Abstract
Inducing long-term protective memory CD8+ T cells is a desirable goal for vaccines against intracellular pathogens. However, the mechanisms of differentiation of CD8+ T cells into long-lived memory cells capable of mediating protection of immunized hosts remain incompletely understood. We have developed an experimental system using mice immunized with WT or mutants of the intracellular bacterium Listeria monocytogenes (Lm) that either do or do not develop protective memory CD8+ T cells. We previously reported that mice immunized with Lm lacking functional SecA2, an auxiliary secretion system of gram-positive bacteria, did not differentiate functional memory CD8+ T cells that protected against a challenge infection with WT Lm. Herein we hypothesized that the p60 and NamA autolysins of Lm, which are major substrates of the SecA2 pathway, account for this phenotype. We generated Lm genetically deficient for genes encoding for the p60 and NamA proteins, ΔiapΔmurA Lm, and further characterized this mutant. Δp60ΔNamA Lm exhibited a strong filamentous phenotype, inefficiently colonized host tissues, and grew mostly outside cells. When Δp60ΔNamA Lm was made single unit (SU), cell invasion was restored to WT levels during vaccination, yet induced memory T cells still did not protect immunized hosts against recall infection. Recruitment of blood phagocytes and antigen-presenting cell activation was close to that of mice immunized with ΔActA Lm which develop protective memory. However, key inflammatory factors involved in optimal T cell-programming such as IL-12 and type I IFN (IFN-I) were lacking, suggesting that cytokine signals may largely account for the observed phenotype. Thus altogether, these results establish that p60 and NamA secreted by Lm promote primary host cell-invasion, the inflammatory response and the differentiation of functional memory CD8+ T cells, by preventing Lm filamentation during growth and subsequent triggering of innate sensing mechanisms.
Introduction
The development of protective vaccines against intracellular pathogens such as the Human Immunodeficiency Virus (HIV) or Mycobacterium tuberculosis, two highly successful health-threatening human pathogens, still requires a more thorough understanding of the cues that regulate the differentiation of long-lived protective memory cells, and in particular that of memory CD8+ T cells (Harty et al., 2008, Sallusto et al., 2010). While many studies are contributing to better defining the key host factors that orchestrate such processes, often through the use of antibody neutralizing molecules or knockout mouse models, the precise sets of events that lead to the development of potent protective pathogen-specific memory during natural infections are usually poorly understood. For instance, immunization of mice with the intracellular bacterium Listeria monocytogenes (Lm) or the lymphochoriomeningitis virus (LCMV Armstrong) are well-established models to study the differentiation of protective memory CD8+ T cells; both vaccination induce life-long host immunity against a challenge infection with otherwise lethal doses of these pathogens, yet the underlying mechanisms are still under intense investigations (Pamer, 2004, Moseman et al., 2013). During immunization, the expression of pathogen-derived molecules plays a critical role to promote pathogen virulence and pathogenesis but also to drive optimal host immune responses. Thus, precisely defining the microbial factors that modulate the initial host immune response, and how, is key to further dissect these complex processes and design innovative vaccination strategies (Iwasaki et al., 2010).
Using Lm genetically deficient for the SecA2 ATPase (ΔSecA2), an auxiliary secretion system found in several pathogenic gram-positive bacteria (Lenz et al., 2003), we have previously established that mice immunized with ΔSecA2 Lm were not protected against a challenge infection with WT Lm contrary to mice immunized with WT or ΔActA Lm (Muraille et al., 2007). We have also found that Lm-specific memory CD8+ T cells from mice immunized with ΔSecA2 but not ΔActA Lm, were unable to transfer protection to primary infected recipient mice (Muraille et al., 2007).
The NamA and p60 autolysins from Lm, a N-acetylmuramidase and a glutamyl-mesodiaminopimelate endopeptidase, are major substrates of the SecA2 secretory pathway and are suggested to digest Lm-peptidoglycans (PGNs) during bacterial growth (Wuenscher et al., 1993, Lenz et al., 2003, Machata et al., 2005). They play an essential role in enabling efficient Lm division and pathogenesis in vivo, and may account for the inability of ΔSecA2 Lm to induce potent immunological memory in immunized mice (Muraille et al., 2007, Narni-Mancinelli et al., 2007). Herein, we hypothesized that p60 and NamA autolysins are key to program CD8+ T cells to become fully functional memory cells. Lm lacking either or both of these autolysins were shown to form bacterial filaments and to inefficiently colonize the host (Machata et al., 2005). p60 and NamA may impact T cell-priming by preventing Lm filamentous phenotype and ease Lm access to antigen-presenting cells (APCs) and/or by releasing PGN products that are known to modulate cytosolic Pattern Recognition Receptor (PRR) triggering and subsequent host APC activation. To investigate these hypotheses, we have generated Lm bearing targeted deletions in genes encoding both the p60 and NamA proteins, Δp60ΔNamA Lm, and further characterized this mutant and its ability to induce an immune response in immunized mice. We found that Δp60ΔNamA Lm were mostly inefficient to invade primary host cells in the spleen of infected mice, likely because of strong filamentation. Subsequently, we show that Δp60ΔNamA Lm failed to induce memory CD8+ T cells that could protect immunized hosts against a challenge infection with WT Lm. Interestingly, rescuing WT single unit (SU) phenotype of Δp60ΔNamA Lm at the time of immunization, restored initial cell invasion and APC activation close to non-filamentous ΔActA Lm, yet failed to restore robust inflammation and long-term protective memory. Thus collectively our results suggest that the p60 and NamA autolysins from Lm control the differentiation of protective memory CD8+ T cells by (i) allowing the access of Lm to host cell cytoplasm and (ii) by promoting efficient cytosolic growth together with the release of immunogenic products essential to promote optimal inflammation such as cyclic di-nucleotides, PGN and others, at the time of T cell priming.
Results
Expression of the p60 and NamA autolysins by Lm during immunization is essential for immunological protection against recall infection with WT Lm
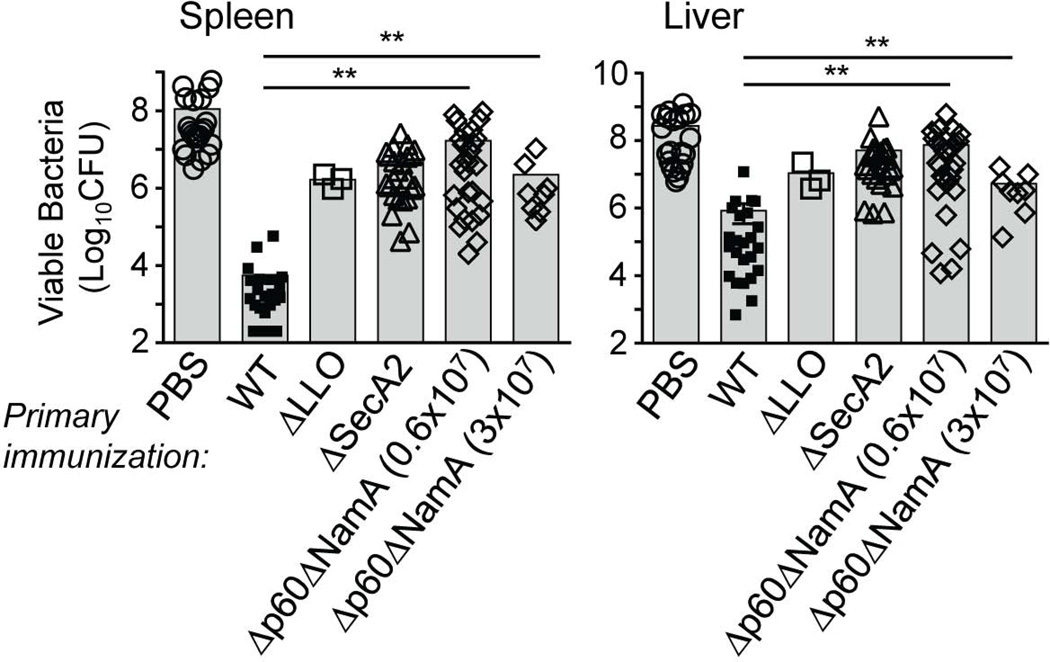
To investigate whether the p60 and NamA autolysins from Lm may account for the inability of ΔSecA2 Lm to induce memory CD8+ T cells that protected immunized mice against challenge infection with WT Lm (Muraille et al., 2007), we generated a knockout mutant of Lm lacking both autolysins. Briefly, ΔNamA Lm bearing a targeted deletion of the gene encoding for the NamA protein (Lenz et al., 2003) were transformed with the pKSV7 transfer vector bearing a p60-targeting knockout construct and ΔNamA Lm knocked-out for the gene encoding the p60 autolysin on its chromosome were selected as previously described (Shen et al., 1995). Deletion was confirmed by PCR using primers outside the knockout portion of the p60 DNA and further sequencing (not shown). We next investigated whether Δp60ΔNamA Lm induced protective immunological memory in mice. For this, we inoculated WT BALB/c mice with two distinct immunizing doses of Δp60ΔNamA Lm, or as controls PBS, WT, ΔSecA2 and ΔLLO Lm (Figure 1). Five weeks later, mice were challenged with 3×105 WT Lm and spleen and liver plated after 48 hrs. As anticipated, while mice immunized with WT Lm averaged ~5.5×103 and 8.4×105 viable bacteria in spleen and liver respectively, Δp60ΔNamA Lm exhibited ~2.2–17 and 10–110 million Lm CFUs, a 3–4 logs higher load of viable bacteria. The numbers of Lm CFUs in mice immunized with Δp60ΔNamA Lm were comparable to that of ΔSecA2 and ΔLLO Lm-immunized groups. Similar results were also obtained when mice were challenged with lower numbers (6×104) of WT Lm (Figure S1), and in WT C57BL/6 mice (not shown). Thus mice immunized with Δp60ΔNamA Lm did not control the challenge infection with WT Lm, establishing that p60 and NamA play an essential role in the development of long-term protective memory.
Figure 1. Mice immunized with Lm lacking p60 and NamA autolysins fail to mount protective memory responses.
WT BALB/c mice were primary immunized i.v. with 0.1×LD50 WT (5,000), ΔLLO (5×108), ΔSecA2 (0.6×106) and two distinct doses of Δp60ΔNamA (0.6×07, 3×107) Lm, or injected with PBS (unimmunized control). Five weeks later, mice were challenged with 10×LD50 WT Lm (3×105), and spleen and liver were harvested 48 hrs later, lysed and plated to determine the number of viable bacterial CFUs. Each symbol represents one individual mouse and shaded bars the average (Mean value). Data result from the pool of 2–6 independent replicate experiments and p-values are indicated ((*) p<0.1; (**) p<0.01; (***) p<0.001; (****) p<0.001; (ns) p>0.1).
Lack of p60 and NamA autolysins induces filamentation of Listeria monocytogenes
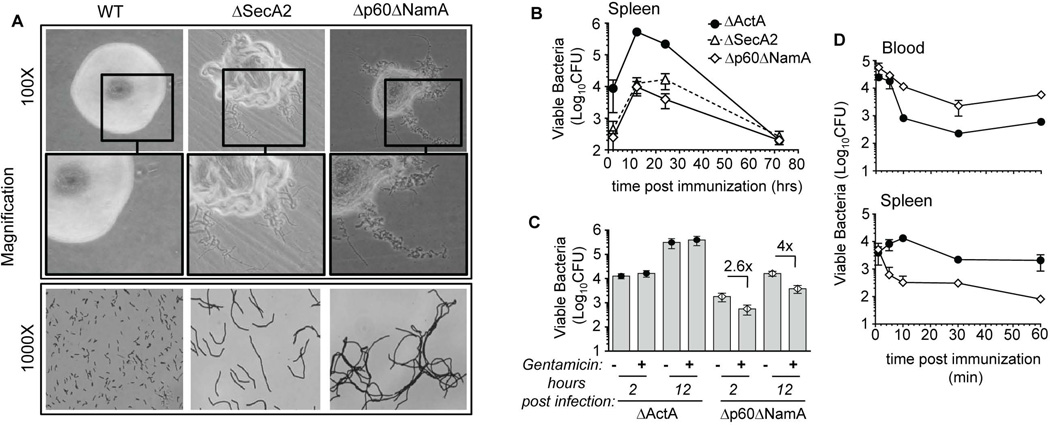
We next characterized the phenotype of mutants lacking both p60 and NamA autolysins compared to WT and ΔSecA2 Lm (Figure 2). While WT Lm formed smooth round-shaped colonies, ΔSecA2 and Δp60ΔNamA Lm exhibited rough, irregular shaped colonies (Figure 2A). Gram staining and microscopic analysis of Δp60ΔNamA Lm revealed that they formed long filamentous chains of bacteria with an average length of several tens of microns, in stark contrast with WT Lm that represented single units of 1–2 microns. These results, consistent with previous studies that had generated a similar mutant of Lm autolysins (Wuenscher et al., 1993, Machata et al., 2005), established that knocking-out the expression of both p60 and NamA autolysins in Lm induced drastic changes in Lm morphology and a bacterial chaining filamentous phenotype.
Figure 2. Lm lacking p60 and NamA autolysins exhibit a filamentous phenotype promoting extracellular growth within infected spleens.
(A) Morphology of Lm colonies grown OVN onto BHI Petri dishes, and corresponding gram staining of bacteria. (B–D) WT C57BL/6 (B6) mice were infected with 0.1×LD50 ΔActA (106), ΔSecA2 (0.6×106) and Δp60ΔNamA (0.6×107) Lm; spleen harvested at the indicated times, lysed and plated. In (C), mice received gentamycin i.p. (2 mg) 1 hr after Lm infection. In (D) immunized mice were bled (200µl/mouse), spleens harvested at indicated times, and Lm CFU determined by plating both cell suspensions after lysis. Data plots and histogram bars average mean values+/−SEM (B-D).
Δp60ΔNamA Lm inefficiently invade cells and colonize mouse tissues
Since Δp60ΔNamA Lm exhibited such a marked filamentous phenotype, we reasoned that they would likely be impaired in their ability to invade cells and tissues, and therefore have lower virulence. To assess this hypothesis, we inoculated WT mice intravenously (i.v.) with 0.6×107 Δp60ΔNamA Lm, a dose that corresponds to ~0.1×LD50 (not shown), and with 0.6×105 ΔSecA2 and 106 ΔActA Lm (Figure 2B), and monitored splenic bacterial growth over a 72 hour time course. ΔActA Lm lacks ActA, an important virulence factor of Lm required for cellular actin polymerization, which confers Lm intracytosolic motility and cell-to-cell spreading. ΔActA Lm exhibits impaired virulence similarly to ΔSecA2 Lm, yet does not form any bacterial chains. While all mutants exhibited comparable growth kinetics, filamentous Lm -ΔSecA2 and Δp60ΔNamA Lm- poorly colonized the spleens early on (2 hrs) compared to non-filamentous ΔActA Lm, and were cleared significantly faster with at least 2 logs less viable bacteria over the first 48 hrs post-inoculation. Together, these results suggested that the low virulence of Δp60ΔNamA Lm is accounted for by inefficient initial invasion of target cells within the spleen and faster clearance.
Filamentation promotes Lm extracellular growth inside infected tissues and survival in blood
While filamentous Lm did not efficiently invade the spleen, they still multiplied by a factor of ~50 over 24 hrs (Figure 2B). We hypothesized that growth of Δp60ΔNamA Lm mostly occurred outside cells yet inside infected spleens. To test this possibility, we injected mice with ΔActA or Δp60ΔNamA Lm, treated them or not with gentamycin 1 hr later to kill extracellular Lm, and plated spleens after 2 and 12 hrs to enumerate viable bacteria (Figure 2C). As expected, most ΔActA Lm were intracellular (>90%) whereas only ~40% (factor of 2.6) and 25% (factor of 4) of viable Δp60ΔNamA Lm were recovered from within cells at 2 and 12 hrs post-immunization, respectively. Moreover, the number of viable Lm CFUs increased in all groups between 2 and 12 hrs post immunization, with the highest in mice immunized with ΔActA Lm (factor of 23) most likely because ΔActA Lm had divided and formed independent CFUs inside infected cells. The number of Δp60ΔNamA Lm CFUs also increased (factor of 12), of which 50–75% was accounted for by extracellular growth. Of note, by 2 hrs post-immunization, the number of viable bacteria recovered from infected spleens was lower by a factor of ~35 in mice that received Δp60ΔNamA compared to ΔActA Lm. Analysis of Lm viability in the blood early after injection, e.g., at 1, 5, 10, 30, 60 and 120 min (Figure 2D), suggested that filamentation increased the persistence of Lm in the blood, likely because of inefficient access to tissues, as observed in the spleen. Thus, collectively, filamentation substantially impairs the ability of Lm to access tissues and invade cells, and to establish a successful infection. Unexpectedly, filamentation promoted the unusual growth of Lm outside cells yet inside infected spleens.
Single unit Δp60ΔNamA Lm efficiently enter cells
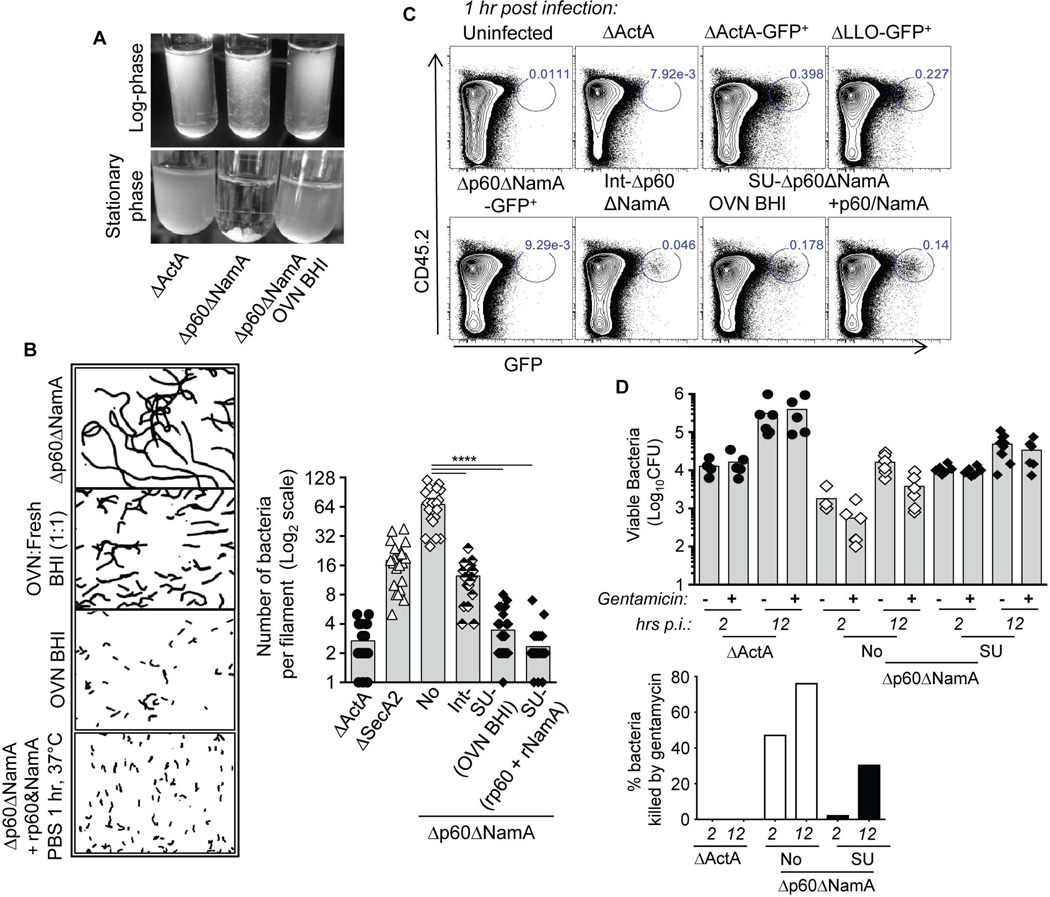
Our results suggest that Lm chaining, which directly results from the lack of p60 and NamA autolysins, impairs the bacterium’s ability to invade cells. Since cytosolic growth of Lm is a prerequisite to prime long-term Lm-specific protective memory (Berche et al., 1987), it is likely that mice immunized with Δp60ΔNamA Lm cannot control recall infection simply because these mutants do not access host cells. Alternately, it is possible that the p60 and NamA autolysins are required independent of their effect on Lm filamentation, possibly through the release of PGN or other products with strong immunomodulatory activities. To explore these hypotheses, we set out to rescue the WT single unit (SU) phenotype in Δp60ΔNamA Lm using two different experimental approaches. First, we supplemented Δp60ΔNamA Lm during growth in vitro using a filtered culture supernatant of ΔLLO Lm grown to stationary phase (e.g., overnight, OVN), which contains the secreted p60 and NamA autolysins but not LLO (Lenz et al., 2003) (Figure 3A, B). Bacteria were inoculated in a 1:1 or 1:0 ratio of OVN:fresh BHI media and grown either to log or stationary phases. While Δp60ΔNamA Lm grown in 100% fresh BHI medium formed long filaments (log-phase) and segregated in ‘cotton-like’ pellets, Δp60ΔNamA Lm grown in p60 and NamA-containing BHI grew as dense cultures (Figure 3A) and formed shorter filaments of intermediate length (1:1 ratio, Int-Δp60ΔNamA) to single unit (1:0 ratio, SU-Δp60ΔNamA) bacteria. As a second approach, recombinant p60 (rp60) and NamA (rNamA) were produced and purified from BL21 Escherichia Coli bacteria. Log-phase grown Δp60ΔNamA Lm incubated with purified autolysins became single bacterial units (Figure 3B). Quantification by microscopy analysis revealed that Δp60ΔNamA and ΔSecA2 Lm exhibited chains with an average of 70 and 16 single units Lm respectively. When cultured in presence of BHI-containing p60 and NamA autolysins, or digested with the purified hydrolases, the length of Δp60ΔNamA Lm was 16 (1:1) or 2 (1:0 and also with purified rp60 and rNamA), with the latter conditions being comparable to ΔActA Lm. These results were also confirmed using cytofluorometry and GFP-expressing Lm to determine the relative sizes (FSC) of the various bacterial suspensions, which varied by at least a factor of 50 (Figure S2A).
Figure 3. Filamentation prevents cell invasion and efficient host spleen colonization.
(A) Pictures of log or stationary phase cultures of indicated Lm mutants grown in fresh BHI or in filtered BHI from OVN grown ΔLLO Lm containing secreted p60 and NamA. (B) Gram staining of log-phase Δp60ΔNamA Lm grown in indicated conditions. (C) WT B6 mice were inoculated with 0.6–1×108 of indicated GFP+ Lm mutants and 1 hr later spleens were harvested, cells stained with cell-surface CD45.2 and analyzed by FACS to determine the frequencies of GFP+ cells (blue numbers) amongst live spleen cells, e.g., cells that contain viable Lm. In (D), mice received gentamycin 1 hr post immunization and spleens were harvested and plated at indicated times to determine Lm titers (upper graph). The lower graph represents the same data as a relative proportion (%) of Lm killed by gentamicin, e.g., extracellular bacteria. All experiments show representative data of 1 of 2 independent replicate experiments; in (D) the pool of the 2 experiments is shown with each symbol representing 1 mouse. Histogram bars average mean values (B, D).
Taking advantage of SU-Δp60ΔNamA Lm obtained upon digestion with purified autolysins, we first tested whether invasion of macrophages could be restored in vitro. We infected WT bone marrow-derived macrophages (BMMP) with SU-Δp60ΔNamA, Δp60ΔNamA, or ΔActA Lm, and measured viable bacterial numbers recovered from these cells 2 hrs later (Figure S2B). Comparable numbers of viable bacterial CFUs (~104) were recovered from 2 hrs-infected macrophages in both conditions, suggesting that shortening to SU-Δp60ΔNamA Lm restored the ability of Δp60ΔNamA Lm to invade BMMP in vitro similarly to ΔActA Lm. Using GFP+ Lm, we further confirmed that reducing the length of Δp60ΔNamA Lm (Int to SU) proportionally improved their ability to invade host cells in vivo, achieving comparable frequencies of GFP+ cells between SU-Δp60ΔNamA, ΔActA and ΔLLO Lm 1 hr post infection (Figure 3C). This was also observed by enumerating Lm CFUs recovered from the spleens of mice 2 hrs post infection wherein SU-Δp60ΔNamA or ΔActA formed equivalent numbers of viable Lm CFUs (~10–12,000), while only ~2,000 Lm CFUs were recovered from mice immunized with Δp60ΔNamA Lm (Figure 3D, upper graph). To provide formal demonstration that SU-Δp60ΔNamA Lm invaded host cells, we inoculated bacterial suspensions as before and one hour later treated mice (or not) with gentamicin for another hour before enumerating Lm splenic CFUs to discriminate between intracellular and extracellular bacteria (Figure 3D). SU-Δp60ΔNamA Lm entered cells as efficiently as ΔActA Lm with ~16,000 CFUs recovered from gentamicin-treated infected spleens, in contrast to ‘full-length’ Δp60ΔNamA Lm with only ~700 CFUs. This further established that filamentation dramatically reduces bacterial ability to enter spleen cells. Of note, between 2 and 12 hrs, SU or full-length -Δp60ΔNamA Lm grew similarly both outside or inside spleen cells. As expected, gentamycin injection mostly decreased Lm loads in mice that receive the filamentous mutant with ~40 (2 hrs) and 80 % (12 hrs) of viable Δp60ΔNamA Lm that were sensitive to gentamicin, correlating with extracellular growth in the host (Figure 3D, lower panel). In summary, our data implicate p60 and NamA autolysins as key regulators of Lm invasion of host tissues and cells, by allowing Lm separation to SU bacteria during growth. Altogether, these results support the idea that the low virulence associated with filamentous Lm may be fully accounted for by such 'physical impairment'.
Rescuing Δp60ΔNamA Lm entry to cells does not restore protective memory in immunized mice
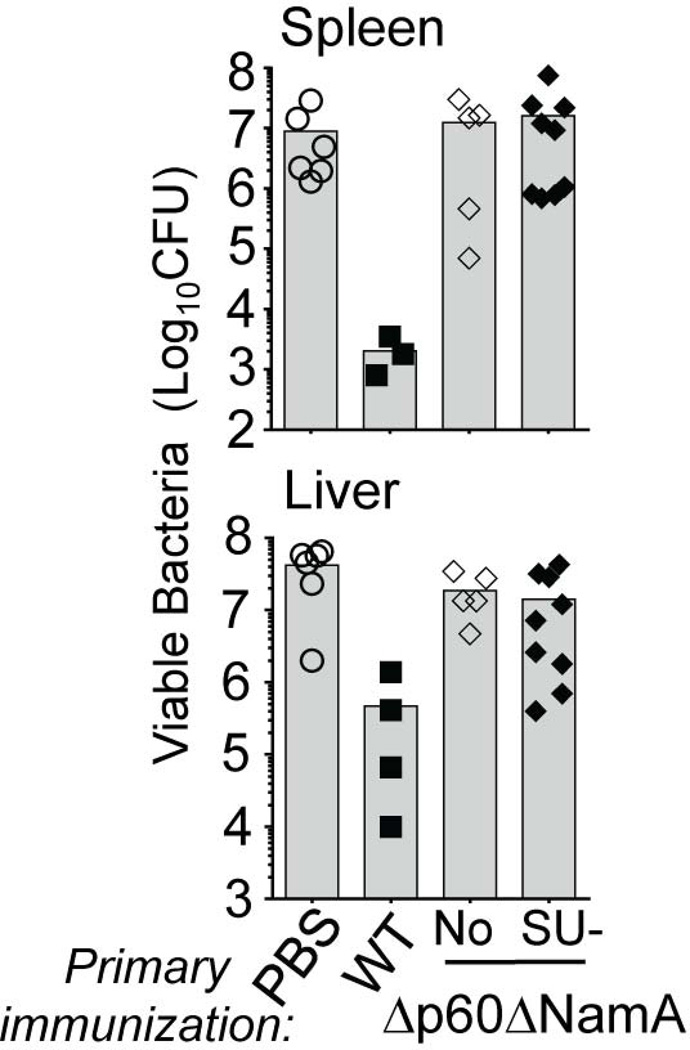
Our results show that the lack of p60 and NamA autolysins acts as a “mechanical brake” to efficient colonization of the host by Lm, subsequently accounting for inadequate T cell priming and the inability of mice immunized with Δp60ΔNamA Lm to resist recall infection with WT Lm (Figure 1). If allowed to access host tissues and cells as SU bacteria, we reasoned that Δp60ΔNamA Lm could indeed prime long-term protective immunity in immunized hosts. Since SU-Δp60ΔNamA Lm could invade host cells as efficiently as ΔActA Lm in vitro and in vivo, we next tested whether WT BALB/c mice immunized with SU Δp60ΔNamA or Δp60ΔNamA and WT Lm as control groups were protected against challenge infection with WT Lm (Figure 4). Data show that mice vaccinated with SU-Δp60ΔNamA Lm exhibited ~100-fold more viable bacteria in their organs -spleen and liver- compared to protected hosts, thus did not control the challenge infection. Therefore, despite efficient entry into host cells, SU-Δp60ΔNamA Lm could not prime long-term protective memory, possibly as a result of poor growth in vivo and/or failure to promote adequate T cell priming environment.
Figure 4. Rescuing cell invasion by filamentous Δp60ΔNamA Lm is not sufficient for protective T cell memory development.
Titers of viable WT Lm CFU in the spleen and liver of mice immunized with 0.1×LD50 of indicated Lm, and challenged 5 weeks later with 3×105 WT Lm for 48 hrs. All experiments show representative data of 1 experiment; each symbol represents 1 mouse and histogram bars average mean values.
Immunization with single-unit Δp60ΔNamA Lm restores robust recruitment of blood-derived Ly6C+ monocytes and neutrophils
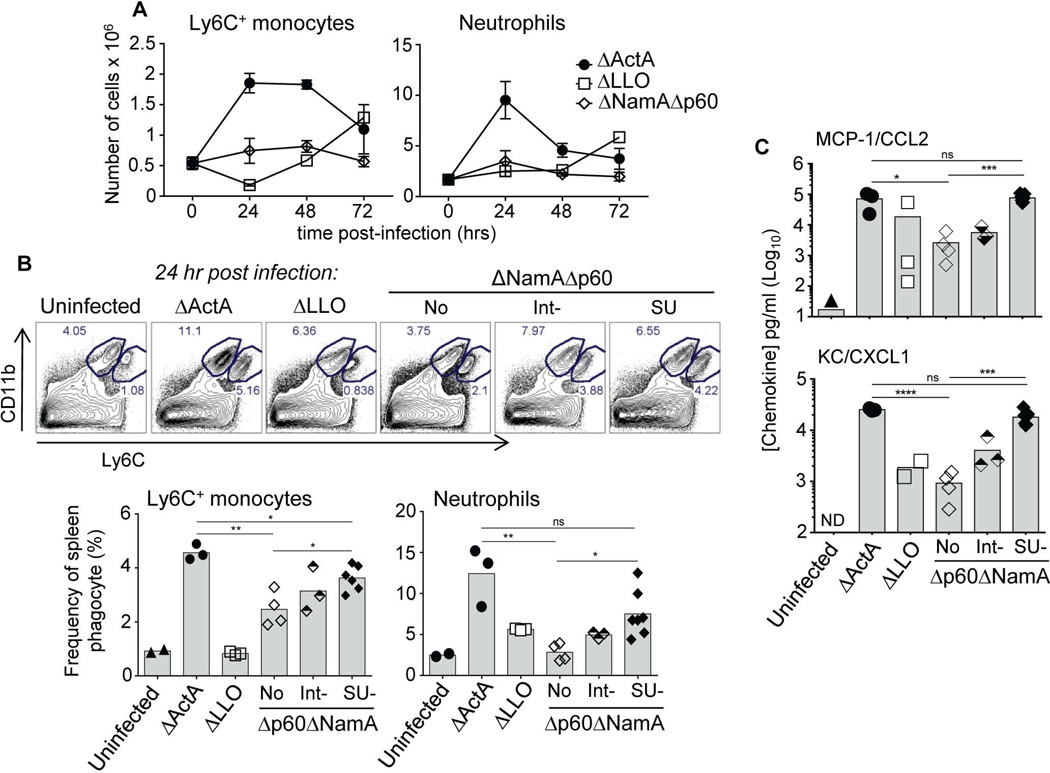
To gain further insights into why mice immunized with SU Δp60ΔNamA Lm were not protected against virulent challenge infection, we next conducted a systematic investigation of the T cell priming environment at the time of vaccination. Optimal T cell priming correlates with strong recruitment and activation of sentinel cells from the innate immune system (Iwasaki et al., 2004, Reis e Sousa, 2006). In mice immunized with ΔActA Lm, which develop strong protective CD8+ T cell memory, blood phagocytes -e.g., Ly6C+ inflammatory monocytes (CD11bhiLy6Chi) and neutrophils (CD11bhiLy6Cdim)- are massively recruited to infected spleens, with peak recruitments between 24–48 hrs after the immunization (Figure 5A). In contrast, in mice that received Δp60ΔNamA Lm or ΔLLO Lm as control, only weak recruitment of these cells was observed, correlating with inefficient priming of long-term immunity. Thus, we next tested whether shortening Δp60ΔNamA Lm still promoted more efficient recruitment of these phagocytes to infected spleens (Figure 5B). We found that Int- and SU- Δp60ΔNamA Lm proportionally increased the frequencies of Ly6C+ monocytes and neutrophils recruited to infected spleens, with frequencies becoming closer to that induced upon ΔActA Lm immunization. In line with these results, CCL2 and CXCL1, two major chemokines respectively regulating Ly6C+ monocytes and neutrophil mobilization during inflammation increased by a factor of ~30–40 (SU-Δp60ΔNamA and ΔActA) compared to mice immunized with full length Δp60ΔNamA or ΔLLO Lm. Thus, in vitro rescue of filamentous Δp60ΔNamA to SU Lm restored their tissue and cell invasiveness in vivo, which subsequently promoted robust recruitment of blood-derived phagocytic effector cells, suggesting that this unlikely accounts for the impaired priming of T cells.
Figure 5. Cytosolic expression of p60 and NamA autolysins by Lm promotes efficient recruitment of blood phagocytes.
(A) Kinetics of Ly6C+ monocyte and neutrophil recruitments to the spleens of WT B6 mice immunized with 0.1×LD50 of indicated mutants of Lm (as before). (B) Comparative analysis of peak recruitments (24 hrs) of blood phagocytes upon the distinct immunizations. Representative dot plots are shown and bar graphs average the frequencies of each subset in 1 of 2 representative experiments. (C) CCL2 and CXCL1/KC chemokine levels measured directly in spleen lysates from mice immunized with indicated mutants of Lm. P-values are indicated. Data plots and histogram bars average mean values +/−SEM and each symbol representing one individual mouse.
Antigen-presenting cell activation, but not overall inflammation, is largely restored by immunization with single-unit Δp60ΔNamA Lm
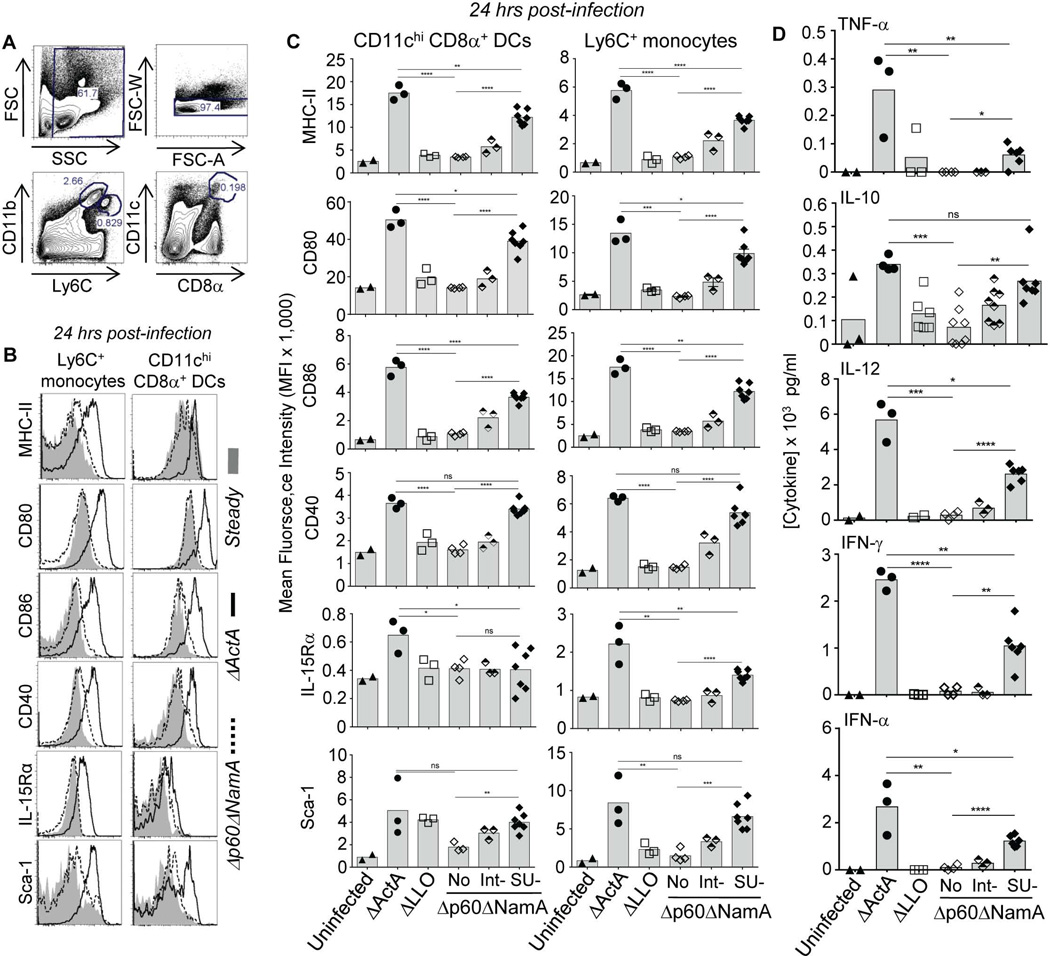
Strong activation of APCs is key to optimal T cell priming and differentiation into functional memory cells (Muraille et al., 2005, Reis e Sousa, 2006). In mice immunized with ΔActA Lm, splenic CD8α+ CD11chi DCs -the major APC subset involved in CD8+ T cell priming (Heath et al., 2004, Hildner et al., 2008)- and Ly6C+ monocytes underwent strong activation peaking between 24–48 hrs post-immunization, as measured by cell-surface upregulation of MHC class II and costimulatory molecules (CD80, CD86, CD40), and Sca-1 and IL-15 receptor alpha (IL-15Rα) two interferon-responsive cell-surface markers (Figure 6B, S2 and not shown) (Beuneu et al., 2011, Soudja et al., 2012). In contrast, only weak activation was found in groups that received Δp60ΔNamA or ΔLLO Lm (Figure 6C). As observed for blood phagocyte recruitments, shortening Δp60ΔNamA Lm proportionally restored strong maturation of APCs with upregulation of MHC-II, costimulatory molecules, the IFN responsive markers Sca-1 and IL-15Rα to levels close to those observed upon ΔActA Lm immunization (~70% of maximum upregulation).
Figure 6. p60 and NamA autolysins secreted by cytosolic Lm promotes robust activation of antigen presenting cells but only limited inflammation.
(A) Gating strategy of spleen Ly6C+ monocytes and CD8α+ DCs. (B, C) WT B6 mice were immunized with various mutants of Lm, or left unimmunized, and 24 hrs later spleens were harvested and cell suspensions stained for expression of indicated cell surface markers. (B) Overlays of representative FACS histograms of resting versus activated monocytes and DCs in mice that received ΔActA and Δp60ΔNamA Lm. (C) The average expression levels (MFI) of indicated cell-surface activation markers on monocytes and DCs in mice immunized with the whole panel of Lm mutants. (D) Cytokine and chemokine levels measured directly in spleen lysates from mice immunized with specified mutants of Lm. All experiments represent the pool of 2 replicate experiments and p-values are indicated. Histogram bars average mean values (C, D) with each symbol representing an individual mouse.
However, while increased compared to full length Δp60ΔNamA Lm, peak levels (24 hrs (Campisi et al., 2011)) of the splenic proinflammatory cytokines TNF-α, IL-12, IFN-γ, IFN-α, but not IL-10, were only partially restored in mice immunized with SU Δp60ΔNamA Lm, with levels only reaching up to 20–45% of those measured in mice immunized with ΔActA Lm (Figure 6D). Therefore, when enabled to access host cell cytoplasm, Δp60ΔNamA Lm could rescue the activation of APCs to substantial extends, yet not inflammatory cytokine levels. Thus while antigen and costimulatory signals were close to those found in mice immunized with ΔActA Lm, inflammatory cytokines remained largely impaired, which may account for the defective T cell priming and differentiation.
Levels of secreted cyclic-di-AMP, a potent bacterial inducer of type I IFN, are comparable between WT and mutant Lm
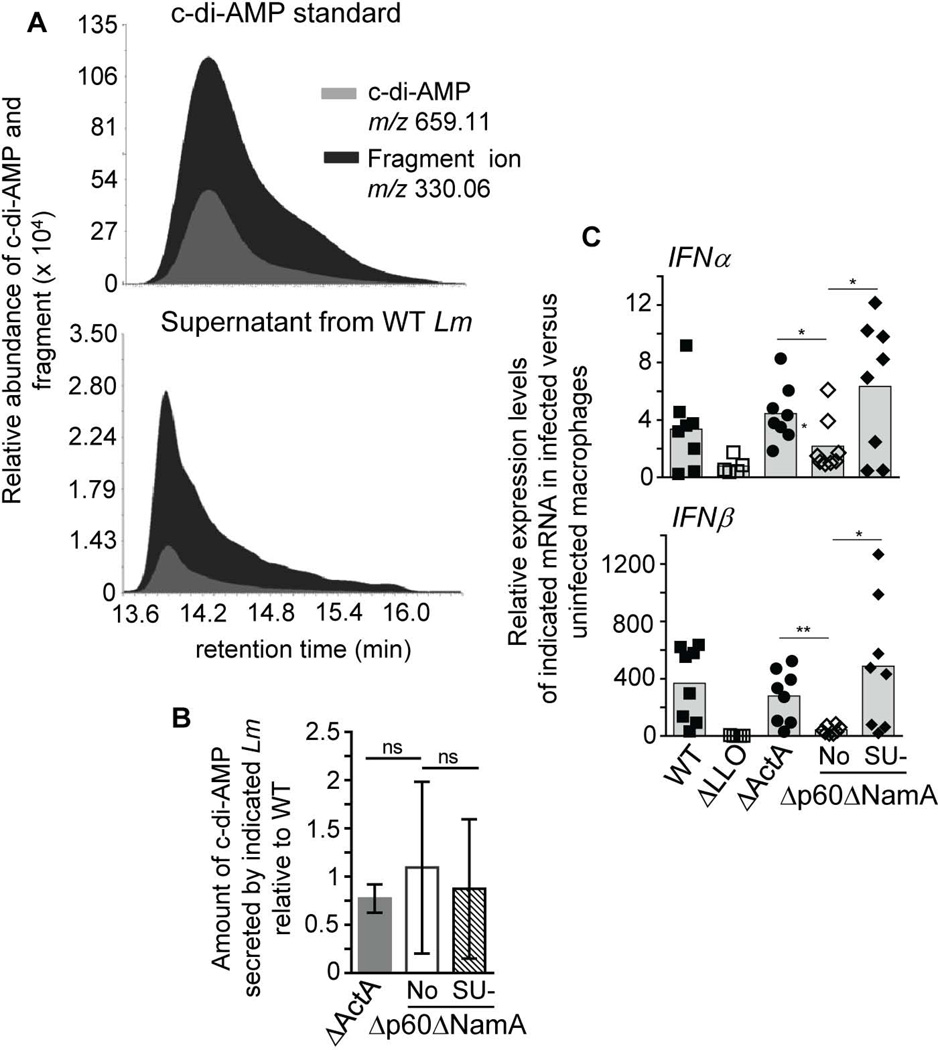
Amongst the inflammatory molecules with impaired secretion in mice immunized with SU Δp60ΔNamA Lm, type I IFN is an important modulator of T cell differentiation (Tough, 2012). Since recent reports provided strong evidence that cyclic diadenosine monophosphate (c-di-AMP) secreted by Lm is a major trigger of the IFN-I pathway (Woodward et al., 2010), we hypothesized that Δp60ΔNamA Lm may secrete a lower amount of this compound. To test this possibility, we analyzed log-phase grown Lm supernatants for the presence of c-di-AMP using ultra-performance liquid chromatography-coupled mass-spectrometry (UPLC-MS). Because the amount of c-di-AMP (659.11 m/z) detected in culture supernatants was very low, we decided to monitor the 330.06 m/z fragment ion, which we could detect at higher levels as seen on representative MS chromatograms (Figure 7A). This ion, a product of in-source fragmentation exhibits the same retention time as intact c-di-AMP but is detected with a ~60% greater intensity, both in a commercial c-di-AMP sample (Invivogen, upper panel) and in a culture supernatant from WT Lm (lower panel). Of note, the quantity detected in the supernatants only represented ~2.5% of the signal detected in the standard (~1.5µg). Using this approach, we compared supernatants from log-phase grown SU-Δp60ΔNamA, Δp60ΔNamA, ΔActA and WT Lm (Figure 7B). As shown from the average of 4 independent replicate quantification experiments, sample variability was high and no significant differences in c-di-AMP abundance could be measured in the culture supernatants from the distinct bacteria. As an additional control, we used supernatants from WT L028 Lm that was reported to over secrete c-di-AMP (Schwartz et al., 2012), and indeed detected substantially greater levels of c-di-AMP secreted by this WT Lm strain compared to WT 10403s (factor >10, not shown). Consistent with this result, infection of bone-marrow macrophages (BMMP) with SU-Δp60ΔNamA, but not ΔLLO or Δp60ΔNamA Lm, induced both IFNα and IFNβ genes to comparable extent as ΔActA or WT Lm (Figure 7C). Thus these results suggest that intrinsic differences in c-di-AMP secretion are unlikely to account for the lower levels of type I IFN measured in the spleen of mice immunized with SU-Δp60ΔNamA Lm, and that the early wave of type I IFN -and possibly other inflammatory cytokines- is equivalent in WT and mutant Lm. Therefore, the lack of overall inflammation in mice vaccinated by SU-Δp60ΔNamA compared to ΔActA Lm is most likely accounted for by inefficient growth of the filamentous mutant in vivo.
Figure 7. Lack of p60 and NamA autolysins neither impairs secretion of cyclic di-AMP, nor early induction of type I IFN genes.
(A) Extracted ion chromatograms for c-di-AMP (m/z 659.11) and its in-source 330.06 m/z fragment ion on commercial c-di-AMP (upper panel) and WT Lm culture supernatant (lower panel). (B) Amount of secreted c-di-AMP 330.06 m/z fragment ion in the supernatant of log-phase grown ΔActA, Δp60ΔNamA and SU Δp60ΔNamA Lm relative to WT in 4 independent replicate experiments. (C) Relative IFNα and IFNβ mRNA expression levels in bone-marrow derived macrophages (WT B6) infected for 6 hrs with indicated mutants or WT Lm (MOI of 5). Histogram bars average mean values with either mean+/− SEM (B) or each symbol representing an individual macrophage tissue culture well replicate (C).
p60 and NamA autolysins contribute to key inflammatory signals required for effective differentiation of protective memory CD8+ T cells in vivo
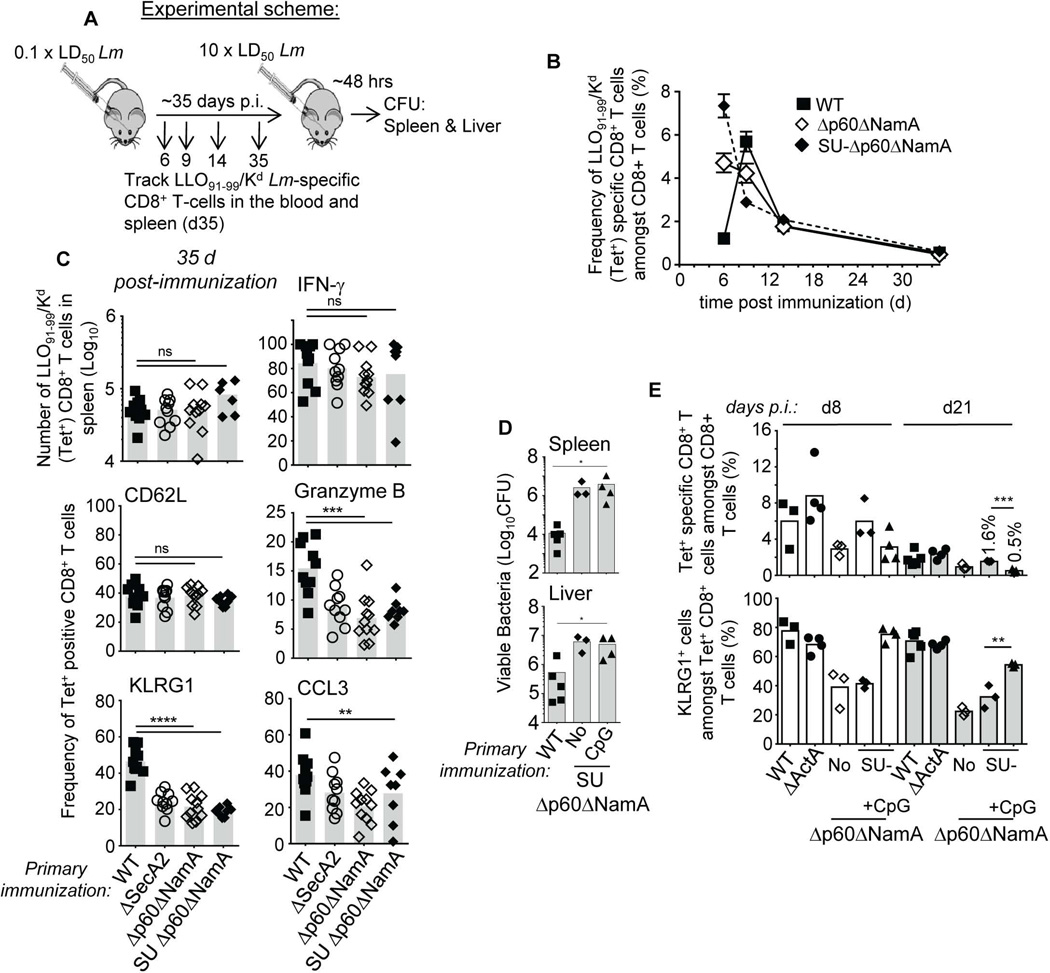
SU-Δp60ΔNamA Lm accessed host cell cytoplasm with comparable efficiency as ΔActA Lm, and promoted robust activation of antigen-presenting cells, yet the inflammatory environment and protective memory development was impaired (Figure 4–Figure 6). While our data favor the idea that the major impact of the p60 and NamA hydrolases on inflammation is related to their direct effect on the ability of Lm to divide and colonize tissues in vivo, it is still possible that the release of PGN digestion fragments or other molecules from Lm by these hydrolases possess PRR-triggering proinflammatory activities. To achieve better understanding of how p60 and NamA autolysins alter CD8+ T cell differentiation, we next monitored CD8+ T cells recognizing the Kd-restricted, naturally presented LLO91–99 epitope from Lm by measuring LLO91–99/Kd (tet+) CD8+ T cell frequencies over time in the blood of WT BALB/c (H-2d) mice immunized with WT, Δp60ΔNamA, SU-Δp60ΔNamA and ΔActA Lm (Figure 8A, B and not shown). We chose to monitor LLO91–99/Kd (tet+) CD8+ T cells as these cells were shown to be sufficient to confer protection to infected host (Harty et al., 1992). While maximum peak frequencies of tet+ cells in mice immunized with WT were measured 7 d post infection, mice that received full length and SU Δp60ΔNamA and ΔActA (not shown) Lm peaked ~2 d earlier, most likely as a result of massive initial antigen loads. In all groups, however, comparable numbers of LLO-specific CD8+ T cells were measured when reaching the memory stage (d 35) (Figure 8C), suggesting that functional differences in LLO-specific memory CD8+ T cells most likely accounted for the lack of effective immunity in mice immunized with SU-Δp60ΔNamA or Δp60ΔNamA Lm compared to those that received WT or ΔActA Lm (Figure 1). Further analysis of LLO-specific memory CD8+ T cells in all groups of immunized mice showed that the proportion of memory CD8+ T cells that expressed effector markers associated with host protection, e.g., CCL3/MIP1Δ, granzyme B (cytolysis) and KLRG1, were significantly diminished in mice that received Δp60ΔNamA, SU Δp60ΔNamA or control ΔSecA2 Lm but not WT or ΔActA Lm (Figure 8C). Consistent with our previous work analyzing mice immunized with ΔSecA2 Lm (Narni-Mancinelli et al., 2007), the proportion of central versus effector memory (CD62Lhi/lo) and of IFN-Δ+ memory CD8+ T cells was comparable between all groups. We could also confirm these observations on the C57BL/6 genetic background, using a transgenic mouse line that expresses H2-Kd on the WT B6 background (Serbina et al., 2003). Altogether, these results supported the idea of an altered pattern of differentiation of Lm-specific memory CD8+ T cells in non-protected immunized mice.
Figure 8. Lm autolysins provide essential inflammatory signals that promote long-term protective memory CD8+ T cells.
(A) Experimental design for (B-E). (B) Kinetics of Lm-specific LLO91–99/Kd+ (tet+) CD8+ T cells in the blood of WT BALB/C mice immunized with indicated Lm. (C) Phenotypic and functional characterization of LLO-specific tet+ splenic memory CD8+ T cells 35 d post immunization with 0.1×LD50 WT, ΔSecA2, Δp60ΔNamA and SU Δp60ΔNamA Lm. In (B, C), blood or spleens were harvested and cells either stained for cell-surface CD8, tet, CD62L, KLRG1 or restimulated ex vivo for 4–6 hrs with the LLO91–99 epitope and stained for intracellular granzyme B, IFN-γ and CCL3 and surface CD8. (D) Titers of viable WT Lm CFU in the spleen and liver of mice immunized with indicated Lm with or without CpG nucleotides, and challenged with WT Lm for 48 hrs. (E) Frequencies of LLO-tet+ CD8+ T cells and expression of KLRG1 in the blood 8 and 21 d after immunization with indicated Lm. Data shown are the pool of 2–4 replicate experiments, each symbol featuring an individual mouse and p-values are indicated. Data plots (B) or histogram bars (C-E) average mean values+/− SEM or mean with each symbol representing an individual mouse.
Results in figure 5C suggested that immunization with SU-Δp60ΔNamA Lm largely fails to rescue strong inflammatory signals at the time of T cell priming, which are known to play key roles in programming T cell differentiation (Haring et al., 2006). As an attempt to rescue protective memory, we provided strong MyD88-dependent inflammatory signals by co-immunizing mice with CpG oligonucleotides and SU-Δp60ΔNamA Lm, challenged these mice five weeks later with WT Lm and determined Lm titers after 2 d (Figure 8D). Whether these mice received CpG or not, bacterial titers in the spleen and the liver were comparable with 2.5–6×106 Lm CFUs, i.e., ~1.5–2 logs more bacteria than in protected mice. Interestingly however, analysis of LLO-specific CD8+ T cells in the blood 8 and 21 d post immunization and before challenging these mice, showed that co-immunization with CpG rescued the differentiation of effector-type KLRG1+ CD8+ T cells, similarly to mice immunized with WT or ΔActA Lm (Figure 8E). However, these cells were quickly lost from the blood of CpG co-immunized mice compared to non-CpG or WT and ΔActA Lm-immunized groups, possibly accounting for the observed lack of protection. In summary, our data suggest that Lm p60 and NamA autolysins promote optimal CD8+ T cell priming by regulating a complex network of inflammatory signals inside infected host cell cytoplasm, resulting from inefficient tissue colonization by filamentous Lm.
Discussion
Our results provide novel evidence that the inability of ΔSecA2 Lm to induce protective memory CD8+ T cells is accounted for by impaired secretion of the p60 and NamA autolysins, two major substrates of this auxiliary secretory pathway. We show that through the control of Lm separation during multiplication, p60 and NamA prevent Lm cording/filamentous phenotype, subsequently promoting efficient invasion of host cells. We also show that p60 and NamA autolysins play a key role in triggering strong inflammation during cytosolic growth of Lm, amongst which type I IFN, IL-12, TNF-α and IFN-γ. Interestingly, once Lm escapes to the cytosol of host cells, whether Lm expressed the autolysins or not, upregulation of cell-surface costimulatory molecules on APCs and recruitment of blood phagocytes were only moderately affected. Altogether, our results suggest that by controlling two essential steps, e.g., cell invasion and cytosolic-driven inflammation, p60 and NamA autolysins act as essential regulators of CD8+ T cell programming and differentiation.
Previous studies have established the requirement for p60 and NamA PGN hydrolases in allowing Lm separation during growth, therefore preventing its filamentation (Wuenscher et al., 1993, Machata et al., 2005). Other reports have also documented that reduced secretion of p60 and NamA accounted for the filamentous, rough colony phenotype of ΔSecA2 Lm, which affected its ability to colonize the host, even though only ~50% of p60 and NamA are secreted through this secretory pathway (Lenz et al., 2002, Lenz et al., 2003). Using ΔSecA2 Lm, we have found that immunized mice did not develop robust CD8+ T cell memory, yet increasing the immunizing dose of ΔSecA2 Lm from 0.1 (usual) to 1×LD50 restored protective T cell responses against challenge infection with WT Lm (Campisi et al., 2011). This suggested that increasing the cytosolic concentration of these Lm proteins that are partially secreted independently of SecA2, could restore signals essential to program protective CD8+ T cell memory. Following up on these early findings, current results show that without p60 and NamA, even with very high immunizing doses of SU Δp60ΔNamA Lm, mice remain unable to mount protective memory responses, consistent with the idea that p60 and NamA account for the inability of ΔSecA2 Lm to induce strong CD8 memory.
Our results highlight two distinct mechanisms by which p60 and NamA Lm autolysins affect T cell differentiation, that are both related to their essential role in allowing separation of Lm bacteria following division. Lack of these enzymes induces remarkable Lm cording with filaments of several tens of microns constituted of ~70–80 units of Lm. Such unusual length represent the first major mechanism that prevents Lm invasion of host cells as a result of a 'physical' impairment, e.g., inability to be efficiently phagocytosed by marginal zone / red pulp macrophages and to be targeted to DCs for efficient shuttling to spleens (Muraille et al., 2005, Neuenhahn et al., 2006, Edelson et al., 2011). In support of this interpretation, filamentous bacteria stay in the blood longer than SU Lm, and mostly grew extracellularly within infected spleens. Interestingly, however, during Mycobacterium tuberculosis (Mtb) infection, a related cording phenotype is indeed essential for bacterial virulence and has been associated with the ability of Mtb to persist within infected hosts (Glickman et al., 2000). In the case of Lm, cytosolic entry and growth is not only critical for successful infection of the host but also for inducing long-term protective memory (Berche et al., 1987). We show that Δp60ΔNamA Lm are unable to enter host cells, therefore providing an explanation as to why mice immunized with this mutant of Lm did not develop effective T cell memory.
The second essential role of p60 and NamA autolysins highlighted by this study relates to their ability to prevent strong inflammation during cytosolic growth, a notion that was already proposed by Lenz and Portnoy (Lenz et al., 2003). By immunizing with single unit (SU) Δp60ΔNamA Lm, we could bypass the initial block in host cell entry and investigate whether the lack of both autolysins during invasion of host cells affects the early innate immune response and T cell priming. In mice immunized with SU -but not full-length- Δp60ΔNamA Lm, secretion of CCL2 and CXCL1, two major chemokines involved in inflammatory Ly6C+ monocyte and neutrophil mobilizations respectively (Kobayashi, 2008, Shi et al., 2011), was restored to similar levels as in mice that received ΔActA Lm. Early recruitment of blood-derived inflammatory monocytes was shown to depend on a first wave of CCL2 secretion, the major ligand involved in Ly6C+ monocytes egress from the bone marrow (Serbina et al., 2006). This initial burst of CCL2 did not require cytosolic growth of Lm but the TLR/MyD88 signaling pathway (Jia et al., 2009). This observation is consistent with our experimental set-up, in which we immunize with high numbers of poorly virulent Lm mutants that enter cells yet grow inefficiently. Of note, Δp60ΔNamA Lm were as hemolytic as WT Lm in vitro, likely ruling out a defect in LLO secretion and escape to the cytosol (not shown). Along the same lines, APC maturation, both splenic CD11chi CD8α+ and CD11b+ DCs and Ly6C+ monocytes, was restored to levels close to those measured in mice immunized with ΔActA but not ΔLLO Lm. Thus, cytosolic escape but not growth, appears sufficient to promote robust blood phagocyte recruitment to infected tissues and strong APC maturation.
Interestingly though, we found that secretion of several inflammatory factors reflecting the triggering of innate immune sensing pathways such as type I IFN were severely impaired. This further suggested that sustained activation of such pathways requires cytosolic growth of Lm as previously reported (O'Riordan et al., 2002). Most importantly, since we neither found reduced secretion of cyclic di-AMP by SU-Δp60ΔNamA or Δp60ΔNamA Lm, nor a defect of early induction of type I IFN genes in infected BMMP, the impaired production of type I IFN measured in vivo is most likely accounted for by inefficient intracellular growth and host tissue colonization of SU-Δp60ΔNamA Lm. Consistent with this interpretation, the secretion of IL-12, IFN-γ and TNF-α in mice immunized with SU-Δp60ΔNamA Lm was not restored to levels observed in mice immunized with ΔActA Lm. Indeed while upon in vitro cutting, access to cells of Δp60ΔNamA Lm could be restored to comparable efficiency than non-filamentous Lm, SU-Δp60ΔNamA Lm most likely still grew as a filament inside infected cells. Thus intracellular growth is probably most important to enhance inflammatory signals in general, which are key for optimal T cell programming (Haring et al., 2006). Overall this impaired triggering of inflammation is accounted for by poor cytosolic growth of filamentous Δp60ΔNamA Lm which is likely associated to the lack of sustained secretion/production of immunogenic products such as cyclic dinucleotides, PGN fragments or others.
Analysis of Lm-induced memory CD8+ T cells in mice that received the distinct immunizations revealed that while frequencies and numbers of LLO-specific effector (CD62Llo) and memory (CD62Lhi) CD8+ T cells were equivalent in all groups, their ability to produce the effector molecules CCL3 and Granzyme B (but not IFN-γ), and to express the marker of strong effector differentiation KLRG1 was diminished in mice immunized with full-length and SU Δp60ΔNamA (or control ΔSecA2) Lm compared to WT or ΔActA Lm. This phenotype correlated with their inability to control the recall infection with WT Lm. Both during SU Δp60ΔNamA and ΔActA Lm immunizations, the CD8+ T cell response against LLO -which is intrinsically protective (Harty et al., 1992)- and upregulation of costimulatory molecules expression on APCs were substantially restored. Inflammation, which provides critical signals during T cell priming, was strongly altered, suggesting that p60 and NamA autolysins trigger cytosolic sensing pathways involved in the secretion of key modulating cytokines. Type I IFN for instance acts as a third signal directly on T cells and sustains their proliferation and differentiation into robust effector and memory cells via anti-apoptotic effects, during various viral infections (Kolumam et al., 2005, Thompson et al., 2006, Kohlmeier et al., 2010). Type I IFN is also a strong inducer of IL-15 trans-presentation by macrophages, monocytes and DCs, which modulates memory T cell homeostatic proliferation and maintenance and/or survival (Mortier et al., 2009). Our data seem to be consistent with type I IFN promoting robust differentiation of effective memory cells, though recent work from the Portnoy group reported that providing increased type I IFN signals via STING/IRF3 during T cell priming by co-immunizing with ΔActA Lm and c-di-AMP prevented the establishment of a strong population of Lm-specific effector and memory CD8+ T cells (Archer et al., 2014), a result supporting an opposite interpretation. KLRG1 expression, however, has not been associated with type I IFN but rather with IL-12 (Joshi et al., 2007). Indeed, IL-12 and subsequent IFN-γ levels in the spleens of mice that received SU Δp60ΔNamA compared to ΔActA Lm remained very low, which may account for the diminished expression of KLRG1 on Lm-specific CD8+ T cells primed in these mice. While as expected (Joshi et al., 2007) co-immunization with CpG, a strong inducer of IL-12, restores KLRG1 expression on these cells, most of these cells were rapidly lost from the spleen of these mice. Altogether, our results and these studies suggests that a fine combination of discrete inflammatory signals, that include type I IFN and IL-12, and likely others, are necessary to generate a highly effective and stable memory CD8+ T cell population.
In summary, results from this study highlight an interesting and novel role for the p60 and NamA Lm autolysins in tuning an appropriate inflammatory balance required to promote long-lived functional memory CD8+ T cells during Lm immunization. It further underlines their potential promise as novel adjuvants for optimal CD8+ T cell-priming in vaccine formulations.
Experimental Procedures
Ethic Statement
Animal studies were conducted in accordance to the National Institutes of Health guidelines in compliance with assurance of the well being of laboratory animals. All protocols used in the study have been approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine (protocol # 20120509). The Albert Einstein College of Medicine of Yeshiva University (Einstein) adheres to all applicable federal, state, local and institutional laws, standards, and guidelines designed to ensure humane use of animals in research, testing, and teaching. Such laws, standards, and guidelines include but are not limited to (i) the Guide for the Care and Use of Laboratory Animals, (ii) the Animal Welfare Act and (iii) PHS Policy on Humane Care and Use of Laboratory Animals.
Mice
WT BALB/c (Harlan) or C57BL/6J (Jackson) 6–8 wk-old male or female mice were used in all experiments as specified in the figure legends. In some experiments, the B6-Kd+/+ (Serbina et al., 2003) transgenic line was used. All mice utilized for experiments were housed and bred in our SPF animal facility at the AECOM).
Generation of recombinant Listeria monocytogenes
Generation of GFP-expressing bacteria
2–5 µg of PEG-purified pNF8 (Fortineau et al., 2000) was electroporated into Δp60NamA Lm that were prepared for electroporation according to standard procedures. Transfected-bacteria were selected onto broth heart infusion (BHI, Sigma Aldrich) plates with 10 µg/ml erythromycin and GFP-expression checked by flow cytometry (FACSCalibur). GFP+ WT, ΔSecA2, ΔLLO Lm were generated previously (Muraille et al., 2007). All experiments were performed with the 10403s strain of Lm.
Generation of Lm lacking p60 and NamA autolysins
Sequences encompassing genomic DNA flanking the gene encoding the p60 protein (iap) and the 5’ (fragment 1) or 3’ (fragment 2) end of the gene were amplified by PCR (HF Advantage 2, Clontech) to generate a deleted p60 chimeric construct that contains targeting sequences for genomic recombination and for knocking out Lm p60 gene. We used Fw1 5’ACGGATCCTGTTGAAGCAATGATAGTGCCG3’ and Rv1 5’ATCGGCACCTGATAGCTAAGCTCATTCTGGCGCACAATACGCTA3’ and, Fw2 5’TGAGCTTAGCTATCAGGTGCCGATAAATGCTGTTACCGCAATCCC3’ and Rev2 5’ACGGATCCCCAAATTCAGAAGCCGTTCCA3’ to amplify both fragments and clone them using the BamHI restriction site directly into the pKSV7 transfer vector. The resulting construct was verified by PCR and sequencing (AECOM sequencing facility). pKSV7 which contains the p60 targeting construct, carries a resistance to ampicillin (in Escherichia coli, 100µg/ml) and to chloramphenicol (Cam) (in Lm, 10 µg/ml) and a temperature-sensitive origin of replication (restrictive temperature of 41°C) and was transformed into ΔmurA/ΔNamA Lm (Lenz et al., 2002). Recombinant ΔNamA Lm mutated for p60 were selected through cycles of excision/recombination that include Cam and temperature restrictive growth as previously described (Rahmoun et al., 2011). Deletion of the p60 gene was checked by PCR using sets of primers outside the targeted area.
Preparation of single unit (SU) ΔNamAΔp60 Lm
Log phase grown ΔNamAΔp60 (ΔiapΔmurA) Lm were pelleted and resuspended in PBS containing 5 µg/ml of purified recombinant p60 (rp60) and NamA (rNamA) and incubated for 1 hour (37C, 100 RPM). Cells were then washed twice in PBS 1X prior to infection. For intermediate (Int-) ΔNamAΔp60 Lm, ΔNamAΔp60 Lm was grown in a 1:1 ratio of fresh BHI media and filtered (0.22 µm) BHI supernatant from OVN culture of ΔLLO Lm.
Cloning, expression and purification of Lm autolysins
DNA encoding for p60 and NamA devoid of the putative sequence encoding for their signal peptides were amplified by PCR (HF Advantage 2, Clontech) using the following primers: for p60, Fw 5’AGTCATATGGTAGTAGTCGAAGCTGGTGATACTC3’ and Rv 5’AGTGGATCCTTATACGCGACCGAAGCC3’; and for NamA, Fw 5’AGTCATATG GACGAAACAGCGCCTG3’ and 5’TCAGGATCC TCACTTAATTGTTAATTTCTGACCAAGA3’ (NamA). Primers include NdeI and BamHI cloning sites and the PCR products were inserted into the IPTG-inducible expression vector pET14b (clontech) in frame (N-terminus) with MCS-encoded 6×histidine tag and a thrombin cutting site. Positive clones were screened by restriction digest and both constructs verified by sequencing. rp60 and rNamA were further produced and purified (AECOM protein core facility) by nickel affinity and size exclusion chromatography from IPTG-induced log-phase grown (OD600~0.8) BL-21 Escherichia coli bacterial pellets expressing either of the 2 constructs. Purified proteins were verified by mass spectrometry, aliquoted and stored in Tris buffer pH7.4 40% glycerol at −80C.
In vitro analysis of Lm mutants
Indicated Lm strains were inoculated into fresh BHI medium from log-phase frozen aliquots at a 1/10th dilution and grown 37C, 220 RPM. Colony morphology was visualized from log phase grown Lm plated on BHI agar plates (37C) for 12 hours. Images were taken using a Nikon light microscope. To visualize bacteria and count the number of Lm single unit per filament, log phase Lm were washed in PBS, heat fixed onto glass slides and Gram stained before imaging. For determination of Lm sizes by FACS, indicated GFP+ Lm were grown to log phase in BHI, washed twice in PBS 1X and acquired on a FACSCalibur flow-cytometer.
Infection of Bone Marrow derived Macrophages (BMMP)
Fresh bone marrow cells were obtained from femurs of 5 weeks (wks) old WT B6 mice. Bone marrow cells were differentiated for 7–10 days into bone-marrow derived macrophages (BMMP) using L929-cell conditioned medium (30% L929 in 1640 RPMI-10% FCS Complete Medium, Gibco) in tissue culture Petri dish. Approximately 106 cells were seeded in 6 well plates until confluent. Cells were then infected with log phase grown Lm (MOI of 10) in RPMI1640 with 2.5% FCS and incubated (37C, 5% CO2) for 1 hour. Excess bacteria were removed by centrifugation, incubated for 1 hr with media supplemented with 100 µg/ml gentamicin to kill extracellular bacteria. At indicated times, MP were lysed in 1% Triton X-100 and dilutions were further plated onto BHI plates to determine Lm titers.
Quantitative RT-qPCR analysis
BMMP were infected as described with 5 bacteria per target cells (MOI of 5) using ΔNamAΔp60, SU-ΔNamAΔp60, ΔLLO, ΔActA and WT Lm and left for 6 hrs (37C, CO2) before harvesting for RNA extraction. RNA was extracted using the RNAqueous Micro Kit (Ambion) and cDNA was synthesized with SuperScript III Reverse Transcriptase (Invitrogen). Quantitative RT-PCR was performed with an Applied Biosystem Prism 7300 fast real time PCR system using Power SYBR green PCR Master Mix (Life Technologies). Primer sequences for Ifnα and Ifnβ genes were chosen according to previous studies (Kim et al., 2012). Briefly, “Universal” primers were designed to target multiple Ifnα variants (GTGAGGAAATACTTCCACAG, GGCTCTCCAGACTTCTGCTC). The following primers were chosen for Actin (GGCTGTATTCCCCTCCATCG, CCAGTTGGTAACAATGCCATGT) and Ifnβ (CAGCTCCAAGAAAGGACGAAC, GGCAGTGTAACTCTTCTGCAT). The cycling threshold (Ct) of gene transcripts was determined by RT-qPCR and normalized to the Ct of beta-actin for calculation of the ΔCt values. The relative expression of genes for each sample was determined by calculation of the 2−ΔΔCt value.
Mice infections and other treatments
All WT Lm were prepared from clones grown to log phase from organs of infected mice and kept at −80°C. For Lm infections, bacteria were grown to a logarithmic phase (OD600~0.05–0.15) in BHI medium, diluted in PBS to infecting concentration (an OD600 of 0.1 corresponds to ~2×108 Lm/ml for WT Lm) and injected i.v. either in the retro-orbital or tail veins. For filamentous mutants, i.e., Δp60ΔNamA and ΔSecA2 Lm, we have established by comparative plating of exponentially growing Δp60ΔNamA with or without in vitro cutting with recombinant p60 and NamA proteins, that an OD600 of 0.1 corresponded to ~1.12×108 Lm/ml (not shown).
Determining Lm titers in organs and blood
To determine bacterial titers, organs were harvested under sterile conditions and dissociated on sterile metal meshes in 0.1% triton X-100 in water. Successive dilutions were plated onto BHI agar plates to determine the total bacterial CFU per organ. For blood, mice were bled retro-orbitally (200 µl), which was lysed vol/vol with in 0.1% triton X-100/water, diluted and plated on BHI Petri dishes.
Other treatments
For gentamicin, mice were injected i.p. 2 mg in 500 µl PBS. For CpG, 50µg of CpG ODN 1826 (Invivogen) were injected i.v. 1 hr post immunization with ΔNamAΔp60 Lm.
UPLC Mass Spectrometry (MS) analysis of secreted c-di-AMP
Preparation of samples for MS analysis
WT, ΔActA, Δp60ΔNamA on 10403s background or WT L028 Lm were inoculated in BHI from −80C frozen stocks and grown OVN (37C, 180RPM). The next day, stationary phase Lm were transferred to fresh BHI medium (1/10) and grown to OD600~0.5, washed once into Improved Minimal Media (IMM) (Phan-Thanh et al., 1997) before growth in IMM for 19 hrs (37°C, 180RPM). Identical numbers of Lm from cultures were corrected based on OD600 measurements, centrifuged, supernatants filtered (0.22µm), and 1 ml was further dried to 1200 µl (speedvac). Protein precipitation was performed by adding 400 µl of methanol and 400 µl of acetonitrile per sample, insoluble material was pelleted by centrifugation (13,000 RPM, 1’), and supernatant was filtered again through a 0.22µm spin column filter.
UPLC-MS analysis
Culture supernatants were analyzed by UPLC-MS to quantitate c-di-AMP using previously reported method (Paglia et al., 2012). A Waters Acquity UPLC system coupled to a Waters Synapt G2 quadrupole-time of flight hybrid mass spectrometer was used to separate and detect metabolites. A Waters Acuity amide column 1.7µm (2.1 × 150mm) was used to separate metabolites in HILIC mode, eluted at 0.5mL/min with 100% acetonitrile, 0.1% formic acid as mobile phase A and 100% water, 0.1% formic acid as mobile phase B. The gradient began with 1% B until 1 min, ramped to 35% B by 14 min, then 60% B by 17 min, held at 60% B for 1 minute, then ramped to 1% B by 19 min and held at 1% B to the end of run at 20 min. The mass spectrometer was operated in positive ion resolution mode with 2kV capillary voltage and 17V cone voltage. Cone gas flow was 20L/hr; desolvation gas flow was 800L/hr. Source temperature was 120°C and desolvation gas temperature was 500°C. Using a scan time of 0.5s, mass spectra were acquired in MSe centroid mode from 50 – 1200 m/z. The lock mass compound leucine enkephalin at 2ng/µL was detected at 556.2771 m/z. An authentic standard of c-di-AMP (Invivogen) was used to determine retention time and high-energy fragmentation pattern under our conditions. Extracted ion chromatograms for 659.11 ± 0.02 m/z and the 330.06 m/z fragment ion peaks corresponding to this retention time (13.9 min) with the same high-energy fragmentation patterns were integrated to determine relative quantities of c-di-AMP secreted by each strain.
Cell preparation for flow-cytometry analysis
For blood, approximately 200 µl was collected from mice via retro-orbital bleeding and lysed for 5 min in 0.83% NH4Cl v/v with culture media. For spleens, they were harvested and dissociated on nylon meshes and incubated (37C, 30') in HBSS (Gibco) medium containing 4000 U/ml of collagenase IV (Roche) and 0.1 mg/ml of DNase (Sigma). Red blood cells were then lysed for 5 min.
Flow cytometry analysis
Cell suspensions prepared as above were recovered in FACS buffer (PBS 1% FCS, 2mM EDTA, 0.02% sodium azide). Briefly, 3–5×106 spleen cells or 105 blood cells were incubated with 2.4G2 for 15 min on ice and further stained with specified antibodies cocktail using directly coupled fluorescent mAbs combinations in 100 µl PBS 0.5% BSA 0.02% NaN3 (FACS buffer). For intracellular staining (IC), cells were incubated for 3–4 hrs at 37°C, 5% CO2 in RPMI1640 (Invitrogen) 5% FCS, Golgi Plug (BD), fixed in IC fixation buffer (eBioscience) for 15 min at 4°C, and permeabilized for 30 min in 1X Perm/Wash buffer (BD or eBioscience) containing the intracellular mAbs. Stained samples were further collected on either FACSCalibur, LSR-II, or Aria III flow cytometers (Becton Dickinson, BD). The following mAbs were used and purchased either from BD Pharmingen or eBioscience: anti-CD8α (53–6.7)-fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyl protein (PcP) or allophycocyanin (APC), anti-CD3ɛ-FITC (145-2C11), anti-CD62L-A700 (MEL-14), anti-CD11c-Pe-Cy7 (N418), anti-CD11b-eFluor 450 or PcP-Cy5.5 (M1/70), anti-Ly-6C-FITC (AL-21), anti-CD45.2-A700 (104), anti-MHC Class II-A700 (M5/114.12.2), anti-CD80-PE (1615A1), anti-CD86-A700 (GL1), anti-CD40-A700 (1C10), anti-ScaI-A700 (D7), anti-IL-15Rα-PE (DNT15Rα), anti-KLRG1-PE-Cy7 (MAFA 2F1), anti-IFNγ-APC or A700 (XMG1.2).
Anti-Granzyme B-APC (mouse and human) and anti-CCL3 (polyclonal goat) were purchased from Invitrogen and R&D systems respectively. H2-Kd/LLO91–99 tetramers coupled to PE were obtained from the NIH tetramer core facility.
Measure of splenic cytokines and chemokines contents
Spleens from immunized mice were harvested, snap-frozen and stored at −80C until further processing. Spleens were then thawed in 150 µl of processing buffer (150mM NaCl, 40mM Tris pH 7.4) containing a cocktail of protease inhibitors (Promega), homogenized with a douncer and froze/thawed once before centrifugation to obtain cleared supernatants to aliquot and store at −80C. Dosing of indicated cytokines/chemokines in cleared supernatants was performed using flow-cytomix (ebioscience) on an Aria III flow cytometer and according to the manufacturer protocol. IL-12 was measured using an IL-12p70 specific ELISA (ebioscience).
Statistics
Statistical significance was calculated using an unpaired Student t test with GraphPad Prism software and two-tailed p values are given as: (*) p<0.1; (**) p<0.01; and (***) p<0.001; (ns) p>0.1. All p values of 0.05 or less were considered significant and are referred to as such in the text.
Supplementary Material
Acknowledgments
H2-Kd/LLO91–99 tetramers were obtained through the NIH Tetramer Facility. This work was supported by institutional funds of the Albert Einstein College of Medicine of Yeshiva University (GL), the National Institute of Health (Grants R21AI095835 and R01AI103338). Core resources that facilitated flow cytometry were supported by the Einstein Cancer Center (NCI cancer center support grant 2P30CA013330).
Footnotes
Author Contributions
C.C. and S.M.S. designed the study, performed most experiments, analyzed the data and contributed to writing of the paper. B.W. ran and interpreted the Mass Spectrometry, and contributed to writing of the paper. M.G. performed some of the protection and T cell assays in vivo. A.F. made the autolysin expression constructs. W.R.J. contributed to critical reading of the paper. G.L. designed the study, contributed to experiments, analyzed the data and wrote the paper.
The authors declare that no conflict of interest exists.
References
- Archer KA, Durack J, Portnoy DA. STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes. PLoS Pathog. 2014;10:e1003861. doi: 10.1371/journal.ppat.1003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berche P, Gaillard JL, Sansonetti PJ. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987;138:2266–2271. [PubMed] [Google Scholar]
- Beuneu H, Deguine J, Bouvier I, Di Santo JP, Albert ML, Bousso P. A dual role for type I IFNs during polyinosinic-polycytidylic acid-induced NK cell activation. J Immunol. 2011;187:2084–2088. doi: 10.4049/jimmunol.1004210. [DOI] [PubMed] [Google Scholar]
- Campisi L, Soudja SM, Cazareth J, Bassand D, Lazzari A, Brau F, et al. Splenic CD8alpha(+) dendritic cells undergo rapid programming by cytosolic bacteria and inflammation to induce protective CD8(+) T-cell memory. Eur J Immunol. 2011;41:1594–1605. doi: 10.1002/eji.201041036. [DOI] [PubMed] [Google Scholar]
- Edelson BT, Bradstreet TR, Hildner K, Carrero JA, Frederick KE, Kc W, et al. CD8alpha(+) Dendritic Cells Are an Obligate Cellular Entry Point for Productive Infection by Listeria monocytogenes. Immunity. 2011;35:236–248. doi: 10.1016/j.immuni.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortineau N, Trieu-Cuot P, Gaillot O, Pellegrini E, Berche P, Gaillard JL. Optimization of green fluorescent protein expression vectors for in vitro and in vivo detection of Listeria monocytogenes. Res Microbiol. 2000;151:353–360. doi: 10.1016/s0923-2508(00)00158-3. [DOI] [PubMed] [Google Scholar]
- Glickman MS, Cox JS, Jacobs WR., Jr A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol Cell. 2000;5:717–727. doi: 10.1016/s1097-2765(00)80250-6. [DOI] [PubMed] [Google Scholar]
- Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Harty JT, Bevan MJ. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992;175:1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia T, Leiner I, Dorothee G, Brandl K, Pamer EG. MyD88 and Type I interferon receptor-mediated chemokine induction and monocyte recruitment during Listeria monocytogenes infection. J Immunol. 2009;183:1271–1278. doi: 10.4049/jimmunol.0900460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CC, Nelson CS, Wilson EB, Hou B, DeFranco AL, DeRisi JL. Splenic red pulp macrophages produce type I interferons as early sentinels of malaria infection but are dispensable for control. PLoS One. 2012;7:e48126. doi: 10.1371/journal.pone.0048126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33:96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz LL, Mohammadi S, Geissler A, Portnoy DA. SecA2-dependent secretion of autolytic enzymes promotes Listeria monocytogenes pathogenesis. Proc Natl Acad Sci U S A. 2003;100:12432–12437. doi: 10.1073/pnas.2133653100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz LL, Portnoy DA. Identification of a second Listeria secA gene associated with protein secretion and the rough phenotype. Mol Microbiol. 2002;45:1043–1056. doi: 10.1046/j.1365-2958.2002.03072.x. [DOI] [PubMed] [Google Scholar]
- Machata S, Hain T, Rohde M, Chakraborty T. Simultaneous deficiency of both MurA and p60 proteins generates a rough phenotype in Listeria monocytogenes. J Bacteriol. 2005;187:8385–8394. doi: 10.1128/JB.187.24.8385-8394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier E, Advincula R, Kim L, Chmura S, Barrera J, Reizis B, et al. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity. 2009;31:811–822. doi: 10.1016/j.immuni.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Moseman EA, McGavern DB. The great balancing act: regulation and fate of antiviral T-cell interactions. Immunol Rev. 2013;255:110–124. doi: 10.1111/imr.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraille E, Giannino R, Guirnalda P, Leiner I, Jung S, Pamer EG, Lauvau G. Distinct in vivo dendritic cell activation by live versus killed Listeria monocytogenes. Eur J Immunol. 2005;35:1463–1471. doi: 10.1002/eji.200526024. [DOI] [PubMed] [Google Scholar]
- Muraille E, Narni-Mancinelli E, Gounon P, Bassand D, Glaichenhaus N, Lenz LL, Lauvau G. Cytosolic expression of SecA2 is a prerequisite for long-term protective immunity. Cell Microbiol. 2007;9:1445–1454. doi: 10.1111/j.1462-5822.2007.00883.x. [DOI] [PubMed] [Google Scholar]
- Narni-Mancinelli E, Campisi L, Bassand D, Cazareth J, Gounon P, Glaichenhaus N, Lauvau G. Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes. J Exp Med. 2007;204:2075–2087. doi: 10.1084/jem.20070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenhahn M, Kerksiek KM, Nauerth M, Suhre MH, Schiemann M, Gebhardt FE, et al. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity. 2006;25:619–630. doi: 10.1016/j.immuni.2006.07.017. [DOI] [PubMed] [Google Scholar]
- O'Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc Natl Acad Sci U S A. 2002;99:13861–13866. doi: 10.1073/pnas.202476699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia G, Hrafnsdottir S, Magnusdottir M, Fleming RM, Thorlacius S, Palsson BO, Thiele I. Monitoring metabolites consumption and secretion in cultured cells using ultra-performance liquid chromatography quadrupole-time of flight mass spectrometry (UPLC-Q-ToF-MS) Anal Bioanal Chem. 2012;402:1183–1198. doi: 10.1007/s00216-011-5556-4. [DOI] [PubMed] [Google Scholar]
- Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- Phan-Thanh L, Gormon T. A chemically defined minimal medium for the optimal culture of Listeria. Int J Food Microbiol. 1997;35:91–95. doi: 10.1016/s0168-1605(96)01205-6. [DOI] [PubMed] [Google Scholar]
- Rahmoun M, Gros M, Campisi L, Bassand D, Lazzari A, Massiera C, et al. Priming of protective anti-Listeria monocytogenes memory CD8+ T cells requires a functional SecA2 secretion system. Infect Immun. 2011;79:2396–2403. doi: 10.1128/IAI.00020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz KT, Carleton JD, Quillin SJ, Rollins SD, Portnoy DA, Leber JH. Hyperinduction of host beta interferon by a Listeria monocytogenes strain naturally overexpressing the multidrug efflux pump MdrT. Infect Immun. 2012;80:1537–1545. doi: 10.1128/IAI.06286-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Shen H, Slifka MK, Matloubian M, Jensen ER, Ahmed R, Miller JF. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci U S A. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory Monocytes Activate Memory CD8(+) T and Innate NK Lymphocytes Independent of Cognate Antigen during Microbial Pathogen Invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol. 2006;177:1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- Tough DF. Modulation of T-cell function by type I interferon. Immunol Cell Biol. 2012;90:492–497. doi: 10.1038/icb.2012.7. [DOI] [PubMed] [Google Scholar]
- Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuenscher MD, Kohler S, Bubert A, Gerike U, Goebel W. The iap gene of Listeria monocytogenes is essential for cell viability, and its gene product, p60, has bacteriolytic activity. J Bacteriol. 1993;175:3491–3501. doi: 10.1128/jb.175.11.3491-3501.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.