Abstract
Background
Future fertility is an important concern for many cancer survivors. Cancer therapies have been shown to adversely impact reproductive function. However, it is difficult to predict the extent to which reproductive dysfunction will occur. The purpose of this study was to compare measures of ovarian reserve (MOR) and pregnancy rates in young female cancer survivors and similar-aged controls.
Procedures
A prospective cohort study was conducted in a university-hospital setting. Participants were followed annually for a mean 25 months to assess reproductive history, the incidence of pregnancy, and MOR (serum follicle-stimulating hormone, luteinizing hormone, estradiol, inhibin B, anti-mullerian hormone (AMH), antral follicle counts and mean ovarian volume).
Results
Eighty-four female survivors (average age 26, and 14 years post-treatment) and 98 similar-aged controls that were sexually active with men were included. At baseline, 27/84 survivors and 42/98 controls reported a prior pregnancy. Adjusted models showed that anti-mullerian hormone (AMH) and antral follicle count (AFC) were impaired in survivors with a prior pregnancy compared to controls with a prior pregnancy (p<0.01, p=0.03). During follow-up in 56 survivors and 74 controls, 19 pregnancies occurred in survivors and 18 in controls. Comparison of MOR between survivors who became pregnant and controls who became pregnant revealed that AMH and AFC were impaired in survivors (p<0.05). Compared to survivors who did not become pregnant, survivors who did were older (p<0.01) and more likely to be cohabitating (p<0.01), but had similar MOR and exposure to alkylators (p=0.34).
Conclusions
Survivors achieved pregnancy at a rate similar to controls despite impaired MOR.
Keywords: fertility, chemotherapy, ovarian reserve, cancer survivors
Introduction
Future fertility is an important concern for many cancer survivors (1, 2). There are currently more than 400,000 reproductive-aged female cancer survivors in the US (3), and many will desire starting or completing their families after cancer therapy. Cancer therapies have an adverse impact on reproductive function, increasing the risk of early menopause and infertility (4, 5). However, based on available literature, it is difficult to predict the extent to which reproductive dysfunction will occur.
Counseling women about their future fertility potential includes an evaluation of measures of ovarian reserve, including ultrasound measures (antral follicle count (AFC) and mean ovarian volume (MOV)), and serum measures (inhibin B (INH), anti-mullerian hormone (AMH), estradiol (E2), lutenizing hormone (LH) and follicle-stimulating hormone (FSH)). These have been used as surrogate markers for fertility potential (6, 7). AMH in particular has been shown to be a sensitive marker for ovarian reserve in survivors of cancer (8-11). Large studies have shown that fertility rates are lower in female cancer survivors (12), and that female cancer survivors may have poorer response to assisted reproductive technologies (13). However, no study has specifically examined the relationship between measures of ovarian reserve and the chance of pregnancy in a group of cancer survivors. A better understanding of the association between measures of ovarian reserve and pregnancy potential in this population will facilitate patient counseling about reproductive risks and fertility options.
The primary objective of this study was to evaluate the incidence of pregnancy and time to pregnancy, and the association between pregnancy and measures of ovarian reserve in young adult cancer survivors compared to similar-aged controls. In addition, this study sought to identify factors associated with pregnancies in cancer survivors. We hypothesized that cancer survivors who achieve pregnancy will have been exposed to less gonadotoxic cancer treatments and will demonstrate less impaired measures of ovarian reserve than those not achieving pregnancy.
Materials and Methods
This study is part of an ongoing prospective cohort study at the University of Pennsylvania (Penn) comparing annual measures of ovarian reserve between females exposed to chemotherapy and similarly-aged healthy unexposed females.
Subjects
Reproductive-age cancer survivors were principally recruited from the Children's Hospital of Philadelphia Survivorship Program and the Transition Program at Penn's Living Well After Cancer Survivorship Program during the years 2006–2010. Inclusion criteria were [1] chemotherapy treatment, [2] at least 1 year from cancer treatment with no evidence of disease, [3] age 15–39 years, [4] postmenarchal, and [5] presence of uterus and both ovaries. Exclusion criteria included history of a brain or ovarian tumor, pregnancy or lactation within 3 months, hormonal contraception or hormone therapy within 4 weeks, and any medical condition other than cancer associated with ovarian dysfunction, such as hypothalamic hypogonadism.
Unexposed controls of similar age to cancer survivors were identified through health practices affiliated with Penn and advertising. Controls were postmenarchal, with regular menstrual cycles (21–35 days), a uterus, and both ovaries. Exclusion criteria were the same as for survivors.
For the purpose of this report, only survivors and controls at risk for pregnancy were included in this analysis, as defined by reporting intercourse with a man during the study. Those who did not report intercourse with a man were not included in this report.
The institutional review board at the University of Pennsylvania approved this study, and informed consent was obtained from all participants. Study visits occurred annually on days 1–4 of the menstrual cycle. For each visit, subjects stopped exogenous hormones for at least 4 weeks and were seen during the subsequent menstrual cycle. Those with irregular cycles or no menses for 6 weeks after stopping hormones were seen any time. Phone interviews were conducted for those unable to appear in person for a scheduled follow-up study visit.
Questionnaires
During a structured interview at each annual visit, detailed information was collected regarding demographics, medical history, menstrual characteristics, pregnancies, infertility history, contraception use, medications, and substance use. In particular, for each pregnancy, subjects were asked about the outcome of the pregnancy, and in the case of live born pregnancies, they were asked about whether the pregnancy was full-term or pre-term and if there were any maternal or child complications. In addition, for each pregnancy, participants were asked about the duration of unprotected intercourse prior to conception, and whether pregnancies were achieved with or without fertility interventions. Contraception use was defined as the use of any contraceptive method, including hormonal contraceptives, intrauterine devices, barrier methods, withdrawal, or rhythm method. Unplanned pregnancies were defined as answering ‘No’ to ‘Was this pregnancy planned?’. Infertility was defined as having unprotected intercourse with a man for longer than 12 months without a conception.
Menstrual Data
Subjects were given a menstrual diary and provided the dates of the two most recent menstrual cycles during the interview. Cycle length was calculated as the interval between the two most recent menstrual cycles. Women were categorized as having menstrual function if they reported menstrual bleeding while not taking exogenous hormones, and categorized as having regular menstrual cycles if they reported regular menses (21–35 days) and no hormone use the previous year.
Physical Examination
Height and weight were measured for calculating body mass index (BMI).
Pelvic Ultrasonography
Uterine volume, ovarian volume, and antral follicle counts (AFC) were determined by ultrasonography. Measurements were performed using a Siemens Sonoline G50 machine, 6.8-MHz probe. Transvaginal ultrasonography was preferred, though transabdominal ultrasound was performed in participants uncomfortable with the transvaginal approach. Uterine and ovarian volumes were calculated using the ellipse formula (A × B × C × 0.5233). Antral follicle count was determined for subjects undergoing transvaginal ultrasonography when both ovaries were visualized and was defined as the number of follicles between 2 and 10 mm in average diameter.
Hormone Analysis
Serum hormones were measured at Penn's Clinical Translational Research Center using FSH and E2 Coat-A-Count kits (Diagnostic Products Corporation) and inhibin B and antimüllerian hormone (AMH) ELISA kits (Diagnostic Systems). The FSH immunoradiometric assay's range is 1.5–100 mIU/mL, with a sensitivity of 0.7 mIU/mL and inter- and intra-assay coefficients of variation (cov) <6% and 4%, respectively. The E2 RIA's range is 20–3,600 pg/mL, with a sensitivity of 7 pg/mL and inter- and intra-assay cov <8.1% and 7%, respectively. The inhibin B ELISA's range is 10–531 pg/mL, with a sensitivity of 7 pg/mL and inter- and intra-assay cov <8% and <6%, respectively. The AMH ELISA's range is 0.050–10.0 ng/mL, with a sensitivity of 0.025 ng/mL and inter- and intra-assay cov <8% and 5%, respectively.
Cancer Therapy
Exposure data were obtained by abstracting medical records. Treatment was summarized for chemotherapeutic type, duration, cumulative dose; radiation dose and location; type of bone marrow transplantation (BMT); and surgery. Alkylating agent dose scores (AAD) were determined by assigning a score ranging from 1 to 3 for each agent received and summing scores over all agents (12).
Data Analysis
Demographic characteristics and log-transformed hormones and ultrasound data were compared between survivors and controls at risk for pregnancy using Pearson χ2 analyses, t tests, and multivariable regression models controlling for age, race, and BMI. Similar comparisons of demographic characteristics and measures of ovarian reserve were then conducted to compare survivors who reported a pregnancy and controls who reported a pregnancy. The risk of pregnancy during the study follow-up between survivors and controls was compared using Kaplan-Meier curves.
Subgroup analysis within the survivor group compared demographic characteristics and measures of ovarian reserve between survivors achieving a pregnancy compared to those who did not achieve a pregnancy. For all analyses hormonal measures were log-transformed. Statistical analysis was performed using STATA v12.0 (StataCorp, College Station, TX).
Results
A total of 96 cancer survivors and 109 controls were enrolled in the study at the time of this analysis. Twelve cancer survivors and 11 controls had never been sexually active with men and were excluded from this report. Thus, 84 female cancer survivors and 98 similar-aged controls at risk for pregnancy were included in this paper. Because women taking contraceptives can become pregnant, this report includes all women who were sexually active with men, regardless of intent to become pregnant. Baseline characteristics for these participants are described in Table 1. The cohort from which these patients were taken has been previously described (6). The mean age of participants included in this analysis was 26.8 years, range 15-39 years. Most were unmarried, nulligravid, and normal weight. Compared to controls, more survivors were Caucasian and fewer graduated from college (p=0.03, p<0.01 respectively). Within the survivor population, 32 cancer survivors had been treated for lymphoma, 26 leukemia, 13 sarcoma, 5 Wilms tumor, 2 breast cancer, and 6 other. Of 84 cancer survivors, 73 (87%) received alkylators, 3 (4%) received pelvic radiation, and 19 had a history of BMT (11 with TBI). Overall, survivors were on average 14 years (95% CI 12-16 years) from the end of cancer therapy. Though similar proportions were using contraceptives in the month before enrollment, survivors were more likely than controls to be using a hormonal method (p=0.02).
Table I.
Baseline demographics
| Survivors n=84 | Controls n=98 | p | |
|---|---|---|---|
| Age (y), mean (95% CI) | 25.6 (24.3-27.0) | 26.9 (26.0-27.9) | 0.10 |
| BMI, mean (95% CI) | 24.1 (22.9-25.3) | 25.2 (24.0-26.5) | 0.21 |
| Race- Caucasian, % (n) | 92 (77/84) | 81 (79/98) | 0.03 |
| Education>College Graduate, % (n) | 55 (46/84) | 74 (70/98) | <0.007 |
| Marital status- single, % (n) | 67 (56/84) | 74 (70/98) | 0.10 |
| Living with partner, % (n) | 34 (30/84) | 24 (24/98) | 0.09 |
| Previous pregnancy, % (n) | 18 (15/84) | 27 (25/98) | 0.15 |
| Gravidity, mean (range) | 1.8 (1-3) | 1.7 (1-5) | |
| Parity, mean | 1.3 | 0.8 | |
| Reported time to pregnancy (months), mean | 3.7 | 0.8 | 0.30 |
| Report current contraceptive use, % (n) | 74 (62/84) | 64 (63/98) | 0.15 |
| Report contraceptive use with a hormonal method, % (n) | 32 (20/62) | 10 (6/63) | 0.02 |
| Menstrual Function, % (n) | 86 (73/84) | 100 (98/98) | <0.001 |
| Report regular menstrual cycles and are not using exogenous hormones, % (n) | 86 (56/65) | ||
| FSH mIU/mL, mean (95%CI)* | 13.9 (10.4-16.4) | 7.2 (6.6-8.0) | <0.001 |
| LH mIU/mL, mean (95% CI)* | 5.3 (4.3-7.2) | 3.8 (3.3-4.3) | 0.02 |
| Inhibin pg/mL, mean (95% CI)* | 27.0 (22.1-36.2) | 39.1 (32.5-47.4) | 0.03 |
| Estradiol pg/mL, mean (95% CI)* | 24.1 (21.5-29.8) | 25.4 (22.7-28.7) | 0.7 |
| AMH ng/mL, mean (95% CI)* | 0.7 (0.5-1.0) | 2.4 (2.0-2.7) | <0.001 |
| AFC, mean (95% CI)* | 11.3 (9.0-14.4) | 22.8 (20.3-26.0) | <0.001 |
| Ovarian volume cm3, mean (95% CI)* | 9.9 (8.9-13.4) | 14.3 (13.1-16.1) | 0.001 |
Geometric mean shown. Adjusted for age, race, and BMI.
Overall, at study enrollment measures of FSH, LH, Inhibin B, AMH, AFC, and MOV were significantly impaired in the survivors compared to the controls. These differences remained significant for FSH, AMH, AFC, and MOV when models adjusted for age, BMI, and race were used (Table 1).
Retrospective Assessment of Prior Pregnancies
At the time of enrollment, 15 cancer survivors and 25 controls reported a prior pregnancy. All of the survivors’ pregnancies had occurred after cancer therapy. Of the 15 survivors and 25 controls who reported pregnancy, there were 27 pregnancies in the survivor group and 42 pregnancies in the control group. The proportion of unplanned pregnancies was similar among groups: 37% (10/27) for the survivors and 40% (17/42) for the controls (p=0.77). For the pregnancies that were planned, the survivors reported requiring a mean 3.7 months to conceive (range 0-12) compared to 0.8 months to conceive (range 0-1; p=0.30) for controls. Four cancer survivors reported using infertility medications in combination with intrauterine insemination in order to achieve pregnancy.
Of the 27 prior pregnancies in the survivors, 70% (19/27) resulted in live births. Eleven percent (3/27) ended in induced abortions, 15% (4/27) in spontaneous miscarriages, and 1 was an ectopic pregnancy. For the controls, 48% (20/42) of the pregnancies resulted in live births. Thirty-eight percent (16/42) ended in induced abortions and 14% ended in spontaneous miscarriage. There was no difference in the distribution of outcomes between groups (p=0.23)
Examination of the ovarian reserve at the time of enrollment for the survivors who had previously achieved a pregnancy compared to the controls who had previously achieved a pregnancy demonstrated that measures of AMH and AFC were impaired in the survivors compared to the controls. The adjusted geometric mean of AMH in the survivors achieving pregnancy was 0.9 mIU/mL (95% CI 0.4-2.1 mIU/mL) compared to 2.4 mIU/mL (95% CI 1.8-3.2 mIU/mL) for the controls who had previously achieved a pregnancy (p=0.008). The mean AFC in the survivors was 12.7 compared to 21.9 in the controls (p=0.02). Importantly, because these measures of ovarian reserve were obtained at the time of enrollment, these values may be remote from the time of the previous pregnancies.
Prospective Assessment of Pregnancy and Ovarian Reserve
In order to assess the association between current ovarian reserve and pregnancy, we compared MOR between cases and controls at risk for pregnancy during the duration of follow-up. The majority of participants- 56 survivors and 74 controls- had follow-up visits. Participants were seen annually and followed prospectively for a mean of 25 months (95% CI 22-28 months); thus, most patients were seen for at least two study visits in addition to their enrollment visit. During this observation period, 19 survivors and 18 controls reported an incident pregnancy. Of the pregnancies in the survivors, 84% (16/19) resulted in live births. Eleven percent (2/19) ended in induced abortions, and 5% (1/19) in spontaneous miscarriages. Table 2 shows measures of ovarian reserve (recorded at the most recent study visit prior to the report of pregnancy) and other characteristics in the survivors who achieved a pregnancy compared to controls who achieved a pregnancy. Ovarian reserve was impaired in the survivors achieving a pregnancy compared to the controls achieving a pregnancy, with diminished AMH and AFC in adjusted models. FSH appeared higher in the survivors, but this did not reach significance (p=0.06). Sixteen percent (3/19) of conceptions in survivors were unplanned, compared to 44% (8/18) in controls (p=0.06). The mean time to conception for the planned pregnancies was 8.6 months (range 0-28) and 3.1 months (range 0-7) for the controls (p=0.30).
Table II.
Characteristics for those reporting pregnancy during the study
| Survivors (n=19) | Controls (n=18) | p | |
|---|---|---|---|
| Age (y), mean (95% CI) | 30.1 (26.8-31.7) | 28.9 (26.7-29.9) | 0.41 |
| BMI, mean (range) | 23.4 (17-31) | 26.3 (18.1-40.6) | 0.12 |
| Race- Caucasian, % (n) | 95 (18/19) | 72 (13/18) | 0.06 |
| Marital status- single, % (n) | 21 (4/19) | 61 (11/18) | 0.02 |
| Living with partner, % (n) | 68 (13/19) | 44 (8/18) | 0.14 |
| Contraceptive use within the past year, % (n) | 68 (13/19) | 39 (7/18) | 0.07 |
| Menstrual Function, % (n) | 89 (17/19) | 94 (17/18) | 0.58 |
| Report regular menstrual cycles and are not using exogenous hormones, % (n) | 88 (14/16) | 94 (15/16) | 0.54 |
| FSH | 12.1 (8.0-18.4) | 7.9 (6.2-10.1) | 0.06 |
| LH | 5.0 (3.4-7.4) | 3.7 (2.6-5.4) | 0.27 |
| Inhibin pg/mL, mean (95% CI)* | 30.0 (23.3-38.8) | 26.7 (23.0-31.0) | 0.39 |
| Estradiol pg/mL, mean (95% CI)* | 34.4 (18.8-62.9) | 36.5 (23.5-56.6) | 0.87 |
| AMH ng/mL, mean (95% CI)* | 0.6 (0.3-1.1) | 1.8 (1.1-3.0) | 0.006 |
| AFC, mean (95% CI)* | 10.2 (6.8-15.3) | 20.7 (16.5-26.0) | 0.003 |
| Ovarian volume cm3, mean (95% CI) | 10.7 (7.7-14.8) | 11.8 (9.8-14.3) | 0.57 |
Geometric mean shown. Adjusted for age, race, and BMI.
Two survivors reported using a combination of medications and intrauterine insemination, and one survivor reported using in vitro fertilization to conceive. No controls reported infertility treatment. Overall 23% (13/56) survivors and 11% (8/74) of controls reported infertility during the study, defined as trying to conceive for >1 year without success (p=0.06). There were no statistically significant differences in the ovarian reserve measures between survivors who reported infertility and controls who reported infertility.
Figure 1 shows the risk of pregnancy over the duration of the study in the survivors compared to the controls. The risk of pregnancy was not statistically different between groups (p=0.35).
FIGURE 1.
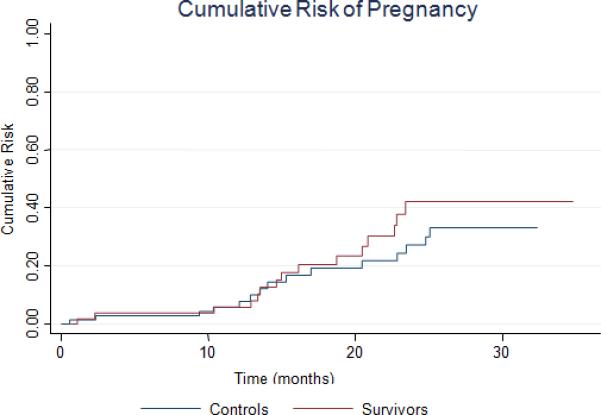
Cumulative risk of pregnancy during study follow-up.
Comparisons of measures of ovarian reserve among survivors who reported pregnancies during the study compared to survivors that did not report a pregnancy revealed no statistically significant differences in ovarian reserve. However, survivors who reported pregnancy were older (mean 30.0 vs. 26.3 in those who became pregnant vs. those who did not, p=0.04) and less likely to be single (21% of survivors who became pregnant vs. 79% of the survivors who did not become pregnant, p<0.01) compared to survivors who did not report pregnancies during the study. Surprisingly, there were no differences in treatment characteristics between survivors who achieved a pregnancy compared to those who did not, including: alkylating score received, percent who received total body irradiation and percent who received bone marrow transplant. One woman who had received total body irradiation, two women who underwent bone marrow transplantation, and five women with alkylator scores ≥4 achieved pregnancies. Importantly, 11% (2/19) of the survivors who became pregnant reported that they did not have regular menses at the study visit prior to the conception of their pregnancy.
Discussion
While cancer therapies have been shown to impair reproductive function in cancer survivors, and ovarian reserve is often used as a proxy measurement for fertility potential, the association between current measures of ovarian reserve at the time of conception in cancer survivors is not well documented. This study provides information that is important to determine the utility of ovarian reserve measures for reproductive counseling in this population.
In this study, a longitudinal assessment of ovarian reserve and reproductive function was completed in young female cancer survivors. For both previous pregnancies reported at the time of enrollment, and pregnancies captured during the study, cancer survivors who reported pregnancies had diminished ovarian reserve compared to controls who reported pregnancies, but not compared to the cancer survivors who did not achieve pregnancies. These findings suggest that predictions about the fertility of a cancer survivor cannot be made on the basis of measures of ovarian reserve alone. These findings have important implications for counseling young cancer survivors about pregnancy risk. Pregnancy may be possible in young survivors even with impaired measures of ovarian reserve and irregular menses.
Indeed, women with very diminished ovarian reserve and those who had received particularly gonadotoxic therapies achieved pregnancies. These data are consistent with other reports that have shown that 22% of cancer patients who suffered chemotherapy-related amenorrhea conceive a child (14) and that cancer survivors with ‘critically low’ AMH (defined as <0.3 ng/mL) can conceive (15). Nonetheless, while we were unable to detect a difference in pregnancy rates between cancer survivors and controls, it is important to note that survivors in this study reported a longer time to conception than controls. Also, more survivors experienced infertility and required infertility treatments compared to controls. While these differences did not reach statistical significance, these findings suggest that cancer survivors may indeed have impaired fertility related to reduced ovarian reserve.
Limited data suggest that AMH measures predict time to pregnancy in non-infertile populations (16), though there is significant inter-individual variation in AMH concentrations in the population (17, 18). In addition, low values of AMH have been determined to be useful in predicting time to menopause in late reproductive age women (19, 20). It is likely that measures of ovarian reserve provide more information about the quantity of the ovarian follicles available rather than quality in a population of young women. Additional studies are needed to better understand the predictive value of measures of ovarian reserve with pregnancy and time to menopause in this population.
It is notable that a number of pregnancies that occurred in survivors in this study were unplanned. Indeed, 37% of pregnancies reported by cancer survivors at the baseline visit and 16% of pregnancies reported during the study were considered unplanned by cancer survivors. This observation emphasizes the need for improved counseling surrounding family planning. All cancer survivors should be counseled to use contraceptives if pregnancy is not desired, regardless of treatment history or current ovarian reserve. Long acting, highly effective, reversal methods of contraception may be particularly appropriate for this population (21).
It is important to emphasize that the women included in this study were young; most were in their mid-20s. Thus, these results may not be applicable to older cohorts of late reproductive age women who may also experience a decline in fertility related to advanced age. Future directions for this study will include continuing to follow this young cohort of cancer survivors as they age, to determine how the time to achieve pregnancy and pregnancy rates change as survivors get older. Additional longitudinal follow-up will also provide more information about the predictive value of measures of ovarian reserve in this population. In addition, in the future we hope to validate the self-report pregnancy outcome data with medical records and publish more detailed information about pregnancy outcomes with a large sample size.
Several limitations should be mentioned. First, slight differences in baseline demographics, specifically race and educational level, were noted in the survivors compared to the controls. While confounding by such factors has the potential to bias results, this is unlikely since adjusted models confirmed the observed associations. In addition, it is important to recognize that this is not a study of couples actively trying to conceive, but rather this study presents data on all women at risk for pregnancy. Therefore intended and unintended pregnancies are reported. This approach is informative and generalizable to this population of young women. However, we acknowledge that several other factors may influence pregnancy rates in this cohort and therefore we included contraceptive use and marital status in our adjusted models to attempt to account for these factors. In addition, because subjects with a variety of diagnoses and treatments were included in this study, it is not possible to compare the effect of specific chemotherapeutic regimens on ovarian reserve and the likelihood of pregnancy.
This study has several strengths. Recall bias was minimized by prospective enrollment, and valid comparisons were made with an unexposed control population of similar age. Confounding has been reduced by clearly defining the population at risk for pregnancy and by restricting the study to nonpregnant, nonlactating females not using hormones and without other causes of ovarian dysfunction. Unlike some studies of ovarian reserve, a comprehensive evaluation of ovarian reserve was performed and hormone variability minimized by obtaining early follicular phase measures. Cancer diagnoses and treatments were validated with medical records to diminish misclassification bias.
This study demonstrates that young cancer survivors with diminished ovarian reserve are still capable of achieving pregnancy. This cohort will continue to provide data regarding time to pregnancy, infertility rates, and association of ovarian reserve with pregnancy in this population. This is crucial information for counseling these patients about family planning; those not wishing to become pregnant should use effective methods of contraception, and those wishing to start a family should be encouraged to attempt pregnancy even if ovarian reserve is diminished. The success of fertility treatments in survivors needs further investigation.
Acknowledgments
Funding/Support: Supported by NIH Grant K01 L:1-CA-133839-03 (CG); 1R01HD062797 (CG, MDS), and the Doris Duke Clinical Research Fellowship (KED).
References
- 1.Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having children after cancer. A pilot survey of survivors' attitudes and experiences. Cancer. 1999;86:697–709. doi: 10.1002/(sici)1097-0142(19990815)86:4<697::aid-cncr20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.Partridge AH, Ruddy KJ. Fertility and adjuvant treatment in young women with breast cancer. Breast. 2007;16(Suppl 2):S175–81. doi: 10.1016/j.breast.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2012-2013 American Cancer Society. Intramural Research. 2012 [Google Scholar]
- 4.Wallace WH, Critchley HO, Anderson RA. Optimizing reproductive outcome in children and young people with cancer. J Clin Oncol. 2012;30:3–5. doi: 10.1200/JCO.2011.38.3877. [DOI] [PubMed] [Google Scholar]
- 5.Wallace WH. Oncofertility and preservation of reproductive capacity in children and young adults. Cancer. 2011;117:2301–10. doi: 10.1002/cncr.26045. [DOI] [PubMed] [Google Scholar]
- 6.Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, et al. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012;97:134, 40, e1. doi: 10.1016/j.fertnstert.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen EC, Muller J, Rechnitzer C, Schmiegelow K, Andersen AN. Diminished ovarian reserve in female childhood cancer survivors with regular menstrual cycles and basal FSH <10 IU/l. Hum Reprod. 2003;18:417–22. doi: 10.1093/humrep/deg073. [DOI] [PubMed] [Google Scholar]
- 8.van Beek RD, van den Heuvel-Eibrink MM, Laven JS, de Jong FH, Themmen AP, Hakvoort-Cammel FG, et al. Anti-Mullerian hormone is a sensitive serum marker for gonadal function in women treated for Hodgkin's lymphoma during childhood. J Clin Endocrinol Metab. 2007;92:3869–74. doi: 10.1210/jc.2006-2374. [DOI] [PubMed] [Google Scholar]
- 9.Lie Fong S, Laven JS, Hakvoort-Cammel FG, Schipper I, Visser JA, Themmen AP, et al. Assessment of ovarian reserve in adult childhood cancer survivors using anti-Mullerian hormone. Hum Reprod. 2009;24:982–90. doi: 10.1093/humrep/den487. [DOI] [PubMed] [Google Scholar]
- 10.Bath LE, Wallace WH, Shaw MP, Fitzpatrick C, Anderson RA. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum Reprod. 2003;18:2368–74. doi: 10.1093/humrep/deg473. [DOI] [PubMed] [Google Scholar]
- 11.Brougham MF, Crofton PM, Johnson EJ, Evans N, Anderson RA, Wallace WH. Anti-Mullerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: a prospective study. J Clin Endocrinol Metab. 2012;97:2059–67. doi: 10.1210/jc.2011-3180. [DOI] [PubMed] [Google Scholar]
- 12.Green DM, Kawashima T, Stovall M, Leisenring W, Sklar CA, Mertens AC, et al. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2009;27:2677–85. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barton SE, Missmer SA, Berry KF, Ginsburg ES. Female cancer survivors are low responders and have reduced success compared with other patients undergoing assisted reproductive technologies. Fertil Steril. 2012;97:381–6. doi: 10.1016/j.fertnstert.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 14.van der Kaaij MA, Heutte N, Meijnders P, Abeilard-Lemoisson E, Spina M, Moser EC, et al. Premature ovarian failure and fertility in long-term survivors of Hodgkin's lymphoma: a European Organisation for Research and Treatment of Cancer Lymphoma Group and Groupe d'Etude des Lymphomes de l'Adulte Cohort Study. J Clin Oncol. 2012;30:291–9. doi: 10.1200/JCO.2011.37.1989. [DOI] [PubMed] [Google Scholar]
- 15.Hamre H, Kiserud CE, Ruud E, Thorsby PM, Fossa SD. Gonadal function and parenthood 20 years after treatment for childhood lymphoma: a cross-sectional study. Pediatr Blood Cancer. 2012;59:271–7. doi: 10.1002/pbc.23363. [DOI] [PubMed] [Google Scholar]
- 16.Steiner AZ, Herring AH, Kesner JS, Meadows JW, Stanczyk FZ, Hoberman S, et al. Antimullerian hormone as a predictor of natural fecundability in women aged 30-42 years. Obstet Gynecol. 2011;117:798–804. doi: 10.1097/AOG.0b013e3182116bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-mullerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97:4650–5. doi: 10.1210/jc.2012-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-mullerian hormone from conception to menopause. PLoS One. 2011;6:e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478–83. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97:1673–80. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarz EB, Hess R, Trussell J. Contraception for cancer survivors. J Gen Intern Med. 2009;24(Suppl 2):S401–6. doi: 10.1007/s11606-009-1023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


