICZN. The publication of the generic name Alexandrium (Halim 1960) as “Alexandrium minutum nov. g. nov. sp.” is valid, even though it does not include a Latin diagnosis. This Latin diagnosis was a mandatory requirement under the ICBN at the time, but not by the ICZN (see Art. 13.1 & 16.4 ICZN). The original ICZN genus description is considered valid under Art. 45.1 of the current ICN which states: ‘If a taxon originally assigned to a group not covered by this Code [current ICN] is treated as belonging to the algae or fungi, any of its names need satisfy only the requirements of the relevant other Code [ICZN] that the author was using for status equivalent to valid publication under this Code [current ICN]’. The publication of Gonyaulax tamarensis (Lebour 1925) is also valid under the ICZN, as was its transfer to the genus Alexandrium (see Art. 12 ICZN). Similarly, A. tamarense (M. Lebour 1925) Balech 1985 and A. fundyense Balech 1985, are considered valid species names under the ICZN at that time, even though their descriptions do not explicitly designate a type, as required by the current ICN (see Art. 13.1 & 16.4 ICZN). As discussed previously, the majority of studies investigating these two species were conducted in the general geographical region where the species were originally isolated. Hence there is good agreement between the species designations and the corresponding genetic Groups for these two species.
In contrast, most of the “A. catenella” Group IV strains have been isolated in the west and south Pacific, even though A. catenella was first described from material collected off the California and Oregon coast (eastern Pacific coast; Balech 1995; Whedon and Kofoid 1936), a region where A. fundyense (Group I) predominates. Consequently, we have proposed rejection of the A. catenella name (John et al. 2014). In this paper, we amend the descriptions of A. fundyense and A. tamarense and describe groups II, IV and V as three new species, A. mediterraneum sp. nov., A. pacificum sp. nov. and A. australiense sp. nov., repectively, under the rules of the ICN.
Alexandrium fundyense Balech 1985 emended D.M. Anderson (Group I)
Historically, the majority of the work conducted on Group I isolates has been carried out along the northeastern coast of the U.S. and southeastern Canada, where the isolates have largely been identified as A. fundyense (e.g., Anderson et al. 1994). This work includes significant information on management of toxic PSP events. Also, the first sequences obtained for this group originated from Bay of Fundy isolates (Scholin et al. 1994), which is the type location for A. fundyense Balech (Balech 1985). Consequently, assigning the name A. fundyense to the Group I isolates provides a clear and logical association between this species and a majority of the previous “A. fundyense” studies. For these reasons, we chose to assign the A. fundyense species name to the Group I isolates.
LECTOTYPE
No type material for this species exists. This designation is required by the ICN prior to designating an epitype that more fully describes the morphology of this species (ICN Art 9.8).
Alexandrium fundyense Balech in Anderson, & al. (editors), Toxic Dinoflagellates: 37, fig. 18, [Balech E (1985) The genus Alexandrium or Gonyaulax of the tamarensis group. In Anderson DM, White AW & Baden DG (eds) Toxic Dinoflagellates, Proceedings of the Third International Conference on Toxic Dinoflagellates. Elsevier, New Yok, pp 33–38.]
Lectotype designated here: figure 18.
EPITYPE
SEM stub of strain SPE10-03 is designated here as epitype for A. fundyense Balech emend. D.M. Anderson (Fig. 4). It is deposited at the Herbarium Senckenbergianum (FR) in the Centre of Excellence for Dinophyte Taxonomy (identification number CEDiT2013E25).
Figure 4.
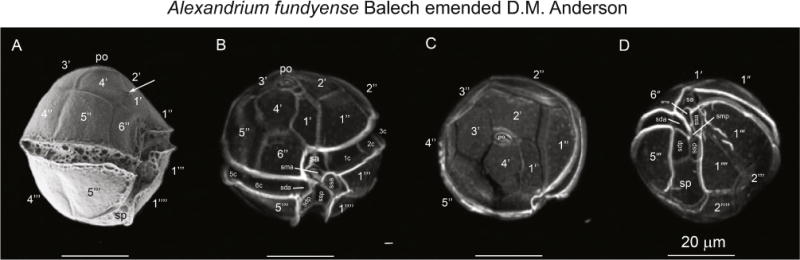
Alexandrium fundyense Balech emended D.M. Anderson. SEM (A) and calcofluor-stained cells (B–D) micrographs of type strain SPE10-03. (A) Right lateral to ventral, B) apical ventral, (C) apical and (D) antapical ventral views. The arrow in panel A indicates the presence of a pore in the 1′ plate which is present in some cells and not others. Scale bars = 20 μm.
ADDITIONAL STRAIN SPE10-03 MATERIALS
Formol-preserved cells of strain SPE10-03 were deposited in the Centre of Excellence for Dinophyte Taxonomy (identification number CEDiT2013RM26). Living cultures of this strain were deposited at the Roscoff Culture Collection (http://www.sb-roscoff.fr/Phyto/RCC/) as strain RCC4086 and at the National Center for Marine Algae and Microbiota (https://ncma.bigelow.org/) as strain CCMP3432. Genomic DNA from strain SPE10-03 was deposited at the Grunelius-Möllgard-Labor in the Senckenberg Research Institute and Natural History Museum, Germany (http://sesam.senckenberg.de/page/suchen/ergebnisliste.asp#) as FIS-6173.
SUPPORTING PUBLICATIONS
The following section contains literature references that provide additional morphological and genetic information for A. fundyense (Group I) as delimited here.
Alexandrium catenella (Whedon & Kof.) Balech sensu Aguilera-Belmonte et. al. (2011), strain PFBX illustrated in figure 2 and the ITS phylogeny in figure 4.
Alexandrium catenella (Whedon & Kof.) Balech sensu Varela et al. (2012), strain morphology illustrated in figure 2, LSU phylogeny in figure 4.
Alexandrium tamarense (Lebour) Balech sensu Baggesen et al. (2012), strain morphology illustrated in figure 2, LSU phylogeny in figure 3.
Alexandrium tamarense (M. Lebour) Balech sensu Touzet et al. (2008), strains BlB10a, BlB10b. BlD10a with a morphology typical of A. tamarense (figure 3), but corresponding genetically to A. fundyense (Group I), figure 1 SSU tree.
Alexandrium fundyense Balech sensu Wang et al. (2014) strain CCMP1719 morphology illustrated in Supplemental table 3, ITS phylogeny in figure 3, the SSU phylogeny figure 4 and the partial LSU phylogeny in figure 5.
Figure 5.
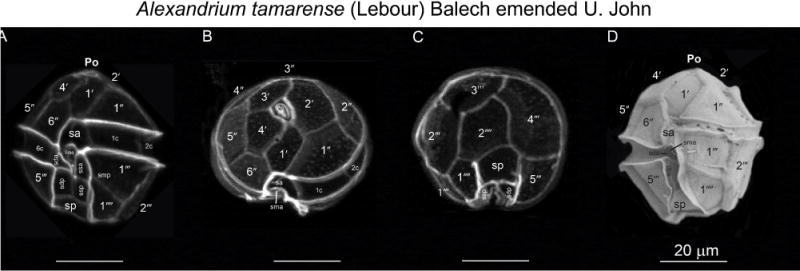
Alexandrium tamarense (M. Lebour) Balech emended U. John. Calcofluor-stained cells (A, B, C) and SEM (D) micrographs of type strain ATSW01-1. (A) Ventral, (B) apical, (C) antapical and (D) ventral views. Arrow in panel A marks the ventral pore in the 1′ plate. Scale bars = 20 μm.
EMENDED SPECIES DESCRIPTION
Cells are as long as wide or slightly longer then wide; cells of strain SPE10-03 are 32.7 ± 2.36 μm (min 30 μm, max 38 μm, n = 20) wide and 34±2.43 μm (min 30 μm, max 38 μm, n = 20) long. Cell contains many golden brown elongated chloroplasts and a horseshoe-shaped nucleus located in the equatorial part of the cell. The epicone is helmet-shaped and the hypocone roughly trapezoidal. The cingulum is descending about one cingular height. The sulcus broadens in its antapical portion and is delimited on both sides by moderately developed sulcal lists. The cell surface is smooth and ornamented with many scattered small pores. The plate formula is: Po, 4′, 6″, 5C, 8–10S, 5‴, 2″″. Po is ornamented by several small pores and presents a comma-shaped foramen. A connecting pore is generally not present on Po. Plate 1′ is irregularly rhomboidal, with longer apical right and antapical left sides; in its apical portion, it contacts Po and in its antapical portion it contacts plate Sa. The ventral pore along the margin between Plate 1′ and 4′ is generally not present. Plate 6″ is as wide as tall or slightly wider than tall. Plate 2″″ variable: it can be transverally extended (prevalent type) or dorsoventrally extended, but transitions exist. Plate sp is pentagonal, and its length:width ratio is ~ 1; a connecting pore is generally not present on the sp plate Cells are almost always single, rarely found in chains of two to eight cells in culture, but can be found in chains in the field. The cyst is ellipsoidal, with a granular dark brown content and is surrounded by a mucous layer.
MOLECULAR DIAGNOSIS
The species is defined by the combined nucleotide sequences of the epitype strain SPE10-03 D1-D2 LSU (GenBank KF908807), ITS/5.8S (GenBank KF908818), and SSU (GenBank KF908795). Maximum likelihood phylogenetic trees showing more detail as to which sequences belong to Group I of the A. tamarense species complex are shown in Figure 2 and Supplementary Material Figs S3–S6. The complete list of diagnostic D1-D2 LSU, ITS/5.8S and SSU GenBank sequences, which can be used as a genetic type for this species, are reported in Supplementary Material Fig. S7.
EPITYPE LOCALITY
The type strain SPE10-03 was isolated from Salt Pond in Eastham, Massachusetts USA, 41.835° N 69.972° W on 05/12/2001 by K. Gribble.
DISTRIBUTION
Based on genetically identified isolates, this species has currently been found in the following regions: Arctic (Greenland), North Atlantic (Canada, Denmark, France, Ireland, Norway, United Kingdom, USA - embayments and coastal regions along the northeast Atlantic (from New England to Long Island New York), and Pacific coasts (from Washington State north including Alaska), South Atlantic (Argentina, South Africa), Mediterranean (France), North Pacific (China, Japan, Russia, South Korea, USA), South Pacific (Chile) (Supplementary Material Figs S8–S10).
ETYMOLOGY
Named after the Bay of Fundy, the type locality of A fundyense Balech (Balech 1985).
TOXICITY
Isolates are almost always toxic (Lilly et al. 2007). A ‘non toxic’ strain has been reported as the result of several parent crosses of Group I strains in mating studies (Cho et al. 2008). The SPE10-03 isolate produces approximately 70 fmol cell−1 of saxitoxin congeners when growing in log phase and in nutrient-replete media. The toxin profile expressed in mole percent of each detected congener is: C1 (1.6%), C2 (55.3%), GTX1 (0.2%), GTX3 (1.1%), GTX4 (13.5%), GTX5 (19.9%), and NEO (8.3%) (D.M. Anderson unpublished). Both toxin content and toxin profiles differ considerably among other A fundyense strains (Anderson et al. 1994; Orlova et al. 2007), and can include the toxin congeners B1, C1, C2, GTX1, 2, 3, 4, 5 and 6, dcSTX, dcGTX3, NEO and STX. Consistent with these toxin data, the conserved gene sxtA4, which is critical to saxitoxin production, was successfully amplified and sequenced from the type isolate SPE10-03 (Accession number: KF985180).
Alexandrium tamarense (M. Lebour 1925) Balech 1985 emended U. John (Group III)
A review of the literature indicates that the majority of Group III isolates have occurred in Northern and Western European waters, where the isolates were generally identified as A. tamarense (Supplementary Materials Figs S8–S10). The first sequences obtained for this group originated from isolates collected in the Tamar River Estuary, England, U.K. (Scholin et al. 1994), which is the type locality of A tamarense (M. Lebour) Balech (Balech 1995). All isolates from that locality as well as other Group III isolates are non-toxic. Consequently, assigning the species name A. tamarenseto the Group III isolates allows a clear and logical association between the new species and a majority of the previous “A. tamarense” studies. For these reasons we choose to assign the A. tamarense species name to the Group III isolates.
BASIONYM
Gonyaulax tamarensis M. Lebour (1925) The dinoflagellates of the Northern Seas. Plymouth Marine Biology Association, p. 95.
LECTOTYPE
Plate XIV, figures 1a–1d (Lebour 1925) is designated here as the lectotype for Gonyaulax tamarensis M. Lebour (ICN Art 9.8).
EPITYPE
SEM stub of strain ATSW01-1 is designated here as epitype for A. tamarense (M. Lebour 1925) Balech 1985 emended U. John (Fig. 5). It is deposited at the Herbarium Senckenbergianum (FR) in the Centre of Excellence for Dinophyte Taxonomy (identification number CEDiT2013E27).
ADDITIONAL STRAIN ATSW01-1 MATERIALS
Formol-preserved cells of strain ATSW01-1 were deposited in the Centre of Excellence for Dinophyte Taxonomy (CEDiT2013RM28). Living cultures of this strain are deposited at the Roscoff Culture Collection (http://www.sb-roscoff.fr/Phyto/RCC/) as strain RCC4087, and at the National Center for Marine Algae and Microbiota (https://ncma.bigelow.org/) as strain CCMP3431. Genomic DNA from strain ATSW01-1 was deposited at the Grunelius-Möllgard-Labor in the Senckenberg Research Institute and Natural History Museum (http://sesam.senckenberg.de/page/suchen/ergebnisliste.asp#) as FIS-6174.
SYNONYMS
Gessnerium tamarensis (Lebour) A.R. Loebl. III & L.A. Loebl. in Loeblich and Loeblich (1979, p. 44).
Protogonyaulax tamarensis (M. Lebour) F.J.R. Taylor in F.J.R. Taylor (1979, p. 51).
Gonyaulax tamarensis (M. Lebour var. excavata) Braarud in Braarud (1945, p. 9).
Gonyaulax excavata (Braarud) Balech in Balech (1971, p. 28).
Protogonyaulax excavata (Braarud) F.J.R. Taylor in F.J.R. Taylor (1979, p. 52).
Alexandrium excavatum (Braarud) Balech & Tangen. Balech and Tangen (1985, p. 334).
SUPPORTING PUBLICATION
The following section contains references which provide additional morphological and genetic information information for A. tamarense (Group III) as delimited here.
Alexandrium tamarense (Lebour 1925) Balech, sensu Wang et al. (2014), strain morphology illustrated in Supplementary Material Table S3 for strains CCAP1119-1, CCAP1119-20, ITS phylogeny in figure 3, the SSU phylogeny in figure 4 and the partial LSU phylogeny in figure 5.
EMENDED SPECIES DESCRIPTION
Cells are as long as wide or slightly longer then wide; cells of strain ATWS01-1 are 33.9 ±2.1 μm (min 30 μm, max 38 μm, n=20) wide and 36±2.34 μm (min 32 μm, max 40 μm, n=20) long. Cell contains many golden brown elongated chloroplasts and a horseshoe-shaped nucleus located in the equatorial part of the cell. The epicone is helmet-shaped and the hypocone roughly trapezoidal. The cingulum is descending about one cingular height. The sulcus broadens in its antapical portion and is delimited on both sides by moderately developed sulcal lists. The cell surface is smooth and ornamented with many scattered small pores. The plate formula is: Po, 4′, 6″, 5C, 8–10S, 5‴, 2″″. Po is ornamented by several small pores and presents a comma-shaped foramen. A connecting pore is generally not present on Po. Plate 1′ is irregularly rhomboidal, with longer apical right and antapical left sides; in its apical portion, it contacts the Po and in its antapical portion it contacts plate Sa. The ventral pore along the margin between Plate 1′ and 4′ is generally present. Plate 6″is as wide as tall or slightly wider than tall. Plate 2″″ variable: it can be transversally extended (prevalent type) or dorsoventrally extended, but transitions exist. Plate sp is pentagonal, and its length: width ratio is ~ 1; a connecting pore is generally not present on sp. Cells are almost always single, rarely found in chains of two cells in cultures. The cyst is ellipsoidal, with a granular dark brown content and is surrounded by a mucous layer.
MOLECULAR DIAGNOSIS
The species is defined by the combined nucleotide sequences of the epitype Strain ATWS01-1 D1-D2 LSU (GenBank KF908805), ITS/5.8S (GenBank KF908813), and SSU (GenBank KF908799). Maximum likelihood phylogenetic trees showing more detail as to which sequences belong to Group III of the A. tamarense species complex are shown in Figure 2 and Supplementary Material Figs S3–S6. The complete list of diagnostic D1–D2 LSU, ITS/5.8S and SSU GenBank sequences which can be used as a genetic type for this species are reported in Supplementary Material Fig. S7.
EPITYPE LOCALITY
Strain ATSW01 was obtained from germinated cysts collected from Gull-mar Fjord, Essvik, Sweden, 58.28° N, 11.58° E, on 14/11/1991 by O. Lindahl. Subsequently, David Kulis isolated a single cell from the ATSW01 culture to establish strain ATSW01-1.
DISTRIBUTION
Based on genetically identified isolates, this species has currently been found in the following regions: North Atlantic (Northern Ireland, Spain, Sweden) Mediterranean (France, Italy, Spain) (Supplementary Material Tables S8–S10).
ETYMOLOGY
From the Tamar River mouth, the type locality of Gonyaulax tamarensis M. Lebour (Lebour 1925).
TOXICITY
Isolates are non-toxic (Higman et al. 2001; John et al. 2003a; Lilly et al. 2007; Orr et al. 2011). This was consistent with our inability to detect the domain sxtA4 in the epitype strain ATWS01-1, which is crucial to saxitoxin production (see Stüken et al. 2011).
Alexandrium pacificum Litaker sp. nov. (Group IV)
Many of the strains belonging to Group IV have slightly flattened cell morphology, form chains and lack the ventral pore; all these are characteristic of the original descripton of A. catenella (Whedon & Kof.) Balech (Fig. 1; Tables 1, 2). However, as illustrated above, the capability to form chains is not a distinctive character of all isolates belonging to Group IV, and this character may become lost in culture (unpubl. observation). Some strains matching the morphological description of A. catenella clustered into Group I, and thus belong to A. fundyense Balech emend. D.M. Anderson (i.e. Aguilera-Belmonte et al. 2011; Varela et al. 2012). This applies also to specimens isolated from California, the type locality of A. catenella (i.e. Ruiz Sebastián et al. 2005). As it is no longer possible to establish the identity of the material originally assigned to A. catenella, we have designated a new species name for Group IV isolates – A. pacificum – and have submitted a proposal to reject the name Alexandrium catenella under Art. 56 of the ICN (John et al. 2014).
HOLOTYPE
SEM stub of strain ACPP01 is designated here as holotype for A. pacificum Litaker (Fig. 6). It is deposited at the Herbarium Senckenbergianum (FR) in the Centre of Excellence for Dinophyte Taxonomy (accession number CEDiT2013H29).
Figure 6.
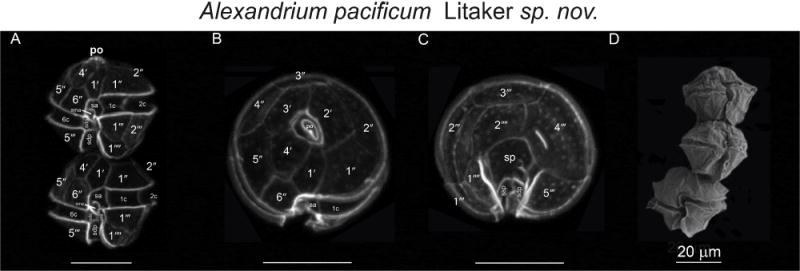
Alexandrium pacificum Litaker sp. nov. Calcofluor-stained cells (A, B, C) and SEM (D) micrographs of the type strain ACPP01. (A) Ventral, (B) apical, (C) antapical and (D) ventral views.
ISOTYPE
Formol preserved cells of strain ACPP01 are deposited at the Herbarium Senckenbergianum (FR) in the Centre of Excellence for Dinophyte Taxonomy (accession number CEDiT2013I30).
ADDITIONAL STRAIN ACPP01 MATERIALS
Living ex-type cultures of this strain were deposited at the Australian National Algae Culture Collection (http://www.csiro.au/Organisation-Structure/National-Facilities/Australian-National-Algae-Culture-Collection.aspx) CS-313/01, at the Roscoff Culture Collection (http://www.sb-roscoff.fr/Phyto/RCC/) as strain RCC4089, at the National Center for Marine Algae and Microbiota (https://ncma.bigelow.org/) as strain CCMP3434. Genomic DNA was deposited at the Grunelius-Möllgard-Labor in the Senckenberg Research Institute and Natural History Museum, Germany (http://sesam.senckenberg.de/page/suchen/ergebnisliste.asp#) as Fis-6170.
SUPPORTING PUBLICATION
The following publication provides additional morphological and genetic information information for A. pacificum (Group IV) as delimited here.
Alexandrium tamarense (Lebour 1925) Balech sensu Wang et al. (2014), morphology illustrated in table 2 for strains ATP ATCI01, ATDH02, ATMJ01, and ASGX01, rDNA ITS phylogeny in figure 3, SSU phylogeny in figure 4 and partial LSU phylogeny in figure 5.
Alexandrium catenella (Whedon & Kof.) Balech sensu Wang et al. (2014), morphology illustrated in table 2 for strains ACDH01, ACHK, ITS phylogeny in figure 3, SSU phylogeny in figure 4 and partial LSU phylogeny in figure 5.
SPECIES DESCRIPTION
Cells are as wide as long or slightly wider than high. In exponentially growing culture, cells of ACPP01 are 34.3 ± 2.92 μm (min 30 μm, max 38 μm, n = 20) wide and 35.7 ± 3.39 μm (min 30 μm, max 40 μm, n = 20) long, with a L:W ratio of 1.04. Cell contains many golden brown elongated chloroplasts and a horseshoe-shaped nucleus located in the equatorial part of the cell. The epicone is helmet-shaped, often presenting more or less noticeable shoulders, and the hypocone is roughly trapezoidal. The cingulum is descending about one cingular height. The sulcus broadens in its antapical portion and is delimited on both sides by moderately developed sulcal lists. The cell surface is smooth and ornamented with many scattered small pores. The plate formula is: Po, 4′, 6″, 5C, 8–10S, 5‴, 2″″. Po is rather wide, ornamented by several small pores and presents a comma-shaped foramen. A connecting pore is generally present on Po. Plate 1′ is irregularly rhomboidal, with longer apical right and antapical left sides; in its apical portion, it contacts Po and in its antapical portion it contacts plate Sa. The ventral pore along the margin between Plate 1 and 4′ is generally not present. Plate 6″ is as wide as tall or slightly wider than tall. Plate 2″″ is generally transversely extended. Plate sp is pentagonal, and its length:width ratio is ~ 1; a connecting pore is generally present on sp. In natural samples, cells can be arranged in chains or single. The cyst is ellipsoidal, with a granular dark brown content and is surrounded by a mucous layer.
MOLECULAR DIAGNOSIS
The species is defined by the combined nucleotide sequences of the holotype strain ACPP01 D1-D2 LSU (GenBank KF908803), ITS/5.8S (GenBank KF908812), and SSU (GenBank KF908800). Maximum likelihood phylogenetic trees showing more detail as to which sequences belong to Group IV of the A. tamarense species complex are shown in Figure 2 and Supplementary Material Figs S3–S6. The complete list of diagnostic D1-D2 LSU, ITS/5.8S and SSU GenBank sequences which can be used as a genetic type for this species are reported in Supplementary Material Fig. S7.
TYPE LOCALITY
Strain ACPP01 was established from a single vegetative cell collected at Port Phillip Bay, Victoria, Australia, 38.1500° S, 144.8667° E on 3/3/1988 by S. Blackburn.
DISTRIBUTION
Based on genetically identified isolates, this species has currently been found in the following regions: Mediterranean (France, Italy, Spain), North Pacific (China, Japan, Singapore, South Korea), South Pacific (Australia, New Zealand), and Antarctic (Drake Passage) (Supplementary Material Figs S8–S10).
ETYMOLOGY
The species name is Latin for ‘from the Pacific Ocean’ and was chosen because the strains found to have this genetic sequence were originally isolated from Japanese, Korean, Australian, and western Pacific Ocean sites (Scholin et al. 1994).
TOXICITY
All isolates examined to date are toxic to some degree (Hallegraeff et al. 1991; Lilly et al. 2007; Negri et al. 2003; Orr et al. 2011). The type strain ACPP01 has a toxin profile of (in molar percent) C1 (3%), C2 (12%), C4 (3.6%), GTX6 (55%), GTX4 (8%), GTX1 (8%), GTX5 (9%) (Negri et al. 2003). Other strains of this species can produce the saxitoxin congeners B1, C1, C2, GTX1, 2, 3, 4, 5 and 6, dcGTX3, NEO and STX (Hallegraeff et al. 1991; MacKenzie et al. 2004; Murray et al. 2011; Negri et al. 2003; Orlova et al. 2007; Orr et al. 2011; Ruiz Sebastián et al. 2005; Scholin et al. 1994). Consistent with this toxicity, sxtA4 sequences were present in the type culture (Accession number: KF985177).
Alexandrium mediterraneum U. John sp. nov. (Group II)
HOLOTYPE
SEM stub of isolate SZN01 is designated here as holotype for A. mediterraneum U. John (Fig. 7). It is deposited at the Herbarium Senckenbergianum (FR) in the Centre of Excellence for Dinophyte Taxonomy (accession number CEDiT2013H31).
Figure 7.
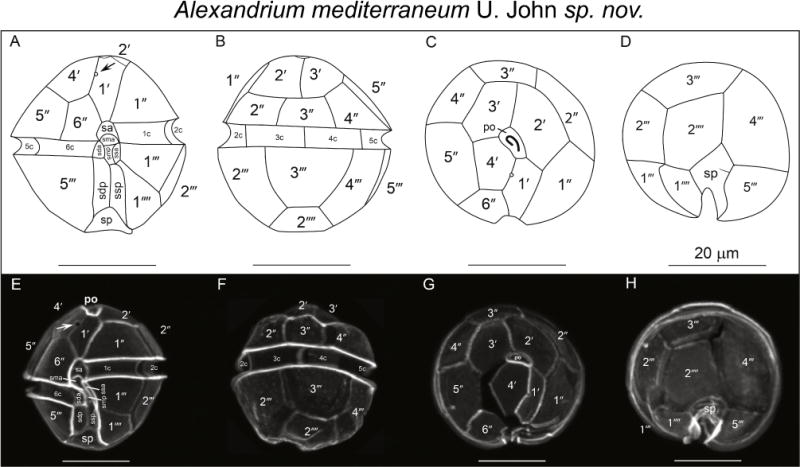
Alexandrium mediterraneum U. John sp. nov. Line drawings (A–D), light micrographs of calcofluor-stained cells of type strain SZN01. (A) Ventral, (B) dorsal, (C) apical, (D) antapical (E) ventral, (F) dorsal, (G) apical and (H) antapical views. Arrows in panels A and E mark the ventral pore in the 1′ plate. Scale bars = 20 μm.
ISOTYPE
Formol-preserved cells of isolate SZN01 are deposited at the Herbarium Senckenbergianum (FR) in the Centre of Excellence for Dinophyte Taxonomy (accession number CEDiT2013I32).
ADDITIONAL STRAIN SZN01 MATERIALS
Living cultures of this strain were deposited at the Roscoff Culture Collection (http://www.sb-roscoff.fr/Phyto/RCC/) as strain RCC4088, and at the National Center for Marine Algae and Microbiota (https://ncma.bigelow.org/) as strain CCMP3433. Genomic DNA was deposited at the Grunelius-Möllgard-Labor in the Senckenberg Research Institute and Natural History Museum, Germany (http://sesam.senckenberg.de/page/suchen/ergebnisliste.asp#) as FIS-6171.
SPECIES DESCRIPTION
Cells are as long as wide or slightly longer then wide; strain SZN01 is 37.6 ± 2.8 μm (min 32 μm, max 40 μm, n = 20) wide and 37.2 ± 2.63 μm (min 32 μm, max 40 μm, n = 20) long. Cell contains many golden brown elongated chloroplasts and a horseshoe-shaped nucleus located in the equatorial part of the cell. The epicone is helmet-shaped and the hypocone roughly trapezoidal. The cingulum is descending about one cingular height. The sulcus broadens in its antapical portion and is delimited on both sides by moderately developed sulcal lists. The cell surface is smooth and ornamented with many scattered small pores. The plate formula is: Po, 4′, 6″, 5C, 8–10S, 5‴, 2″″. Po is ornamented by several small pores and presents a comma-shaped foramen. A connecting pore is generally not present on Po. Plate 1′ is irregularly rhomboidal, with longer apical right and antapical left sides; in its apical portion, it contacts Po and in its antapical portion it contacts plate Sa. The ventral pore along the margin between Plate 1′ and 4′ is generally present. Plate 6″ is as wide as tall or slightly wider than tall. Plate 2″″ is transversely extended. Plate sp is pentagonal, and its length:width ratio is ~ 1; a connecting pore is generally not present on Sp. Cells are almost always single, rarely found in chains of two cells in cultures. The cyst is ellipsoidal, with a granular dark brown content and is surrounded by a mucous layer.
MOLECULAR DIAGNOSIS
The species is defined by the combined nucleotide sequences of the holotype strain SZN01 D1-D2 LSU (GenBank KF908808), ITS/5.8S (GenBank KF908815), and SSU (GenBank KF908797). Maximum likelihood phylogenetic trees showing more detail as to which sequences belong to Group II of the A. tamarense species complex are shown in Figure 2 and Supplementary Material Figs S3–S6. The complete list of diagnostic D1-D2 LSU, ITS/5.8S and SSU GenBank sequences which can be used as a genetic type for this species are reported in Supplementary Material Fig. S7.
TYPE LOCALITY
Strain SZN01 was established from a single vegetative cell collected at LTER station MareChiara in the Gulf of Naples (Tyrrhenian Sea, Mediterranean Sea), 40.48.5° N 14.15° E on 16/06/1999 by M. Montresor.
DISTRIBUTION
Based on genetically identified isolates, this species has only been found in the Mediterranean from the coastal waters of Greece, Italy, France and Spain (John et al. 2003a; Lilly et al. 2007; Penna et al. 2008) (Supplementary Material Tables S8–S10).
ETYMOLOGY
Latin for an Alexandrium from the Mediterranean Sea in recognition that the first sequences available for this species derived from isolates obtained from the Mediterranean Sea (John et al. 2003a).
TOXICITY
Isolates are described to be non-toxic at levels of current detection capabilities (John et al. 2003a; Lilly et al. 2007; Orr et al. 2011; Penna et al. 2008). Consistent with this observation, the saxitoxin gene domain sxtA4, which is indicative of the ability to produce saxitoxins, could not be amplified from SZNB01 and SZNB08.
Alexandrium australiense Sh. Murray sp. nov. (Group V)
HOLOTYPE
SEM stub of strain ATBB01 is designated here as holotype for A. australiense (Fig. 8). It is deposited at the Herbarium Senckenbergianum (FR) in the Centre of Excellence for Dinophyte Taxonomy (accession number CEDiT2013H33).
Figure 8.
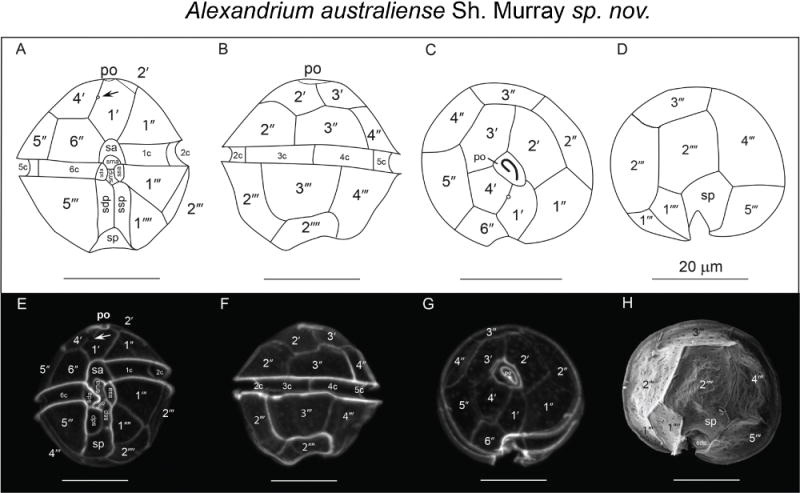
Alexandrium australiense Sh. Murray sp. nov. Line drawings (A–D) light micrographs of calcofluor-stained cells (E–G) and SEM micrograph (H) of the type strain ATBB01. (A) ventral, (B) dorsal, (C) apical, (D) antapical, (E) ventral, (F) dorsal, (G) apical and (H) antapical view. Panel H is from Murray et al. (2012) fig. 1D. Arrows in panels A and E mark the ventral pore in the 1′ plate. Scale bars = 20 μm.
ISOTYPE
Formol-preserved cells of strain ATBB01 are deposited at the Herbarium Senckenbergianum (FR) in the Centre of Excellence for Dinophyte Taxonomy (accession number CEDiT2013I34).
ADDITIONAL STRAIN ATBB01 MATERIALS
Live cultures of this strain are deposited at the Australian National Algae Culture Collection (http://www.csiro.au/Organisation-Structure/National-Facilities/Australian-National-Algae-Culture-Collection.aspx) as strain CS-298, at the Roscoff Culture Collection (http://www.sb-roscoff.fr/Phyto/RCC/) as strain RCC4090, at the National Center for Marine Algae and Microbiota (https://ncma.bigelow.org/) as strain CCMP3435. Genomic DNA was deposited at the Grunelius-Möllgard-Labor in the Senckenberg Research Institute and Natural History Museum, Germany (http://sesam.senckenberg.de/page/suchen/ergebnisliste.asp#) as FIS-6172.
SUPPORTING PUBLICATION
The following publication provides additional morphological and genetic information for A. australiense (Group V) as delimited here.
Alexandrium tamarense (Lebour) Balech sensu Murray et al. (2012), figures 1 and 2.
SPECIES DESCRIPTION
Cells are as long as wide or slightly longer then wide; strain ATBB01 is 35±3.15 μm (min 30 μm, max 40 μm, n = 20) wide and 35±2.93 μm (min 30 μm, max 40 μm, n=20) long. Cell contains many golden brown elongated chloroplasts and a horseshoe-shaped nucleus located in the equatorial part of the cell. The epicone is helmet-shaped and the hypocone roughly trapezoidal. The cingulum is descending about one cingular height. The sulcus broadens in its antapical portion and is delimited on both sides by moderately developed sulcal lists. The cell surface is smooth and ornamented with many scattered small pores. The plate formula is: Po, 4′, 6″, 5C, 8–10S, 5‴, 2″″. Po is ornamented by several small pores and presents a comma-shaped foramen. A connecting pore is generally not present on Po. Plate 1′ is irregularly rhomboidal, with longer apical right and antapical left sides; in its apical portion, it contacts Po and in its antapical portion it contacts plate sa The ventral pore along the margin between Plate 1′ and 4′ is generally present. Plate 6″ is as wide as high or slightly wider than high. Plate 2″″ is transversely extended. Plate sp is pentagonal, and its length:width ratio is ~ 1; a connecting pore may or may not be present on sp. Cells are almost always single, rarely found in chains of two cells in cultures. The cyst is ellipsoidal, with a granular dark brown content and is surrounded by a mucous layer.
MOLECULAR DIAGNOSIS
The species is defined by the combined nucleotide sequences of the holotype strain ATBB01 D1-D2 LSU (GenBank KF908810), ITS/5.8S (GenBank KF908817), and SSU (GenBank KF908802). Maximum likelihood phylogenetic trees showing more detail as to which sequences belong to Group V of the A. tamarense species complex are shown in Figure 2 and Supplementary Material Figs S3–S6. The complete list of diagnostic D1-D2 LSU, ITS/5.8S and SSU GenBank sequences which can be used as a genetic type for this species are reported in Supplementary Material Fig. S7.
TYPE LOCALITY
The Group V isolate ATBB01 was established from single vegetative cells collected at Bell Bay, Tasmania, Australia 41.14° S 146.86° E on 01/01/1987 by C Bolch.
DISTRIBUTION
Based on genetically identified isolates, this species has been found in the South (Australia: Tasmania and South Australia) and North Pacific (Japan) (Supplementary Material Figs S8–S10).
ETYMOLOGY
The species name is Latin for an Alexandrium from Australia in recognition of the fact that the first strain recognized to have this genotype was isolated from Tasmania, Australia.
TOXICITY
The strain ATBB01 has been found non-toxic (Hallegraeff et al. 1991; Orr et al. 2011) using HPLC-FL and the Lawrence method, but did show some response in the sensitive sax-iphilin binding assay, suggesting it may contain trace amounts of toxin (Negri et al. 2003; Scholin et al. 1994). Of the four strains that have been tested for saxitoxin production using the standard Lawrence method (Lawrence and Niedzwiadek 2001; Lawrence et al. 2004), three were found to be non-toxic or producing saxitoxins at levels below current detection limits. One strain, ATNWB01, was toxic with a toxin profile that included GTX5, STX, C1, 2 and deSTX (Murray et al. 2012). Results from this study also confirmed that the sxtA4 domain was present. These data indicate that at least some strains of this species are toxic.
Conclusions
The goal of this study was to formalize the taxonomic status of the A. tamarense species complex Groups I–V, which were previously proposed as ad interim species. A comprehensive phylogeny was constructed using a broad dataset of ribosomal operon sequences, including concatenated alignments of the SSU, ITS and the D1-D2 regions of the LSU. The resulting ribosomal phylogenetic trees always recovered five distinct clades (Groups), consistent with previous studies. The genetic distances between these Groups were calculated from ITS/5.8S sequences, and were equal to or exceeded the genetic distances previously observed among other closely related dinoflagellate species. Additionally, compensatory base changes (CBCs) were observed in the secondary structure of the ITS2 region among several of the Groups. When present, a CBC consistently indicates that the sequences being compared come from genetically isolated species. Similarly, evidence from inter-Group mating studies also supports the classification of Groups as species according to the ‘biological species complex’ sensu Mayr. The Groups also differ in the production of saxitoxins and the presence/absence of sxtA4, a critical component of saxitoxin biosynthesis pathway. The latter results underscore important differences in the physiology of Groups I–V that will impact management of seafood hazards. In contrast to the genetic results, a detailed examination of the morphological criteria previously proposed for taxonomic identification of A. tamarense species-complex species demonstrated that all features are shared among the five respective ribosomal Groups. The combination of all available data supports the segregation of the Alexandrium species complex into five distinct species according to its ribosomal phylogeny. Consequently, we have amended the diagnosis of A. fundyense (Group I) and A. tamarense (Group III) and described three new species, A. mediterraneum (Group II), A. pacificum (Group IV) and A. australiense (Group V).
Methods
Morphological species descriptions
A complete review of the morphological data used to establish A. catenella, A. fundyense, and A. tamarense as species was undertaken to evaluate whether the original characteristics (cell size and shape, presence or absence of the ventral pore in the 1′ plate, presence or absence of chain formation) used to distinguish species were valid characters (Table 1, Fig. 1). The morphological characteristics were collated from original descriptions (e.g. Balech 1971, 1985, 1995; Lebour 1925; Loeblich III and Loeblich 1979; Parke and Dixon 1976; Taylor 1979; Whedon and Kofoid 1936) and compared with more recent morphological observations reported in the literature (Aguilera-Belmonte et al. 2011; Cho and Lee 2001; Faust and Gulledge 2002; Fukuyo et al. 1985; Gayoso and Fulco 2006; Hallegraeff et al. 1991; Kim et al. 2002; Larsen and Nguyen 2004; Leaw et al. 2005; MacKenzie et al. 2004; Murray et al. 2012; Orlova et al. 2007), as well as those in AlgaeBase (Guiry and Guiry 2014).
Algal cultures
The clonal strains ACQH01, SPE10-03 (Group I), SZN01, SZN08 (Group II), ATSW01-1 (Group III), ACPP01 (also known as CS 313), ATTL01, (Group IV) and ATBB01, (also known as CS 298; a Group V strain) were cultured for use as epitype or type material (see the following section and Table 2) and for obtaining additional sequence data for analysis. The isolates were grown in K-medium (Keller et al. 1987) prepared from filter-sterilized (0.2 μm VacuCap 90 filter units, Pall Corporation, Port Washington) North Sea water (salinity 33) at 15°C, 14:10 light:dark cycle, with an irradiance of 150 μmol photons m−2 s−1. The morphological and genetic characterization of these isolates is described below.
Morphological analyses
All material used for the morphological characterizations of the A. tamarense species complex types came from cultures sampled in mid-exponential growth phase. Cells were fixed with formaldehyde at 1.6% final concentration. The length and width of 20 cells were measured by light microscopy at 400x magnification (i.e. 10x ocular and 40x objective; Zeiss Axioplan, Carl Zeiss, Oberkochen, Germany). Fixed cells were also stained with Calcofluor White (Sigma-Aldrich, Hamburg, Germany) according to the protocol of Fritz and Triemer (1985) and examined by epifluorescence microscopy (Zeiss Axioplan, Carl Zeiss, Oberkochen, Germany) to establish the morphology of diagnostic thecal plates and the presence or absence of cell chains. Additionally, an LSM confocal microscope was used to take 1–3 μm z-stack images of calcofluor stained cells. Z-stacks were reviewed using Zen Black software (Carl Zeiss, Oberkochen, Germany) and 3-D image reconstructions were exported as flat images to document the thecal structure of the epitype/holotype strains. Scanning electron microscope images of each of the strains were made from formaldehyde- or gluteraldehyde-fixed samples that were dehydrated via serial washes in ethanol (25%, 50%, 75%, 90% and 2×100%), then critical point dried and sputter coated with gold for observation under a JEOL JSM 6700F microscope or platinum for observation using a JEOL 840 microscope (JEOL Ltd., Tokyo, Japan). Cells were preserved for epitype/holotype deposits at the Senckenberg Collection at the Centre of Excellence for Dinophyte Taxonomy both as stubs and preserved material (2% formalin-fixed).
Phylogenetic analyses
Ribosomal DNA sequence data for the phylogenetic analyses were obtained from the Alexandrium cultures listed above using the following protocol. Initially, 50 ml samples of exponentially growing cells were collected by centrifugation at 3,220 × g for 15 min at room temperature (Eppendorf 5810R, Hamburg, Germany). The cell pellets were frozen at −20°C for 20 min before extraction of total DNA with the DNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The purity and quantity of the DNA was assessed by UV-spectroscopy with a NanoDrop ND-1000 system (Peqlab, Erlangen, Germany) and the integrity of DNA was confirmed using 1% agarose gel electrophoresis where a majority of the extracted genomic DNA exceeded 20 kilobases.
The following ribosomal DNA loci were amplified from each total DNA extract by polymerase chain reaction (PCR): the 18S small subunit (SSU), the D1/D2 region of the 28S large subunit (LSU), and the internal transcribed spacer region (including the ITS1, 5.8S subunit, and ITS2 sequences; referred to hereafter as ITS/5.8S). The forward and reverse primers for SSU amplification were: 1F (5′- AAC CTG GTT GAT CCT GCC AGT - 3′) and 1528R (5′ - TGA TCC TTC TGC AGG TTC ACC TAC - 3′), respectively. The forward and reverse primers for LSU amplification were: D1R-F (5′ -ACC CGC TGA ATT TAA GCA TA- 3′) and D2C-R (5′-CCTTGGTCCGTGTTTCAAGA-3′), respectively. The forward and reverse primers for ITS/5.8 amplification were: ITS a (5′ -CCA AGC TTC TAG ATC GTA ACA AGG (ACT)TC CGT AGG T- 3′) and ITS b (5′ -CCT GCA GTC GAC A(GT)A TGC TTA A(AG)T TCA GC(AG) GG- 3′), respectively. Reaction conditions were as follows: HotMasterTaq® (5Prime, Hamburg, Germany) buffer 1×, 0.1 mM of dNTPs, 0.1 mM of each forward and reverse primer and 1.25 units of Taq polymerase were added to 10–30 ng of the extracted genomic DNA in total reaction volumes of 50 μL. For 18S and 28S rDNA amplifications, the reactions were subjected to the following thermocycling conditions: one cycle of 95°C 7min; 35 cycles 94°C for 45 s, 54°C for 2 min, 70°C for 1.5 min; and a final extension 70°C 5 min. The thermocycling conditions for the ITS/5.8 amplifications were: one cycle 94°C for 4 min; 9 cycles 94°C for 50 s, 60°C for 40 s and 70°C for 1 min; 29 cycles 94°C for 45 s, 50°C for 45 s, 70°C for 1 min; and a final 5 min extension step at 70°C Samples were kept at 10°C until prior to analysis on 1% agarose gel to ensure the expected amplification products were present. After PCR amplification and subsequent cloning into the vector provided with the TOPO TA Cloning® kit (Invitrogen, Carlsbad, California, USA), 3 – 8 clones per amplicon were sequenced using the M13 vector primers supplied with the kit. Sequencing was conducted with a standard cycle sequencing chemistry ABI 3.1 (Applied Biosystems, Darmstadt, Germany) and additional internal primers were used for SSU sequencing (Montresor et al. 2004). Cycle sequencing products were analyzed on an ABI 3130 XL capillary sequencer (Applied Biosystems, Darmstadt, Germany) and the generated sequences were assembled with CLC main workbench version 6.0 (www.CLCbio.com). The resulting sequences were submitted to GenBank (Accession numbers: KF908795-KF908818).
The SSU, ITS/5.8S, and LSU sequences were then combined with those available in GenBank and different alignments constructed in order to conduct phylogenetic analyses using either single SSU (Supplementary Material Figs S1–S3), ITS/5.8 (Supplementary Materials Figs files S1–2, S4), or LSU (Supplementary Material Figs S1–2, S5) data sets or a concatenated SSU-ITS/5.8-LSU data set (Supplementary Material Figs S1–2, S6). For the concatenated phylogenetic analyses, only the combined sequences from the type species SPE10-03, SZN01, ATBB01, ACPP01, ATSW01-1 as well as those for CCMP115, ACQH01, SZN08, ATTL0, ATNWB01 were included in the analysis plus outgroup sequences from Alexandrium affine (H. Inoue & Fukuyo) Balech (CCMP 112). The individual SSU, LSU and ITS/5.8S data sets were separately aligned with MAFFT using the q-insi option (Katoh et al. 2005) and concatenated afterwards (Supplementary Material Fig. S2). The full ML analysis (GTRGAMMA, 1000 bootstrap replicates) was conducted with RAxML (Stamatakis et al. 2005). In the case for the concatenated dataset the RAxML analysis used three partitions for each gene.
In order to generate a representative phylogeny, all the D1–D2 LSU Alexandrium rDNA sequences available in GenBank were aligned using ClustalX. The sequences were then sorted into species groups. This ensured any that sequences whose species identification were incorrect in GenBank were assigned to the correct species group. If the D1–D2 sequences varied within species, they were further sorted into subgroups that differed from one another by more than 2 bp. For several species, within species sequence divergences were significantly larger and fell into easily identifiable subclades (labeled 1, 2, 3, etc.). In these cases, it was easier to identify sequences varying by less than 2bp by analyzing the sequences belonging to each subclade separately. Specifically, the sequences belonging to each subclade were transferred to separate alignment files and sorted based on the <2 bp variation criterion. Each of the resulting <2 bp subgroups were then given a letter desgination (e.g. 2a, 2b, etc. for subgroups within subclade 2). For the final analysis, a single sequence from every <2 bp subgroup was selected for construction of the D1–D2 phylogeny. This filtering procedure captured a majority of the within-species variation while reducing the total number of sequences in the analysis for all species from 638 to 40. These D1–D2 sequences were then combined with a representiative sequence from each of the five Alexandrium type or epitype isolates. The combined data set was then aligned using ClustalX using an open gap penalty of 10 and extended gap penalty of 5. Obvious misalignments were corrected manually (Supplmentary Material Fig. S1) and the final alignment analysed by RAxML CAT, 1000 bootstrap replicates as described above (Supplementary Material Fig. S2).
ITS analysis
The ITS/5.8S sequence data were partitioned into species groups, aligned using ClustalW, and genetic distances (substitutions per site) between each possible sequence combination was estimated using PAUP* (Swofford 1998). The procedure was essentially identical to that employed by Litaker et al. (2007) except that no attempt was made to identify and eliminate pseudogenes (i.e., all GenBank sequences were used; see Supplementary Material Fig. S7). The inclusion of these pseudogenes would tend to increase the apparent genetic distance estimates over those measured in the original studies. The mean substitutions per site, both within the five species and between them were then calculated along with the minimum and maximum values observed in each within- or between-group comparison.
ITS2 rRNA secondary structure modeling
The presence or absence of CBCs was determined as follows. The sequences of the type strains were aligned with those of other A. tamarense complex taxa and strains identified via BLAST searches in GenBank. The beginning and end of the ITS2 region in these strains were identified via homology with two strains of Alexandrium affine (one misidentified as “A. tamarense strain CCMP116”) available in the ITS2 database (Koetschan et al. 2010), and were confirmed by comparison with a broader alignment of dinoflagellates. The ITS2 region consisted of approximately 180bp in our Alexandrium strains. The ITS2 secondary structure was predicted using the existing structural “a” model for the A. affine ITS2 region (Koetschan et al. 2010), which was in agreement with the general dinoflagellate structures predicted by Gottschling and Plötner (2004). Structures were visualized using VARNA (Darty et al. 2009). The compensatory base changes in the predicted secondary structure models were identified using the software 4SALE (Seibel et al. 2006).
Toxicity
Because the production of saxitoxins is variable among Alexandrium strains/species, the current literature was reviewed to determine what is known about toxin production by isolates belonging to each of the five genetic groups (sensu species) within the A. tamarense species complex. In addition, except for ATBB01 (Stüken et al. 2011) the type isolates SPE10-03 (Group I), SZN01 (Group II), ATSW01-1 (Group III), and ACPP01 (Group IV) were screened using the following PCR procedure for the presence of the sxtA4, a conserved region in the sxtA gene which is a key component of the saxitoxin synthesis pathway. Briefly, the primer pair used was sxt007 (5′ -ATGCTCAACATGGGAGTCATCC- 3′) and sxt008 (5′-GGGTCCAGTAGATGTTGACGATG-3′; Stüken et al. 2011). For each 20 μl PCR reaction, HotMasterTaq® (5Prime, Hamburg, Germany) buffer 1X, 0.1 mM of dNTPs, 0.1 mM of each forward and reverse primer and 0.75 units of Taq polymerase were added to 10–30 ng of the extracted genomic DNA. The following thermocycling conditions were used: one cycle of 95°C for 2 min; 35 cycles 94°C for 30 s, 55°C for 30 s, 70°C for 1.5 min; and a final extension 70°C for 10 min. Amplicons were purified, cloned, sequenced and analysed as described above. The resulting sequences were submitted to GenBank (accession numbers: KF985177- KF985182).
Supplementary Material
Acknowledgments
We give our sincere thanks to John McNeill for guiding us through the ICN, to Mona Hoppenrath for her very helpful and precise advice and shared expertise, as well as to three anonymous reviewers who helped shape and finalize this ambitious project. We also thank Nancy Kühne for her assistance in the laboratory, David Kulis for maintenance and distribution of cultures, and Steve Kibler and Mark Vandersea who reviewed the manuscript and provided helpful suggestions. Financial support to U.J. was provided by the PACES research program of the Alfred-Wegener-Institute Helmholtz-Zentrum für Polar- und Meeresforschung. Partial support for W.L. was provided by North Pacific Research Board Project 1021 publication number 503 and the NOAA Coastal Science Board, for M.M. by the Stazione Zoologica Anton Dohrn, and for S.M. and U.J. by the Australian Research council grant number DP120103199, an Australian Academy of Science DAAD German-Australian mobility grant and BMBF PT/DLR IB FKZ 01DR14006 projectn (NSF) Grants OCE- 1128041 and OCE-1314642; and National Institute of Environmental Health Sciences (NIEHS) Grant 1-P50-ES021923-01.
Appendix A. Supplementary Data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.protis.2014.10.001.
References
- Adachi M, Sako Y, Ishida Y. Restriction fragment length polymorphism of ribosomal DNA internal transcribed spacer and 5. 8S regions in Japanese Alexandrium species (Dinophyceae) J Phycol. 1994;30:857–863. [Google Scholar]
- Adachi M, Sako Y, Ishida Y. Analysis of Alexandrium (Dinophyceae) species using sequences of the 5. 8S ribosomal DNA and internal transcribed spacer regions. J Phycol. 1996;32:424–432. [Google Scholar]
- Aguilera-Belmonte A, Inostroza I, Franco JM, Riobó P, Gómez PI. The growth, toxicity and genetic characterization of seven strains of Alexandrium catenella (Whedon and Kofoid) Balech 1985 (Dinophyceae) isolated during the 2009 summer outbreak in southern Chile. Harmful Algae. 2011;12:105–112. [Google Scholar]
- Anderson DM, Wall D. Potential importance of benthic cysts of Gonyaulaxtamarensis and G. excavata in initiating toxic dinoflagellate blooms. J Phycol. 1978;13:224–234. [Google Scholar]
- Anderson DM, Kulis DM, Doucette GJ, Gallager JC, Balech E. Biogeography of toxic dinoflagellates in the genus Alexandrium from the northeast United States and Canada as determined by morphology, bioluminescence, toxin composition, and mating compatibility. Mar Biol. 1994;120:467–478. [Google Scholar]
- Anderson DM, Alpermann TJ, Cembella AD, Collos Y, Masseret E, Montresor M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae. 2012;14:10–35. doi: 10.1016/j.hal.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Kulis DM, Keafer BA, Gribble KE, Marin R, Scholin CA. Identification and enumeration of Alexandrium spp. from the Gulf of Maine using molecular probes. Deep-Sea Res Pt II. 2005;52:2467–2490. [Google Scholar]
- Baggesen C, Moestrup Ø, Daugbjerg N, Krock B, Cembella AD, Madsen S. Molecular phylogeny and toxin profiles of Alexandrium tamarense (Lebour) Balech (Dinophyceae) from the west coast of Greenland. Harmful Algae. 2012;19:108–116. [Google Scholar]
- Balech E. Microplancton de la campana oceanografica Productividad III. Revista del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”. Hidrobiologia. 1971;3:1–202. [Google Scholar]
- Balech E. The Genus Alexandrium or Gonyaulax of the tamarensis Group. In: Anderson DM, White AW, Baden DG, editors. Toxic Dinoflagellates, Proceedings of the Third International Conference on Toxic Dinoflagellates. Elsevier; New York: 1985. pp. 33–38. [Google Scholar]
- Balech E. The Genus Alexandrium Halim (Dinoflagellata) Sherkin Island Marine Station Publication, Sherkin Island, Co; Cork, Ireland: 1995. [Google Scholar]
- Balech E, Tangen K. Morphology and taxonomy of toxic species in the tamarensis group (Dinophyceae): Alexandrium excavatum (Braarud) comb. nov. and Alexandrium ostenfeldii (Paulsen) comb. nov. Sarsia. 1985;70:333–343. [Google Scholar]
- Bolch CJS, de Salas MF. A review of the molecular evidence for ballast water introduction of the toxic dinoflagellates Gymnodinium catenatum and the Alexandrium “tamarensis complex” to Australia. Harmful Algae. 2007;6:465–485. [Google Scholar]
- Braarud T. Morphological observations on marine dinoflagellate cultures (Porella perforata, Gonyaulax tamarensis, Protoceratium reticulatum) Avh Utgitt Nor Vidensk Akad Oslo, Mat - Naturvidensk Kl 1944. 1945;11:1–18. [Google Scholar]
- Brosnahan ML, Kulis DM, Solow AR, Erdner DL, Percy L, Lewis J, Anderson DM. Outbreeding lethality between toxic Group I and nontoxic Group III Alexandrium tamarense spp. isolates: Predominance of heterotypic encystment and implications for mating interactions and biogeography. Deep-Sea Res Pt II. 2010;57:175–189. doi: 10.1016/j.dsr2.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cembella AD, Taylor FJR. Electrophoretic variability within the Protogonyaulax tamarensis/catenella species complex: Pyridine-linked dehydrogenases. Biochem Syst Ecol. 1986;14:311–323. [Google Scholar]
- Cho ES, Lee HJ. Thecal plates, toxin content and growth of five clones of Alexandrium tamarense (Dinophyceae) isolated from Jinhae Bay, Korea. Phycologia. 2001;40:435–439. [Google Scholar]
- Cho Y, Hiramatsu K, Ogawa M, Omura T, Ishimaru T, Oshima Y. Non-toxic and toxic subclones obtained from a toxic clonal culture of Alexandrium tamarense (Dinophyceae): Toxicity and molecular biological feature. Harmful Algae. 2008;7:740–751. [Google Scholar]
- Coleman AW. ITS2 is a double-edged toll for eukaryotic evolutionary comparison. Trends Genet. 2003;19:370–375. doi: 10.1016/S0168-9525(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Coleman AW. Is there a molecular key to the level of “biological species” in eukaryotes? A DNA guide. Mol Phylogenet Evol. 2009;50:197–203. doi: 10.1016/j.ympev.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Collins C, Graham J, Brown L, Bresnan E, Lacaze JP, Turrell EA. Identification and toxicity of Alexandrium tamarense (Dinophyceae) in Scottish waters. J Phycol. 2009;45:692–703. doi: 10.1111/j.1529-8817.2009.00678.x. [DOI] [PubMed] [Google Scholar]
- Darty K, Denise A, Ponty Y. VARNA: Interactive drawing and editing of the RNA secondary structure. Bioinformatics. 2009;25:1974–1975. doi: 10.1093/bioinformatics/btp250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destombe C, Cembella AD, Murphy CA, Ragan MA. Nucleotide sequence of the 18S ribosomal RNA genes from the marine dinoflagellate Alexandrium tamarense (Gonyaulacales, Dinophyta) Phycologia. 1992;31:121–124. [Google Scholar]
- Dyhrman ST, Erdner D, La Du J, Galac M, Anderson DM. Molecular quantification of toxic Alexandrium fundyense in the Gulf of Maine using real-time PCR. Harmful Algae. 2006;5:242–250. doi: 10.2307/1543269. [DOI] [PubMed] [Google Scholar]
- Dyhrman ST, Haley ST, Borchert JA, Lona B, Kollars N, Erdner DL. Parallel analyses of Alexandrium catenella cell concentrations and shellfish toxicity in the Puget Sound. Appl Environ Microbiol. 2010;76:4647–4654. doi: 10.1128/AEM.03095-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdner DL, Percy L, Keafer B, Lewis J, Anderson DM. A quantitative real-time PCR assay for the identification and enumeration of Alexandrium cysts in marine sediments. Deep-Sea Res Part II. 2010;57:279–287. doi: 10.1016/j.dsr2.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry S, Köhler A, Coleman AW. Intraspecies analysis: comparison of ITS sequence data and gene intron sequence data with breeding data for a worldwide collection of Gonium pectorale. J Mol Evol. 1999;48:94–101. doi: 10.1007/pl00006449. [DOI] [PubMed] [Google Scholar]
- Faust MA, Gulledge RA. Identifying Harmful Marine Dinoflagellates. Smithsonian Institution; Washington, DC: 2002. p. 144. [Google Scholar]
- Fritz L, Triemer RE. A rapid simple technique utilizing Calcofluor white M2R for the visualization of dinoflagellate thecal plates. J Phycol. 1985;21:662–664. [Google Scholar]
- Fukuyo Y, Yoshida K, Inoue H. Protogonyaulax in Japanese Coastal Waters. In: Anderson DM, White AW, Baden DG, editors. Toxic Dinoflagellates, Proceedings of the Third International Conference on Toxic Dinoflagellates. Elsevier-North Holland; New York: 1985. pp. 27–32. [Google Scholar]
- Garneau ME, Schnetzer A, Countway PD, Jones AC, Seubert EL, Caron DA. Examination of the seasonal dynamics of the toxic dinoflagellate Alexandrium catenella at Redondo Beach, California, by quantitative PCR. Appl Environ Microbiol. 2011;77:7669–7680. doi: 10.1128/AEM.06174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayoso AM, Fulco VK. Occurrence patterns of Alexandrium tamarense (Lebour) Balech populations in the Golfo Nuevo (Patagonia, Argentina), with observations on ventral pore occurrence in natural and cultured cells. Harmful Algae. 2006;5:233–241. [Google Scholar]
- Genovesi B, Shin-Grzebyk MS, Grzebyk D, Laabir M, Gagnaire PA, Vaquer A, Pastoureaud A, Lasserre B, Collos Y, Berrebi P, Masseret E. Assessment of cryptic species diversity within blooms and cyst bank of the Alexandrium tamarense complex (Dinophyceae) in a Mediterranean lagoon facilitated by semi-multiplex PCR. J Plankton Res. 2011;33:405–414. [Google Scholar]
- Godhe A, Cusack C, Pedersen J, Andersen P, Anderson DM, Bresnan E, Cembella A, Dahl E, Diercks S, Elbraechter M, Edler L, Galluzzi L, Gescher C, Gladstone M, Karlson B, Kulis D, LeGresley M, Lindahl O, Marin R, McDermott G, Medlin LK, Naustvoll L-J, Penna A, Toebe K. Intercalibration of classical and molecular techniques for identification of Alexandrium fundyense (Dinophyceae) and estimation of cell densities. Harmful Algae. 2007;6:56–72. [Google Scholar]
- Gottschling M, Plötner J. Secondary structure models of the nuclear internal transcribed spacer regions and 5. 8S rRNA in Calciodinellaoideae (Peridiniaceae) and other dinoflagellates. Nucleic Acids Res. 2004;32:307–315. doi: 10.1093/nar/gkh168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling M, Keupp H, Plotner J, Knop R, Willems H, Kirsch M. Phylogeny of calcareous dinoflagellates as inferred from ITS and ribosomal sequence data. Mol Phylogenet Evol. 2005;36:444–455. doi: 10.1016/j.ympev.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Gu H, Zeng N, Lui T, Yang W, Müller A, Krock B. Morphology, toxicity, and phylogeny of Alexandrium (Dinophyceae) species along the coast of China. Harmful Algae. 2013;27:68–681. [Google Scholar]
- Guillou L, Nézan E, Cueff V, Erard-Le Denn E, Cambon-Bonavita M-A, Gentien P, Barbier G. Genetic diversity and molecular detection of three toxic dinoflagellate genera (Alexandrium, Dinophysis, and Karenia) from French Coasts. Protist. 2002;153:223–238. doi: 10.1078/1434-4610-00100. [DOI] [PubMed] [Google Scholar]
- Guiry MD, Guiry GM. AlgaeBase World-wide electronic publication. National University of Ireland; Galway: 2014. http://www.algaebase.org: accessed on 13 March 2014. [Google Scholar]
- Hallegraeff GM, Bolch CJ, Blackburn SI, Oshima Y. Species of the toxigenic dinoflagellate genus Alexandrium in south eastern Australian waters. Bot Mar. 1991;34:575–587. [Google Scholar]
- Higman WA, Stone DM, Lewis JM. Sequence comparisons of toxic and non-toxic Alexandrium tamarense (Dinophyceae) isolates from UK waters. Phycologia. 2001;40:256–262. [Google Scholar]
- Halim Y. Alexandrium minutum nov. g. nov. sp. dinoflagellé provocant des ‘eaux rouges’. Vie Milieu. 1960;11:102–105. [Google Scholar]
- Ho KC, Lee TCH, Kwok OT, Lee FWF. Phylogenetic analysis on a strain of Alexandrium tamarense collected from Antarctic Ocean. Harmful Algae. 2012;15:100–108. [Google Scholar]
- Jedlicki A, Fernández G, Astorga M, Oyarzún P, Toro JE, Navarro JM, Martínez V. Molecular detection and species identification of Alexandrium (Dinophyceae) causing harmful algal blooms along the Chilean coastline. AoB Plants pls033. 2012 doi: 10.1093/aobpla/pls033. http://aob.doi.org/10.1093/aobpla/pls033. [DOI] [PMC free article] [PubMed]
- Jester RJ, Baugh KA, Lefebvre KA. Presence of Alexandrium catenella and paralytic shellfish toxins, shellfish and rock crabs in Montego Bay, California, USA. Mar Biol. 2009;156:493–504. doi: 10.1007/s00227-008-1103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John U, Fensome RA, Medlin LK. The application of a molecular clock based on molecular sequences and the fossil record to explain biogeographic distributions within the Alexandrium tamarense “species complex” (Dinophyceae) Mol Biol Evol. 2003a;20:1015–1027. doi: 10.1093/molbev/msg105. [DOI] [PubMed] [Google Scholar]
- John U, Cembella A, Hummert C, Elbrächter M, Groben R, Medlin L. Discrimination of the toxigenic dinoflagellates Alexandrium tamarense and A. ostenfeldii in co-occurring natural populations from Scottish coastal waters. Eur J Phycol. 2003b;38:25–40. [Google Scholar]
- John U, Medlin LK, Groben R. Development of specific rRNA probes to distinguish between geographic clades of the Alexandrium tamarense species complex. J Plankton Res. 2005;27:199–204. [Google Scholar]
- John U, Litaker W, Montresor M, Murray S, Brosnahan ML, Anderson DM. Proposal to reject the name Alexandrium catenella (Dinophyceae) Taxon. 2014;63:932–933. doi: 10.12705/634.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikawa R, Nagai S, Hosoi-Tanabe S, Itakura S, Yamaguchi M, Uchida Y, Baba T, Sako Y. Application of real-time PCR assay for detection and quantification of Alexandrium tamarense and Alexandrium catenella cysts from marine sediments. Harmful Algae. 2007;6:413–420. [Google Scholar]
- Katoh K, Kuma K, Toh H, Miyata T. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005;33:511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MD, Selvin RC, Claus W, Guillard RRL. Media for the culture of oceanic ultraphytoplankton. J Phycol. 1987;23:633–638. [Google Scholar]
- Kim KY, Yoshida M, Fukuyo Y, Kim CH. Morphological observation of Alexandrium tamarense (Lebour) Balech, A. catenella (Whedon et Kofoid) Balech and one related morphotype (Dinophyceae) in Korea. Algae. 2002;17:11–19. [Google Scholar]
- Klöpper S, John U, Zingone A, Mangoni O, Kooistra WHCF, Cembella A. Phylogeny and morphology of a Chattonella (Raphidophyceae) species from the Mediterranean Sea: what is C. subsalsa? Eur J Phycol. 2013;48:79–92. [Google Scholar]
- Koetschan C, Förster F, Keller A, Schleicher T, Ruderisch B, Schwarz R, Müller T, Wolf M, Schultz J. The ITS2 Database III-sequences and structures for phylogeny. Nucleic Acids Res. 2010;38:275–276. doi: 10.1093/nar/gkp966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J, Nguyen NL. Potentially toxic microalgae of Vietnamese waters. Opera Bot. 2004;140:5–216. [Google Scholar]
- Lawrence JF, Niedzwiadek B. Quantitative determination of paralytic shellfish poisoning toxins in shellfish by using prechromatographic oxidation and liquid chromatography with fluorescence detection. AOAC Int. 2001;84:1099–1108. [PubMed] [Google Scholar]
- Lawrence JF, Niedzwiadek B, Menard CJ. Quantitative determination of paralytic shellfish poisoning toxins in shellfish using prechromatographic oxidation and liquid chromatography with fluorescence detection: interlaboratory study. AOAC Int. 2004;87:83–100. [PubMed] [Google Scholar]
- Leaw CP, Lim PT, Ng BK, Cheah MY, Ahmad A, Usup G. Phylogenetic analysis of Alexandrium species and Pyrodinium bahamense (Dinophyceae) based on theca morphology and nuclear ribosomal gene sequence. Phycologia. 2005;44:550–565. [Google Scholar]
- Lebour MV. The Dinoflagellates of the Northern Seas. Marine Biological Association of the United Kingdom; Plymouth: 1925. p. 150. [Google Scholar]
- Lilly EL. Ph D dissertation. Massachusetts Institute of Technology; Cambridge: 2003. Phylogeny and Biogeography of the Toxic dinoflagellate Alexandrium. [Google Scholar]
- Lilly EL, Halanych KM, Anderson DM. Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae) J Phycol. 2007;43:1329–1338. [Google Scholar]
- Lilly EL, Kulis DM, Gentien P, Anderson DM. Paralytic shellfish poisoning toxins in France linked to a human-introduced strain of Alexandrium catenella from the western Pacific: Evidence from DNA and toxin analysis. J Plankton Res. 2002;24:443–452. [Google Scholar]
- Litaker RW, Vandersea MW, Kibler SR, Reece KS, Stokes NA, Lutzoni FM, Yonish BA, West MA, Black MND, Tester PA. Recognizing dinoflagellate species using ITS rDNA sequences. J Phycol. 2007;43:344–355. [Google Scholar]
- Loeblich AR, III, Loeblich LA. The Systematics of Gonyaulax with Special Reference to the Toxic Species. In: Taylor DL, Seliger HH, editors. Toxic Dinoflagellate Blooms, Proc Second International Conference on Toxic Dinoflagellate Blooms. Elsevier-North Holland; New Yok: 1979. pp. 41–46. [Google Scholar]
- Loeblich LA, Loeblich AR., III In: LoCicero VR, editor. The Organism Causing New England Red Tides: Gonyaulaxexcavata; Proceedings of the First International Conference on Toxic Dinoflagellate Blooms Massachusettes Science Technology Foundation; Wakefield, MA. 1975. pp. 207–224. [Google Scholar]
- Loeblich LA, Winn GJ, Loeblich AR., III Comparative studies on Gonyaulax tamarensis and related red-tide species. J Phycol. 1975;11(Suppl):21. [Google Scholar]
- MacKenzie L, de Salas M, Adamson J, Beuzenberg V. The dinoflagellate genus Alexandrium (Halim) in New Zealand coastal waters: comparative morphology, toxicity and molecular genetics. Harmful Algae. 2004;3:71–92. [Google Scholar]
- Masseret E, Grzebyk D, Nagai S, Genovesi B, Lasserre B, Laabir M, Collos Y, Vaquer A, Berrebi P. Unexpected genetic diversity among and within populations of the toxic dinoflagellate Alexandrium catenella as revealed by nuclear microsatellite markers. Appl Environ Microbiol. 2009;75:2037–2045. doi: 10.1128/AEM.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Marhold K, Prado J, Prud’homme van Reine WF, Smith GF, Wiersema JH, Turland NJ. International Code of Nomenclature for algae, fungi and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. Regnum Vegetabile. 2012;154:1–240. [Google Scholar]
- Medlin L, Lange M, Wellbrock U, Donner G, Elbrächter M, Hummert C, Luckas B. Sequence comparisons link toxic European isolates of Alexandrium tamarense from the Orkney Islands to toxic North American stocks. Eur J Protistol. 1998;34:329–335. [Google Scholar]
- Miranda LN, Zhuang YY, Zhang H, Lin S. Phylogenetic analysis guided by intragenomic SSU rDNA polymorphism refines classification of “Alexandrium tamarense” species complex. Harmful Algae. 2012;16:35–48. [Google Scholar]
- Montresor M, John U, Beran A, Medlin LK. Alexandrium tamutum sp. nov. (Dinophyceae): A new nontoxic species in the genus Alexandrium. J Phycol. 2004;40:398–411. [Google Scholar]
- Murray S, Wiese M, Stüken A, Brett S, Kellmann R, Hallegraeff G, Neilan B. sxtA-based quantitive molecular assay to identify saxitoxin producing harmful algal blooms in marine waters. Appl Environ Microbiol. 2011;77:7050–7057. doi: 10.1128/AEM.05308-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S, Wiese M, Neilan BA, Orr RJS, Salas M, Brett S, Hallegraeff G. A reinvestigation of saxitoxin production and sxtA in the ‘non-toxic’ Alexandrium tamarense Group V clade. Harmful Algae. 2012;18:96–104. [Google Scholar]
- Negri A, Llewellyn L, Doyle J, Webster N, Frampton D, Blackburn S. Paralytic shellfish toxins are restricted to few species among Australia’s taxonomic diversity of cultured microalgae. J Phycol. 2003;39:663–667. [Google Scholar]
- Orlova TY, Selina MS, Lilly EL, Kulis DM, Anderson DM. Morphogenetic and toxin composition variability of Alexandrium tamarense (Dinophyceae) from the east coast of Russia. Phycologia. 2007;46:534–548. [Google Scholar]
- Orr RJS, Stüken A, Murray SA, Jakobsen KS. Evolutionary acquisition and loss of saxitoxin biosynthesis in dinoflagellates: the second “core” gene, sxtG. Appl Environ Microbiol. 2013;79:2128–2136. doi: 10.1128/AEM.03279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr RJS, Stüken A, Rundberget T, Eikrem W, Jakobsen KS. Improved phylogenetic resolution of toxic and non-toxic Alexandrium strains using a concatenated rDNA approach. Harmful Algae. 2011;10:676–688. [Google Scholar]
- Parke M, Dixon PS. Check-list of British marine algae – Third revision. J Mar Biol Assoc UK. 1976;56:527–594. [Google Scholar]
- Penna A, Garcés E, Vila M, Giacobbe MG, Fraga S, Lugliè A, Bravo I, Bertozzini E, Vernesi C. Alexandrium catenella (Dinophyceae), a toxic ribotype expanding in the NW Mediterranean Sea. Mar Biol. 2005;148:13–23. [Google Scholar]
- Penna A, Fraga S, Maso M, Giacobbe MG, Bravo I, Garces E, Vila M, Bertozzini E, Andreoni F, Luglie A, Vernesi C. Phylogenetic relationships among the Mediterranean Alexandrium (Dinophyceae) species based on sequences of 5. 8S gene and Internal Transcript Spacers of the rRNA operon. Eur J Phycol. 2008;43:163–178. [Google Scholar]
- Pfiester LA, Anderson DM. Dinoflagellate Life Cycles and their Environmental Control. In: Taylor FJR, editor. The Biology of Dinoflagellates. Blackwell Scientific Publications Ltd; Oxford: 1987. pp. 611–648. [Google Scholar]
- Ride WDL, Cogger HG, Dupuis C, Kraus O, Minelli A, Thompson FC, Tubbs PK. International Code of Zoological Nomenclature. Cromwell; London: 1999. [Google Scholar]
- Ruhl MW, Wolf M, Jenkins TM. Compensatory base changes illuminate taxonomically difficult taxonomy. Mol Phylogent Evol. 2009;54:664–669. doi: 10.1016/j.ympev.2009.07.036. [DOI] [PubMed] [Google Scholar]
- Ruiz Sebastián C, Etheridge SM, Cook PA, O’Ryan C, Pitcher GC. Phylogenetic analysis of toxic Alexandrium (Dinophyceae) isolates from South Africa: Implications for the global phylogeography of the Alexandrium tamarense species complex. Phycologia. 2005;44:49–60. [Google Scholar]
- Scholin C, Herzog AM, Sogin M, Anderson DM. Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rDNA gene. J Phycol. 1994;30:999–1011. [Google Scholar]
- Scholin CA, Hallegraeff GM, Anderson DM. Molecular evolution of the Alexandrium tamarense ‘species complex’ (Dinophyceae): Dispersal in the North American and West Pacific regions. Phycologia. 1995;34:472–485. [Google Scholar]
- Seibel PN, Müller T, Dandekar T, Schultz J, Wolf M. 4SALE – A tool for synchronous RNA sequence and secondary structure alignment and editing. BMC Bioinformatics. 2006;7:498. doi: 10.1186/1471-2105-7-498. http://www.biomedcentral.com/1471-2105/7/498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smayda TJ. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol Oceanogr. 1997;42:1137–1153. [Google Scholar]
- Stamatakis A, Ludwig T, Meier H. RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics. 2005;21:456–463. doi: 10.1093/bioinformatics/bti191. [DOI] [PubMed] [Google Scholar]
- Stüken A, Orr RJS, Kellmann R, Murray SA, Neilan BA, Jakobsen KS. Discovery of nuclear-encoded genes for the neurotoxin saxitoxin in dinoflagellates. PLoS ONE. 2011;6:e20096. doi: 10.1371/journal.pone.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunda WG, Granéli E, Gobler CJ. Positive feedback and the development and persistence of ecosystem disruptive algal blooms. J Phycol. 2006;42:963–974. [Google Scholar]
- Swofford DL. PAUP* Phylogenetic Analysis Using Parsimony (* and other methods) Version 4. Sinauer Associates, Sunderland; Massachusetts: 1998. [Google Scholar]
- Taylor FJR. The Toxigenic Gonyaulacoid Dinoflagellates. In: Taylor DL, Seliger HH, editors. Toxic Dinoflagellate Blooms, Proceedings of the Second International Conference on Toxic Dinoflagellate Blooms. Elsevier; New York: 1979. pp. 47–56. [Google Scholar]
- Taylor FJR. Toxic Dinoflagellates: Taxonomic and Biogeographic Aspects with Emphasis on Protogonyaulax. In: Ragelis EP, editor. Seafood Toxins. American Chemical Society; Washington, D.C: 1984. pp. 77–97. [Google Scholar]
- Toebe K, Alpermann TJ, Tillmann U, Krock B, Cembella AD, John U. Molecular discrimination of toxic and non-toxic Alexandrium species (Dinophyta) in natural phytoplankton assemblages from the Scottish coast of the North Sea. Eur J Phycol. 2013;48:12–26. [Google Scholar]
- Touzet N, Franco JM, Raine R. Morphogenetic diversity and biotoxin composition of Alexandrium (Dinophyceae) in Irish coastal waters. Harmful Algae. 2008;7:782–797. [Google Scholar]
- Touzet N, Davidson K, Pete R, Flanagan K, McCoy GR, Amzil Z, Maher M, Chapelle A, Raine R. Co-occurrence of the West European (Gr. III) and North American (Gr.I) ribotypes of Alexandrium tamarense (Dinophyceae) in Shetland, Scotland. Protist. 2010;161:370–384. doi: 10.1016/j.protis.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Vanormelingen P, Hegewald E, Braband A, Kitschke M, Friedel T, Sabbe K, Vyverman W. The systematics of a small spineless Desmodesmus species, D. costato-granulatus (Sphaeropleales, Chlorophyceae), based on ITS2 rDNA sequence analyses and cell wall morphology. J Phycol. 2007;43:378–396. [Google Scholar]
- Varela D, Paredes J, Alves-de-Souza C, Seguel M, Sfeir A, Frangopulos M. Intraregional variation among Alexandrium catenella (Dinophyceae) strains from southern Chile: Morphological, toxicological and genetic diversity. Harmful Algae. 2012;15:8–18. [Google Scholar]
- Vila M, Garcés E, Maso M, Camp J. Is the distribution of the toxic dinoflagellate Alexandrium catenella expanding along the NW Mediterranean coast? Mar Ecol Prog Ser. 2001;222:73–83. [Google Scholar]
- Wang L, Zhuang YY, Zhang H, Lin X, Lin S. DNA barcoding species in Alexandrium tamarense complex using ITS and proposing designation of five species. Harmful Algae. 2014;31:100–113. doi: 10.1016/j.hal.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Whedon WF, Kofoid CA. Dinoflagellata of the San Francisco Region. I. On the skeletal morphology of two new species, Gonyaulax catenella and G. acatenella. Univ Calif Publ Zool. 1936;41:25–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


