Abstract
Skeletal muscle from sedentary obese patients is characterized by depressed electron transport activity, reduced expression of genes required for oxidative metabolism, altered mitochondrial morphology and lower overall mitochondrial content. These findings imply that obesity, or more likely the metabolic imbalance that causes obesity, leads to a progressive decline in mitochondrial function, eventually culminating in mitochondrial dissolution or mitoptosis. A decrease in the sensitivity of skeletal muscle to insulin represents one of the earliest maladies associated with high dietary fat intake and weight gain. Considerable evidence has accumulated to suggest that the cytosolic ectopic accumulation of fatty acid metabolites, including diacylglycerol and ceramides, underlies the development of insulin resistance in skeletal muscle. However, an alternative mechanism has recently been evolving, which places the etiology of insulin resistance in the context of cellular/mitochondrial bioenergetics and redox systems biology. Overnutrition, particularly from high-fat diets, generates fuel overload within the mitochondria, resulting in the accumulation of partially oxidized acylcarnitines, increased mitochondrial hydrogen peroxide (H2O2) emission and a shift to a more oxidized intracellular redox environment. Blocking H2O2 emission prevents the shift in redox environment and preserves insulin sensitivity, providing evidence that the mitochondrial respiratory system is able to sense and respond to cellular metabolic imbalance. Mitochondrial H2O2 emission is a major regulator of protein redox state, as well as the overall cellular redox environment, raising the intriguing possibility that elevated H2O2 emission from nutrient overload may represent the underlying basis for the development of insulin resistance due to disruption of normal redox control mechanisms regulating protein function, including the insulin signaling and glucose transport processes.
Keywords: mitochondria, intramyocellular fat, inflammation, H2O2 emission, redox environment, hexokinase
Introduction
Deciphering the underlying mechanism(s) responsible for the development of insulin resistance in peripheral tissues is one of the cornerstones to understanding the etiology of type 2 diabetes. A gradual decrease in the sensitivity of skeletal muscle to insulin is considered a primary event in the disease process and, as such, likely holds the key to devising more effective prevention and treatment strategies. By virtue of its high percentage of total body mass and sensitivity to insulin, skeletal muscle accounts for the vast majority (∼80%) of glucose disposal.1 The control of glucose uptake is distributed across delivery, transport and phosphorylation, any one of which may be rate limiting depending on the physiological circumstances.2 Insulin increases glucose delivery via relaxation of resistance vessels to increase total blood flow and relaxation of precapillary arterioles to increase microvascular surface area perfusion within muscle, thereby increasing the trans-endothelial transport of insulin and glucose.3 Although there is some evidence that insulin resistance induced by a high-fat diet compromises glucose delivery,4 more research is needed to fully define this potential mechanism of action. The glucose transport process adds further potential control points beginning with insulin binding to and activating its receptor, and progressing through activation of downstream intracellular signaling events leading to the translocation (that is, budding, transport, tethering, docking, fusion and endocytosis) and activation of the GLUT4 transporter protein.5, 6 There is compelling evidence that phosphorylation of glucose, the third potential control point, is the rate-limiting step for glucose uptake in response to insulin or exercise in skeletal muscle with normal insulin sensitivity;7, 8 however, the functional barrier appears to shift to transport in the insulin-resistant state.7, 9, 10 GLUT4 translocation process does not appear to be defective in insulin-resistant muscle, as both contraction and hypoxia, which utilize a signaling pathway different from insulin, stimulate GLUT4 translocation and glucose uptake normally.11, 12 Thus, most of the research since the mid-1990s has focused on deciphering the mechanism(s) by which the insulin signaling pathway is inhibited by a high-fat diet. The present review provides a brief overview of this research and then presents an alternative hypothesis to link mitochondrial H2O2 emission with insulin resistance during nutrient overload.
Intramyocellular fat accumulation
Triacylglycerol
Skeletal muscle of obese and diabetic individuals is characterized by a greater size and number of lipid droplets.13 Once assumed to be relatively inert, lipid droplets are now known to be coated with phospholipids and a variety of proteins, to exist in a regulated equilibrium of triglyceride synthesis and degradation and to participate in other cellular processes including vesicle trafficking and cell signaling.14, 15, 16 One possibility is that fat deposition in tissues not specialized for fat storage can promote lipotoxicity.17 Numerous initial studies in both humans and rodents reported a strong correlation between intramuscular triglyceride content and insulin resistance.18, 19, 20, 21, 22, 23 However, endurance-trained athletes also have high intramuscular triglyceride content, as well as high insulin sensitivity,24 indicating that triglyceride accumulation per se is not an underlying cause of insulin resistance. In fact, mice with skeletal muscle-specific overexpression of diacylglycerol acyltransferase (DGAT1), the final enzyme in the TAG synthesis pathway, are protected from high-fat diet-induced insulin resistance despite higher muscle TAG content.25 Nevertheless, given the dynamic interplay between triglyceride synthesis and storage, it has recently been suggested that a mismatch between these processes could give rise to potential insulin-desensitizing lipid intermediates.14, 15
Diacylglycerol (DAG) and long-chain acyl-coAs (LCACoAs)
Further clues as to the mechanism by which fat accumulation in muscle may lead to insulin resistance have come from studies of the signaling pathway acting downstream of the insulin receptor. Under normal circumstances, activation of the insulin receptor tyrosine kinase and subsequent tyrosine phosphorylation of the insulin receptor substrate (IRS) docking proteins leads to the recruitment and activation of phosphoinositide 3-kinase (PI3K). The major substrate for PI3K is the membrane lipid phosphatidylinositol-4,5-bisphosphate (PI(4,5,)P2), which is phosphorylated to produce phosphatidylinositol-3,4,5-trisphosphate (PI(3,4,5,)P3). The rise in PI(3,4,5,)P3 provides a lipid-based platform that attracts downstream pleckstrin homology domain-containing signaling proteins, including the serine/threonine kinases phosphoinositide-dependent protein kinase-1 (PDK1) and Akt.6 PDK1 phosphorylates Akt and atypical protein kinase C isoforms λ and ζ (aPKC-λ/ζ). Both Akt and aPKC-λ/ζ have been linked to insulin-stimulated GLUT4 translocation and glucose uptake.
In addition to tyrosine residues, phosphorylation of IRS proteins on serine residues has emerged as a major control point for the insulin signaling pathway. Of the ∼70 serine residues at potential consensus phosphorylation sites, more than 20 have been identified by proteomics to be phosphorylated by insulin,26 and more than a dozen of these sites are subject to reversible phosphorylation by at least 16 different kinases, including 2 of the novel PKC isoforms (PKC δ and θ).27 Given that both LCACoAs and DAG are well-established activators of PKCs,28 much attention has been given to the possibility that novel PKCs may mediate the serine phosphorylation and inactivation of IRS proteins. LCACoAs and DAGs both accumulate in muscle with lipid infusion or high-fat feeding29 and are associated with membrane translocation and activation of PKC-θ, increased IRS-1 serine phosphorylation and decreased insulin-stimulated IRS-1 tyrosine phosphorylation, IRS-1-associated PI3K activity and muscle glucose uptake.30, 31, 32 In cultured myocytes, expression of active PKC-θ increases phosphorylation of IRS-1 at Ser1101 and blocks insulin-stimulated IRS-1 tyrosine phosphorylation and activation of Akt, whereas mutation of Ser1101 to alanine renders IRS-1 insensitive to PKC-θ and restores downstream insulin signaling.33 Similarly, PKC-θ kinase activity has recently been shown to mediate the serine phosphorylation and inactivation of PDK1 in mouse embryonic fibroblast cells exposed to palmitate.34 Finally, and most compelling, PKC-θ knockout mice were found to be completely protected against lipid infusion-induced disruptions in insulin signaling and muscle glucose uptake.35
Evidence specifically linking PKC-θ to insulin resistance, however, has also been challenged. Mice expressing a kinase-dead, dominant-negative mutant form of PKC-θ specifically in skeletal muscle, rather than being protected from fat-induced insulin resistance, have impaired insulin signaling in muscle by 4 months of age and develop insulin resistance and obesity on a chow diet by 6 to 7 months of age.36 PKC-θ may mediate its effects indirectly via other signaling kinases, such as PDK1, JNK and/or IKK, or may be compensated for by the highly homologous PKCδ.33 Curiously, no studies have been conducted on either PKC-θ knockout or muscle-specific, dominant-negative mice on a high-fat diet, the more physiologically relevant model of insulin resistance, nor have mice expressing a constitutively active or inducible PKC-θ specifically in skeletal muscle been generated.
Sphingosines and ceramides
In addition to LCACoA and DAG, the accumulation of the sphingolipid intermediate ceramide has also been suggested to have a role in lipid-induced insulin resistance.37, 38 Intracellular accumulation of ceramide occurs in response to numerous cellular stressors (for example, cytokines, hypoxia and ROS) via the stress-induced activation of sphingomyelinases/glucosidases and/or the suppression of ceramide clearance.39 High-fat diets and lipid infusion have been shown to increase intracellular ceramide, impair insulin signaling and decrease glucose uptake in skeletal muscle of rodents40 and humans,41 as well as in cultured myotubes.42 Inhibition of de novo ceramide synthesis prevents the development of muscle insulin resistance in response to lipid infusion and in various diet-induced obesity models,43, 44, 45 providing fairly compelling evidence that ceramide accumulation may be a key factor in the etiology of insulin resistance. However, no increase in ceramide content was detected in at least two other studies using lipid infusion to induce insulin resistance in rats or humans,46, 47 and no differences in ceramide levels were found in the skeletal muscle from type 2 diabetic versus healthy individuals.48 In addition, at least one of the inhibitors used to inhibit ceramide (myriocin) has also been shown to increase energy expenditure in treated animals,44 potentially complicating the interpretation of these studies.49 Thus, it appears that further work is required to determine whether ceramide accumulation is necessary and/or sufficient for the induction of diet-induced insulin resistance in skeletal muscle.
Inflammation
Numerous lines of evidence have established a link between elevated systemic, as well as tissue-derived, inflammation with the development of insulin resistance (for review see Heilbronn and Campbell50 and Schenk et al.51). As such, elevations in proinflammatory cytokines (tumor necrosis factor-α interleukin-6) and C-reactive protein have repeatedly been observed in the plasma, as well as within peripheral insulin target tissues (for example, liver, adipose tissue and skeletal muscle), of both animal (high-fat diet) and human (obesity and type 2 diabetes) models of insulin resistance (for review see de Luca and Olefsky52 and Shoelson et al.53). Regardless of the origin/site of inflammation, the effectors of insulin resistance within peripheral tissues in the context of high-fat diet-induced inflammation are believed to involve hyperactivation of stress-sensitive Ser/Thr kinases, such as JNK54 and IKKβ,55 due in large part to increased signaling through the JNK/activator protein 1 (AP1) and IKK/NF-κB pathways. Overactivation of these signaling pathways and their associated Ser/Thr kinases is expected to induce reductions in insulin sensitivity through a similar mechanism as that described previously for intracellular lipid accumulation (for example, inhibitory serine phosphorylation of the insulin receptor:IRS1 axis).27
At present, it would appear that the eventual manifestation of an insulin-resistant phenotype likely results from, or is exacerbated by, the summation of proinflammatory responses that occur systemically,56 as well as within each peripheral tissue bed (adipose,57, 58 skeletal muscle59, 60) as a consequence of high-fat diet exposure. In other words, activation of proinflammatory cascades seems to be a general response within all cells under conditions of nutrient overload. Although the association between inflammation and high-fat diet-mediated insulin resistance appears to be well established, a central question remains: what is the mechanism by which excessive nutrient/lipid supply serves to activate proinflammatory cascades within peripheral tissues? Suggested mechanisms have included the following: (1) microhypoxic conditions within engorged adipocyte depots,61 (2) adipocyte cell death/necrosis,62 (3) endoplasmic reticulum stress63 and (4) activation of cytotoxic T cells following ligation of T-cell receptors via specific fatty acid species.58 An additional potential unifying mechanism involves the induction of intrinsic proinflammatory cascades within peripheral cells as a consequence of elevated H2O2 production.64, 65, 66 That is, activation of the aforementioned proinflammatory pathways (JNK/AP1 and IKK/NF-κB) is also possible within peripheral tissues following an oxidative shift in the cellular redox environment, without any need for macrophage activation/infiltration.67 This later hypothesis is intriguing given that mitochondrial-derived hydrogen peroxide (H2O2), a major regulator of the cellular redox environment, has recently been linked to the etiology of diet-induced insulin resistance.68, 69, 70 To understand the mechanisms by which mitochondrial-derived ROS are believed to interact with and ultimately impair cellular signaling in response to insulin, a brief discussion of the factors governing mitochondrial H2O2 generation is necessary.
Mitochondrial H2O2 emission, the redox environment and insulin resistance
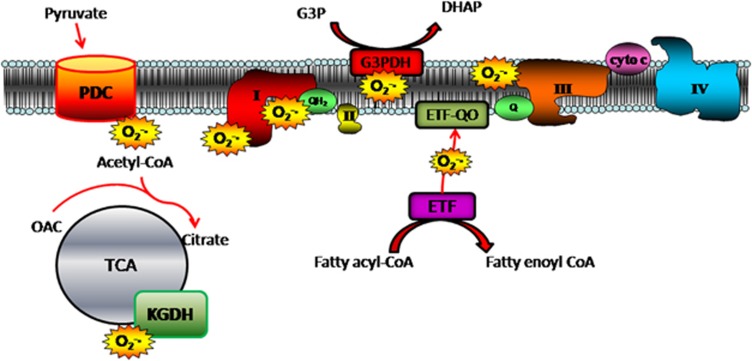
H2O2 is the nonradical dismutation product of superoxide (O2•−), the short-lived parent molecule of all ROS. Although there are numerous sources of H2O2, mitochondrial-derived H2O2 is considered the major source in biological systems.71 Mitochondrial H2O2 emission reflects the balance between the rate of O2•−/H2O2 formation and scavenging within the matrix, with emitted H2O2 serving to regulate the intracellular redox environment in favor of greater reducing (↓ H2O2) or oxidizing (↑ H2O2) conditions.72 Currently, identified sites of electron leak within the respiratory chain include the flavin mononucleotide (site IF) and ubiquinone-binding site (site IQ) within complex I, the quinone at centre ‘o' within complex III (site IIIQo), the quinone-binding site within glycerol-3-phosphate dehydrogenase (G3PDH) and electron-transferring flavoprotein Q oxidoreductase (ETF-QOR).73 Additional nonrespiratory chain sites include two matrix dehydrogenase enzyme complexes, pyruvate dehydrogenase (PDH) and α-ketoglutarate dehydrogenase (KGDH)74 (Figure 1).
Figure 1.
Potential sites of mitochondrial superoxide (O2•−) generation. Mitochondrial-derived O2•− can arise from any one of seven known sites of electron leak, including the flavin mononucleotide- and ubiquinone-binding site within complex I, the quinone at centre ‘o' within complex III, the quinone-binding site within G3PDC, the ETF-QOR complex and the matrix dehydrogenase enzyme complexes PDC and KGDH. Cyto c, cytochrome c; OAC, oxaloacetate; QH2, ubiquinol; Q, ubiquinone.
Regardless of the site, mitochondrial H2O2 is strongly dependent on overall metabolic balance, as maximal rates of electron leak occur under elevated reducing conditions (↑ NAD(P)H/NAD(P)+, QH2/Q), in which mitochondrial membrane potential (Δψ) is highly negative and the overall demand for ATP synthesis is low.75, 76 These criteria are met under conditions that mimic state 4 respiration, where respiring mitochondria are fully saturated with substrate, yet not actively phosphorylating ADP.77 Although it is unlikely that cells in vivo are ever truly engaged in state 4 respiration, near state 4 conditions likely occur during periods of nutrient overload combined with minimal ATP demand (that is, high caloric intake combined with a sedentary lifestyle). These conditions would be expected to elevate the reducing pressure within the respiratory chain, accelerate mitochondrial O2•− generation/H2O2 emission and trigger an oxidative shift in the redox environment. In support of this notion, high dietary fat intake generates an increase in partially oxidized lipid intermediates indicative of mitochondrial overload78 and decreases the GSH/GSSG ratio in muscle, indicative of a shift in the intracellular redox environment to a more oxidized state.68 This appears to be mediated, at least in part, by a remarkable increase in the propensity for mitochondrial H2O2 emission, implying some type of alteration in the governance of H2O2 production/emission in response to the lipid overload.68 Treatment of high-fat-fed rodents with SS31 (mitochondrial-targeted small antioxidant peptide), as well as the transgenic expression of the human catalase gene within muscle mitochondria (mCAT), completely blocked the development of insulin resistance, as well as the associated increase in H2O2-emitting potential and oxidative shift in the redox environment. Interestingly, mCAT mice fed a standard chow diet exhibit improved skeletal muscle insulin sensitivity, as well as reduced H2O2-emitting potential, compared with chow-fed WT mice.68
Collectively, these findings suggest that the degree of skeletal muscle insulin sensitivity within a cell may rely on the degree of reduction or oxidation within the intracellular redox environment. In this context, H2O2 emission by the mitochondria and the resulting oxidative shift in the cellular redox environment during nutrient overload is viewed as a metabolic feedback sensor to decrease insulin sensitivity.79 The mechanism(s) by which H2O2-mediated redox control regulates insulin sensitivity is unknown. Given the sensitivity of cellular phosphatases to redox state,80 it has recently been suggested that elevated mitochondrial H2O2 emission may lead to a decrease in the normally dominant global phosphatase tone in cells, increasing the susceptibility of insulin signaling proteins to inhibitory serine/threonine phosphorylation by stress-sensitive kinases (that is, JNK/AP1 and IKK/NF-κB; for review see Fisher–Wellman and Neufer79). Another possibility is that elevated H2O2 emission may directly target a key component of the glucose uptake process itself.
Alterations in hexokinase (HK) during nutrient overload
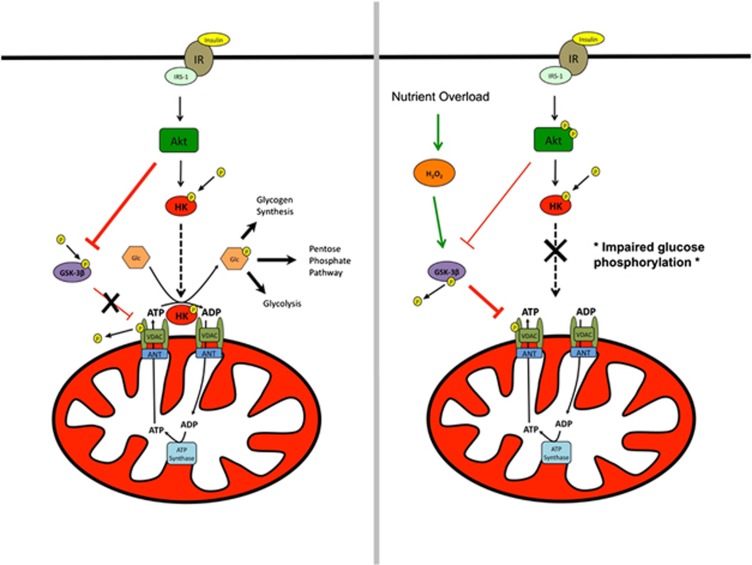
Glucose phosphorylation is an integral step for insulin-stimulated glucose uptake and has been shown to be functionally impaired during nutrient overload induced by a high-fat diet81, 82 and in association with type 2 diabetes.83 Glucose phosphorylation is catalyzed by HK, and gene and protein expression of HKII, the predominant isoform found in skeletal muscle, is increased in response to insulin84 but depressed in patients with type 2 diabetes.85, 86 HKI and HKII can also bind to the mitochondrial outer membrane through interactions with mitochondrial porin in skeletal muscle.87 Intriguingly, overexpression of HKII in insulin-resistant mice fed a high-fat diet improves exercise-stimulated, but not insulin-stimulated, glucose uptake.7 This presents a quandary: why does not increasing glucose phosphorylating capacity (via HKII overexpression) improve glucose uptake if glucose phosphorylation is rate limiting? One possibility is that glucose phosphorylation is not solely a function of HKII content, but also dependent upon the subcellular localization of HK with mitochondria. When bound to mitochondria in skeletal muscle, HK displays greater sensitivity for ATP derived from mitochondria than exogenous ATP,88 suggesting that HK association with mitochondria provides a bioenergetic advantage to glucose phosphorylation. Insulin promotes HKII association with the mitochondrial outer membrane in both rodent89 and human90 striated muscle, and may do so via Akt in two ways: (1) direct phosphorylation of HKII91 and (2) inhibition of glycogen synthase kinase-3β (GSK-3β),92 a basally active negative regulator of glycogen synthesis. Inhibition of GSK-3β decreases phosphorylation tone on VDAC, which increases the binding affinity between VDAC and HK.91 GSK-3β activity is increased during nutrient overload93 and oxidative stress,94 whereas muscle-specific overexpression of GSK-3β is casually linked to insulin resistance.95 In addition, exogenous H2O2 has been shown to dissociate HKII from mitochondria in cultured cardiomyocytes,96 providing a potential direct link between mitochondrial/cellular redox control and HK association with mitochondria (Figure 2). Whether elevated mitochondrial H2O2 emission leads to dissociation of HK from mitochondria and thereby contributes to high-fat diet-induced insulin resistance awaits further investigation.
Figure 2.
Effects of insulin and H2O2 on HK association with mitochondria. Left panel: HK association with mitochondria is promoted by insulin-mediated Akt phosphorylation of HK. Concurrently, Akt phosphorylation of GSK-3β relieves tonic phosphorylation of VDAC, resulting in increased HK/VDAC-binding affinity. When bound to mitochondria, HK is thought to gain a bioenergetic advantage via coupling with oxidative phosphorylation by virtue of the fusion of the outer and inner mitochondrial membranes through interaction between VDAC/ANT. Right panel: exogenous H2O2 dissociates HK from the mitochondria in cardiomyocytes through an unknown mechanism. GSK-3β activity is increased by exogenous H2O2, which is hypothesized to decrease binding of HK to VDAC, leading to HK dissociation from mitochondria, which is proposed to contribute to the etiology of diet-induced insulin resistance by impairing glucose phosphorylation in skeletal muscle.
Concluding remarks
Research conducted over the last several decades has firmly established a link between positive metabolic balance and the development of metabolic disease (for example, insulin resistance, type 2 diabetes). However, deciphering the mechanism(s) by which chronic excess nutrient supply actually interacts with and impairs insulin signaling, ultimately leading to the clinical manifestation of insulin resistance, has proven difficult. Evidence is accumulating that adaptations within the mitochondria, either in ‘response to' or ‘as a consequence of' excessive nutrients, likely underlie this process. Further elucidation of the complex relationships between metabolic balance, H2O2 emission and cellular redox environment will be necessary to allow for the eventual design/development of pharmacological/dietary interventions designed to restore/prevent metabolic disease.
Acknowledgments
Support from the National Institutes of Health (R01-DK073488 and RO1-DK074825, PDN) is acknowledged.
The authors declare no conflict of interest.
Footnotes
This article was published as part of a supplement funded with an unrestricted educational contribution from Desjardins Sécurité Financière.
References
- DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981; 30: 1000–1007. [DOI] [PubMed] [Google Scholar]
- Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab 2009; 296: E11–E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W et al. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 2009; 52: 752–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 2007; 56: 1025–1033. [DOI] [PubMed] [Google Scholar]
- Larance M, Ramm G, James DE. The GLUT4 code. Mol Endocrinol 2008; 22: 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thong FS, Dugani CB, Klip A. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology (Bethesda) 2005; 20: 271–284. [DOI] [PubMed] [Google Scholar]
- Fueger PT, Bracy DP, Malabanan CM, Pencek RR, Granner DK, Wasserman DH. Hexokinase II overexpression improves exercise-stimulated but not insulin-stimulated muscle glucose uptake in high-fat-fed C57BL/6J mice. Diabetes 2004; 53: 306–314. [DOI] [PubMed] [Google Scholar]
- Halseth AE, Bracy DP, Wasserman DH. Overexpression of hexokinase II increases insulinand exercise-stimulated muscle glucose uptake in vivo. Am J Physiol 1999; 276: E70–E77. [DOI] [PubMed] [Google Scholar]
- Roden M. How free fatty acids inhibit glucose utilization in human skeletal muscle. News Physiol Sci 2004; 19: 92–96. [DOI] [PubMed] [Google Scholar]
- Shulman GI. Unraveling the cellular mechanism of insulin resistance in humans: new insights from magnetic resonance spectroscopy. Physiology (Bethesda) 2004; 19: 183–190. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Douen AG, Ramlal T, Klip A, Holloszy JO. Stimulation of glucose transport in skeletal muscle by hypoxia. J Appl Physiol 1991; 70: 1593–1600. [DOI] [PubMed] [Google Scholar]
- Zierath JR, Houseknecht KL, Gnudi L, Kahn BB. High-fat feeding impairs insulin-stimulated GLUT4 recruitment via an early insulin-signaling defect. Diabetes 1997; 46: 215–223. [DOI] [PubMed] [Google Scholar]
- Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 2000; 49: 677–683. [DOI] [PubMed] [Google Scholar]
- Moro C, Bajpeyi S, Smith SR. Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab 2008; 294: E203–E213. [DOI] [PubMed] [Google Scholar]
- Meex RC, Schrauwen P, Hesselink MK. Modulation of myocellular fat stores: lipid droplet dynamics in health and disease. Am J Physiol Regul Integr Comp Physiol 2009; 297: R913–R924. [DOI] [PubMed] [Google Scholar]
- Digel M, Ehehalt R, Fullekrug J. Lipid droplets lighting up: insights from live microscopy. FEBS Lett 2010; 584: 2168–2175. [DOI] [PubMed] [Google Scholar]
- Unger RH. Lipotoxic diseases. Annu Rev Med 2002; 53: 319–336. [DOI] [PubMed] [Google Scholar]
- Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1 H NMR spectroscopy study. Diabetologia 1999; 42: 113–116. [DOI] [PubMed] [Google Scholar]
- Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 1997; 46: 983–988. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Caddy S, Ilic V, Fielding BA, Frayn KN, Borthwick AC et al. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabolism 1996; 45: 947–950. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Kelley DE. Skeletal muscle triglyceride: marker or mediator of obesity-induced insulin resistance in type 2 diabetes mellitus? Curr Diab Rep 2002; 2: 216–222. [DOI] [PubMed] [Google Scholar]
- Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes 1991; 40: 1397–1403. [DOI] [PubMed] [Google Scholar]
- Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes 1991; 40: 280–289. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 2001; 86: 5755–5761. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest 2007; 117: 1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z, Langlais P, De Filippis EA, Luo M, Flynn CR, Schroeder S et al. Global assessment of regulation of phosphorylation of insulin receptor substrate-1 by insulin in vivo in human muscle. Diabetes 2007; 56: 1508–1516. [DOI] [PubMed] [Google Scholar]
- Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab 2009; 296: E581–E591. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000; 404: 787–790. [DOI] [PubMed] [Google Scholar]
- Hegarty BD, Furler SM, Ye J, Cooney GJ, Kraegen EW. The role of intramuscular lipid in insulin resistance. Acta Physiol Scand 2003; 178: 373–383. [DOI] [PubMed] [Google Scholar]
- Griffin M, Marcucci M, Cline G, Bell K, Barucci N, Lee D et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 1999; 48: 1270–1274. [DOI] [PubMed] [Google Scholar]
- Yu C, Chen Y, Zong H, Wang Y, Bergeron R, Kim JK et al. Mechanism by which fatty acids inhibit insulin activation of IRS-1 associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 2002; 277: 50230–50236. [DOI] [PubMed] [Google Scholar]
- Schmitz-Peiffer C, Oakes ND, Browne CL, Kraegen EW, Biden TJ. Reversal of chronic alterations of skeletal muscle protein kinase C from fat-fed rats by BRL-49653. Am J Physiol 1997; 273: E915–E921. [DOI] [PubMed] [Google Scholar]
- Li Y, Soos TJ, Li X, Wu J, DeGennaro M, Sun X et al. Protein kinase C {theta} inhibits insulin signaling by phosphorylating IRS1 at ser1101. J Biol Chem 2004; 279: 45304–45307. [DOI] [PubMed] [Google Scholar]
- Wang C, Liu M, Riojas RA, Xin X, Gao Z, Zeng R et al. Protein kinase C theta (PKCtheta)-dependent phosphorylation of PDK1 at Ser504 and Ser532 contributes to palmitate-induced insulin resistance. J Biol Chem 2009; 284: 2038–2044. [DOI] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest 2004; 114: 823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra C, Federici M, Buongiorno A, Senni MI, Morelli S, Segratella E et al. Transgenic mice with dominant negative PKC-theta in skeletal muscle: a new model of insulin resistance and obesity. J Cell Physiol 2003; 196: 89–97. [DOI] [PubMed] [Google Scholar]
- Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 2008; 29: 381–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turinsky J, O'Sullivan DM, Bayly BP. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem 1990; 265: 16880–16885. [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem 2002; 277: 25847–25850. [DOI] [PubMed] [Google Scholar]
- Gorska M, Dobrzyn A, Zendzian-Piotrowska M, Gorski J. Effect of streptozotocin-diabetes on the functioning of the sphingomyelin-signalling pathway in skeletal muscles of the rat. Horm Metab Res 2004; 36: 14–21. [DOI] [PubMed] [Google Scholar]
- Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Kinalska I, Baranowski M et al. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes 2004; 53: 1215–1221. [DOI] [PubMed] [Google Scholar]
- Hajduch E, Balendran A, Batty IH, Litherland GJ, Blair AS, Downes CP et al. Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia 2001; 44: 173–183. [DOI] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 2007; 5: 167–179. [DOI] [PubMed] [Google Scholar]
- Ussher JR, Koves TR, Cadete VJJ, Zhang L, Jaswal JS, Swyrd SJ et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 2010; 59: 2453–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 2011; 121: 1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2002; 51: 2005–2011. [DOI] [PubMed] [Google Scholar]
- Serlie MJ, Meijer AJ, Groener JE, Duran M, Endert E, Fliers E et al. Short-term manipulation of plasma free fatty acids does not change skeletal muscle concentrations of ceramide and glucosylceramide in lean and overweight subjects. J Clin Endocrinol Metab 2007; 92: 1524–1529. [DOI] [PubMed] [Google Scholar]
- Skovbro M, Baranowski M, Skov-Jensen C, Flint A, Dela F, Gorski J et al. Human skeletal muscle ceramide content is not a major factor in muscle insulin sensitivity. Diabetologia 2008; 51: 1253–1260. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 2012; 15: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des 2008; 14: 1225–1230. [DOI] [PubMed] [Google Scholar]
- Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 2008; 118: 2992–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett 2008; 582: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007; 132: 2169–2180. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K et al. A central role for JNK in obesity and insulin resistance. Nature 2002; 420: 333–336. [DOI] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 2001; 293: 1673–1677. [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005; 11: 191–198. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xu A, Chung SK, Cresser JH, Sweeney G, Wong RL et al. Selective inactivation of c-Jun NH2-terminal kinase in adipose tissue protects against diet-induced obesity and improves insulin sensitivity in both liver and skeletal muscle in mice. Diabetes 2011; 60: 486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15: 914–920. [DOI] [PubMed] [Google Scholar]
- Sabio G, Kennedy NJ, Cavanagh-Kyros J, Jung DY, Ko HJ, Ong H et al. Role of muscle c-Jun NH2-terminal kinase 1 in obesity-induced insulin resistance. Mol Cell Biol 2010; 30: 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EG, Ko HJ, Cho YR, Kim HJ, Ma Z, Yu TY et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes 2009; 58: 2525–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007; 56: 901–911. [DOI] [PubMed] [Google Scholar]
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005; 46: 2347–2355. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004; 306: 457–461. [DOI] [PubMed] [Google Scholar]
- Dasgupta J, Kar S, Liu R, Joseph J, Kalyanaraman B, Remington SJ et al. Reactive oxygen species control senescence-associated matrix metalloproteinase-1 through c-Jun-N-terminal kinase. J Cell Physiol 2010; 225: 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005; 120: 649–661. [DOI] [PubMed] [Google Scholar]
- Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 2011; 208: 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal 2005; 7: 395–403. [DOI] [PubMed] [Google Scholar]
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009; 119: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Na R, Gu M, Salmon AB, Liu Y, Liang H et al. Reduction of mitochondrial H2O2 by overexpressing peroxiredoxin 3 improves glucose tolerance in mice. Aging Cell 2008; 7: 866–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ et al. Targeted Expression of Catalase to Mitochondria Prevents Age-Associated Reductions in Mitochondrial Function and Insulin Resistance. Cell Metab 2010; 12: 668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman HJ, Maiorino M, Ursini F. Signaling Functions of Reactive Oxygen Species. Biochemistry 2010; 49: 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 2001; 30: 1191–1212. [DOI] [PubMed] [Google Scholar]
- Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol 2010; 45: 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS et al. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci 2004; 24: 7779–7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem 2003; 86: 1101–1107. [DOI] [PubMed] [Google Scholar]
- Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 1997; 416: 15–18. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 2002; 80: 780–787. [DOI] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008; 7: 45–56. [DOI] [PubMed] [Google Scholar]
- Fisher-Wellman KH, Neufer PD. Linking mitochondrial bioenergetics to the etiology of insulin resistance via redox biology. Trends Endocrinol Metab 2012; 23: 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright VP, Reiser PJ, Clanton TL. Redox modulation of global phosphatase activity and protein phosphorylation in intact skeletal muscle. J Physiol 2009; 587: 5767–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furler SM, Oakes ND, Watkinson AL, Kraegen EW. A high-fat diet influences insulin-stimulated posttransport muscle glucose metabolism in rats. Metabolism 1997; 46: 1101–1106. [DOI] [PubMed] [Google Scholar]
- Halseth AE, Bracy DP, Wasserman DH. Limitations to basal and insulin-stimulated skeletal muscle glucose uptake in the high-fat-fed rat. Am J Physiol Endocrinol Metab 2000; 279: E1064–E1071. [DOI] [PubMed] [Google Scholar]
- Bonadonna RC, Del Prato S, Bonora E, Saccomani MP, Gulli G, Natali A et al. Roles of glucose transport and glucose phosphorylation in muscle insulin resistance of NIDDM. Diabetes 1996; 45: 915–925. [DOI] [PubMed] [Google Scholar]
- Vogt C, Ardehali H, Iozzo P, Yki-Jarvinen H, Koval J, Maezono K et al. Regulation of hexokinase II expression in human skeletal muscle in vivo. Metabolism 2000; 49: 814–818. [DOI] [PubMed] [Google Scholar]
- Kruszynska YT, Mulford MI, Baloga J, Yu JG, Olefsky JM. Regulation of skeletal muscle hexokinase II by insulin in nondiabetic and NIDDM subjects. Diabetes 1998; 47: 1107–1113. [DOI] [PubMed] [Google Scholar]
- Pendergrass M, Koval J, Vogt C, Yki-Jarvinen H, Iozzo P, Pipek R et al. Insulin-induced hexokinase II expression is reduced in obesity and NIDDM. Diabetes 1998; 47: 387–394. [DOI] [PubMed] [Google Scholar]
- Anflous-Pharayra K, Cai ZJ, Craigen WJ. VDAC1 serves as a mitochondrial binding site for hexokinase in oxidative muscles. Biochim Biophys Acta 2007; 1767: 136–142. [DOI] [PubMed] [Google Scholar]
- Viitanen PV, Geiger PJ, Erickson-Viitanen S, Bessman SP. Evidence for functional hexokinase compartmentation in rat skeletal muscle mitochondria. J Biol Chem 1984; 259: 9679–9686. [PubMed] [Google Scholar]
- Chen-Zion M, Bassukevitz Y, Beitner R. Sequence of insulin effects on cytoskeletal and cytosolic phosphofructokinase, mitochondrial hexokinase, glucose 1,6-bisphosphate and fructose 2,6-bisphosphate levels, and the antagonistic action of calmodulin inhibitors, in diaphragm muscle. Int J Biochem 1992; 24: 1661–1667. [DOI] [PubMed] [Google Scholar]
- Vogt C, Yki-Jarvinen H, Iozzo P, Pipek R, Pendergrass M, Koval J et al. Effects of insulin on subcellular localization of hexokinase II in human skeletal muscle in vivo. J Clin Endocrinol Metab 1998; 83: 230–234. [DOI] [PubMed] [Google Scholar]
- Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res 2005; 65: 10545–10554. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995; 378: 785–789. [DOI] [PubMed] [Google Scholar]
- Eldar-Finkelman H, Schreyer SA, Shinohara MM, LeBoeuf RC, Krebs EG. Increased glycogen synthase kinase-3 activity in diabetes- and obesity-prone C57BL/6J mice. Diabetes 1999; 48: 1662–1666. [DOI] [PubMed] [Google Scholar]
- Dokken BB, Saengsirisuwan V, Kim JS, Teachey MK, Henriksen EJ. Oxidative stress-induced insulin resistance in rat skeletal muscle: role of glycogen synthase kinase-3. Am J Physiol Endocrinol Metab 2008; 294: E615–E621. [DOI] [PubMed] [Google Scholar]
- Pearce NJ, Arch JR, Clapham JC, Coghlan MP, Corcoran SL, Lister CA et al. Development of glucose intolerance in male transgenic mice overexpressing human glycogen synthase kinase-3beta on a muscle-specific promoter. Metabolism 2004; 53: 1322–1330. [DOI] [PubMed] [Google Scholar]
- Wu R, Smeele KM, Wyatt E, Ichikawa Y, Eerbeek O, Sun L et al. Reduction in hexokinase II levels results in decreased cardiac function and altered remodeling after ischemia/reperfusion injury. Circ Res 2011; 108: 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]