Abstract
Background
It is important to study differential inflammatory cellular migration, particularly of eosinophils and neutrophils, in asthma and how this is influenced by environmental stimuli such as allergen exposure and the effects of anti asthma therapy.
Methods
We isolated blood neutrophils and eosinophils from 12 atopic asthmatic human volunteers (Group 1 — four Early Allergic Responders unchallenged (EAR); Group 2 — four Early and Late Allergic Responders (LAR) challenged; Group 3 — four EAR and LAR challenged and treated with systemic corticosteroids) using cGMP CD16 CliniMACS. Cells were isolated prior to allergen challenge where applicable, labelled with 99mTc-HMPAO and then re-infused intravenously. The kinetics of cellular influx/efflux into the lungs and other organs were imaged via scintigraphy over 4 h, starting at 5 to 6 h following allergen challenge where applicable.
Results
Neutrophils and eosinophils were isolated to a mean (SD) purity of 98.36% (1.09) and 96.31% (3.0), respectively. Asthmatic neutrophils were activated at baseline, mean (SD) CD11bHigh cells 46 (10.50) %. Isolation and radiolabelling significantly increased their activation to > 98%. Eosinophils were not activated at baseline, CD69+ cells 1.9 (0.6) %, increasing to 38 (3.46) % following isolation and labelling. Analysis of the kinetics of net eosinophil and neutrophil lung influx/efflux conformed to a net exponential clearance with respective mean half times of clearance 6.98 (2.18) and 14.01 (2.63) minutes for Group 1, 6.03 (0.72) and 16.04 (2.0) minutes for Group 2 and 5.63 (1.20) and 14.56 (3.36) minutes for Group 3. These did not significantly differ between the three asthma groups (p > 0.05).
Conclusions
Isolation and radiolabelling significantly increased activation of eosinophils (CD69) and completely activated neutrophils (CD11bHigh) in all asthma groups. Net lung neutrophil efflux was significantly slower than that of eosinophils in all asthma study groups. There was a trend for pre-treatment with systemic corticosteroids to reduce lung retention of eosinophils following allergen challenge.
Keywords: Asthma, Eosinophils, Neutrophils, Migration kinetics, Radioisotope, Allergen challenge
Highlights
-
•
Isolation and radiolabelling of eosinophils or neutrophils from asthmatics significantly activates them.
-
•
Corticosteroids alter eosinophil, but not neutrophil 99mTc-HMPAO radiolabelling efficiency.
-
•
Leukocyte activation as a result of manipulation ex vivo does not measurably alter lung sequestration.
-
•
Eosinophil migration through the lungs of asthmatic patients is slower than in healthy volunteers.
1. Introduction
Asthma is a complex, heterogeneous and chronic inflammatory lung disease characterised by reversible bronchoconstriction, airway inflammation and hyperresponsiveness (Lemanske and Busse, 2010). Differential net infiltration of effector granulocytes, particularly eosinophils and neutrophils into the airways forms the basis of current definitions of asthma phenotypes and endotypes (Wenzel, 2006, Simpson et al., 2006).
There remains controversy, however, as to the precise mechanism of genesis of such phenotypes (Cieslewicz et al., 1999). One of the major stumbling blocks here is lack of data concerning the kinetics of lung granulocyte immigration and emigration in asthma. Studies addressing the differential migration of key effector cells such as eosinophils and neutrophils have hitherto relied on static, indirect techniques such as induced sputum, bronchoalveolar lavage and biopsy (Metzger et al., 1986, Metzger et al., 1987). Such strategies do not however address the question of spatiotemporal kinetics and provide only a “snapshot” sample of the cells in various compartments of the airways. Furthermore, they do not inform on the cells that may temporarily marginate in the lungs or their fate at later time points. Finally, they require artificial intervention (sputum induction, BAL) which could be argued and alter the characteristics of cellular migration in its own right. Thanks to the recent introduction of magnetic bead isolation it has now become possible to separate pure eosinophils and neutrophils and track their net ingress and egress in the lungs and other organs in real time, in vivo.
The concept of asthma phenotyping and endotyping is underpinned partly by the postulated contributions which eosinophils and neutrophils make to various clinical manifestations of the disease. Disease instability and airways structural changes are associated with eosinophilic inflammation (Gleich, 2000). Nevertheless, eosinophilic inflammation is present only in about 50% of asthmatics (Douwes et al., 2002), and its presence in clinical syndromes such as eosinophilic bronchitis does not necessarily lead to asthma symptoms (Brightling et al., 2003). Equally, neutrophilic inflammation is also believed to play a significant role in the pathophysiology of this condition. Activated neutrophils are able to release mediators that promote and prolong asthma symptoms (Kamath et al., 2005).
The neutrophilic phenotype/endotype has been associated in various studies with severe asthma (Little et al., 2002), corticosteroid resistant asthma (Green et al., 2002), nocturnal asthma (Martin et al., 1991), smokers' asthma (Chalmers et al., 2001), and “sudden onset fatal” asthma (Sur et al., 1993).
We have previously defined the kinetics of net eosinophil and neutrophil influx into, and efflux from the lungs and other organs of healthy volunteers by infusing autologous 99mTc-HMPAO radiolabelled purified populations of cells followed by gamma camera scanning (Lukawska et al., 2014). Our aim in this study was to employ the same technique to examine these kinetics in the lungs of patients with mild/moderate atopic asthma, further to study the impact of asthma exacerbation and anti-asthma therapy on this process. To do this we employed the classical model of bronchial allergen challenge, which induces an immediate or “early phase reaction” (EAR), characterised by acute bronchoconstriction maximal around 30 min in suitably sensitised patients and attributed to the acute release of mediators from mast cells in the airways followed in some patients after recovery by a second “late phase reaction” (LAR) characterised by a late period of bronchoconstriction (maximal around 3–9 h) attributed to local release of further mediators including Th2 type cytokines and ongoing cellular infiltration. It is not clear why only a proportion of atopic asthmatics exhibit late phase bronchoconstriction under these conditions, but this may partly reflect individual clinical sensitivity to allergen challenge, limiting the dosage of allergen which can be administered to produce a “safe” early response. Both phases are associated with eosinophil and neutrophil influx into the bronchial mucosa, but only the late phase reaction, reflecting IL-5 and other mediator release from T cells, is susceptible to corticosteroid inhibition (O'Byrne et al., 1987). We hypothesised that late phase, allergen-induced bronchoconstriction in atopic asthmatics, which is known to be associated with neutrophil and eosinophil influx into the bronchial mucosa, is associated with modification of the kinetics of net eosinophil and neutrophil influx/efflux into and out of the lungs and other organs, and that this is further modified in the presence of systemic corticosteroid. To address this we compared, in real time, the kinetics of this net influx/efflux in lungs, liver, spleen and bone marrow in asthmatics who had previously demonstrated an isolated EAR in response to allergen challenge, but were not challenged on this occasion (Group 1), to allergen-challenged atopic asthmatics who had previously exhibited dual EAR and LAR reactions to allergen and had been re-challenged (Group 2 and Group 3). A proportion of the patients (Group 3) were pre-administered systemic corticosteroid. Because of the demanding nature of the protocol it was not possible to monitor lung function changes concomitantly with gamma camera imaging. For the same reason we were obliged to venesect the patients for blood eosinophil and neutrophil isolation prior to allergen challenge. We compared the data with retrospective, previously reported data from normal controls obtained using an identical protocol (Lukawska et al., 2014).
2. Methods
2.1. Clinical Protocol
This study was approved by the South East London Ethics Committee REC 3 and by ARSAC (Administration of Radioactive Substances Advisory Commitee) as well as the Department of Research and Development at Guy's and St Thomas' NHS Foundation Trust.
Twelve mild/moderate asthmatics (FEV1 ≥ 65%) were studied on 4 separate occasions (screening, dose-ranging allergen challenge and either no further challenge (Group 1) or 2 full dosage allergen challenges followed by eosinophil and neutrophil tracking on separate occasions). Asthma was defined as a clinical history of typical symptoms and documented ≥ 15% variability of the FEV1 within the previous 2 years. Atopy was defined as a positive skin prick test (Aquagen SQ, ALK, Horsholm, Denmark) to one or more of the challenge aeroallergens (grass pollen, cat dander, D. pteronyssinus) in the presence of valid positive histamine and negative saline controls. All asthmatics were treated with inhaled, short acting bronchodilator only and had been free of exacerbations and overt upper respiratory tract infections for at least 6 weeks. Their peripheral blood eosinophil and neutrophil counts were within the normal laboratory range. All volunteers provided informed, written consent to participate in the study.
2.2. Incremental Aeroallergen Challenge
This was performed with grass, house dust mite or cat allergen (Aquagen SQ, ALK, Horsholm, Denmark) according to departmental Standing Operating Procedure based on well established protocols (Singh et al., 2007, Ketchell et al., 2002). Volunteers who demonstrated EAR only following incremental aeroallergen challenge were not challenged again prior to their gamma camera studies (Group 1). Volunteers who exhibited both EAR and LAR were challenged with full dose aeroallergen prior to each subsequent study (Groups 2 and 3) (See Table 1 for allergen challenge data).
Table 1.
Incremental allergen challenge.
| Sex/Age | FEV1 L | Pred% | EAR% | LAR% | Allergen | |
|---|---|---|---|---|---|---|
| Group 1 EAR Not challenged |
M/44 | 2.33 | 69 | 29 | Absent | HDM |
| M/32 | 4.06 | 82 | 20 | Absent | HDM | |
| F/47 | 2.13 | 97 | 22 | Absent | Grass | |
| M/61 | 2.12 | 66 | 20 | Absent | Grass | |
| Group2 EAR & LAR Challenged |
M/25 | 3.76 | 83 | 35 | 44 | Grass |
| M/24 | 5.59 | 105 | 27 | 29 | HDM | |
| F/21 | 3.14 | 82 | 24 | 27 | Cat | |
| M/28 | 4.72 | 119 | 31 | 22 | HDM | |
| Group 3 EAR & LAR Challenged/Pretreated with steroids |
F/26 | 3.21 | 110 | 20 | 20 | Cat |
| M/40 | 3.04 | 71 | 29 | 34 | HDM | |
| M/27 | 3.93 | 95 | 20 | 36 | HDM | |
| M/21 | 3.69 | 87 | 25 | 18 | HDM |
HDM — House Dust Mite; FEV1 — Forced Expiratory Volume in 1 s; Pred % — FEV1 percentage predicted; EAR — Early Asthmatic Response defined as at least 15% decrease in FEV1 within 30 min following allergen challenge; LAR — Late Asthmatic Response defined as at least 15% decrease in FEV1 three to 9 h following allergen challenge.
2.3. Full Dosage Aeroallergen Challenge
On two further occasions separated by a minimum period of 28 days, 8 volunteers showing dual responses (EAR and LAR) were re-challenged with the same cumulative dosage of allergen which had previously caused these responses but as a single bolus. They were split into Group 2 and Group 3. Volunteers in Group 2 were allergen challenged with no premedication. Volunteers in Group 3 were premedicated with oral prednisolone at a dosage of 5 mg per kg administered every day for 5 days prior to challenge. FEV1 was again measured following a baseline saline challenge and subsequently at 30 min intervals following bolus allergen challenge, for a total of 10 h. At the end of the experiment, volunteers were administered nebulised salbutamol ad libitum until FEV1 had improved to at least 90% of the baseline value. The patient was then issued with an action plan and rescue medication (single oral dose of prednisolone at 5 mg per kg).
2.4. GMP Neutrophil and Eosinophil Isolation and Radiolabelling
All open manipulations were carried out in a Grade A environment in a pharmaceutical isolator located in a Grade D background GMP facility in the Department of Nuclear Medicine at Guy's Hospital, UK. As explained above, each volunteer in groups 1, 2 and 3 attended on two separate occasions to undergo reinfusion of eosinophils on one occasion and neutrophils on the other. Volunteers in Group 2 and 3 underwent bolus allergen challenge 5 to 6 h prior to reinfusion. Volunteers in Group 3 on each occasion were pre treated with Prednisolone for five days prior to the challenge, but excluding the challenge day. At each visit the volunteer donated a 110 mL sample of venous blood and eosinophils and neutrophils were cGMP isolated using a CliniMACS (Miltenyi Biotec, Bisley, UK) and radiolabelled with 99mTc-HMPAO (Ceretec, GE Healthcare, Amersham, UK) as previously described (Lukawska et al., 2014). Volunteers who underwent bolus allergen challenge (Group 2 and 3) provided their whole blood sample before they were challenged. Volunteers in Group 1 were not challenged prior to their scintigraphy studies.
2.5. Viability, Phenotype and Purity of Purified Radiolabelled Neutrophils and Eosinophils
Viability, phenotype, activation and purity of purified radiolabelled neutrophils and eosinophils were measured as previously described (Lukawska et al., 2014).
2.6. Imaging Protocol and Data Analysis
Between 6 and 7 h following peripheral blood sampling, and 5 to 6 h following allergen challenge where applicable, the volunteer was positioned in a dual-headed gamma camera (Symbia, Siemens Medical Solutions, UK). 100 (96 ± 27) MBq of labelled autologous eosinophils or neutrophils suspended in 2.5 mL autologous plasma were injected intravenously as a bolus. See Lukawska, Livieratos et al. for the full account of radiolabelling, imaging and data analysis protocols (Lukawska et al., 2014).
2.7. Statistical Analysis
To test for significant differences between the net eosinophil and neutrophil influx/efflux profiles in the various organs between the study groups a Kruskall–Wallis One Way Anova was performed, with a p value of < 0.05 being considered significant. If the Kruskal–Wallis test was significant, then a post hoc analysis was performed by Student–Newman–Keuls pair wise comparison; again p-values of < 0.05 were considered significant. The unpaired Student's t test was used to compare eosinophil and neutrophil activation and numbers between the different study groups.
3. Results
3.1. Radiolabelling Efficiency
In Group 1 and 2 volunteers, the Tc 99 m HMPAO labelling of eosinophils was significantly more efficient than that of neutrophils (p = 0.014 and p = 0.01 respectively). In contrast in Group 3 volunteers the efficiency of eosinophil labelling was significantly (p = 0.0021) reduced compared to that in Groups 1 and 2, and there was a trend towards an increase in neutrophil radiolabelling efficiency although not significant (p = 0.0537) (Table 2). Overall leukocyte radiolabelling efficiencies in Groups 1 and 2 were comparable to those previously published in healthy subjects (Lukawska et al., 2014).
Table 2.
Mean radiolabelling efficiencies of eosinophils and neutrophils in the 3 asthmatic study groups. Standard deviations given in brackets. Radiolabelling efficiency was measured by dividing the activity of the neutrophil/eosinophil pellet (in MBq) by the total activity (pellet and supernatant) × 100% following 99mTc-HMPAO incubation.
| Mean radiolabelling efficiency (%) | Group 1 EAR not challenged |
Group 2 EAR & LAR challenged |
Group 3 EAR & LAR challenged & pre-treated steroids |
|---|---|---|---|
| Eosinophil | 80.18 (3.32) | 69.18 (7.1) | 46.58 (17.82) |
| Neutrophils | 38.87 (6.28) | 24.80 (4.57) | 54.68 (17.01) |
3.2. Viability, Phenotype and Purity of Purified Radiolabelled Neutrophils and Eosinophils
Following the CliniMACS purification and radiolabelling procedures the mean percentage purity of isolated neutrophils, defined as CD66+/CD16+ cells, exceeded 98%, while the mean percentage purity of eosinophils, defined as CD66+/CD16− cells exceeded 96% (Table 3, Fig. 1). Importantly the neutrophil fraction contained < 0.2% of CD66+/CD16− eosinophils and < 0.15% of mononuclear cells (CD3+, CD19+, CD56+ or CD80+), while the eosinophil fraction contained < 0.5% neutrophils and < 1% mononuclear cells. The mean percentages of activated CD11bHigh neutrophils in whole blood, as defined in Lukawska et al were elevated in the asthmatics at baseline compared to healthy volunteers with means of 36% in Group 1, 45% in Group 2 and 57% in Group 3, as compared with ~ 0.5% previously reported in healthy volunteers (Lukawska et al., 2014). Following cellular isolation and radiolabelling of neutrophils the percentages of CD11bHigh cells were further significantly elevated as compared with the ex vivo measurements, to > 98% in all 3 groups (Table 3). The mean percentages of activated eosinophils in whole blood at baseline were 1.9% in Group 1, 3% in Group 2 and 2.5% in Group 3, comparable to a mean of ~ 1.8% in previously reported healthy volunteers (Lukawska et al., 2014), suggesting little or no activation of eosinophils prior to isolation. Again, following cellular isolation and radiolabelling of eosinophils, the percentages of eosinophils expressing CD69 were significantly increased, as compared with untouched eosinophils in whole blood, to means of 40% in Group1, 40% in Group 2 and 34% in Group 3 (Table 3). Cellular viability was well maintained in all groups (Table 3).
Table 3.
In vitro characterisation of purified human neutrophils and eosinophils.
| Group 1 EAR not challenged |
Group 2 EAR & LAR challenged |
Group 3 EAR & LAR challenged & pre-treated steroids |
|
|---|---|---|---|
| Neutrophils (CD66+, CD16+a) | |||
| Purityb (%) | 98.5 (± 1.1) | 98.3 (± 1.5) | 98.2 (± 0.9) |
| % CD11b+ ex vivo whole blood | 35.7 (± 13.7) | 44.6 (± 34.4) | 56.8 (± 44.0) |
| % CD11b+ after radiolabelling | 99.8 (± 0.2) | 98 (± 1.8) | 99.2 (± 0.5) |
| Viability (%) | 97.0 (± 3.3) | 98.2 (± 3.2) | 92.7 (± 2.3) |
| Eosinophils (CD66+, CD16−a) | |||
| Purityb (%) | 96.1 (± 4.2) | 96.5 (± 2.4) | 96.4 (± 3.5) |
| % CD69+ ex vivo whole blood | 1.9 (± 1.0) | 3.0 (± 2.1) | 2.5 (± 2.7) |
| % CD69+ after radiolabelling | 39.6 (± 25.2) | 39.7 (± 28.1) | 33.7 (± 27.0) |
| Viability (%) | 96.0 (± 4.0) | 95.5 (2.08) | 93.4 (± 3.8) |
Phenotype following CD16 CliniMACS isolation.
Contaminants were defined as CD3, CD19, CD56 or CD80 positive mononuclear cells and eosinophils (CD66+/CD16− cells) in the neutrophil fraction and mononuclear cells and neutrophils (CD66+/CD16+ cells) in the eosinophil fraction. Figures represent mean ± SD.
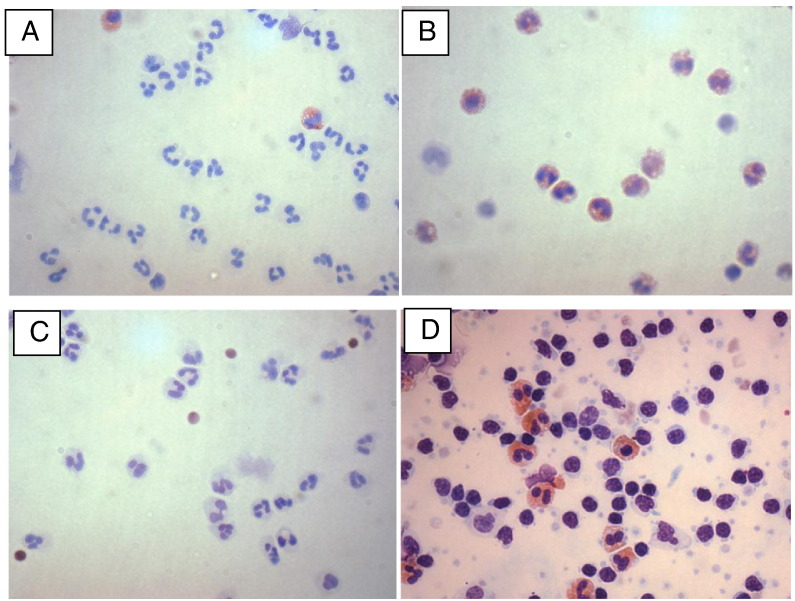
Fig. 1.
Blood neutrophils and eosinophils following CliniMACS separation (magnification × 40). A) Pure neutrophils from an asthmatic subject. B) Pure eosinophils from an asthmatic subject. C) Pure neutrophils from an asthmatic subject following corticosteroid pre-treatment. D) Pure eosinophils from an asthmatic subject following corticosteroid pre-treatment.
3.3. In Vivo Imaging of Neutrophils and Eosinophils
We previously showed that, in healthy individuals, the mean half-life (t1/2) of net clearance of eosinophils from the lungs differed significantly from that of neutrophils, with eosinophils showing much faster clearance compared with neutrophils (Lukawska et al., 2014). This difference was maintained and statistically significant in all 3 asthma groups in the present study (Group 1 p = 0.022, Group 2 p = 0.0028, Group 3 p = 0.005) (Table 4).
Table 4.
Mean half times T1/2 of clearance (minutes) of eosinophils and neutrophils from the lungs of volunteers in 3 asthma study groups. Standard deviations shown in brackets.
| Lung T (1/2) | Group 1 EAR not challenged |
Group 2 EAR & LAR challenged |
Group 3 EAR & LAR challenged & pretreated steroids |
|---|---|---|---|
| Eosinophil | 6.98 (2.18) | 6.03 (0.72) | 5.63 (1.20) |
| Neutrophils | 14.01 (2.63) | 16.04 (2.00) | 14.56 (3.36) |
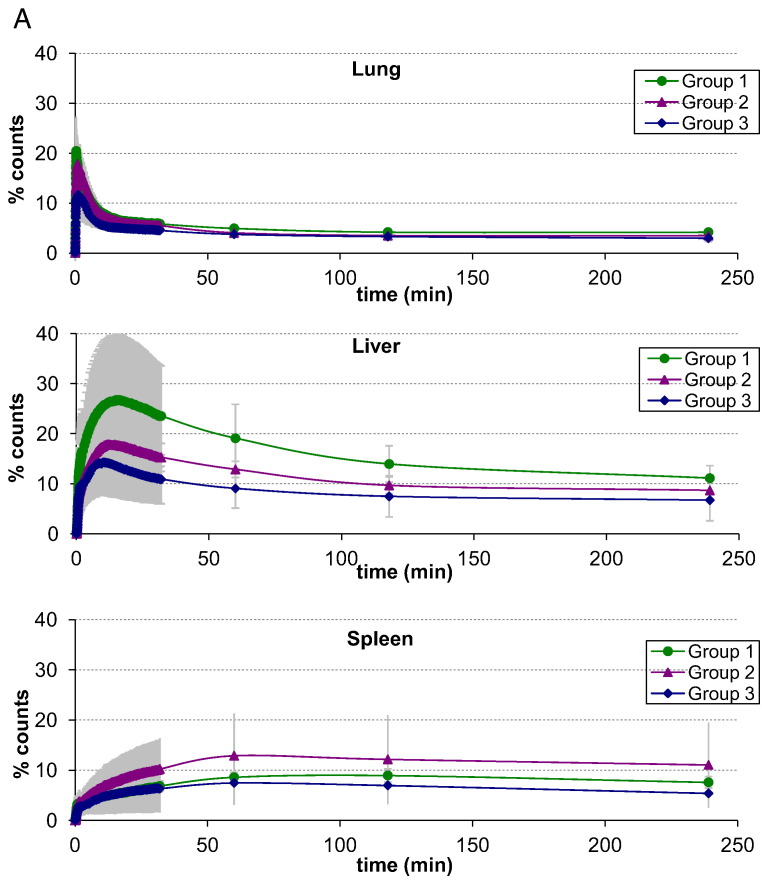
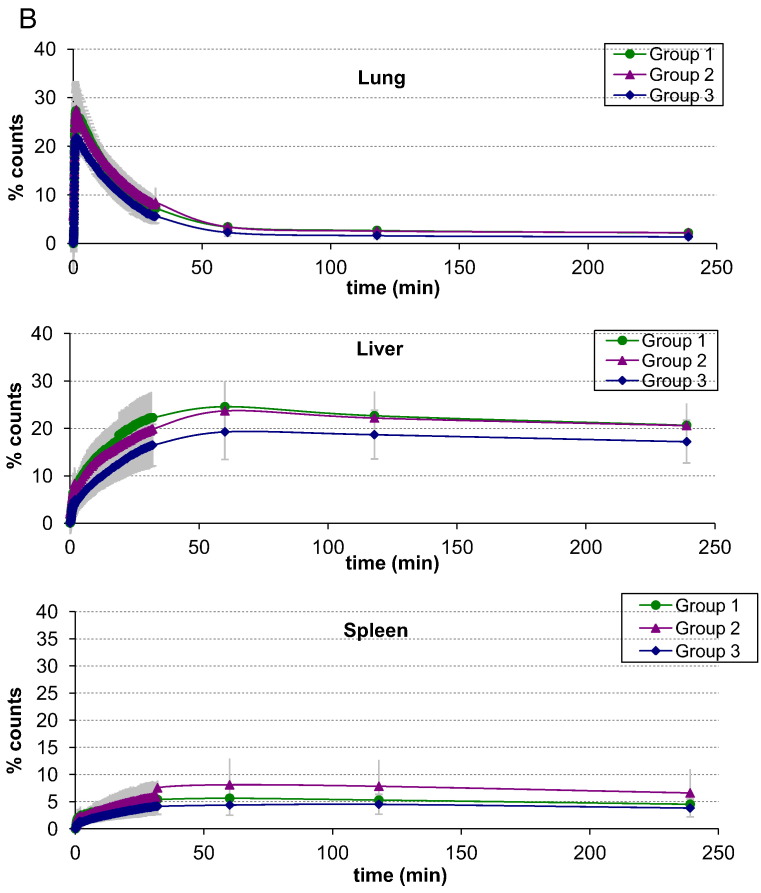
There were no significant differences in the mean half-times of net clearance of radiolabelled eosinophils or neutrophils from the lungs of the asthmatics previously demonstrating an isolated EAR but not challenged (Group 1), and asthmatics with EAR and LAR who were allergen challenged without (Group 2) or with (Group 3) pre-treatment with corticosteroid (p > 0.05) (Fig. 2, Table 4). Retrospective comparison of these kinetics with those in healthy volunteers using an identical protocol (Lukawska et al., 2014) suggested that the half time of clearance of eosinophils from the lungs of the unchallenged asthmatics (Group 1) was significantly higher compared with normal controls (p = 0.0428).
Fig. 2.
A. Eosinophil organ specific asthma time activity curves for: lung, liver, spleen. Error bars represent ± one standard deviation.
B. Neutrophil organ specific asthma time activity curves for: lung, liver, spleen. Error bars represent ± one standard deviation.
Combined time activity curves for eosinophil and neutrophil mean counts over the lungs, liver and spleen are shown in Fig. 2. There were no significant differences in net eosinophil migration into and out of the spleen between the three asthma groups. There was also no significant difference between the asthma groups and previously published kinetic data from normal volunteers (Lukawska et al., 2014). Further comparison with these retrospective data showed that significantly fewer neutrophils were retained in the spleen at 1 h in corticosteroid pre-treated asthmatics (Group 3) compared to healthy volunteers (p = 0.012). Significantly fewer eosinophils were retained in the liver at 1 h in the corticosteroid pre-treated volunteers (Group 3) when compared to Group 1 (p = 0.042). A greater proportion of eosinophils than neutrophils were retained in the bone marrow at 4 h (p = 0.0287) in all asthma study groups. This was consistent with the data from healthy volunteers (Lukawska et al., 2014).
3.4. Safety and Tolerability
In order to be repeatable, for example to study potential changes in leukocyte migration following therapy or over time, the procedure must be tolerable. In terms of exposure to radiation, we used less than half the dose per imaging study than is typically used for a clinical white blood cell scan. So, in principle this could be used for repeated examinations. Our volunteers were required to lie flat and still in the scanner for periods of up to 30 min, some of them while experiencing late phase bronchoconstriction following the allergen challenge. In practice, all of our volunteers tolerated this as well as can be expected on two separate occasions and, at least informally, reported that they would be prepared to undergo repeated examinations in the future.
4. Discussion
Recently we validated the technique of cGMP isolation of eosinophils and neutrophils from peripheral blood of normal volunteers, radiolabelling and re-infusing them and then using gamma scintigraphy to track their migration. The resulting accumulation and decay of the radioactivity in entire body organs, which reflects the time course of net influx and efflux of these cells into and out of the target organs was reported (Lukawska et al., 2014). We found that the decay of net retention of pure eosinophils and neutrophils in the lungs conformed to a single exponential model time activity curve, and that the mean half time of lung net clearance of eosinophils was significantly reduced compared with that of neutrophils.
In the present study we have utilised this technique in an attempt to track perturbations in net differential influx/efflux of eosinophils and neutrophils from the lungs and other organs during the course of the late phase bronchoconstrictor response to allergen challenge of atopic asthmatics showing both EAR and LAR (Groups 2 and 3), some of whom were pretreated with systemic corticosteroids (Group 3), and comparing with asthmatics previously displaying an isolated EAR who were not challenged (Group 1). Each volunteer was imaged on two separate occasions. We were able to confirm our previous findings in non-diseased controls that the kinetics of net influx/efflux of eosinophils and neutrophils into and out of the lungs are fundamentally different (Lukawska et al., 2014). Comparison of these kinetics with our previously reported data in normal controls using an identical technique also revealed a significantly elevated half time of retention of eosinophils in the lungs of the unchallenged asthmatics (t1/2 = 6.98) compared with the normal controls (t1/2 = 4.16).
We hypothesised that we would be able to observe significant prolongation of t1/2 of the net kinetics of eosinophil influx/efflux in the lungs of allergen challenged asthmatics during the peak of the late-phase asthmatic response when compared to unchallenged stable asthmatics, and that these kinetics would be further modified in the presence of systemic corticosteroid, which is known to attenuate this late response. In this study we were unable to do so, but we did however observe the following trends. It was notable that systemic corticosteroid therapy was associated with a trend for faster clearance of eosinophils from the lungs of the treated asthmatics, with rates (t1/2 of 5.63) approaching those previously observed in non-diseased healthy individuals (t1/2 of 4.16) (Lukawska et al., 2014). There was a trend for retention of eosinophils in the lungs of challenged volunteers (Group 2) (t1/2 = 6.03) compared to asthmatics recently treated with corticosteroids and healthy volunteers. There was no similar trend observed in neutrophil studies of the same groups of subjects.
What we found interesting was the increased t1/2 of eosinophil lung retention in Group 1 patients (EAR, no challenge) when compared to healthy volunteers, but the lack of evidence of any such effect in allergen challenged volunteers in Groups 2 and 3. This we expected to be greatest in Group 2. We propose several possible explanations for this. Firstly, as this study involved the use of radioactive tracer and a demanding protocol, our volunteer numbers in each study group were modest (4 participants). It is possible therefore that the study was underpowered statistically, although this in turn implies that any differences, had they existed, must have been very subtle. Secondly, and we believe possibly most importantly, the data from patients in Groups 2 and 3 may have been confounded by our inability to maintain haemodynamic stability. Since our protocol for patients in Groups 2 and 3 involved their lying flat and immobile in the scanner during the phase of considerable bronchoconstriction associated with the LAR this almost certainly induced sinus tachycardia (British Thoracic Society/Scottish Intercollegiate Guidelines Network, 2003), which in turn may have resulted in increased blood flow through the pulmonary circulation, diluting any tendency for eosinophil retention introduced by the allergen challenge. Thirdly, it is also possible that measurements made in this relatively low resolution scanning technique may have been confounded by changes in the ventilation/perfusion dynamics in allergen challenged asthmatic airways: there exists evidence that, during asthmatic bronchoconstriction defects in ventilation are spatially associated with defects in perfusion (i.e. hypoventilated regions are also hypoperfused) (Harris et al., 2006). This may arise from mechanical compression of blood vessels of the pulmonary circulation and/or effects of local hypoxia, further promoting vasoconstriction of the pulmonary vessels resulting in hypoxic pulmonary vasoconstriction (HPV). HPV is believed to be an adaptive mechanism unique to the pulmonary circulation that allows redirection of blood flow to alveoli with higher oxygen tension, thereby reducing ventilation/perfusion mismatch (Hunter et al., 2004). Consequently blood flow in the pulmonary circulation in these experiments may have been diverted away from regions most affected by allergen challenge, which would again tend to dilute any measured effect on the migration of blood cells such as eosinophils and neutrophils.
In future studies involving allergen challenge, we plan to scan patients in an upright sitting position. This we believe will improve their haemodynamic stability, HPV and overall comfort during the investigation.
Finally, there are some other caveats to be considered. Because of the time required to purify and radiolabel blood eosinophils and neutrophils ex vivo it was logistically impossible to reinfuse the labelled cells earlier than 5–6 h after blood sampling. Full processing and labelling of reinfused cells prior to commencing challenge would have required a 24 h protocol. Consequently we timed our scans to coincide with the period during the peak of LAR, commencing 6 h after allergen challenge, but we were unable to gather any data earlier than this. It is conceivable that precise timing of the scans may be critical here: there is some evidence, for example, that neutrophil influx into allergen-challenged airways is early and transient (Koh et al., 1993, Kelly et al., 2000, Nocker et al., 1999). For the same reason, the whole blood used for neutrophil/eosinophil isolation from the subjects was obtained just prior to, rather than after allergen challenge. It is possible that the challenge procedure itself might have altered the functions of populations of circulating eosinophils and neutrophils available for labelling. In designing future studies of real time tracking of net granulocyte influx and efflux from the lungs of asthmatics, and especially those involving allergen challenge or other forms of induced bronchoconstriction, these considerations must be taken into account.
As a readout of neutrophil activation, both ex vivo and following the isolation procedure, we monitored the percentages of these cells expressing CD11b (CD11bHigh) above a predetermined fluorescence intensity. We chose CD11b because it is an integrin component implicated in cellular adhesion, leukoaggregation and pulmonary sequestration of granulocytes although neutrophil migration, as distinct from adhesion more clearly depends on co-expression of CD18 in the integrin heterodimer (Vedder and Harlan, 1988). What we found was that the mean percentages of blood neutrophils expressing CD11b above this threshold directly ex vivo were already elevated in the three groups of patients with asthma (to 36% in Group 1, 45% in Group 2 and 57% in Group 3) as compared (retrospectively (Lukawska et al., 2014)) with healthy volunteers (~ 0.5%) even following isolation. This is likely to reflect priming by underlying chronic inflammation (Mann and Chung, 2006, Kämpe et al., 2011). Following the isolation and radiolabelling processes, these increased further to > 98% in all three groups of asthmatics. Nevertheless, this had no significant effect, within the resolution of our measurements, on the net kinetics of influx and efflux of these cells into and out of the lungs, not only in the patients with asthma but also (retrospectively (Lukawska et al., 2014)) in the healthy volunteers: when we compared the kinetics of net lung neutrophil influx/efflux in healthy volunteers (CD11bHigh 1.1 ± 0.8%) and all three asthma groups (CD11bHigh > 98%) we uncovered no significant differences. Similarly, as a readout of eosinophil activation we monitored the percentages of these cells expressing CD69. CD69 is an activation marker on subgroups of T cells as well as eosinophils, and has also been implicated in delaying the egress of T cells from lymph nodes (Zhi et al., 2011). In this case we found that the mean percentages of eosinophils expressing CD69 were not significantly different in the 3 groups of asthmatic patients (1.9% in Group 1, 3% in Group 2 and 2.5% in Group 3) and retrospectively (Lukawska et al., 2014) in healthy volunteers (~ 1.8%) directly ex vivo, while the process of isolation and radiolabelling significantly increased the mean percentages of eosinophils expressing CD69 in all 3 groups of asthmatic patients (to 40% in Group 1, 40% in Group 2 and 34% in Group 3) but not healthy volunteers (~ 4.8%). One could speculate that this might have been higher still if the procedures had been performed in the asthmatics following allergen challenge. As with neutrophils, this had no significant effect, within the resolution of our measurements, on the net kinetics of influx and efflux of these cells into and out of the lungs in the asthmatic patients. The only significant difference we were able to observe (retrospectively) was a greater net retention time of eosinophils in the Group 1 asthmatic patients as compared with the healthy volunteers. Consequently, although it is appropriate to be concerned about the possible effects of cellular isolation and labelling on cellular functions and properties in vivo, and although we chose what we perceived as likely relevant functional markers, expression of neither CD11b on neutrophils nor CD69 on eosinophils appeared to alter their migration kinetics in this study. The factors which govern net cellular migration into and out of organs in vivo are likely highly complex and variable, and as yet relatively poorly understood, and in time it may be possible to identify more appropriate markers to “screen” for possible effects of cellular isolation on these processes. We do not believe, however, that this reservation detracts from the intrinsic value of our observations and of the process, and indeed further experiments may result in more appropriate markers of “activation” which influence cellular migration to be defined.
As previously reported, the radiolabelling data indicated that eosinophils radiolabel with 99mTc-HMPAO with a far higher specific activity (10 to 25 times higher) than neutrophils and thus are likely to contribute to more than 50% of the signal in unseparated granulocyte populations in vivo (Lukawska et al., 2014). It was notable in the present study that prior corticosteroid therapy of the asthmatics reduced the radiolabelling efficiency of pure eosinophils (p = 0.002) and tended to increase that of neutrophils, although not significantly (p = 0.0537). This likely results from the mechanism by which 99mTc-HMPAO is normally preferentially taken into the small secretory vesicles of eosinophils and less so into the vesicles of neutrophils (Moberg et al., 2001). We hypothesise that the numbers of secretory vesicles in corticosteroid pre-treated eosinophils are diminished, resulting in poorer radiolabelling efficiency. This would have an impact on scanning data from mixed granulocyte populations, but should not affect a pure eosinophil or neutrophil scan.
In conclusion, we have demonstrated that differential, in vivo real time tracking of eosinophil and neutrophil migration into the lungs is feasible in asthmatics, even following experimental allergen challenge, as well as healthy volunteers. Given the complexity of the procedures involved in this work, it might be more appropriately regarded as a proof of concept study (the concept that asthmatics can be challenged, reinfused with labelled granulocytes and scanned within the time frame of the late phase reaction) than a definitely statistically powered investigation. While we failed to demonstrate differences between eosinophil or neutrophil efflux/influx in the three asthma groups using the current protocol, we have identified a number of critical issues that, with modification of the protocol and treatment of bronchoconstriction in challenged individuals should allow us to develop the technique as a key tool for phenotyping asthma and as an in vivo biomarker for the directing of novel asthma therapies. Data from this study are being used to determine suitable sample sizes required to power a Phase 1/2 clinical study.
Author Contribution
GM — study design, data interpretation, manuscript. JL — study design, recruitment, data collection and interpretation, manuscript. LL — study design, data interpretation, statistics, figures. BS — data collection, data interpretation. MK — data collection, clinical protocol design. JB — study design, data interpretation. MO — study design. PB — study design. CC — study design, manuscript. TL — study design. E S-P — data interpretation, statistics. GG — study design.
Role of the Funding Source
Biomedical Research Centre and Lee Lu Cheung Fund had no role in study design, data collection, data interpretation and writing of the manuscript.
Acknowledgements
The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (DOH/NIHR) comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust and Lee Lu Cheung Fund. The authors also wish to acknowledge the Department of Nuclear Medicine at Guys and St. Thomas' Hospital for providing radiopharmacy, medical physics and technical/radiographer support for the study.
References
- Brightling C.E., Symon F.A., Birring S.S. Comparison of airway immunopathology of eosinophilic bronchitis and asthma. Thorax. 2003;58:528–532. doi: 10.1136/thorax.58.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British guideline on the management of asthma: management of acute asthma. Thorax. 2003;58(Suppl. I):i32–i50. doi: 10.1136/thorax.58.suppl_1.1i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers G.W., Macleod K.J., Thomson L. Smoking and airway inflammation in patients with mild asthma. Chest. 2001;120:1917–1922. doi: 10.1378/chest.120.6.1917. [DOI] [PubMed] [Google Scholar]
- Cieslewicz G., Tomkinson A., Adler A. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J. Clin. Invest. 1999;104:301–308. doi: 10.1172/JCI7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douwes J., Gibson P., Pekkanen J. Noneosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57:643–648. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich G.J. Mechanisms of eosinophil-associated inflammation. JACI. 2000;105:651. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- Green R.H., Brightling C.E., Woltmann G. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57:875–879. doi: 10.1136/thorax.57.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, Winkler, Tgavalekos Regional pulmonary perfusion, inflation, and ventilation defects in bronchoconstricted patients with asthma. Am. J. Respir. Crit. Care Med. 2006;174:245–253. doi: 10.1164/rccm.200510-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C.J., Dejam A., Blood A.B. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat. Med. Oct. 2004;10(10):1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- Kamath A.V. Is the neutrophil the key effector cell in severe asthma? Thorax. 2005;60:529–530. doi: 10.1136/thx.2005.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kämpe M., Stolt I., Lampinen M., Janson C., Stålenheim G., Carlson M. Patients with allergic rhinitis and allergic asthma share the same pattern of eosinophil and neutrophil degranulation after allergen challenge. Clin. Mol. Allergy. 2011;9:3. doi: 10.1186/1476-7961-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E.A., Busse W.W., Jarjour N.N. Increased matrix metalloproteinase-9 in the airway after allergen challenge. Am. J. Respir. Crit. Care Med. 2000;162:1157. doi: 10.1164/ajrccm.162.3.9908016. [DOI] [PubMed] [Google Scholar]
- Ketchell R.I., D'Amato M., Jensen M.W., O'Connor B.J. Contrasting effects of allergen challenge on airway responsiveness to cysteinyl leukotriene D4 and methacholine in mild asthma. Thorax. 2002;57:575–580. doi: 10.1136/thorax.57.7.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh Y.Y., Dupuis R., Pollice M., Albertine K.H., Fish J.E., Peters S.P. Neutrophils recruited to the lungs of humans by segmental allergen challenge display a reduced chemotactic response to leukotriene B4. Am. J. Respir. Cell Mol. Biol. 1993;8:493–499. doi: 10.1165/ajrcmb/8.5.493. [DOI] [PubMed] [Google Scholar]
- Lemanske R.F., Jr., Busse W.W. Asthma: clinical expression and molecular mechanisms. J. Allergy Clin. Immunol. 2010;125(2):S95–S102. doi: 10.1016/j.jaci.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S.A., Macleod K.J., Chalmers G.W. Association of forced expiratory volume with disease duration and sputum neutrophils in chronic asthma. Am. J. Med. 2002;112:446–452. doi: 10.1016/s0002-9343(02)01047-1. [DOI] [PubMed] [Google Scholar]
- Lukawska J., Livieratos L., Sawyer B., Lee T. Real time differentia tracking of human neutrophil and eosinophil migration in vivo. JACI. 2014;133(1):233–239. doi: 10.1016/j.jaci.2013.06.031. [DOI] [PubMed] [Google Scholar]
- Mann B.S., Chung K.F. Blood neutrophil activation markers in severe asthma: lack of inhibition by prednisolone therapy. Respir. Res. 2006;7:59. doi: 10.1186/1465-9921-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.J., Cicutto L.C., Smith H.R. Airways inflammation in nocturnal asthma. Am. Rev. Respir. Dis. 1991;143:351–357. doi: 10.1164/ajrccm/143.2.351. [DOI] [PubMed] [Google Scholar]
- Metzger W.J., Richerson H.B., Worden K., Monick M., Hunninghake G.W. Bronchoalveolar lavage of allergic asthmatic patients following allergen bronchoprovocation. Chest. 1986;89:477–483. doi: 10.1378/chest.89.4.477. [DOI] [PubMed] [Google Scholar]
- Metzger W.J., Zavala D., Richerson H.B., Moseley P., Iwamota P., Monick M. Local allergen challenge and bronchoalveolar lavage of allergic asthmatic lungs: description of the model and local airway inflammation. Am. Rev. Respir. Dis. 1987;135:433–440. doi: 10.1164/arrd.1987.135.2.433. [DOI] [PubMed] [Google Scholar]
- Moberg L., Karawajczyk M., Venge P. 99mTc-HMPAO (Ceretec) is stored in and released from the granules of eosinophil granulocytes. Br. J. Haematol. 2001;114:185–190. doi: 10.1046/j.1365-2141.2001.02889.x. [DOI] [PubMed] [Google Scholar]
- Nocker R.E., Out T.A., Weller F.R., Mul E.P., Jansen H.M., van der Zee J.S. Influx of neutrophils into the airway lumen at 4 h after segmental allergen challenge in asthma. Int. Arch. Allergy Immunol. 1999;119:45–53. doi: 10.1159/000024174. [DOI] [PubMed] [Google Scholar]
- O'Byrne P.M., Dolovich J., Hargreave F.E. State of the art: late asthmatic responses. Am. Rev. Respir. Dis. 1987;136:740–751. doi: 10.1164/ajrccm/136.3.740. [DOI] [PubMed] [Google Scholar]
- Simpson J.L., Scott R., Boyle M.J. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- Singh D., Richards D., Knowles R.G., Schwartz S., Woodcock A., Langley S., O'Connor B.J. Selective inducible nitric oxide synthase inhibition has no effect on allergen challenge in asthma. Am. J. Respir. Crit. Care Med. 2007;176:988–993. doi: 10.1164/rccm.200704-588OC. [DOI] [PubMed] [Google Scholar]
- Sur S., Crotty T.B., Kephart G.M. Sudden-onset fatal asthma: a distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am. Rev. Respir. Dis. 1993;148:713–719. doi: 10.1164/ajrccm/148.3.713. [DOI] [PubMed] [Google Scholar]
- Vedder N.B., Harlan J.M. Increased surface expression on CD11b/CD18 (MAC-1) is not required for stimulated neutrophil adherence to cultured epithelium. J. Clin. Invest. 1988;81(3):676–682. doi: 10.1172/JCI113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel S.E. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- Zhi L., Kim P., Thompson B.D. FTY720 blocks egress of t cells in part by abrogation of their adhesion on the lymph node sinus. J. Immunol. 2011;187:2244–2251. doi: 10.4049/jimmunol.1100670. [DOI] [PMC free article] [PubMed] [Google Scholar]