Abstract
Objective
Implicit skill learning is hypothesized to depend on nondeclarative memory that operates independent of the medial temporal lobe (MTL) memory system and instead depends on cortico-striatal circuits between the basal ganglia and cortical areas supporting motor function and planning. Research with the Serial Reaction Time (SRT) task suggests that patients with memory-disorders due to MTL damage exhibit normal implicit sequence learning. However, reports of intact learning rely on observations of no group differences, leading to speculation whether implicit sequence learning is fully intact in these patients. Patients with Parkinson's Disease (PD) often exhibit impaired sequence learning, but this impairment is not universally observed.
Method
Implicit perceptual-motor sequence learning was examined using the Serial Interception Sequence Learning (SISL) task in patients with amnestic Mild Cognitive Impairment (MCI; n=11) and patients with PD (n=15). Sequence learning in SISL is resistant to explicit learning and individually adapted task difficulty controls for baseline performance differences.
Results
Patients with MCI exhibited robust sequence learning, equivalent to healthy older adults (n=20), supporting the hypothesis that the MTL does not contribute to learning in this task. In contrast, the majority of patients with PD exhibited no sequence-specific learning in spite of matched overall task performance. Two patients with PD exhibited performance indicative of an explicit compensatory strategy suggesting that impaired implicit learning may lead to greater reliance on explicit memory in some individuals.
Conclusion
The differences in learning between patient groups provides strong evidence in favor of implicit sequence learning depending solely on intact basal ganglia function with no contribution from the MTL memory system.
Keywords: implicit memory, Parkinson's disease, mild cognitive impairment, sequence learning, skill learning
The process by which we learn skills through practice depends materially on implicit learning mechanisms. Performance becomes increasingly fluid, more accurate, and less effortful, but the exact information that contributes to this is often outside of awareness. The neural basis of this kind of learning has been hypothesized to depend on brain regions outside the medial temporal lobe (MTL) memory system since the observation of normal procedural learning (e.g., rotary pursuit) in the famous amnesic patient H.M. (Corkin, 1968; Milner, Corkin, & Teuber, 1968). It is probably not adequate to simply say that all procedural skill learning is intact following MTL damage (e.g., Beatty et al. 1987), as some explicit knowledge of the task goals or initial instructions likely contributes to many forms of complex skill learning. When first attempting a new complex skill, initial performance likely depends on conscious, declarative task knowledge that would be impaired by damage to the MTL memory system. However, once practice is begun and repeated, further improvements in performance continue to accrue due to implicit learning within brain regions supporting performance. Practice with a repeating or structured sequence of actions leads to learning that produces increasingly effective and efficient processing and overall improvement in execution.
The canonical task for examining implicit learning of perceptual-motor sequencing has been the Serial Reaction Time (SRT) task. This task was first described by Nissen & Bullemer (1987) and uses perceptual cues to direct participants through a repeating sequence of motor responses. Improvements in performance are measured through gradually decreasing reaction times (RTs) and if the underlying sequence of cues is changed, a disruption in the speed of responding indicates acquired knowledge of the sequence. The SRT task is typically found to be learned at a normal rate by patients with MTL damage due to Korsakoff's syndrome (Nissen & Bullemer, 1987), anterograde amnesia (Reber & Squire, 1994) or Alzheimer's disease (AD; Knopman & Nissen, 1987). In a review of 23 studies of implicit motor-skill learning (van Hlateren-van Tilbourg et al. 2007), consistent learning was found for practiced motor skills trained implicitly. These findings suggested that incidental practice of activities of daily life could contribute materially to maintenance of activities of daily life in patients early in the progression of AD by relying on implicit learning to establish daily habits. However, it has also been reported that higher-order associations among stimulus elements may be learned more slowly by amnesic patients (Curran, 1997). In addition, findings of intact learning rely on a non-significant difference between conditions that may mask a subtle learning deficit (Shanks & St. John, 1994). One of the challenges of using the SRT task to assess sequence learning is that reliable learning, even in healthy participants, often produces a very modest change in RT scores (10-20ms). Another is the tendency for healthy participants to acquire some explicit knowledge of the repeating sequence (e.g., Willingham, Greely, & Bardone, 1989) that may occasionally contribute to better performance and make patient group comparisons difficult. For example, Ferraro, Balota, and Connor (1993) found impaired learning in early AD; however, their experiment used a paradigm that can lead to explicit sequence knowledge, making it difficult to tell if the higher learning scores in non-memory impaired control participants was due to a contribution from explicit memory.
Learning during the SRT task has also been studied extensively with patients with Parkinson's Disease (PD) to test the hypothesis that their learning is impaired due to PD-associated dysfunction in the basal ganglia. The overall pattern across studies is that patients with PD are impaired at sequence learning (see Siegert et al., 2006 for a meta-analysis of a number of related studies), although there is some variability in the findings. The specific motor demands of the task may affect the success of sequence learning. For example, Smith et al. (2001) reported unimpaired learning in patients with PD when the response was made verbally and Werheid et al. (2003) reported unimpaired learning when the associated motor responses were not spatially organized to be compatible with cues. However, Price & Shin (2009) reported sequence learning impairments for spatial patterns incidental to response location even though spatial attention was not impaired. A consistent challenge in this research is that the motor impairments typically found in PD lead to very slow and variable RTs, potentially making it difficult to estimate the magnitude of any learning effects with precision. Even in cases of apparently unimpaired learning, slow and variable responses may lead to non-reliable differences between groups that mask a potential impairment. More significant impairments are frequently seen later in disease progression (Stephan et al., 2011; Siegert et al., 2006) which may indicate that sequence learning deficits become more prevalent and more easily detected in more impaired patients, although concerns about basic motor performance also become more important for these patients.
To examine sequence learning while controlling for differences in overall level of motor control, we assessed sequence-specific performance benefits using the Serial Interception Sequence Learning (SISL) task. The SISL task requires a precisely timed response rather than a maximally rapid one and the task can be adapted in difficulty to suit individual participants. Learning during SISL practice depends relatively selectively on implicit learning and is often detectable in individual participants, not just group averages (Sanchez, Gobel, & Reber, 2010). After practice with SISL, task performance becomes increasingly fluid and efficient, which is associated with reductions in evoked activity in cortical performance networks and may depend on dopaminergic function in the ventral striatum (Gobel, Parrish, & Reber, 2011), suggesting that learning in the SISL task depends on corticostriatal circuits. Learning by patients with PD, who have basal ganglia dysfunction, was compared with learning by patients with amnestic Mild Cognitive Impairment (MCI), who have impaired explicit memory presumed to be due to AD neurpathologic changes (Albert et al. 2011) predominantly in the MTL. Although their explicit memory is impaired, amnestic MCI patients do not exhibit generalized cognitive deficits that might interfere with understanding task instructions or goals during training. While their memory deficits are not as severe as patients with probable Alzheimer's disease, implicit sequence learning with a relatively rapidly moving task like the SISL has not been previously examined in patients with MTL dysfunction. In addition, the covertly embedded 12-item repeating sequence used here requires learning of higher-order associations among stimulus elements, which could provide a challenge to these patients. To make the task as accessible as possible for the patients with PD, the SISL task was modified so that responses were made with the index, middle and ring fingers of the participant's preferred hand to allow patients with PD to use the hand with the least expression of motor symptoms. Based on our hypothesis that implicit sequence learning depends on cortico-striatal circuits that are impaired in PD, we predicted that the patients with MCI would exhibit intact sequence learning while patients with PD would exhibit impaired sequence learning.
Method
Participants
Twenty cognitively healthy older adults (14 women, 6 men) and 13 patients (8 women, 5 men) with a diagnosis of probable amnestic Mild Cognitive Impairment (MCI) were recruited from the Cognitive Neurology and Alzheimer's Disease Center at Northwestern University. All participants are engaged in longitudinal research involving annual cognitive assessments. Healthy controls (HC) all scored within normal range on the UDS neuropsychological battery (Weintraub et al. 2009) supplemented with more extensive neuropsychological testing. The diagnosis of amnestic MCI followed current guidelines (Petersen 2004) and required a clinical dementia rating of 0 or 0.5 and no deficits in Activities of Daily Living according to the Functional Assessment Questionnaire (FAQ; Pfeffer, et al., 1982). Classification of the amnestic subtype without any other cognitive impairment indicated a score of 1.5 standard deviations or more below age, gender and education corrected norms on one of more tests of declarative memory (not every memory test is given to every patient and Table 1 reports commonly available scores). Two MCI patients were subsequently excluded for exhibiting signs of motor dysfunction (one had a UPDRS score of 35 and the other had significant difficulties with the motor task elements). Seventeen patients diagnosed with mild to moderate Parkinson's disease (6 women, 11 men; Hoehn-Yahr stages 2.5 or less) were recruited from the Movement Disorders Clinic at Northwestern University. Neuropsychological evaluation beyond diagnostic criteria (UPDRS, Hoehn & Yahr) was not available for these patients. Clinic patients are only referred for neuropsychological assessment if there is evidence of cognitive impairment and none of the participating patients were judged to need this assessment by the referring clinicians. All patients with PD were on regular anti-Parkinsonian medications and tested in an ON state. Two PD patients were unable to complete the training task and were excluded from further analysis. The study was approved by the Institutional Review Board at Northwestern University. Written informed consent was obtained from all participants. Participants were paid $10/hour for their participation.
Table 1. Demographic and Neuropsychological Data for Included Participants.
| Test | Healthy older adults Mean (SD) [Range] |
Patients with MCI Mean (SD) [Range] |
Patients with PD Mean (SD) [Range] |
|---|---|---|---|
| Age | 71.2 (5.7) [61-86] | 76.0 (8.1) [62-91] | 63.3 (5.7) b [55-74] |
| Years of Education | 16.5 (1.9) [12-20] | 16.1 (3.0) [12-20] | 15.9 (2.5) [12-19] |
| MMSE | 29.1 (0.9) [28-30] | 28.3 (2.2) [23-30] | n/a |
| WMS-R Delayed Recall | 16.0 (3.9) [10-22] | 8.25 (3.8) a [4-18] | n/a |
| UPDRS | 0.9 (1.8) [0-7] | 2.6 (4.2) [0-11] | 33.2 (12.6) b [13.5-51] |
Indicates a significant difference (p < .05) between healthy older adults and the patients with MCI.
Indicates a significant difference (p < .05) between patients with PD and the other two groups.
Age, education, and relevant neuropsychological characteristics of included participants are described for all three participant groups in Table 1. Scores on MMSE and WMS-R delayed memory were not available for the PD patient group and for one healthy older adult. UPDRS scores were not available for three of the PD patients and one healthy older adult.
Materials
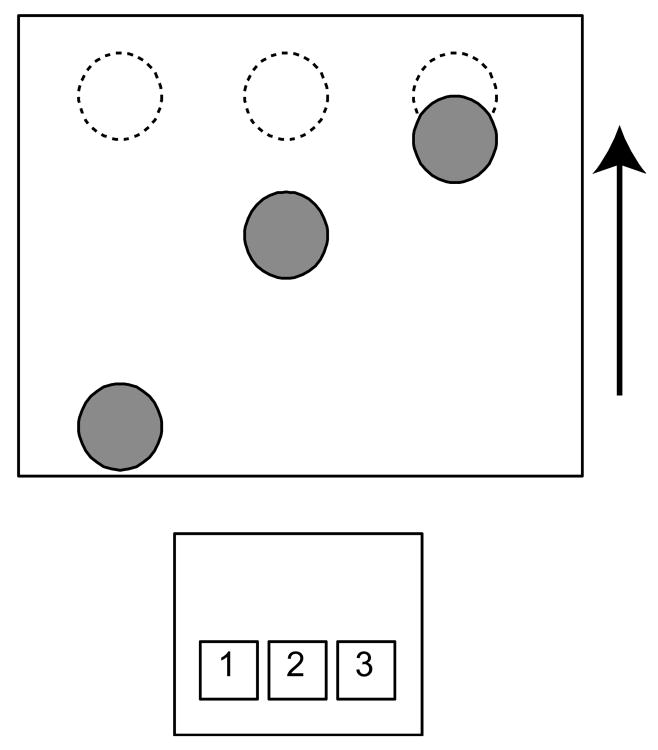
The Serial Interception Sequence Learning (SISL) task requires participants to make a precisely timed motor response to coincide with a cue (circle) moving through a target zone at the top of a computer screen (Figure 1). The SISL task was adapted from prior research (Sanchez et al., 2010) and simplified to use only three keys corresponding to three columns in which the cues move vertically upwards. All participants viewed the task via laptop computer screen and made responses using the index, middle and ring fingers of their preferred hand and a wireless keypad placed comfortably and such that the 1, 2 and 3 keys were spatially compatible with the left, middle and right columns, respectively. As can be seen in Figure 1, multiple cues appear on the screen simultaneously and participants responded to the highest cue, timing the key press as the cue moved through the target zone. The height of the cues and the target zone was 1.96 cm and cues moved 11.8 cm from first appearance to the target zone.
Figure 1.
The Serial Interception Sequence Learning (SISL) task. Cues (filled circles) move vertically up the screen and the participant is asked to make a precisely timed motor response to coincide with the cue crossing the target region (dashed circles at the top of the screen). Responses are made with the participant's preferred hand on a comfortably placed wireless keypad using the keys 1, 2, and 3. In the figure, the participant would be making the response ‘3’ to coincide with the rightmost cue and planning to make responses ‘2’ and then ‘1’ as the next cues moved up the screen. The speed of the task is adaptively adjusted for each individual to keep participants performing between 75% and 92% correct during training.
The speed of the moving cues was initially set during a pre-training assessment consisting of a single moving cue, then two and three cues in succession. On the first attempt, the cue moved across the screen at a rate of 7.86 cm/sec (1.5 s travel time) and the speed was adjusted (by the experimenter) until the participant could perform the initial practice successfully. During training, the cue speed continued to be adaptively adjusted to keep performance in the range of 75% - 92% correct. After every 12 trials, if the participant made correct and well-timed responses to all 12 cues (100% correct), the speed was increased by 5%. If the participant made more than 3 errors (<75% correct) the speed was reduced by 5%. The speed remained constant if 1 to 3 errors were made.
Participants were not told that the cues followed a repeating sequence of 12 items on 80% of the training trials. The embedded repeating sequence contained each of the three cue locations four times each without successive locations repeating and was constructed as to require identification of third-order conditionals to predict the next item in the sequence, e.g., 3-2-1-2-1-3-1-2-3-2-3-1 (each possible trigram sub-sequence occurs once within the sequence). In addition, the sequence had a consistent timing structure with half the inter-cue intervals being short (initially 350ms) and the other half being longer (initially 700ms). The inter-cue intervals were adjusted consistently with the change in overall speed but the ratio was maintained, providing the appearance of a slightly slowing or speeding player piano scroll. Each participant was given a randomly selected repeating sequence from a set of 120 possible sequences. Four additional sequences were set aside to be used as foils for a post-training recognition test. The remaining sequences that shared neither the order nor timing structure were introduced as the 20% non-repeating trials during training. In every block of 60 trials during training, the target sequence would repeat four times (48 trials) and a novel 12-item sequence would be randomly inserted in between repetitions. Non-repeating segments were chosen to avoid repeats in location of consecutive cues and the starting location of the target sequence was randomly selected after each novel sequence. The addition of these non-repeating segments and random starting locations during training reduces the amount of explicit sequence knowledge acquired by participants during practice.
Procedure
Participants, who were not informed of the existence of any sequential structure, first completed a training phase of the task consisting of 1440 trials (96 repetitions of the sequence and 24 novel non-repeating segments) taking approximately 30 minutes. After every 360 trials, participants were given a short self-timed break to reduce fatigue. After the fourth break (after 1440 trials), participants completed an implicit test of sequence knowledge. No information about the change in task structure was provided. During the implicit test, participants completed twelve 60-item blocks in which four blocks contained repetitions of the trained repeating sequence (no non-repeating segments) and the other eight blocks contained repetitions of two of the novel foil sequences (four blocks each). No adaptive adjustments to speed were made during the test. The key measure of implicit sequence learning was the difference in overall percentage of correct responses during performance of the trained and foil sequences during this test.
Following the implicit test, the participants completed two tests of explicit sequence knowledge. Participants were told that a repeating sequence was present during the majority of the task they had just completed and were then shown five sequences in a recognition test that contained the trained sequence and four foils (including the two used as foils during the implicit sequence test). Each sequence was shown in full and the participant then rated on a scale from 1 to 10 whether they thought it was the trained sequence. This was followed by a free recall test in which the participants attempted to produce the trained sequence by pressing the keypad keys to the best of their ability. Recognition performance was measured as the difference in rating given to the trained sequence and the average of the four foils. The sequence produced during the free recall test was compared to the trained sequence and foils and the longest matching sub-sequence was identified. Successful recall would lead to producing a sequence that matched the trained sequence better than the foils.
Results
The primary measure of sequence-specific learning was the difference in performance between the trained sequence and foils on the implicit test following training. During the test, two healthy older adults and two patients with PD exhibited unusually low levels of overall performance. The patients with PD will be considered separately below due to being atypical in performance on a number of measures. The two healthy controls were eliminated from further analysis since their task performance level of less than 60% correct indicated either fatigue or non-compliance with task instructions1.
Performance on the implicit learning test is shown in Figure 2A for the three groups of participants. The healthy older adults and the patients with MCI both exhibited reliably higher rates of performance during the test when the cues followed the trained sequence than during the blocks containing the unpracticed foil sequences. The healthy older adults exhibited a significant mean sequence-specific performance advantage for the trained sequence of 4.8% correct (SE = 2.1%), t(17) = 2.32, p = .03. The patients with MCI exhibited a significant mean sequence-specific advantage of 7.5% (SE = 1.6%), t(10) = 4.68, p < .001. In contrast, while the majority of the patients with PD were able to perform the task in the target range, these patients exhibited no advantage during blocks in which the cues followed the practiced sequence compared with the foils. The average sequence-specific performance advantage for the patients with PD was 1.2% (SE = 1.4%), t(12) = 0.91.
Figure 2.
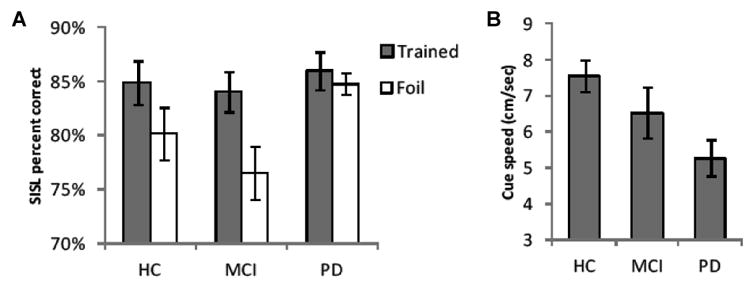
(A) Performance during the implicit test of sequence knowledge following training. The task speed was no longer adjusted adaptively but maintained at the same speed at which participants had been performing between 75% and 92% correct at the end of training. The test contained randomly ordered blocks of the trained sequence and two repeating but unfamiliar foil sequences. SISL task performance during the foils dropped significantly for health older adults (HC) and patients with MCI, reflecting the fact that they had acquired sequence-specific knowledge of the trained sequence. In contrast, the patients with PD performed at a high and equal rate for both the trained and foil sequences. (B) Cue speed at the end of training and during the implicit test. In order to perform the task in the target range, patients with PD required the cues to be moving at a slower rate than the healthy older adults, reflecting their motor impairment. The patients with MCI required an intermediate speed to achieve the target performance rate.
While the patients with PD did not exhibit significant knowledge of the trained sequence, the sequence-specific performance advantage only marginally differed across the three groups, F(2,39) = 2.5, p < .10. However, across the 39 participants with UPDRS measures, the individual learning score was found to correlate reliably with UPDRS score, r = -.36, p = .02, indicating that sequence-specific learning was impaired as evidence of PD symptoms increased. In addition, a 2×2 ANOVA restricted to just the MCI and PD groups (sequence type X group) found a reliable interaction, F(1,22)=9.04, p<.01, η2 = .29, reflecting a smaller sequence performance difference for the patients with PD (i.e., no advantage for the trained sequence) compared to the patients with MCI. The fact that the contrast between the two patient groups without the healthy control group shows a more robust dissociation suggests that there was more variability in sequence learning in the healthy controls than in the patients with MCI.
The adaptive administration of the SISL task means that the cue speed was adjusted to keep performance during the repeating sequence at around 83% correct. For this reason, on the test when the foil sequences are introduced, the sequence-specific learning advantage will be expressed as a decrease in the percentage of correct responses (i.e., an increase in errors). This has the advantage of controlling for task performance characteristics so that learning can be assessed. General task performance level is shown by the cue speed that was used to produce task performance in the 75%-92% range at the end of training and during the implicit test (Figure 2B) and varied reliably across groups F(2,39) = 4.48, p < .02. Post-hoc group comparisons indicated that patients with PD required the cues to be moving at a slower rate than the healthy older adults in order to perform the SISL task, t(29)=3.35, p=.01.
On the explicit sequence knowledge tests following the implicit test, all groups exhibited very little recognition or recall of the trained sequence. For the recognition test, sequence knowledge was measured as the difference between the rating (1-10) given to the trained sequence and the four untrained foils. The healthy older adults had an average recognition score of -0.68 (SE = 0.6), indicating very slightly higher ratings (but not significantly) of familiarity given to the foils over the trained sequence. The patients with MCI had average recognition scores of -0.09 (SE = 0.7), likewise exhibiting essentially no recognition of the sequence. The patients with PD had an average recognition score of 0.44 (SE = 0.41) and similarly exhibited no reliable evidence of sequence recognition, t(10) = 1.08, p = .30. Given the lack of recognition on this relatively sensitive test, it was not surprising that the sequences produced during the attempt to explicitly recall the sequence tended to match the targets as well as the foils (M = 5.5 and 5.6 items, respectively) with no hint of a difference between groups, F(2,39) = 0.25.
Atypical Patients With PD
Two of the patients with PD exhibited a pattern of performance on the implicit and explicit tests that was clearly different from the rest of the group. Both of these patients exhibited extremely low performance during the foil blocks on the implicit test that was due to high rates of non-responding to cues (23% and 12% missed trials compared to the average rate of missing trials of 4.2%). In addition, these patients had two of the highest recognition scores in this group, rating the target sequence at 8 and 10 (of 10) in familiarity and the foils at an average of 3.75 and 6.25 thus giving them the second and third highest scores among all participants. These two patients were also performing the task at the fastest speed among all the patients with PD (9.2 and 9.0 cm/sec, compared to an average of 5.3 cm/sec for all others). Low task performance at test is often a signal of non-compliance or fatigue (e.g., in healthy young participants), but for these patients it appears to reflect the adoption of an explicit strategy to perform the SISL task that led to effective performance for the repeating sequence but led to very poor performance during the blocks that did not contain the repeating sequence (2/3 of the implicit test). Because of their knowledge of the trained sequence, they may have made less effort to respond when the cue sequence changed or had difficulty keeping up with the rapidly moving task (which was quite rapid because the speed had been adjusted to match their good performance during the trained sequence) due to the fact that the cues did not follow the expected sequence. Of note, this type of performance was not observed to occur with any of the amnestic MCI patients who showed consistent responding and a consistent small performance advantage for the trained sequence. These two patients were not obviously different from the other patients with PD by available demographical or diagnostic data (age: 65, 67; disease duration 5, 10 years; UPDRS 28, n/a; Hoehn & Yahr stage: 2.0, 2.0).
Discussion
Healthy older adults and patients with MCI exhibited similarly robust levels of learning of the embedded repeating sequence during SISL task performance. This finding reinforces previous findings of intact perceptual-motor sequence learning in patients with memory impairments associated with damage to the MTL using the SRT task (Nissen & Bullemer, 1987; Reber & Squire, 1994; Reber & Squire, 1998). The current results extend this finding to the SISL task, which requires relatively rapid and precisely timed responses to moving cues. In this study, learning the sequential structure of the responses required learning higher-order associations and patients with MCI showed no evidence of a deficit in high-order associations (in contrast to Curran, 1997). The SISL task produces relatively more robust learning in individual participants and low levels of explicit sequence knowledge (Sanchez et al., 2010) likely due to the need to continuously attend to and prepare for responding to the next cue. The relative absence of explicit knowledge in the healthy older adults may further support the finding that the patients with MCI exhibited no hint of impairment and in fact, exhibited numerically superior sequence-specific learning. While the memory disruption associated with MCI is relatively milder than the dense anterograde amnesia observed in probable AD or following MTL damage, the patients with MCI tested here were quite significantly impaired on standard assessments of memory (WMS-R delayed story recall) with no apparent effect on their implicit learning.
The learning performance of patients with PD was more complex. The lack of sequence-specific learning exhibited by the majority of the patients with PD supports our hypothesis that implicit perceptual-motor sequence learning depends on corticostriatal circuits between the basal ganglia and motor cortical regions. Our hypothesized basis of sequence learning is consistent with other findings (e.g., Wilkinson et al. 2009) but it should be noted that we cannot rule out the possibility of another element of cognitive dysfunction in the patients here due to the absence of a comprehensive neuropsychological screening battery. Goldman & Litvan (2011) have reported fairly high rates of MCI in patients with PD and although our amnestic MCI patients exhibited strong learning, it is possible the patients with PD had an undetected cognitive deficit affecting learning. It is clearly the case that dysfunction associated with PD affects sequence learning, while amnestic MCI does not, but the precise neural basis of the deficit observed in PD may depend on a better understanding of exactly how the progression of PD affects cognition.
In general, patients with PD have been previously found to be impaired when sequence learning was assessed with the SRT task (Siegert et al., 2006). It is notable that the variability in performance meant that this deficit is most clearly seen in a meta-analysis across six studies and in particular when disease severity is considered. We found that the degree of impairment was correlated with UPDRS measures, consistent with other analyses of this type (Stephan et al. 2011). One of the challenges of assessing sequence learning with the SRT task in patients with PD is that the overall slowing in reaction time by these patients produces high variability in estimates of performance and learning. This variability can make it difficult to statistically detect learning impairments. Here, by using the SISL task, which does not depend on variable RT measures, we observed good task performance and a general lack of sequence-specific learning in patients who had early stage and fairly mild PD. By individually adapting the speed of the task, the overall task performance of the patients with PD was brought into the same range as the healthy controls and patients with MCI, so that sequence-specific learning could be directly compared across groups. However, the performance of the two atypical patients indicates that it cannot simply be concluded that patients with PD are always impaired at all forms of sequence learning. These patients acquired some sequence knowledge and while the interpretation of their performance is necessarily speculative, they appear to have succeeded at the task by relying on a more explicit approach. These patients may have been able to guide their sequential motor responses explicitly, or found a strategy by which their explicit memory could support implicit learning allowing them to compensate for an implicit learning deficit.
Compensatory explicit learning in patients with PD has been reported previously in studies of probabilistic classification learning. In that task, patients with PD were initially reported to be impaired at early learning but not late learning (Knowlton, Mangels, & Squire, 1996), apparently because they became able to employ explicit strategies to support task performance. Subsequent findings reported evidence of learning in some patients with PD and a neuroimaging study suggested that patients with PD succeeded at learning by relying more on their intact MTL memory system (Moody, Bookheimer, Vanek, & Knowlton, 2004). A similar effect may occur in some sequence learning tasks as well. Carbon et al. (2010) assessed changes in neural activity longitudinally in patients with PD and found that patients who achieved good learning performance tended to exhibit increased activity in MTL regions that appeared to compensate for reductions in evoked activity in parietal and temporo-occipital areas.
In the current study, the majority of patients with PD did not exhibit evidence of explicit sequence knowledge via some compensatory mechanism. Wilkinson, Shah, and Jahanshahi (2009) found impaired sequence learning in both implicit and explicit conditions and it may be that spontaneous use of explicit strategies only occurs for some patients with PD. The patients with PD required the SISL task to be administered at a slower rate than healthy controls, but at this rate were still able to perform the task at around 85% correct. However, no advantage for the trained sequence was seen, suggesting that the disruption in basal ganglia function associated with dopaminergic cell loss in PD led to a significant impairment in sequence learning. While a number of studies have also found impaired sequence learning in patients with PD (Siegert et al., 2006), Price and Shin (2009) found unimpaired sequence learning in mild PD. While the lack of a difference between patients and controls might reflect variability in responses, Muslimovic, Post, Speelman and Schmand (2007) also found that early-stage PD patients were not reliably impaired at sequence learning: never-medicated patients with PD exhibited no evidence of impairment and performed with a normal range of reaction times. While this could be due to disease severity (milder in these patients), this finding raises the intriguing possibility that the sequence-specific learning impairment could be related to dopamine replacement therapy. Impairments observed in sequence learning for patients with PD here should not be due to the absence of systemic dopamine, as all the patients were optimally medicated with dopamine replacement or agonist therapy. However, increases in basal dopamine levels via medication may not allow for the proper phasic dopaminergic signaling that we believe to be critical for implicit perceptual-motor sequence learning.
Conclusion
Healthy older adults and patients with explicit memory impairments associated with MCI exhibited similarly robust levels of sequence-specific learning in the SISL implicit perceptual-motor skill learning task. This result extends the previous findings of intact implicit perceptual-motor sequence learning in memory-disordered patients to tasks requiring the sequencing of precisely timed responses to a moving cue. In addition, the statistical structure of the repeating sequence required higher-order associations to be learned among sequence elements but this did not impair the learning in patients with MCI. In contrast, the majority of patients with PD exhibited no sequence-specific learning although they were able to perform the task at the same overall accuracy level in general (with a slower overall task speed). The lack of learning by patients with PD supports the hypothesis that this form of implicit learning depends critically on intact function in corticostriatal circuits between the basal ganglia and perceptual and motor planning areas of cortex. Two of the patients with PD exhibited an atypical behavioral profile that was suggestive of a strategy based on using explicit sequence knowledge to compensate for their impaired implicit learning. The ability to clearly dissociate implicit and explicit learning in the patient groups is facilitated by the SISL task, which leads to robust implicit learning in most cognitively healthy and memory-disordered individuals and high general levels of performance due to the individually adjusted task difficulty. Intact implicit learning in memory-disordered patients together with impaired sequence-specific learning in patients with PD supports the hypothesis that perceptual-motor skill learning depends materially on intact function in the basal ganglia but not on the medial temporal lobe.
Acknowledgments
Thanks to Alejandra K. Balen and Mallory R Swift at the CNADC for help with data collection with the healthy older adults and patients with MCI and Karen Williams at the Movement Disorders Clinic for help with data collection for the patients with PD. The authors would also like to thank Column MacKinnon, Daniel J. Sanchez, and Kathryn L. Gigler for feedback and advice on the project and manuscript. This research was supported by a pilot project award (PJR) funded by P30 AG13854-16 and, in part, directly by the Alzheimer's Disease Core Center grant (AG-13854) to Northwestern University.
Footnotes
Both of these participants performed particularly poorly on the test blocks overall but actually exhibited greater apparent sequence learning than the average for the group. However the particularly low performance rates suggests that this estimate of sequence learning may not be reliable.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's and Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Salmon DP, Bernstein N, Martone M, Lyon L, Butters N. Procedural learning in a patient with amnesia due to hypoxia. Brain and Cognition. 1987;6:386–402. doi: 10.1016/0278-2626(87)90135-7. [DOI] [PubMed] [Google Scholar]
- Carbom M, Reetz J, Chilardi MF, Dawan V, Eidelberg D. Early Parkinson's disease: longitudinal changes in brain activity during sequence learning. Neurobiology of Disease. 2010;37:455–460. doi: 10.1016/j.nbd.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S. Acquisition of motor skill after bilateral medial temporal-lobe excision. Neuropsychologia. 1968;6:255–265. [Google Scholar]
- Curran T. Higher-order associative learning in amnesia: Evidence from the serial reaction time task. Journal of Cognitive Neuroscience. 1997;9:522–533. doi: 10.1162/jocn.1997.9.4.522. [DOI] [PubMed] [Google Scholar]
- Gobel EW, Parrish TB, Reber PJ. Neural correlates of skill acquisition: Decreased cortical activity during a serial interception sequence learning task. NeuroImage. 2011;58:1150–1157. doi: 10.1016/j.neuroimage.2011.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel EW, Sanchez DJ, Reber PJ. Integration of temporal and ordinal information during serial interception sequence learning. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2011;37:994–1000. doi: 10.1037/a0022959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JG, Litvan I. Mild cognitive impairment in Parkinson's disease. Minerva medicine. 2011;102:441–459. [PMC free article] [PubMed] [Google Scholar]
- Ferraro FR, Balota DA, Connor LT. Implicit memory and the formation of new associations in nondemented Parkinson's disease individuals and individuals with senile dementia of the Alzheimer type: A serial reaction time (SRT) investigation. Brain and Cognition. 1993;21:163–180. doi: 10.1006/brcg.1993.1013. [DOI] [PubMed] [Google Scholar]
- Knopman D, Nissen MJ. Implicit learning in patients with probable Alzheimer's Disease. Neurology. 1987;37:784–788. doi: 10.1212/wnl.37.5.784. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Milner B, Corkin S, Teuber HL. Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of H.M. Neuropsychologia. 1968;6:215–234. [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Moody TD, Bookheimer SY, Vanek Z, Knowlton BJ. An implicit learning task activates medial temporal lobe in patients with Parkinson's disease. Behavioral Neuroscience. 2004;118:438–442. doi: 10.1037/0735-7044.118.2.438. [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B. Motor procedural learning in Parkinson's disease. Brain. 2007;130:2887–2897. doi: 10.1093/brain/awm211. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. The Journal of Gerontology. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Price A, Shin JC. The impact of Parkinson's disease on sequence learning: Perceptual pattern learning and executive function. Brain and Cognition. 2009;69:252–261. doi: 10.1016/j.bandc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Parallel brain systems for learning with and without awareness. Learning & Memory. 1994;1:217–229. [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Encapsulation of implicit and explicit memory in sequence learning. Journal of Cognitive Neuroscience. 1998;10:248–263. doi: 10.1162/089892998562681. [DOI] [PubMed] [Google Scholar]
- Sanchez DJ, Gobel EW, Reber PJ. Performing the unexplainable: Implicit task performance reveals individually reliable sequence learning without explicit knowledge. Psychonomic Bulletin & Review. 2010;17:790–796. doi: 10.3758/PBR.17.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks DR, John MF., St Characteristics of dissociable human learning systems. Behavioral and Brain Sciences. 1994;17:367–447. [Google Scholar]
- Siegert RJ, Taylor KD, Weatherall M, Abernethy DA. Is implicit sequence learning impaired in Parkinson's disease's A meta-analysis. Neuropsychology. 2006;20:490–495. doi: 10.1037/0894-4105.20.4.490. [DOI] [PubMed] [Google Scholar]
- Smith J, Siegert RJ, McDowall J, Abernethy D. Preserved implicit learning on both the serial reaction time task and artificial grammar in patients with Parkinson's Disease. Brain and Cognition. 2001;45:378–391. doi: 10.1006/brcg.2001.1286. [DOI] [PubMed] [Google Scholar]
- Stephan M, Meier B, Zaugg SW, Kaelin-Lang A. Motor sequence learning performance in Parkinson's disease patients depends on the stage of the disease. Brain and Cognition. 2011;75:135–140. doi: 10.1016/j.bandc.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer's disease centers’ Uniform Data Set (UDS): The neuropsychological test battery. Alzheimer's Disease and Associated Disorders. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werheid K, Ziessler M, Natkemper D, von Cramon DY. Sequence learning in Parkinson's disease: The effect of spatial stimulus-response compatibility. Brain and Cognition. 2003;52:239–249. doi: 10.1016/s0278-2626(03)00076-9. [DOI] [PubMed] [Google Scholar]
- Wilkinson, et al. 2009 [Google Scholar]
- Willingham DB, Greely T, Bardone AM. Dissociation in a serial response time task using a recognition measure: comment on Perruchet and Amorim (1992) Journal of Experimental Psychology: Learning, Memory and Cognition. 1989;19:1424–1430. [Google Scholar]