Abstract
Traumatic optic neuropathy (TON) is an uncommon cause of visual loss following blunt or penetrating head trauma, but the consequences can be devastating, especially in cases with bilateral optic nerve involvement. Although the majority of patients are young adult males, about 20% of cases occur during childhood. A diagnosis of TON is usually straightforward based on the clinical history and examination findings indicative of an optic neuropathy. However, the assessment can be difficult when the patient's mental status is impaired owing to severe trauma. TON frequently results in profound loss of central vision, and the final visual outcome is largely dictated by the patient's baseline visual acuities. Other poor prognostic factors include loss of consciousness, no improvement in vision after 48 hours, the absence of visual evoked responses, and evidence of optic canal fractures on neuroimaging. The management of TON remains controversial. Some clinicians favor observation alone, whereas others opt to intervene with systemic steroids, surgical decompression of the optic canal, or both. The evidence base for these various treatment options is weak, and the routine use of high-dose steroids or surgery in TON is not without any attendant risks. There is a relatively high rate of spontaneous visual recovery among patients managed conservatively, and the possible adverse effects of intervention therefore need to be even more carefully considered in the balance.
Keywords: head injury, optic canal fracture, optic nerve, steroids, traumatic optic neuropathy
1. Classification
Traumatic optic neuropathy (TON) refers to any insult to the optic nerve secondary to trauma. It can be classified depending on the site of injury (optic nerve head, intraorbital, intracanalicular, or intracranial) or according to the mode of injury (direct or indirect).1,2 In direct TON, there is significant anatomical disruption to the optic nerve, for example, from a projectile penetrating the orbit at high velocity (Fig. 1), or as a result of optic nerve avulsion (Fig. 2). Indirect TON is caused by the transmission of forces to the optic nerve from a distant site, without any overt damage to the surrounding tissue structures. The deformative stress transmitted to the skull from blunt trauma is concentrated in the region of the optic canal. The intracanalicular segment of the optic nerve is particularly susceptible to this form of injury, because the dural sheath is tightly adherent to the periosteum at this specific location.3,4 The intracranial portion of the optic nerve in close proximity to the falciform dural fold is the next most common site at risk of injury.5 In one report using computerized tomography (CT) imaging, about half of all TON cases were found to have an associated sphenoidal bone fracture, an indirect measure of the significant compressive forces involved at impact.6 However, both direct and indirect mechanisms can contribute to optic nerve damage, and a clear distinction is not always possible.
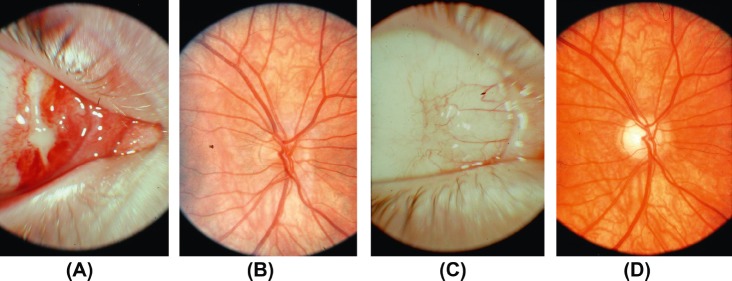
Fig. 1.
Direct traumatic optic neuropathy. (A) Entry site of a projectile in the medial canthal region of the right eye. (B) The patient's posterior pole was normal when he was assessed shortly after the accident. His visual acuity at that time was no perception of light. (C) Conjunctival scar over the entry site. (D) Optic disc pallor, more marked temporally, was apparent 6 weeks later. The patient's visual acuity had not improved and he was subsequently lost to follow-up. (Courtesy of Professor David Taylor, Institute of Child Health, London, UK.)
Fig. 2.
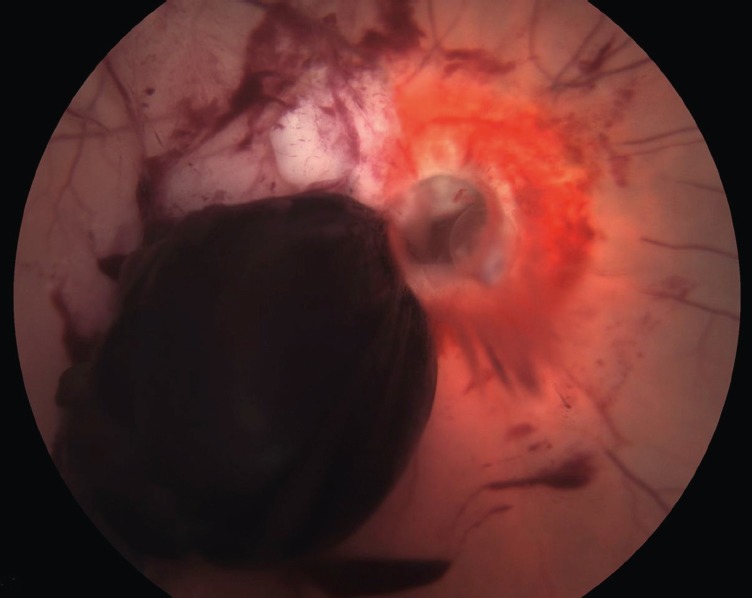
Traumatic optic nerve avulsion following a road traffic accident. (Courtesy of Dr Scott Schoenberger, Vanderbilt Eye Institute, Nashville, TN, USA.)
2. Pathophysiology
The pathophysiology of indirect TON is likely to be multifactorial, and the concept of primary and secondary injury has been proposed.7,8 Following trauma, there is an immediate shearing of aproportion of retinal ganglion cell axons, an irreversible process that results in neuronal loss. There is then a degree of optic nerve swelling within the tight confines of the optic canal secondary to direct mechanical trauma and vascular ischemia. The ensuing compartment syndrome further impairs the already compromised blood supply to surviving retinal ganglion cells, setting up a downward spiral toward apoptotic cell death. This two-stage model of TON forms the basis for optic nerve decompression by medical or surgical means, in order to break this vicious cycle and to preserve the remaining retinal ganglion cells that survived the initial insult.
3. Epidemiology
TON is an uncommon cause of visual loss following blunt or penetrating head trauma with a reported incidence of 0.7–2.5% in published case series.9,10,11,12 A recent national epidemiological survey of TON in the United Kingdom found a minimum prevalence in the general population of one in 1,000,000.13 The vast majority of affected patients are young adult males (79–85%) in their early 30s. The most common causes of TON in this patient group are motor vehicle and bicycle accidents (49%), falls (27%), and assaults (13%).13,14 In the pediatric population, the majority of TON cases are secondary to falls (50%) and road traffic accidents (40%).15
4. Clinical assessment
TON is a clinical diagnosis supported by a history of direct or indirect trauma to the head or face. The injury can sometimes be trivial, and a careful history of the incident must be elicited from the patient and any other witnesses that might have been present especially when dealing with children or unconscious patients. A detailed record should also be kept as cases of TON are not infrequently the subject of future medicolegal proceedings. Although usually straightforward, the clinical assessment can sometimes prove difficult in the setting of severe trauma when the patient's level of consciousness is impaired. In this scenario, it is essential to exclude possible reversible causes of visual loss that require immediate attention, for example, a retrobulbar hemorrhage. The patient's baseline visual acuity should be clearly documented in the notes and even if only a bedside examination is possible, this can still be achieved with a portable vision chart and the use of a pinhole occluder. A thorough examination of the eye and the adnexal structures is mandatory, with particular care taken to exclude associated orbital or facial fractures requiring more specialized maxillofacial input. Except when neurosurgical monitoring of the pupil is required, a detailed dilated examination of the posterior pole must be carried out to document the state of the optic disc, any associated retinal or vitreous hemorrhages, and the possibility of an intraocular foreign body in cases of penetrating trauma. A high degree of clinical vigilance must also be maintained because TON can infrequently be associated with delayed visual loss secondary to the development of an optic nerve sheath hematoma (Fig. 3). The following features are consistent with a diagnosis of TON. (1) Unilateral or bilateral ocular involvement. (2) A relative afferent pupillary defect except in bilateral symmetric cases. A relative afferent pupillary defect is an important clinical sign, and in patients with mild TON, it can be the only objective evidence of optic nerve dysfunction prior to the development of overt optic atrophy. (3) Variable loss of visual acuity ranging from normal to no light perception. Between 40% and 60% of patients present with severe visual loss of light perception or worse at baseline.13,14,15,16,17 (4) Impairment of color vision. (5) Variable visual field defects.
Fig. 3.
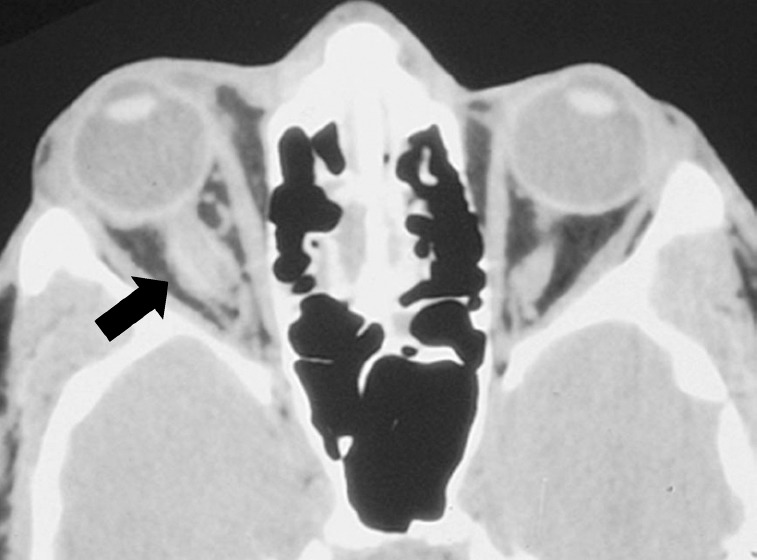
Right optic nerve sheath hematoma. (Courtesy of Dr Peter Savino, Wills Eye Institute, Philadelphia, PA, USA.)
The optic disc appearance will depend on the anatomical site and the timing of injury. With injuries to the optic nerve anterior to the entry point of the central retinal vessels, there is optic disc swelling with associated retinal hemorrhages. With more posterior injuries, which are more common, the fundus can look entirely normal. Optic disc pallor usually develops about 6 weeks following the initial injury (Fig. 1).
5. Neuroimaging
There is a wide variation in practice worldwide regarding the use of neuroimaging in TON. Some clinicians request CT or magnetic resonance imaging or both for all cases, whereas others limit these investigations to patients with progressive visual deterioration or when therapeutic interventions are being considered.6,18,19 Before a magnetic resonance imaging is carried out, it is essential to exclude the possibility of an intraorbital or intraocular metallic foreign body by conventional radiography. CT is the best imaging modality for delineating optic canal fractures and their full extent in preparation for possible surgical intervention (Fig. 4). However, the clinical usefulness of universal neuroimaging in TON remains debatable as there is no consistent correlation between the finding of an optic canal fracture, the severity of visual loss, and the prognosis for visual recovery.20,21
Fig. 4.
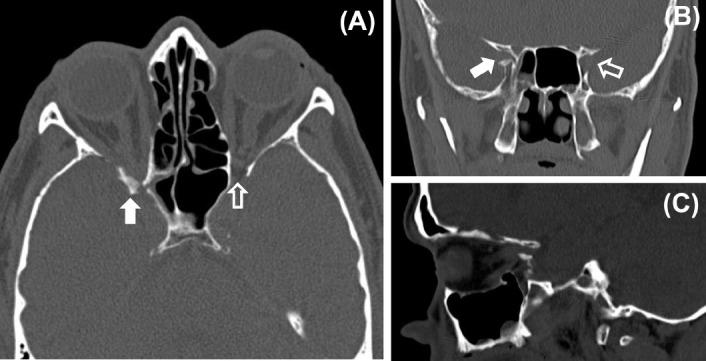
Optic canal fracture and optic nerve compression. (A) Axial computerized tomography (CT) scan through the optic nerves showing a fracture in the posterior part of the lateral wall of the right orbit. The bone fragment is causing compression of the right optic nerve within the optic canal (white arrow). A normal wide optic canal can be seen on the left side (clear arrow). (B) Coronal CT scan showing narrowing of the right optic foramen (white arrow) compared with the left side (clear arrow). (C) Sagittal CT scan showing compression of the right optic nerve by the bone fragment. (Courtesy of Dr Manjunatha YC, Sri Devaraj Urs Medical College, Tamaka, Kolar, India.)
6. Prognostic factors
A visual recovery rate of 40–60% has been reported for indirect TON cases managed conservatively, with baseline visual acuity being the most important predictor of final outcome.14,22,23,24,25,26 There is a significant correlation between initial and final visual acuities, and patients reporting no light perception at presentation invariably have limited or no visual improvement. Other poor prognostic factors include loss of consciousness, lack of visual recovery after 48 hours, and absence of visual evoked responses.26,27 The presence of an optic canal fracture was found to predict a poor visual outcome in some, but not all, case series.16,22,26,28,29 Direct TON is a distinct category that results in severe, irreversible visual loss with little likelihood for recovery, and no intervention is of proven benefit.
7. Management
The controversies surrounding the optimal management of TON have been the subject of recent Cochrane systematic reviews.20,21 Despite these persisting uncertainties, the main treatment options in current use for TON are as follows: (1) systemic steroids of varying doses, duration, and mode of administration; (2) surgical decompression of the optic canal; (3) a combination of steroids and surgery; and (4) observation alone (i.e., conservative management).
8. Steroids
The pharmacological rationale for using steroids in TON first arose from their perceived benefits when applied to various animal models of central nervous system injuries.30,31 The observed neuroprotective effect has been ascribed to the antioxidant properties of steroids and to the inhibition of free radical-induced lipid peroxidation.32,33 This hypothesis was further reinforced following the clinical introduction of steroids to the treatment of traumatic spinal cord injuries. The second National Acute Spinal Cord Injury Study (NASCIS-II) was a multicenter, randomized, double-blind, placebo-controlled trial set up to assess the benefits of megadose steroids in patients with acute spinal cord injury.34 The treatment regimen consisted of an initial bolus dose of 30 mg/kg, followed by an infusion at 5.4 mg/kg/h for a total duration of 23 hours. Patients who received steroids within 8 hours of their injury had significantly better improvement in neurological functions compared to those in the placebo group or those who were treated after 8 hours.34 In the third NASCIS (NASCIS–III), patients who received steroids 3–8 hours after their injury experienced greater motor and functional recovery when this regimen was maintained for 48 hours instead of 24 hours.35 For those patients who were treated within 3 hours of injury, the neurological outcomes in the 24- and 48-hour arms of the trial were similar. Unsurprisingly, the findings of the NASCIS trials have heavily influenced clinical practice, leading to the increased use of steroids in TON from the mid-1990s onward.
9. Steroid regimens
Steroids have been used both on its own and in combination with surgical optic nerve decompression either pre-, intra-, or postoperatively.20,21 Based on the initial daily dose of methyl-prednisolone used, steroid regimens can be classified as: (1) low dose (< 100 mg), (2) moderate dose (100–499 mg), (3) high dose (500–1999 mg), (4) very high dose (2000–5399 mg), or (5) megadose (> 5400 mg). The most commonly used steroid protocol in TON is a course of intravenous methylprednisolone in the very high-dose to megadose range.
10. Critical analysis
All the published case series in TON suffer from several methodological flaws.13,14,16,22,23,36 The majority are small, retrospective studies that lack the sample size for rigorous statistical analysis, and the absence of adequate randomization introduces the added possibility of selection bias. It is also very difficult to compare the results, even qualitatively, because of the wide range of steroid regimens used and the variable time allowed prior to the initiation of treatment. A Cochrane systematic review identified only one double-blind, randomized controlled trial comparing high-dose intravenous steroids to placebo in patients with indirect TON diagnosed within 7 days of their initial injuries.21 Although this study was relatively small, with only 16 patients in the treated group and 15 patients in the placebo group, there was no significant difference in the final visual outcome between these two groups, precluding a major therapeutic effect from the use of steroids.37
11. International Optic Nerve Trauma Study
The International Optic Nerve Trauma Study (IONTS) is the largest, prospective, multicenter study of TON published to date.14 It was intended to be a randomized controlled trial, but it had to be converted to an observational study after 2 years owing to recruitment failure. The analysis included a total of 133 people with indirect TON treated within 7 days of injury and categorized into three groups: untreated (n = 9), steroids (n = 85), or optic canal decompression surgery (n = 33). The majority of patients in the steroid group had either a megadose (40%) or very high-dose regimen (18%), and all the participants in the surgical group, except for one, also received steroids. Follow-up data were available for 104 cases at 1 month and for 40 cases at 6 months. After adjustment for baseline visual acuity, no significant differences were found between the three treatment groups. A three-line increase in visual acuity or more occurred in 57% of the untreated group, 52% of the steroid group, and 32% of the surgery group.14 Interestingly, there was no trend suggesting an increased probability of visual recovery with higher doses of steroids or with earlier initiation of treatment. Although some case series have reported higher improvement rates with steroids, most published figures (44–62%) are comparable with IONTS.13,16,22,23,36 Crucially, none of these studies have demonstrated any convincing functional visual benefit following treatment with steroids.
12. NASCIS
The application of steroids to TON relies heavily on extrapolation made from the NASCIS trials, and therefore a critical appraisal of their results is appropriate.34,35 There is ongoing debate in the literature regarding the significance of the neurological benefit reported by NASCIS among patients treated within the 8-hour window of sustaining a spinal cord injury.38,39,40,41 The mean difference between the steroid and the placebo groups indicated a significant neurological benefit for motor scores, but not for sensory scores. Critics of the NASCIS trials have argued that the finding of a beneficial effect for the early treatment group is relatively weak, being based on a post hoc subgroup analysis.38,39,40,41 Concerns have also been raised on possible randomization imbalance between the treatment arms, which might have biased the results in favor of the steroid group. For clinicians, perhaps the most compelling argument is that although statistically significant, the relatively small change in motor scores might not actually translate into any functional benefit. A Cochrane systematic review on the use of steroids following acute spinal cord injury has not resolved the controversy surrounding the NASCIS trials.42 The optic nerve is a predominantly white matter tract, and it differs histologically from the spinal cord both in terms of its cellular environment and organization. There is also no comparative data on the actual concentration of the active metabolites that are achieved locally within the optic nerve and the spinal cord following intravenous steroid injections. Several fundamental questions therefore remain whether extrapolating experimental and clinical data from the spinal cord to the optic nerve is biologically plausible.
13. Adverse effects
High-dose to megadose steroids are relatively safe, but serious complications can occur and these need to be considered, especially if preexisting susceptibility factors are present.43,44,45 The CRASH (Corticosteroid Randomisation After Significant Head injury) study was a large randomized controlled trial investigating the effectiveness and safety of steroids in patients with acute traumatic brain injury.45 Patients presenting within 8 hours of head trauma were allocated to either placebo or megadose intravenous methylprednisolone (2 g over 1 hour, followed by 0.4 g/h for 48 hours). The initial protocol was to recruit 20,000 participants, but the study was terminated prematurely after 10,008 people had been enrolled because an interim analysis revealed a detrimental effect in the steroid arm of the trial. At 6 months of follow-up, the risk of death was significantly higher in the steroid group (25.7%) than in the placebo group (22.3%), as was the risk of death or severe disability (38.1% vs. 36.3%, respectively). This landmark trial provides convincing evidence that steroids should no longer be used in patients with traumatic brain injury, a conclusion that was also reached by a recent Cochrane systematic review on this topic.45,46 The recommendations from the CRASH study must therefore be considered seriously in the subgroup of TON patients who have sustained significant head injuries.21,41
A number of animal models of TON have been developed, and the most widely used experimental paradigm involves a direct, mechanical crush injury to the rat's optic nerve.7 In three studies, rats treated with various regimens of methylprednisolone were compared with sham controls following an optic nerve crush injury.47,48,49 Two studies failed to show any difference in retinal ganglion cell survival and axonal regeneration between these two groups.47,48 However, in the third study, steroids exacerbated retinal ganglion cell loss and there was a significant, dose-dependent decline in axonal counts with increasing doses of steroids.49 Although some caution is needed when extrapolating evidence from animal studies, supraphysiological doses of steroids ould exert a negative effect on neuronal survival by suppressing key endogenous neuroprotective pathways.50 For this reason, a maximum daily dose of 1 g intravenous methylprednisolone has been advocated in TON to minimize the risk of neurotoxicity when a decision has been made to initiate treatment.51
14. Surgery
A wide range of intra- and extracranial surgical techniques have been used to achieve optic nerve decompression in TON.52,53 Although the favored intervention is largely dictated by the expertise available locally and the surgeon's preference, there has been a shift toward minimally invasive extracranial approaches employing the transethmoidal, endonasal, or sublabial outes.16,29,54,55,56,57
15. Timing of surgery
The timing of surgery is a relevant issue in the context of trauma where life-threatening injuries often lead to unavoidable delays before a formal ophthalmological assessment can be carried out. Intuitively, the longer the delay, the less likely optic canal decompression would be expected to salvage compromised retinal ganglion cells and restore visual function. Based on the limited data available, there is conflicting evidence whether the length of time between the initial insult and surgical intervention actually impacts on visual recovery in TON.20,51
16. Optic canal fracture
Some authorities argue that the optic canal should be imaged in all TON cases, and if a fracture is identified with a bone fragment impinging on the optic nerve (Fig. 5), prompt surgical intervention should be advocated.51,58 The counterargument is that some studies have actually identified the presence of an optic canal fracture as a poor prognostic factor for visual recovery, irrespective of the treatment modality used.16,22,26,28,29 This makes biological sense, because a bone fragment is likely to transect a large proportion of retinal ganglion cell axons resulting in immediate irreversible injury and decompressing the optic canal in this situation is unlikely to restore significant visual function.
Fig. 5.
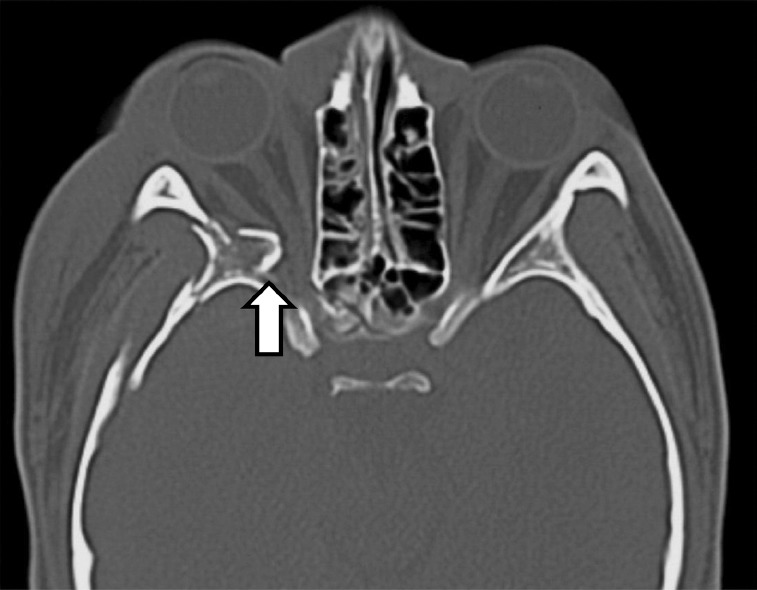
Axial computerized tomography scan showing bone fragments compressing the right optic nerve in the posterior orbital region. Multiple fractures of the lateral orbital wall and of the greater wing of the sphenoid can also be noted on the right side. (Courtesy of Professor Kazuo Shimozato, Aichi-Gakuin University, Nagoya, Japan.)
17. Critical analysis
There are no randomized controlled trials on the effectiveness of surgical optic nerve decompression in TON.20 As part of the IONTS cohort, three out of 33 patients (10%) who underwent external surgical decompression suffered postoperative cerebrospinal fluid leak, with one patient developing meningitis.14 Another case series reported accidental dural exposure in 5% of patients who underwent endoscopic optic canal decompression.57 Given the relatively high rate of spontaneous visual improvement in indirect TON, the decision to subject a patient to a surgical intervention with potentially serious complications must be even more circumspect.
18. Practical considerations
There are practical limitations in applying the NASCIS findings given the narrow window of opportunity available for initiating treatment. There are often unavoidable delays in diagnosing TON when patients have life-threatening injuries that justifiably take precedence before an ophthalmological opinion is sought. If the patient is unconscious for a prolonged period, visual loss is likely to be reported late, and even if a clinical diagnosis is made within the 8-hour window, there are obvious ethical considerations to initiating potentially controversial treatment without proper informed consent. Recent animal studies and the CRASH trial have also highlighted significant gaps in our understanding of central nervous system injuries, and there is an urgent need for further research into the role of steroids in modulating neuronal recovery following trauma. The logistics required for an adequately powered randomized controlled trial in TON are daunting, and practically, it is unclear whether the resources needed for such a major undertaking are feasible, both in terms of patient recruitment and standardization of treatment. There is a relatively high rate of spontaneous visual recovery in TON, and there is no convincing evidence that steroids or surgical optic nerve decompression provides any additional benefit over conservative management alone. Each case therefore needs to be assessed on an individual basis, and the patient needs to be made fully aware of both the theoretical risks suggested by recent studies, and the real risks, albeit rare, of a serious adverse event with active intervention.
Acknowledgments
PYWM is a Medical Research Council (MRC, UK) clinician scientist. PYWM also receives funding from Fight for Sight (UK) and the UK National Institute of Health Research (NIHR) as part of the Rare Diseases Translational Research Collaboration. We gratefully acknowledge the support of the Cochrane Eyes and Vision Group, in particular, Ms Anupa Shah and Mr Richard Wormald.
Footnotes
Conflicts of interest: PYWM reports no relevant financial disclosures or conflicts of interest.
References
- 1.Sarkies N. Traumatic optic neuropathy. Eye. 2004;18:1122–1125. doi: 10.1038/sj.eye.6701571. [DOI] [PubMed] [Google Scholar]
- 2.Steinsapir KD, Goldberg RA. Traumatic optic neuropathy. Surv Ophthalmol. 1994;38:487–518. doi: 10.1016/0039-6257(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RL, Panje WR, Gross CE. Optic-nerve blindness following blunt forehead trauma. Ophthalmology. 1982;89:445–455. doi: 10.1016/s0161-6420(82)34769-7. [DOI] [PubMed] [Google Scholar]
- 4.Gross CE, Dekock JR, Panje WR, Hershkowitz N, Newman J. Evidence for orbital deformation that may contribute to monocular blindness following minor frontal head trauma. J Neurosurg. 1981;55:963–966. doi: 10.3171/jns.1981.55.6.0963. [DOI] [PubMed] [Google Scholar]
- 5.Crompton MR. Visual lesions in closed head injury. Brain. 1970;93:785–792. doi: 10.1093/brain/93.4.785. [DOI] [PubMed] [Google Scholar]
- 6.Seiff SR, Berger MS, Guyon J, Pitts LH. Computed tomographic evaluation of the optic canal in sudden traumatic blindness. Am J Ophthalmol. 1984;98:751–755. doi: 10.1016/0002-9394(84)90693-7. [DOI] [PubMed] [Google Scholar]
- 7.Levkovitch-Verbin H. Animal models of optic nerve diseases. Eye. 2004;18:1066–1074. doi: 10.1038/sj.eye.6701576. [DOI] [PubMed] [Google Scholar]
- 8.Osborne NN, Chidlow G, Layton CJ, Wood JP, Casson RJ, Melena J. Optic nerve and neuroprotection strategies. Eye (Lond) 2004;18:1075–1084. doi: 10.1038/sj.eye.6701588. [DOI] [PubMed] [Google Scholar]
- 9.Cockerham GC, Goodrich GL, Weichel ED, et al. Eye and visual function in traumatic brain injury. J Rehabil Res Dev. 2009;46:811–818. doi: 10.1682/jrrd.2008.08.0109. [DOI] [PubMed] [Google Scholar]
- 10.Edmund J, Godtfredsen E. Unilateral optic atrophy following head injury. Acta Ophthalmol. 1963;41:693–697. doi: 10.1111/j.1755-3768.1963.tb03588.x. [DOI] [PubMed] [Google Scholar]
- 11.Nau HE, Gerhard L, Foerster M, Nahser HC, Reinhardt V, Joka T. Optic-nerve trauma — clinical, electrophysiological and histological remarks. Acta Neurochirurg. 1987;89:16–27. doi: 10.1007/BF01406662. [DOI] [PubMed] [Google Scholar]
- 12.Pirouzmand F. Epidemiological trends of traumatic optic nerve injuries in the largest Canadian adult trauma center. J Craniofac Surg. 2012;23:516–520. doi: 10.1097/SCS.0b013e31824cd4a7. [DOI] [PubMed] [Google Scholar]
- 13.Lee V, Ford RL, Xing W, Bunce C, Foot B. Surveillance of traumatic optic neuropathy in the UK. Eye. 2010;24:240–250. doi: 10.1038/eye.2009.79. [DOI] [PubMed] [Google Scholar]
- 14.Levin LA, Beck RW, Joseph MP, Seiff S, Kraker R. The treatment of traumatic optic neuropathy — the International Optic Nerve Trauma Study. Ophthalmology. 1999;106:1268–1277. doi: 10.1016/s0161-6420(99)00707-1. [DOI] [PubMed] [Google Scholar]
- 15.Mahapatra AK, Tandon DA. Traumatic optic neuropathy in children — a prospective-study. Pediatr Neurosurg. 1993;19:34–39. doi: 10.1159/000120698. [DOI] [PubMed] [Google Scholar]
- 16.Wang BH, Robertson BC, Girotto JA, et al. Traumatic optic neuropathy: a review of 61 patients. Plast Reconstr Surg. 2001;107:1655–1664. doi: 10.1097/00006534-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Lessell S. Indirect optic-nerve trauma. Arch Ophthalmol. 1989;107:382–386. doi: 10.1001/archopht.1989.01070010392031. [DOI] [PubMed] [Google Scholar]
- 18.Manfredi SJ, Raji MR, Sprinkle PM, Weinstein GW, Minardi LM, Swanson TJ. Computerized tomographic scan findings in facial fractures associated with blindness. Plast ReconstrSurg. 1981;68(4):479–490. doi: 10.1097/00006534-198110000-00001. PMID: 7280095 [PubMed – indexed for MEDLINE] [DOI] [PubMed] [Google Scholar]
- 19.Takehara S, Tanaka T, Uemura K, et al. Optic-nerve injury demonstrated by MRI with STIR sequences. Neuroradiology. 1994;36:512–514. doi: 10.1007/BF00593510. [DOI] [PubMed] [Google Scholar]
- 20.Yu-Wai-Man P, Griffiths PG. Surgery for traumatic optic neuropathy. Cochrane Database Syst Rev. 2005:CD005024. doi: 10.1002/14651858.CD005024.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Yu-Wai-Man P, Griffiths PG. Steroids for traumatic optic neuropathy. Cochrane Database Syst Rev. 2011:CD006032. doi: 10.1002/14651858.CD006032.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Chou PI, Sadun AA, Chen YC, Su WY, Lin SZ, Lee CC. Clinical experiences in the management of traumatic optic neuropathy. Neuro-Ophthalmology. 1996;16:325–336. [Google Scholar]
- 23.Cook MW, Levin LA, Joseph MP, Pinczower EF. Traumatic optic neuropathy — a meta-analysis. Arch Otolaryngol Head Neck Surg. 1996;122:389–392. doi: 10.1001/archotol.1996.01890160031006. [DOI] [PubMed] [Google Scholar]
- 24.Seiff SR. High-dose corticosteroids for treatment of vision loss due to indirect injury to the optic-nerve. Ophthalmic Surg Lasers. 1990;21:389–395. [PubMed] [Google Scholar]
- 25.Steinsapir KD. Treatment of traumatic optic neuropathy with high-dose corticosteroid. J Neuro-Ophthalmol. 2006;26:65–67. doi: 10.1097/01.wno.0000204646.94991.68. [DOI] [PubMed] [Google Scholar]
- 26.Carta A, Ferrigno L, Salvo M, Bianchi-Marzoli S, Boschi A, Carta F. Visual prognosis after indirect traumatic optic neuropathy. J Neurol Neurosurg Psychiatry. 2003;74:246–248. doi: 10.1136/jnnp.74.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes MD, Sires BS. Flash visual evoked potentials predict visual outcome in traumatic optic neuropathy. Ophthalmic Plast Reconstr Surg. 2004;20:342–346. doi: 10.1097/01.iop.0000134272.55294.4c. [DOI] [PubMed] [Google Scholar]
- 28.Rajiniganth MG, Gupta AK, Gupta A, Bapuraj JR. Traumatic optic neuropathy visual outcome following combined therapy protocol — visual outcome following combined therapy protocol. Arch Otolaryngol Head Neck Surg. 2003;129:1203–1206. doi: 10.1001/archotol.129.11.1203. [DOI] [PubMed] [Google Scholar]
- 29.Yang WG, Chen CT, Tsay PK, de Villa GH, Tsai YJ, Chen YR. Outcome for traumatic optic neuropathy — surgical versus nonsurgical treatment. Ann Plast Surg. 2004;52:36–42. doi: 10.1097/01.sap.0000096442.82059.6d. [DOI] [PubMed] [Google Scholar]
- 30.Braughler JM, Hall ED, Means ED, Waters TR, Anderson DK. Evaluation of an intensive methylprednisolone sodium succinate dosing regimen in experimental spinal-cord injury. J Neurosurg. 1987;67:102–105. doi: 10.3171/jns.1987.67.1.0102. [DOI] [PubMed] [Google Scholar]
- 31.Hall ED, Braughler JM. Corticosteroid-therapy in experimental cord injury. J Neurosurg. 1984;61:805–806. [PubMed] [Google Scholar]
- 32.Bains M, Hall ED. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta Mol Basis Dis. 2012;1822:675–684. doi: 10.1016/j.bbadis.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall ED. The neuroprotective pharmacology of methylprednisolone. J Neurosurg. 1992;76:13–22. doi: 10.3171/jns.1992.76.1.0013. [DOI] [PubMed] [Google Scholar]
- 34.Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury — results of the 2nd National Acute Spinal-Cord Injury Study. New Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 35.Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury — results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. J Am Med Assoc. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 36.Spoor TC, Hartel WC, Lensink DB, Wilkinson MJ. Treatment of traumatic optic neuropathy with corticosteroids. Am J Ophthalmol. 1990;110:665–669. doi: 10.1016/s0002-9394(14)77065-5. [DOI] [PubMed] [Google Scholar]
- 37.Entezari M, Rajavi Z, Sedighi N, Daftarian N, Sanagoo M. High-dose intravenous methylprednisolone in recent traumatic optic neuropathy; a randomized double-masked placebo-controlled clinical trial. Graefes Arch Clin Exp Ophthalmol. 2007;245:1267–1271. doi: 10.1007/s00417-006-0441-0. [DOI] [PubMed] [Google Scholar]
- 38.Geisler FH, Coleman WP, Benzel E, Ducker T, Hurlbert RJ. Spinal cord injury. Lancet. 2002;360:1883–1883. doi: 10.1016/s0140-6736(02)11744-2. [DOI] [PubMed] [Google Scholar]
- 39.Hurlbert RJ. Strategies of medical intervention in the management of acute spinal cord injury. Spine. 2006;31:S16–S21. doi: 10.1097/01.brs.0000218264.37914.2c. [DOI] [PubMed] [Google Scholar]
- 40.Spencer MT, Bazarian JJ. Evidence-based emergency medicine/systematic review abstract. Are corticosteroids effective in traumatic spinal cord injury? Ann Emerg Med. 2003;41:410–413. doi: 10.1067/mem.2003.84. [DOI] [PubMed] [Google Scholar]
- 41.Steinsapir KD, Goldberg RA. Traumatic optic neuropathy: an evolving understanding. Am J Ophthalmol. 2011;151:928–933. doi: 10.1016/j.ajo.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Bracken MB. Steroids for acute spinal cord injury. Cochrane Database System Rev. 2012:CD001046. doi: 10.1002/14651858.CD001046. [DOI] [PubMed] [Google Scholar]
- 43.Beck RW, Cleary PA, Anderson MM, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. New Engl J Med. 1992;326:581–588. doi: 10.1056/NEJM199202273260901. [DOI] [PubMed] [Google Scholar]
- 44.Sauerland S, Nagelschmidt M, Mallmann P, Neugebauer EAM. Risks and benefits of preoperative high dose methylprednisolone in surgical patients — a systematic review. Drug Saf. 2000;23:449–459. doi: 10.2165/00002018-200023050-00007. [DOI] [PubMed] [Google Scholar]
- 45.Edwards P, Arango M, Balica L, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury — outcomes at 6 months. Lancet. 2005;365:1957–1959. doi: 10.1016/S0140-6736(05)66552-X. [DOI] [PubMed] [Google Scholar]
- 46.Alderson P, Roberts I. Corticosteroids for acute traumatic brain injury. Cochrane Database SystRev (Online) 2005;1:CD000196. doi: 10.1002/14651858.CD000196.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang TL, Chang CH, Lin KH, Sheu MM, Tsai RK. Lack of protective effect of local administration of triamcinolone or systemic treatment with methylprednisolone against damages caused by optic nerve crush in rats. Exp Eye Res. 2011;92:112–119. doi: 10.1016/j.exer.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Ohlsson M, Westerlund U, Langmoen IA, Svensson M. Methylprednisolone treatment does not influence axonal regeneration or degeneration following optic nerve injury in the adult rat. J Neuro-Ophthalmol. 2004;24:11–18. doi: 10.1097/00041327-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Steinsapir KD, Goldberg RA, Sinha S, Hovda DA. Methylprednisolone exacerbates axonal loss following optic nerve trauma in rats. Restor Neurol Neurosci. 2000;17:157–163. [PubMed] [Google Scholar]
- 50.Diem R, Hobom M, Maier K, et al. Methylprednisolone increases neuronal apoptosis during autoimmune CNS inflammation by inhibition of an endogenous neuroprotective pathway. J Neurosci. 2003;23:6993–7000. doi: 10.1523/JNEUROSCI.23-18-06993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volpe NJ, Levin LA. How should patients with indirect traumatic optic neuropathy be treated? J Neuro-Ophthalmol. 2011;31:169–174. doi: 10.1097/WNO.0b013e31821c9b11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levin LA, Joseph MP, Rizzo JF, Lessell S. Optic canal decompression in indirect optic-nerve trauma. Ophthalmology. 1994;101:566–569. doi: 10.1016/s0161-6420(94)31299-1. [DOI] [PubMed] [Google Scholar]
- 53.Goldberg RA, Steinsapir KD. Extracranial optic canal decompression: indications and technique. Ophthal Plast Reconstr Surg. 1996;12:163–170. doi: 10.1097/00002341-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Li H, Zhou B, Shi J, Cheng L, Wen W, Xu G. Treatment of traumatic optic neuropathy: our experience of endoscopic optic nerve decompression. J Laryngol Otol. 2008;122:1325–1329. doi: 10.1017/S0022215108002296. [DOI] [PubMed] [Google Scholar]
- 55.Wang DH, Zheng CQ, Qian J, Barr JJ, Anderson AG., Jr Endoscopic optic nerve decompression for the treatment of traumatic optic nerve neuropathy. ORL J Otorhinolaryngol Relat Spec. 2008;70:130–133. doi: 10.1159/000114537. [DOI] [PubMed] [Google Scholar]
- 56.Yang QT, Zhang GH, Liu X, Ye J, Li Y. The therapeutic efficacy of endoscopic optic nerve decompression and its effects on the prognoses of 96 cases of traumatic optic neuropathy. J Trauma Acute Care Surg. 2012;72:1350–1355. doi: 10.1097/TA.0b013e3182493c70. [DOI] [PubMed] [Google Scholar]
- 57.Jiang RS, Hsu CY, Shen BH. Endoscopic optic nerve decompression for the treatment of traumatic optic neuropathy. Rhinology. 2001;39:71–74. [PubMed] [Google Scholar]
- 58.Levin LA, Baker RS. Management of traumatic optic neuropathy. J Neuro-Ophthalmol. 2003;23:72–75. doi: 10.1097/00041327-200303000-00013. [DOI] [PubMed] [Google Scholar]