Abstract
Anti-tuberculosis drugs have some adverse effects such as anti-tuberculosis drug-induced liver injury (ATDILI) and mental disorders. The involvement of glutathione S-transferase (GST) genes in pathogenesis of ATDILI or schizophrenia (SCZ) has been reported. Therefore, GST genes may exemplify molecular connectors between ATDILI and SCZ. However, association studies of GSTM1/T1 polymorphisms with these two diseases have yielded conflicting results. After searching case-control association studies in PubMed, ISI Web of Science, EMBASE, Chinese National Knowledge Infrastructure (CNKI), and Chinese BioMedical Literature Database, we performed meta-analyses across a total of 20 published association studies on 3146 subjects for the association of GSTM1 and ATDILI, 2587 for the GSTT1-ATDILI association, 2283 for GSTM1-SCZ and 1116 for GSTT1-SCZ to test the associations of GSTM1/T1 polymorphisms with ATDILI and SCZ. The GSTM1 present genotype was significantly associated with decreased risks of ATDILI (risk ratio(RR): 0.81, 95% confidence interval (CI): 0.75–0.88, P < 0.0001) and SCZ (RR: 0.88, 95%CI: 0.80–0.96, P = 0.004) according to the fixed-effect model, while the GSTT1 present genotype was significantly associated only with a high risk of SCZ (RR: 1.17, 95%CI: 1.04–1.32, P = 0.01) according to both the random- and fixed-effect models, but not with ATDILI (P = 0.82) according to the fixed-effect model. Moreover, these significant results were supported with moderate evidence according to the Venice criteria. These results indicate that GSTM1 represents a genetic connection between ATDILI and SCZ, and suggest that ATDILI and SCZ may be co-occurring for the subjects with GSTM1 null genotype.
Introduction
Tuberculosis (TB) remains a devastating disease and the major leading cause of death worldwide. In 2011, an estimated 8.7 million new TB cases were reported, and 1.4 million people died from TB [1]. First-line therapeutic agents, such as isoniazid (INH), rifampin (RIF) and pyrazinamide (PZA) are effective treatments for TB [2]. However, these drugs can induce various adverse effects, among which anti-tuberculosis drug-induced liver injury (ATDILI) is the most common and serious side effect [3–5]. ATDILI, caused by the drugs’ reactive metabolites instead of their direct toxicities, has been widely suggested to be a Glutathione S-transferases (GSTs) related disease [6–8].
Schizophrenia (SCZ) is a severe, disabling and chronic mental disorder affecting approximately 1% of the general population [9,10]. Oxidative metabolite damage to neuronal cells is considered to be one of the risk factors for the development of Schizophrenia [11]. Several lines of evidence have suggested that GSTs can modulate the progress of SCZ, given that GSTs and glutathione-related enzymes can detoxify oxidative damage products [12–14]. Thus, GST genes are hypothesized to play an important role in both ATDILI and SCZ, acting as ‘molecular bridges’ between these two diseases.
As phase II detoxification enzymes, GSTs have important protective effects of detoxification of anti-TB drugs’ reactive metabolites and products of oxidative stress through conjugating glutathione with target toxic substances and facilitating their elimination from the body [12,15]. GSTs comprise a superfamily of ubiquitous, multifunctional enzymes with two main genes being the glutathione S-transferase Mu-1 (GSTM1) gene, located on chromosome 1p13.3,which codes for cytosolic GST class Mu 1 enzyme, and the glutathione S-transferase theta-1 (GSTT1) gene, located on chromosome 22q11.2, which codes for cytosolic GST class theta 1 enzyme [16,17]. Both GSTM1 and GSTT1 have a null mutation consisting of the complete deletion of the respective gene through homologous unequal crossing over [18–20]. Homozygous null mutations of GSTM1 and GSTT1 gene can cause the absence of GST activity, and thus may induce different diseases, including ATDILI and SCZ. However, so far the studies testing the association between GSTM1/T1 polymorphisms and SCZ or ATDILI have produced conflicting results [21–23].
To assess whether the GST genes represent ‘molecular connectors’ between ATDILI and SCZ, we have performed meta-analyses of published studies that test the association between GSTM1/T1 polymorphisms and ATDILI or SCZ.
Materials and Methods
Literature search strategy
The digital medical databases PubMed, ISI Web of Science, EMBASE, Chinese National Knowledge Infrastructure (CNKI) and Chinese BioMedical Literature Database were searched for studies with publication date up to Nov. 30 2013 using the following keywords: (‘anti-tuberculosis drug-induced liver injury’, ‘anti-tuberculosis drug-induced hepatotoxicity’, ‘ATDILI’ or ‘ATDILI’) and (‘Schizophrenia’), respectively, combined with (‘glutathione S-transferase’, ‘GST’, ‘GSTM’, ‘GSTM1’, ‘GSTT’, or ‘GSTT1’). Furthermore, references of retrieved articles were also reviewed for the literature that they cite.
Inclusion and exclusion criteria
Articles included in the meta-analysis complied with the following criteria: 1) original case-control association studies with complete data were based on unrelated, randomly selected individuals; 2) cases were TB patients with ATDILI and controls were TB patients without ATDILI, or cases were Schizophrenic patients and controls were healthy subjects; 3) both cases and controls were matched for sex and age; 4) association studies of GSTM1/T1 polymorphisms with SCZ or ATDILI. Other studies, such as: case-only studies, duplications, animal studies, comparisons of laboratory methods, editorials, and review articles were excluded.
Data extraction
Data extraction was performed independently by two reviewers using a standardized protocol and reporting form. The discrepancy between the two reviewers was resolved by further discussion with a third party. For overlapping studies, the study with the larger sample size was retained for the meta-analysis. The recorded study characteristics included: 1) first author’s name; 2) publication year; 3) sample ethnicity; 4) number of cases and controls for GSTM1/T1 null and present genotypes; 5) control and case characteristics; 6) sex proportion; 7) mean age of cases and controls; 8) methods used for genotyping; and 9) PCR primers and amplification regions.
Statistical analysis
The strengths of the associations between GSTM1 and GSTT1 polymorphisms and the risks of ATDILI or SCZ were measured using risk ratios (RRs) with corresponding 95% confidence intervals (CIs)[24]. Pooled RRs were calculated for null vs. non-null (present) genotypes. Two models of meta-analysis for calculating the pooled RRs were applied, the random-effect model and the fixed-effect model, using the DerSimonian–Laird and Mantel-Haenszel methods, respectively [25,26]. The former assumes that the study samples are taken from populations with varying effect sizes, calculating the study weights both from within-study and between-study variances, while the latter assumes that the study samples are drawn from populations with the same effect size, making an adjustment to the study weights on the basis of the within-study variance [27]. Between-study heterogeneity was assessed with the Chi-square-based Q-test (Cochran’s Q statistic), and P <0.1 was considered statistically significant [28]. The I² statistic was also calculated to quantify the proportion of the total variation due to heterogeneity, and I² >50% was considered to be statistically significant [29]. If the P value of the heterogeneity test was > 0.1, pooled RRs were evaluated according to the fixed-effect model. Otherwise, the pooled RRs were calculated according to the random-effect model. Subgroup analyses were performed to evaluate racial effects. Power analysis was performed using the Power and Sample Size Program with α = 0.05 as the level of significance and the effects sizes estimated from the meta-analyses [24]. A sensitivity analysis in which one study at a time was removed and the remainder analyzed was conducted to evaluate whether the results could have been affected significantly by a single study. Publication bias was assessed through the Egger weighted regression method and Begg’s test, and P < 0.05 was considered representative of statistically significant publication bias [30]. All statistical analyses were performed using Review Manger 5.2 (The Nordic Cochrane Centre, Copenhagen, Denmark) and Stata version 11.2 (Stata Corporation, College Station, TX, USA).
Credibility of meta-analysis results
The cumulative evidence for the genetic association of GSTM1 and GSTT1 present genotypes with ATDILI/SCZ, respectively, was assessed according to the Venice interim criteria including amount of evidence, replication of results and protection from bias [31]. With regard to assessment of amount of evidence, grade A was given for nminor >1,000, grade B for 100≤nminor≤1,000 and grade C for nminor <100. Here, the nminor referred to the total number of cases and controls with the least frequent genotype. To assessment of replication, grade A was given for I²<25%, grade B for 25%≤I²≤50% and grade C for I²>50%. To assess bias protection, any of following criteria were required: 1) clear phenotype definition; 2) high genotyping quality with a low genotyping error rate; 3) no loss of significance when the first published study was excluded; and 4) no evidence of small-study effects based on a Harbord regression test (significance, P<0.05) [32].
Results
Characteristics of the included studies
A flow diagram summarizing the study selection process was shown in Fig 1. A total of 15 and 19 potentially relevant studies on the associations between GSTM1/T1 polymorphisms and respective risk of ATDILI and SCZ, respectively, were identified after firstly screening based on the titles and abstracts of the candidate articles. After the second screening, totally, there were 965 cases and 1881 controls in 13 studies of GSTM1 and ATDILI, 811 cases and 1476 controls in 11 studies of GSTT1 and ATDILI, 1101 cases and 1182 controls in 5 studies of GSTM1 and SCZ, and 568 cases and 548 controls in 3 studies of GSTT1 and SCZ. The detailed characteristics of each study are listed in S1 Table. All these studies were confirmed to relate to the same complete loss of gene mutation of GSTM1 or GSTT1. The genotype distributions across all studies for cases and controls of ATDILI and SCZ are shown in Table 1 and Table 2, respectively.
Fig 1. A flow diagram of the study selection process.
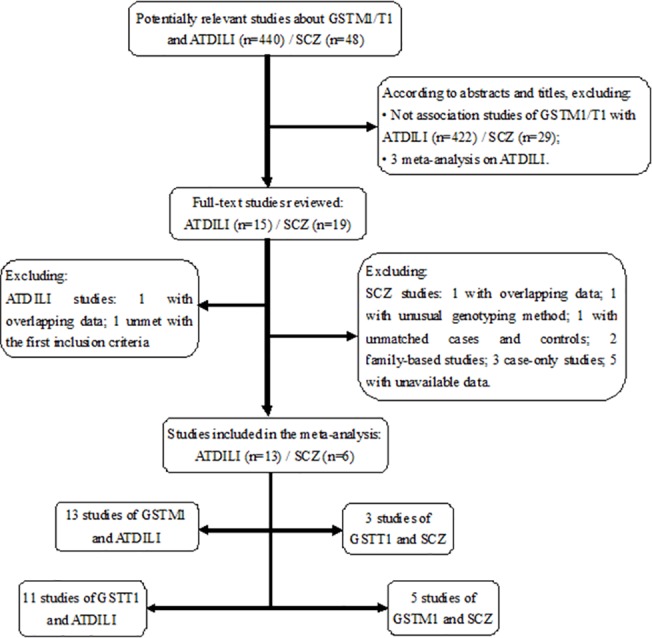
Table 1. Genotype distributions of GSTM1/T1 polymorphisms among ATDILI as cases and non-ATDILI as controls.
| First author, Publication year | Race | Cases | Controls | ||||
|---|---|---|---|---|---|---|---|
| Null | Present | Total | Null | Present | Total | ||
| An, 2010 | East Asian | 64 | 37 | 101 | 55 | 52 | 107 |
| Chatterjee, 2009 | Caucasian | 25 | 26 | 51 | 49 | 51 | 100 |
| Guo, 2009 | East Asian | 50 | 56 | 106 | 25 | 81 | 106 |
| Huang, 2007 | East Asian | 42 | 21 | 63 | 29 | 34 | 63 |
| Leiro, 2008 | Caucasian | 12 | 23 | 35 | 25 | 35 | 60 |
| Monteiro, 2012 | Caucasian | 21 | 38 | 59 | 34 | 84 | 118 |
| Roy, 2001 | Caucasian | 17 | 16 | 33 | 8 | 25 | 33 |
| Sotsuka, 2011 | East Asian | 12 | 8 | 20 | 50 | 42 | 92 |
| Tang, 2012 | East Asian | 55 | 34 | 89 | 203 | 153 | 356 |
| Teixeira, 2011 | Caucasian | 11 | 15 | 26 | 61 | 80 | 141 |
| Wang, 2010 | East Asian | 63 | 41 | 104 | 54 | 57 | 111 |
| Zhu, 2011 | East Asian | 133 | 95 | 228 | 152 | 148 | 300 |
| Zhu, 2004 | East Asian | 21 | 29 | 50 | 53 | 241 | 294 |
| An, 2010 | East Asian | 48 | 53 | 101 | 49 | 58 | 107 |
| Chatterjee, 2009 | Caucasian | 3 | 48 | 51 | 3 | 97 | 100 |
| Guo, 2009 | East Asian | 53 | 53 | 106 | 44 | 62 | 106 |
| Huang, 2007 | East Asian | 24 | 39 | 63 | 25 | 38 | 63 |
| Leiro, 2008 | Caucasian | 17 | 18 | 35 | 16 | 44 | 60 |
| Monteiro, 2012 | Caucasian | 11 | 48 | 59 | 28 | 90 | 118 |
| Roy, 2001 | Caucasian | 5 | 28 | 33 | 1 | 32 | 33 |
| Sotsuka, 2011 | East Asian | 7 | 13 | 20 | 40 | 52 | 92 |
| Tang, 2012 | East Asian | 40 | 49 | 89 | 164 | 192 | 356 |
| Teixeira, 2011 | Caucasian | 4 | 22 | 26 | 27 | 114 | 141 |
| Zhu, 2011 | East Asian | 103 | 125 | 228 | 148 | 152 | 300 |
Table 2. Genotype distributions of GSTM1/T1 polymorphisms among SCZ and healthy control.
| First author, Publication year | Race | Cases | Controls | ||||
|---|---|---|---|---|---|---|---|
| Null | Present | Total | Null | Present | Total | ||
| GSTM1 polymorphism | |||||||
| Gravina, 2011 | Caucasian | 82 | 56 | 138 | 70 | 63 | 133 |
| Harada, 2001 | East Asian | 57 | 30 | 87 | 87 | 89 | 176 |
| Pae, 2004 | East Asian | 70 | 41 | 111 | 61 | 69 | 130 |
| Raffa, 2013 | Caucasian | 79 | 59 | 138 | 63 | 60 | 123 |
| Watanabe, 2010 | East Asian | 339 | 288 | 627 | 322 | 298 | 620 |
| GSTT1 polymorphism | |||||||
| Gravina, 2011 | Caucasian | 25 | 113 | 138 | 30 | 103 | 133 |
| Raffa, 2013 | Caucasian | 59 | 79 | 138 | 67 | 56 | 123 |
| Saadat, 2007 | Caucasian | 52 | 240 | 292 | 99 | 193 | 292 |
Association of GST polymorphisms with ATDILI
The evaluation of the associations between GSTM1/T1 polymorphisms and risk of ATDILI is summarized in Fig 2. Because no significant heterogeneity was found in either analysis (P = 0.22 and I 2 = 22% for GSTM1, P = 0.37 and I 2 = 8% for GSTT1), the fixed-effect model was used to analyze both GSTM1 and GSTT1. The frequencies of both GSTM1 and GSTT1 null genotype were higher among cases than among controls (54.51% vs. 42.42% for GSTM1 and 38.84% vs. 36.92% for GSTT1). This finding indicated that the GSTM1 present genotype was significantly associated with a decreased risk of ATDILI (RR: 0.81, 95%CI: 0.75–0.88, P < 0.0001) (Fig 2A), whereas no significant association was found between the GSTT1 present genotype and ATDILI (RR: 0.99, 95%CI: 0.93–1.06, P = 0.82) (Fig 2B).
Fig 2. Forest plots for meta-analysis of GSTM1/T1 polymorphisms and ATDILI.
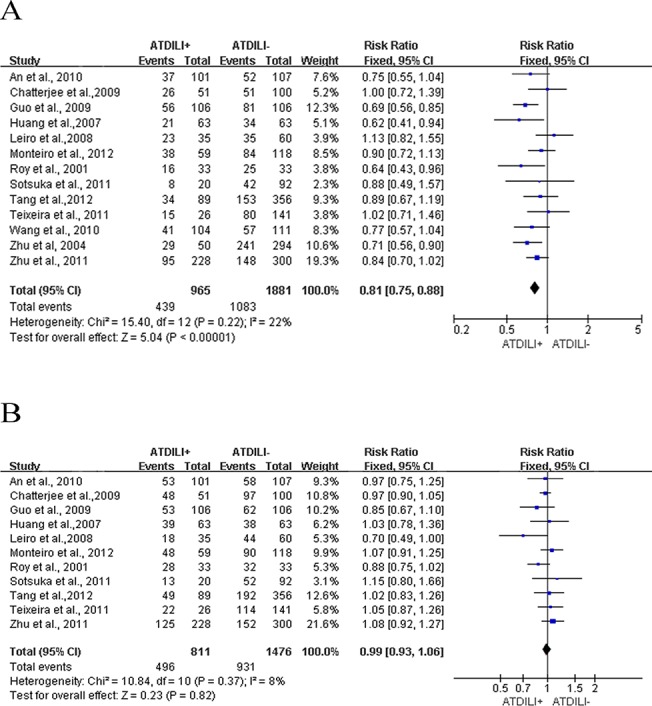
A. the summary of RRs with 95% CIs for GSTM1 present genotype; B. the summary of RRs with 95% CIs for GSTT1 present genotype.
In subgroup analyses of the association between GSTM1 and GSTT1 genotypes and ATDILI (S1A and S1B Fig), a significant association was identified between the GSTM1 present genotype and a decreased risk of ATDILI among an East Asian population under a fixed-effect model (RR: 0.77, 95%CI: 0.70–0.85, P = 0.68 for heterogeneity). The regression model indicated strong evidence for an association between the race and the effects of the GSTM1 present genotype (P = 0.033). However, although the total sample size power for detecting a significant effect between the GSTM1 present genotype and ATDILI exceeded 99%, the sample size power in the East Asian group exceeded 99%, whereas that in the Caucasian group was only 14.8%. Given the low power value in the Caucasian group, we are unable to definitively conclude on the association between GST genes and ATDILI in the Caucasian populations.
Association of GST polymorphisms with SCZ
The combined analysis of the associations between GSTM1/T1 polymorphisms and risk of SCZ are shown in Fig 3. Because no significant heterogeneity was observed in analysis of GSTM1 (P = 0.22 and I 2 = 41%), the fixed-effect model was used; because mildly significant heterogeneity was observed in analysis of GSTT1 (P = 0.10, I 2 = 57%), the random-effect model was used. The GSTM1 null genotype and GSTT1 present genotype frequencies were higher among cases than among controls (56.95% vs. 51.02% for GSTM1 and 23.94% vs. 35.77% for GSTT1). This finding indicated that statistically significant associations between the GSTM1 present genotype and a decreased risk of SCZ (RR: 0.88, 95%CI: 0.80–0.96, P = 0.004) in the fixed-effect model (Fig 3A) as well as between the GSTT1 present genotype and the risk of SCZ (RR: 1.17, 95%CI: 1.04–1.32, P = 0.01) in the random-effect model (Fig 3B).
Fig 3. Forest plots for meta-analysis of GSTM1/T1 polymorphisms and SCZ.
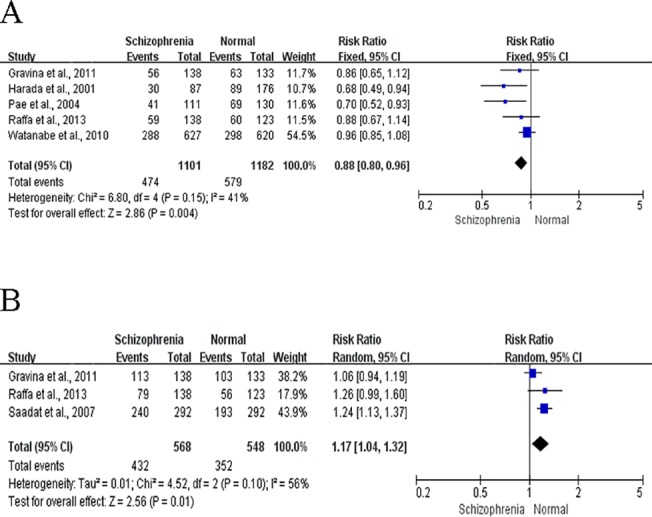
A. the summary of RRs with 95% CIs for GSTM1 present genotype; B. the summary of RRs with 95% CIs for GSTT1 present genotype.
Because only three papers concerning the GSTT1 present genotype were included in the meta-analysis, a subgroup analysis was not performed. No significant associations were found in subgroup analyses of the relationship between the GSTM1 present genotype with SCZ (S1C Fig). However, the regression model indicated no evidence for an association between race and effects of the GSTM1 present genotype (P = 0.72). The total sample size power for detecting a significant association between GSTM1 present genotype and SCZ was 83.5%; in contrast, sample size powers were 68.2% for the East Asian group and 32.1% for the Caucasian group.
Sensitivity analyses and publication bias
A sensitivity analysis via re-analysis after leaving one study out was performed to assess the effect of individual studies on the overall meta-analysis estimate. The P values for testing the overall effects after excluding one study ranged from <0.00001 to <0.0001 and from 0.37 to 0.90 in the GSTM1 and GSTT1 and ATDILI analyses, respectively; the P values for testing overall effects ranged from 0.0006 to 0.03 in the GSTM1 and SCZ analyses. These results indicated that the analyses were stable. However, in GSTT1 and SCZ analysis, the summarized RRs ranged from 1.17 (95%CI: 1.04–1.32) to 1.12 (95%CI: 0.94–1.34) and the P values from 0.01 to 0.20 after excluding a study by Saadat (13); this indicated that this study was the main source of the observed mild heterogeneity. Furthermore, there was no indication of significant publication bias in the overall meta-analysis according to both Egger’s and Begg’s tests (P = 0.766 and 0.951, respectively, for GSTM1 vs. ATDILI; P = 0.137 and 0.35, respectively, for GSTT1 vs. ATDILI; P = 0.066 and 0.221, respectively, for GSTM1 vs.SCZ; and P = 0.114 and 0.296, respectively, for GSTT1 vs. SCZ).
Credibility of meta-analysis results
In this meta-analysis, strict inclusion criteria were used to address phenotype definitions and genotyping quality. For the meta-analysis of ATDILI studies, the nminor for GSTM1 null genotype was 1321, indicating a grade of A. The I 2 for the GSTM1 null genotype was 22%, and a grade of A was given. After excluding study by Roy conducted in 2001 [21], a significant association remained between GSTM1 present genotype and ATDILI (P<0.0001), and the Harbord test P value was 0.679. For the meta-analysis of SCZ studies, nminor for the GSTM1 present genotype was 1053, and a grade of A was given. The I 2 for the GSTM1 null genotype was 41%, resulting in a grade of B. After excluding the study by Harada conducted in 2001[13], a significant association remained between the GSTM1 present genotype and SCZ (P = 0.03), and the Harbord test P value was 0.0716. Therefore, ‘moderate’ cumulative evidence supported significant associations of the GSTM1 present genotype with both ATDILI and SCZ.
Discussion
Previously, ATDILI was recognized as the primary adverse effect of anti-TB drugs. Although mental disorders, of which SCZ is an example, have been considered an additional adverse effect of anti-TB drugs [33], a clear picture of whether there is sharing of common biological determinants among ATDILI and Schizophrenia has yet to emerge. To our knowledge, the current study is the first to explore the molecular connection between SCZ and ATDILI by GST genes via large-scale meta-analyses [28]. We found that the GSTM1 present genotype was significantly associated with decreased risks of ATDILI (P < 0.0001) and SCZ (P = 0.004), whereas the GSTT1 present genotype was only significantly associated with a high risk of SCZ (P = 0.01), but not ATDILI (P = 0.82); these significant results were supported by ‘moderate’ evidence according to the Venice criteria. Because a dependent conclusion may have resulted from the evidence that a number of studies simultaneously tested the associations of both GSTT1 and GSTM1 with single disorder, a Bonferroni correction method was used to correct the P value. After correction, the GSTM1 present genotype remained significantly associated with decreased risks of both ATDILI (P < 0.0001) and SCZ (P = 0.008), and the GSTT1 present genotype remained significantly associated only with a high risk of SCZ (P = 0.02), but not ATDILI (P = 1). These results indicate that GSTM1, rather than GSTT1, may be a ‘molecular connector’ between ATDILI and SCZ.
GSTs, acting as free radical scavengers through glutathione conjugation to reduce target substances’ potential toxicity, have varied tissue-specific expression patterns [34]. GSTM1 is mainly expressed in the liver and brain [35], which offers some support for the significant association found here with both ATDILI and SCZ. Furthermore, GSTM1 is found to not only detoxify anti-TB drugs’ toxic metabolites generated by CYP2E1 in the liver, but also catalyze the conjugation of glutathione with the aminochrome and the dopa-o-quinone metabolite of oxidized dopamine in the brain [36]. In the brain, reactive oxygen species are generated at high rates, and the redox mechanism that controls a balance between neuro destructive oxidants and neuro protective antioxidants partly regulating growth and pruning of neurons [37]. Thus, the inactive GSTM1 caused by the GSTM1 null genotype can cause not only liver injury but also accumulation of neuro destructive oxidants leading to Schizophrenia [11]. In contrast, GSTT1 has considerable presence in the brain, it has only trace expression in the liver [38], and it is not involved in the detoxification of the oxidized o-quinone dopamine metabolite[36]. Furthermore, besides its role as a beneficial scavenger towards electrophiles, mammalian GSTT1 can act as a deleterious metabolic activator for halogenated compounds to produce a variety of intermediate materials potentially dangerous for DNA and cells [39]. In the current meta-analysis, the results did not show an association between the GSTT1 gene and ATDILI, but indicated that the GSTT1 present genotype increases the risk of SCZ.
A meta-analysis with a larger sample size is widely considered to provide greater statistical power to identify the effect of a genetic polymorphism on a disease than a single association study [40]. An assessment of heterogeneity is critical to ensure the credibility of a meta-analysis because studies are pooled based on an assumption of etiological homogeneity across studies. In this meta-analysis, both P value and I 2 were estimated for the heterogeneity test. No significant heterogeneity was observed in the meta-analyses, except for mildly significant heterogeneity in the analysis of GSTT1 with SCZ. Interestingly, in the subgroup analyses of studies concerning GSTM1 and ATDILI, a significant association was found in the East Asian group, but not in the Caucasian group. This may be because of the low power value in the latter group (14.8%), which reduces the ability to detect significant effects. Although race was significant associated with the effects GSTM1 on ATDILI, the biological impact of GSTM1 gene on the risk of ATDILI may be consistent across racial groups, and this significant result may instead be because of the effects of different cultural and living habits among different races, such as: diet [41]. A sensitivity analysis was also performed to explore the source of the heterogeneity identified in the analysis of GSTT1 with SCZ and indicated that the study by Saadat greatly affected this analysis; accordingly, these results should be treated with caution. Two meta-analyses have been previously published on GSTM1/T1 and ATDILI: Cai in 2012 and Tang in 2013 [42,43]. Although the current study yielded similar results, particularly that the GSTM1 null genotype was significantly associated with an increased risk of ATDILI, a study by Kim was included in those previous meta-analyses, but was excluded from the current analysis[44]. In Kim’s study, the cases with ATD-induced adverse cutaneous reactions are enrolled, and subjects without skin diseases are as controls. We removed this study at the second screening of our study due to its different study design compared to the other studies.
Some potential limitations of our meta-analysis deserve consideration. First, diseases are caused by both genetic and environmental factors; however, we did not include an environmental factor analysis. We attempted to obtain complete information regarding dietary habits and substance abuse, among other factors. However, the collected literature seldom provided data in addition to genotypic data. As a result, the current meta-analysis solely focused on genetic factors. Second, the sample size in the current analysis was limited. Additional association studies focused on the co-morbidities of ATDILI and SCZ, and involving large-scale samples will be required to confirm the current results.
In summary, the current meta-analysis results demonstrate that GSTM1, rather than GSTT1, may act as a molecular link between ATDILI and SCZ and suggest that ATDILI and SCZ may be concurrent adverse effects of anti-TB drugs in subjects harboring the GSTM1 null genotype.
Supporting Information
A. the summary of RRs with 95% CIs for GSTM1 present genotype and ATDILI; B. the summary of RRs with 95% CIs for GSTT1 present genotype and ATDILI; C. the summary of RRs with 95% CIs for GSTM1 present genotype and Schizophrenia.
(DOCX)
(XLS)
Acknowledgments
We thank the anonymous reviewers for their constructive comments. We also appreciate Dr. Paul F. O’Reilly and Dr. Simone de Jong for their edition and suggestions.
Funding Statement
This work was supported by the 973 Program (grant numbers 2012CB910102, 2010CB529600), the Chinese National Science Foundation (No. 31101015, 81271486, 31100252 and 81421061) and the scientific research foundation for the returned overseas, the Chinese Ministry of Education (No. 12Z102050009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. WHO (2012) World Health Organization: Global tuberculosis report. [Google Scholar]
- 2. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. (2003) American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 167: 603–662. [DOI] [PubMed] [Google Scholar]
- 3. Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D (2003) Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med 167: 1472–1477. [DOI] [PubMed] [Google Scholar]
- 4. Thompson NP, Caplin ME, Hamilton MI, Gillespie SH, Clarke SW, Burroughs AK, et al. (1995) Anti-tuberculosis medication and the liver: dangers and recommendations in management. Eur Respir J 8: 1384–1388. [DOI] [PubMed] [Google Scholar]
- 5. Nathwani RA, Kaplowitz N (2006) Drug hepatotoxicity. Clin Liver Dis 10: 207–217, vii. [DOI] [PubMed] [Google Scholar]
- 6. Huang YS (2007) Genetic polymorphisms of drug-metabolizing enzymes and the susceptibility to antituberculosis drug-induced liver injury. Expert Opin Drug Metab Toxicol 3: 1–8. [DOI] [PubMed] [Google Scholar]
- 7. Roy PD, Majumder M, Roy B (2008) Pharmacogenomics of anti-TB drugs-related hepatotoxicity. Pharmacogenomics 9: 311–321. 10.2217/14622416.9.3.311 [DOI] [PubMed] [Google Scholar]
- 8. Yew WW, Leung CC (2006) Antituberculosis drugs and hepatotoxicity. Respirology 11: 699–707. [DOI] [PubMed] [Google Scholar]
- 9. Sullivan PF, Kendler KS, Neale MC (2003) Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry 60: 1187–1192. [DOI] [PubMed] [Google Scholar]
- 10. Saadat M, Mobayen F, Farrashbandi H (2007) Genetic polymorphism of glutathione S-transferase T1: a candidate genetic modifier of individual susceptibility to schizophrenia. Psychiatry Res 153: 87–91. [DOI] [PubMed] [Google Scholar]
- 11. Yao JK, Reddy RD, van Kammen DP (2001) Oxidative damage and schizophrenia: an overview of the evidence and its therapeutic implications. CNS Drugs 15: 287–310. [DOI] [PubMed] [Google Scholar]
- 12. Valentini A, Gravina P, Bernardini S, Federici G (2008) Role of glutathione Stransferase in the cellular antioxidant defence In: Eleuteri, A.M: Research Signpost, Kerala, India. [Google Scholar]
- 13. Harada S, Tachikawa H, Kawanishi Y (2001) Glutathione S-transferase M1 gene deletion may be associated with susceptibility to certain forms of schizophrenia. Biochem Biophys Res Commun 281: 267–271. [DOI] [PubMed] [Google Scholar]
- 14. Gravina P, Spoletini I, Masini S, Valentini A, Vanni D, Paladini E, et al. (2011) Genetic polymorphisms of glutathione S-transferases GSTM1, GSTT1, GSTP1 and GSTA1 as risk factors for schizophrenia. Psychiatry Res 187: 454–456. 10.1016/j.psychres.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 15. Sodhi CP, Rana SV, Mehta SK, Vaiphei K, Attri S, Thakur S, et al. (1996) Study of oxidative stress in isoniazid-induced hepatic injury in young rats with and without protein-energy malnutrition. J Biochem Toxicol 11: 139–146. [DOI] [PubMed] [Google Scholar]
- 16. Pearson WR, Vorachek WR, Xu SJ, Berger R, Hart I, Vannais D, et al. (1993) Identification of class-mu glutathione transferase genes GSTM1-GSTM5 on human chromosome 1p13. Am J Hum Genet 53: 220–233. [PMC free article] [PubMed] [Google Scholar]
- 17. Webb G, Vaska V, Coggan M, Board P (1996) Chromosomal localization of the gene for the human theta class glutathione transferase (GSTT1). Genomics 33: 121–123. [DOI] [PubMed] [Google Scholar]
- 18. Kerb R, Brockmoller J, Sachse C, Roots I (1999) Detection of the GSTM1*0 allele by long polymerase chain reaction. Pharmacogenetics 9: 89–94. [PubMed] [Google Scholar]
- 19. Huang RS, Chen P, Wisel S, Duan S, Zhang W, Cook EH, et al. (2009) Population-specific GSTM1 copy number variation. Hum Mol Genet 18: 366–372. 10.1093/hmg/ddn345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sprenger R, Schlagenhaufer R, Kerb R, Bruhn C, Brockmoller J, Roots I, et al. (2000) Characterization of the glutathione S-transferase GSTT1 deletion: discrimination of all genotypes by polymerase chain reaction indicates a trimodular genotype-phenotype correlation. Pharmacogenetics 10: 557–565. [DOI] [PubMed] [Google Scholar]
- 21. Roy B, Chowdhury A, Kundu S, Santra A, Dey B, Chakraborty M, et al. (2001) Increased risk of antituberculosis drug-induced hepatotoxicity in individuals with glutathione S-transferase M1 'null' mutation. J Gastroenterol Hepatol 16: 1033–1037. [DOI] [PubMed] [Google Scholar]
- 22. Teixeira RL, Morato RG, Cabello PH, Muniz LM, Moreira Ada S, Kritski AL, et al. (2011) Genetic polymorphisms of NAT2, CYP2E1 and GST enzymes and the occurrence of antituberculosis drug-induced hepatitis in Brazilian TB patients. Mem Inst Oswaldo Cruz 106: 716–724. [DOI] [PubMed] [Google Scholar]
- 23. Watanabe Y, Nunokawa A, Kaneko N, Someya T (2010) A case-control study and meta-analysis of association between a common copy number variation of the glutathione S-transferase mu 1 (GSTM1) gene and schizophrenia. Schizophr Res 124: 236–237. 10.1016/j.schres.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 24. Cai L, Deng SL, Liang L, Pan H, Zhou J, Wang MY, et al. (2013) Identification of genetic associations of SP110/MYBBP1A/RELA with pulmonary tuberculosis in the Chinese Han population. Hum Genet 132: 265–273. 10.1007/s00439-012-1244-5 [DOI] [PubMed] [Google Scholar]
- 25. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 26. Sutton A, Abrams KR, Jones DR, Sheldon TA, Song F (2000) Methods for meta-analysis in medical research: Wiley, Chichester. [Google Scholar]
- 27. Li C, Long J, Hu X, Zhou Y (2013) GSTM1 and GSTT1 genetic polymorphisms and risk of anti-tuberculosis drug-induced hepatotoxicity: an updated meta-analysis. Eur J Clin Microbiol Infect Dis 32: 859–868. 10.1007/s10096-013-1831-y [DOI] [PubMed] [Google Scholar]
- 28. Cochran W (1954) The combination of estimates from different experiments. Biometrics 10: 101–129. [Google Scholar]
- 29. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Bmj 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Bmj 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ioannidis JP, Boffetta P, Little J, O'Brien TR, Uitterlinden AG, Vineis P, et al. (2008) Assessment of cumulative evidence on genetic associations: interim guidelines. Int J Epidemiol 37: 120–132. [DOI] [PubMed] [Google Scholar]
- 32. Harbord RM, Egger M, Sterne JA (2006) A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25: 3443–3457. [DOI] [PubMed] [Google Scholar]
- 33. Doherty AM, Kelly J, McDonald C, O'Dywer AM, Keane J, Cooney J (2013) A review of the interplay between tuberculosis and mental health. Gen Hosp Psychiatry 35: 398–406. 10.1016/j.genhosppsych.2013.03.018 [DOI] [PubMed] [Google Scholar]
- 34. Boyer TD (1989) The glutathione S-transferases: an update. Hepatology 9: 486–496. [DOI] [PubMed] [Google Scholar]
- 35. Rowe JD, Nieves E, Listowsky I (1997) Subunit diversity and tissue distribution of human glutathione S-transferases: interpretations based on electrospray ionization-MS and peptide sequence-specific antisera. Biochem J 325 (Pt 2): 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B (1997) Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J 324 (Pt 1): 25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smythies J (1999) Redox mechanisms at the glutamate synapse and their significance: a review. Eur J Pharmacol 370: 1–7. [DOI] [PubMed] [Google Scholar]
- 38. Juronen E, Tasa G, Uuskula M, Pooga M, Mikelsaar AV (1996) Purification, characterization and tissue distribution of human class theta glutathione S-transferase T1-1. Biochem Mol Biol Int 39: 21–29. [DOI] [PubMed] [Google Scholar]
- 39. Landi S (2000) Mammalian class theta GST and differential susceptibility to carcinogens: a review. Mutat Res 463: 247–283. [DOI] [PubMed] [Google Scholar]
- 40. Petitti DB (1994) Meta-analysis, decision analysis and cost-effectiveness New York, NY, USA: Oxford University Press. [Google Scholar]
- 41. Ioannidis JP, Ntzani EE, Trikalinos TA (2004) 'Racial' differences in genetic effects for complex diseases. Nat Genet 36: 1312–1318. [DOI] [PubMed] [Google Scholar]
- 42. Cai Y, Yi J, Zhou C, Shen X (2012) Pharmacogenetic study of drug-metabolising enzyme polymorphisms on the risk of anti-tuberculosis drug-induced liver injury: a meta-analysis. PLoS One 7: e47769 10.1371/journal.pone.0047769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tang N, Deng R, Wang Y, Lin M, Li H, Qiu Y, et al. (2013) GSTM1 and GSTT1 null polymorphisms and susceptibility to anti-tuberculosis drug-induced liver injury: a meta-analysis. Int J Tuberc Lung Dis 17: 17–25. 10.5588/ijtld.12.0447 [DOI] [PubMed] [Google Scholar]
- 44. Kim SH, Kim SH, Yoon HJ, Shin DH, Park SS, Kim YS, et al. (2010) GSTT1 and GSTM1 null mutations and adverse reactions induced by antituberculosis drugs in Koreans. Tuberculosis (Edinb) 90: 39–43. 10.1016/j.tube.2009.12.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. the summary of RRs with 95% CIs for GSTM1 present genotype and ATDILI; B. the summary of RRs with 95% CIs for GSTT1 present genotype and ATDILI; C. the summary of RRs with 95% CIs for GSTM1 present genotype and Schizophrenia.
(DOCX)
(XLS)


