Abstract
Monocytes are blood-derived mononuclear phagocytic cells that traffic throughout the body and can provide rapid innate immune effector responses in response to microbial pathogen infections. Amongst blood monocytes, the most abundant subset in mice is represented by inflammatory Ly6C+ CCR2+ monocytes and is the functional equivalent of the CD14+ monocytes in humans. Herein we focus on published evidence describing the exquisite functional plasticity of these cells, and we extend this overview to their multiples roles in vivo during host immune defenses against microbial pathogen infections, as antigen-presenting cells, inflammatory cells or Trojan horse cells.
Keywords: Inflammatory Ly6C+ monocytes, Microbial pathogens, Effector functions, Antigen presentation, CCR2
Introduction
Monocytes are blood-derived myeloid cells that belong to the mononuclear phagocytic system (MPS), a specialized system of phagocytic cells localized throughout the body (1, 2). The cells of this system provide innate immune responses, support the adaptive immune response and play a role in the maintenance of tissue homeostasis. Monocytes are a critical component of the MPS and are important in many diseases with an inflammatory component, such as infection, cardiovascular disease, type I diabetes and cancer. Circulating monocytes, like most dendritic cells (DCs) and some tissue-associated macrophages, originate in vivo from hematopoietic stem cell-derived progenitors with myeloid-restricted potential. In the bone-marrow, the successive commitment steps toward monocyte differentiation include common myeloid progenitors (CMPs), granulocyte-macrophage precursors (GMPs) and the macrophage/DC progenitors (MDPs). Finally, the MDPs give rise to the common DC progenitor (CDP) and the common monocyte progenitor (cMoP) found in bone marrow and spleen (3, 4). The cMoP was suggested to be restricted to monocytes and monocyte-derived macrophages (4).
In mice, at least two major subsets of blood monocytes have been defined: the Ly6C+ monocytes (CX3CR1intCCR2+) also called 'inflammatory' monocytes and the Ly6C− monocytes (CX3CR1hiCCR2−) also known as 'patrolling' monocytes that both express the M-CSF receptor (M-CSFR/CD115) (5, 6). While inflammatory monocytes are most crucial during acute inflammation, and undergo CCR2-dependent bone-marrow mobilization, the patrolling subset has mostly been defined by its ability to survey blood vessels, a behavior qualified as ‘patrolling’. In humans, complete understanding of monocyte subsets remains to be investigated. Today, three of them have been proposed, i.e., the CD16+CD14+ and the CD14+CD16int/low monocytes which functionally resemble Ly6C+ inflammatory murine monocytes, and the CD14dimCD16+ which are equivalent to the murine Ly6C− subset and exhibit a patrolling behavior (7). Some of the overlapping functional features of these subsets of monocytes in mice and humans include important molecules involved in trafficking (CX3CR1, CCR2, CD62L, LFA1), cellular functions (phagocytosis, innate sensing, antigen presentation) as well as the expression of antimicrobial and cytokine effector functions upon activation (TNFα, IL-1β, NO/RO). A detailed overview and comparison of mouse and human monocyte subsets is the subject of another review of this volume.
The current review focuses on the multiple 'effector mechanisms' expressed by murine monocytes that are essential in host immune defenses and microbial pathogen elimination. We first provide an overview of the known functional features of these cells allowing for their rapid sensing of microbial pathogens, mobilization and microbicidal effector mechanisms. Second, we discuss how these characteristics are linked to either protective immunity or deleterious immune responses. We only summarize in vivo evidence provided from mouse models and when possible, we refer to human studies.
1. Cell-intrinsic functional characteristics of Ly6C+ monocytes
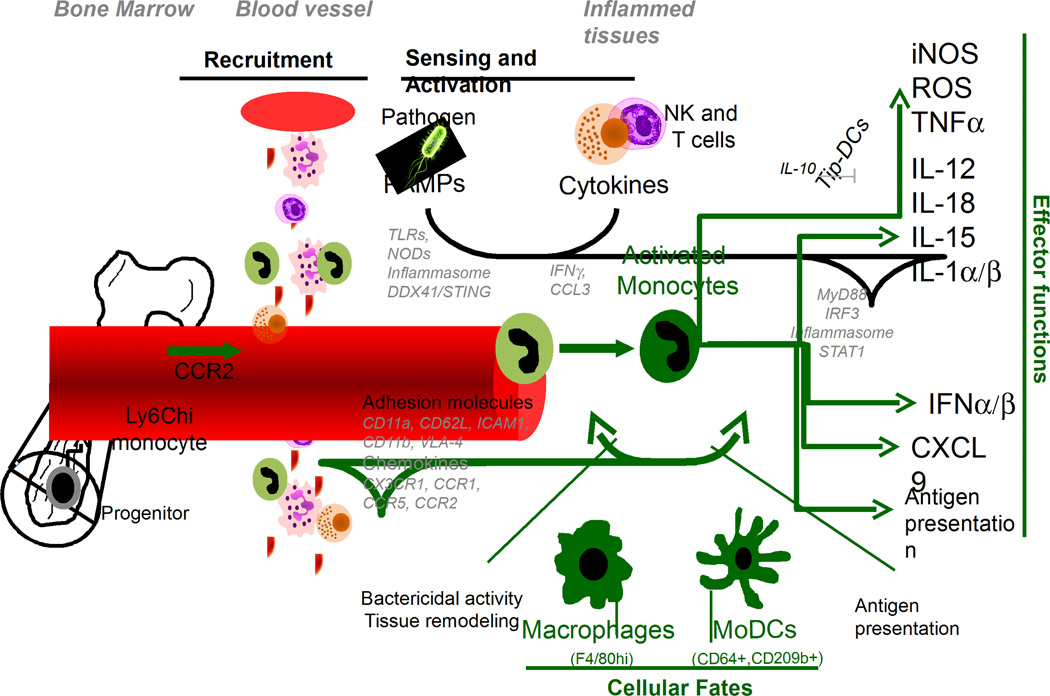
Ly6C+ monocytes are blood circulating mononuclear leucocytes with short half-life (~19 hours, (8)) which represent >80% of the blood monocytes at steady state (2). Both under sterile and microbial inflammatory conditions, Ly6C+ monocytes can differentiate into a progeny of distinct functional subtypes of effector cells, commonly defined as 'inflammatory Ly6C+ monocytes'. Acquisition of multiple functional features by these cells is largely regulated by inflammatory cues from the environment. Seminal studies from the Pamer lab using the mouse model of infection by the intracellular bacterium Listeria monocytogenes (Lm), had defined differentiating Ly6C+ monocytes based on their functions, i.e., their ability to rapidly produce the microbicidal molecule nitric oxid (NO) and secrete the potent inflammatory mediator TNF-α. This led to defining these cells as 'Tip-DCs' for TNF-α and NO producing dendritic cells (9, 10). While such denomination was functionally very relevant, specifically in the context of Lm clearance in mice, it did not account for the complexity of effector functions that may be expressed by these cells and their very high plasticity (See Figure). For these reasons, and to discriminate Ly6C+ monocyte-derived cells from conventional DCs (cDCs) inside tissues, subsequent studies have attempted to define more universal cell-surface markers such as the C-type lectin CD209/DC-SIGN (11) and the high-affinity IgG receptor FcγRI CD64 (12). Recently too, Jung and colleagues have proposed an interesting model to incorporate different fates of activated Ly6C+ monocytes into either effector monocytes, monocyte-derived DCs (Mo-DCs) or monocytederived macrophages (Mo-MP), that is largely based on the nature and timing of inflammatory cues (13). Yet the fate of Ly6C+ monocytes is beyond the scope of this section, in which we are focusing on intrinsic functional features of 'undifferentiated’ / ‘differentiating' Ly6C+ monocytes accounting for their ability to express distinct effector functions after microbial pathogen-mediated activation in vivo.
Figure. The Multi-Potency of Ly6Chi Monocytes.
Upon microbial infection, Ly6Chi monocytes are rapidly mobilized from the bone-morrow to the blood. Via chemokine and adhesion processes, they reach infected tissues. Through sensing of microbial products and lymphoyte-derived cytokines and chemokines, they can differentiate into multi-effector cells, macrophages and/or monocyte-derived DCs (MoDCs).
1.1. Sensing machineries
How to sense danger signals?
Ly6C+ monocytes are equipped with sets of innate scavenging and sensing receptors and functional activation pathways that allow for efficient detection of microbial-pathogen derived molecules as well as molecules released as a result of tissue injury and cell death. Unraveling the molecular basis for this exceptional plasticity is the subject of intense investigations. Important studies from the Geissmann lab and the Randolph lab have conducted in depth genetic expression profiling comparisons between mouse and human monocyte subsets yet these were performed at homeostasis with the major focus on defining developmental pathways of these cells (7, 14). Through the study of many murine models of infection that include viruses, bacteria, fungi and parasites, series of reports discussed below have nonetheless established specific pattern recognition receptors (PRRs) and activation pathways that can be expressed by Ly6C+ monocytes, and involved in orchestrating their functional differentiation.
TLRs and MyD88
Early studies from the Pamer lab using Lm in mice, suggested that Ly6C+ monocytes differentiated into Tip-DCs in a MyD88-dependent manner, supporting the functional involvement of MyD88 in their ability to secrete TNFα and express iNOS (10). Interesting work from the Muraille group using mice infected with Brucella melitensis bacteria, the causing agent of Brucellosis in humans, as a relevant model of gram− bacterial infection, added further support to the functional importance of MyD88 but not TRIF in promoting Ly6C+ monocytes differentiation into iNOS+ cells (15). This study involved TLR4 as initiating sensor, yet only partially accounted for the MyD88−/− phenotype. Investigating further the role of Ly6C+ monocytes during infection of mice with the intracellular parasite Leishmania major, the same group also reported the key role of MyD88, in part via TLR9, in generating iNOS+ inflammatory monocytes (16). In a recent work, the Steinman lab found that upon injection of purified LPS or Escherichia coli, Ly6C+ monocytes rapidly differentiated into antigen-presenting cells (APCs) exhibiting comparable potency as conventional dendritic cells (cDCs), through TLR4 and TRIF but not MyD88 (11). Of note, while all these studies clearly demonstrate the presence of specific TLRs and functional activation pathways intrinsically present in Ly6C+ monocytes and involved in regulating their differentiation into iNOS+ and/or TNFα+ cells, and potent APCs, none of them formally established their cell-intrinsic requirements. In line with this interpretation, a very recent report by the Sparwasser group utilized elegant gain of function experiments in which a 'floxed-STOP' cassette was inserted upstream of the MyD88 adaptor, preventing its expression, unless when the Cre recombinase was expressed (17). Functional MyD88 expression in CD11c+ DCs only, with lack of MyD88 in all other cells, was sufficient to orchestrate Ly6C+ monocytes differentiation into Tip-DCs during Lm infection, establishing that cell-intrinsic expression of MyD88 by Ly6C+ monocytes may not be required. In addition to becoming Tip-DCs, multiple reports have provided compelling evidence that inflammatory monocytes can secrete important amounts of bioactive IL-12 following various microbial pathogen infections in mice, that include Lm (18) and Citrobacter rodentium bacteria (19, 20), Leishmania major and Toxoplasma gondii parasites, influenza virus {Leon, 2007 #1560}{Goldszmid, 2012 #1163;Nakano, 2009 #206}, though the exact sensing pathway has not been elucidated.
Barton and colleagues also reported that influenza virus derived products specifically trigger Ly6C+ monocytes to secrete type I interferon in a Toll-like receptor 2 (TLR2) dependent manner, an unexpected finding given that type I interferon during viral infection is usually produced via recognition of nucleic acids by TLR7 and TLR9 in plasmacytoid DCs (pDCs), a subset of DCs that produces high levels of type I IFN (24). While evidence for TLR2 involvement in early sensing of viruses and subsequent non-type I IFN proinflammatory response was documented (25, 26), the connection between TLR2 and type I IFN secretion by activated Ly6C+ monocytes suggested the uniqueness of this pathway inside these cells. Along similar lines, Jung and colleagues have also found that Ly6C+ monocytes can express TLR2 in a model of induced colitis (27).
Nonetheless, and even though inefficient maturation of inflammatory monocytes was reported in MyD88−/− mice in most of these infections (15, 16, 28), except in the case of T. gondii for which MyD88 was not important (22), both the sensor(s) and activation pathways would remain to be formally defined using adoptive transfers of knockout Ly6C+ monocytes or mice bearing cellspecific knocked out pathways.
Cytosolic sensors
-DDX41 and STING
Independent works from the Scheu and the Lienenklaus groups using Lm as a model system and IFNβ-reporter or cell-specific IFNβ-knockout mice suggested that activated Ly6C+ monocytes represent the major source of type I IFN during this infection (29, 30). Multiple in vitro and in vivo studies over the years, in particular from the Portnoy lab, provided compelling evidence that cyclic di-nucleotides, c-di-AMP and c-di-GMP, secreted by this bacterium is the major trigger of type I IFN production, a process that occurs inside infected cell cytosol when Lm escapes from early phagosomes to cell cytoplasm (31, 32). Cyclic dinucleotide sensing inside myeloid cells required DDX41, a cytosolic DNA binding helicase that binds cyclic dinucleotides, and further associates with the endoplasmic reticulum sensor STING to induce TBK1/IRF3-mediated transcription of type I IFN (33, 34). Elegant in vivo experiments using STING−/− mice, mutants of Lm secreting variable amounts of c-di-AMP and co-immunization with synthetic cyclic di-AMPs as adjuvant, further confirmed that STING represented the major pathway of type I IFN induction during Lm infection (35). While not yet formally proven, all these data collectively suggest that Ly6C+ monocytes indeed possess unique pathways of activation involving specific sets of cytosolic nucleic acid sensors leading to type I IFN production.
-Inflammasomes
The functional expression of different inflammasomes complexes by Ly6C+ monocytes and human CD14+ monocytes is supported by numerous studies from several groups, both in mice challenged with bacteria (Lm, Francisella tularensis, Mycobacterium tuberculosis (Mtb)), viruses (Vaccinia, murine cytomegalovirus), fungi (Candida albicans) (36–38) and on freshly isolated human blood monocytes (36, 39). These studies provided evidence for the presence of both precursor and/or bioactive IL-1 and/or IL-18 effector cytokines as a result of expression of functional inflammasome complexes inside inflammatory monocytes.
-Nod1 and Nod2
Lastly, Jung and colleagues reported that Ly6C+ monocytes expressed Nod2 and could contribute to colitis through this sensing pathway (27). The cytosolic PRRs Nod1 and Nod2 may have evolved to enable the detection of cytosolic invasive bacteria, and in particular bacterial cell wall peptidoglycans (PGNs) compounds such as d-glutamyl-mesodiaminopimelic acid (iE-DAP) and muramyl dipeptide (MDP), as shown by many studies using model cell lines and bone-marrow macrophages in vitro (40). However, Nod2−/− mice increased susceptibility to oral but not intravenous Lm and to other intestinal bacteria such as Helicobacter pilori or Citrobacter rodentium seems to be accounted for by both impaired detection of invading bacteria by non-hematopoietic cells and altered intestinal myeloid cell responsiveness and homeostasis (19). This altogether suggested that, at least in vivo, the importance of Nod1 and Nod2 sensing inside monocytes is complex and requires further investigations.
In summary, Ly6C+ monocytes exhibit tremendous plasticity in their multiple possible functional fates, which allows them to act as highly efficient sensing cells of the innate immune system. This is accounted for by their constitutive or induced expression of impressive sets of sensors and pathways of activation. Further studies will be important to continue characterizing the full potential of these cells and precisely understand how they integrate distinct pattern recognition receptors (PRRs) signals.
Activating cytokines and chemokines
Inflammatory cytokines and chemokines are usually secreted as a consequence of early PRRs triggering and can act both in an autocrine and a paracrine manner. Work from the Locksley lab for instance provided evidence that the expression of iNOS by inflammatory monocytes is dependent on lymphocyte-derived IFNγ during mouse listeriosis (41). Similarly, in mice inoculated with cysts of the parasite T. gondii, Sher and colleagues documented that IFNγ from NK cells orchestrates Ly6C+ monocyte differentiation into F4/80+ macrophages and IL-12-producing Mo-DCs (22). Along these lines, we found that memory T cell-derived MIP1α/CCL3 acted as a key signal to induce Ly6C+ monocytes to secrete high levels of TNFα, which could act in an autocrine manner to promote their own reactive oxygen species (ROS) production (42). Recently, we also reported that IFNγ, which is massively produced by memory T cells independently of cognate antigen recognition, indeed represents a key ignition signal for triggering a robust microbicidal effector program inside Ly6C+ monocytes in vivo (43). Finally, Ly6C+ monocytes were shown to respond to type I IFN and respectively trans-present bioactive IL-15 (37) or turn off IL-1 secretion (44) during murine Lm or Mtb infections. Thus altogether the current body of evidence suggests that other cytokines and chemokines are likely to shape monocyte differentiation, and further studies will be required to formally establish such possibility.
1.2. Trafficking machineries
How to traffic to injured tissues? Chemokine receptors and adhesion molecules
Chemokines represent essential orchestrators of cell mobilization and relocation inside tissues as was recently reviewed (45). Because monocytes constantly traffic inside tissues, they can be rapidly mobilized during microbial or sterile inflammation.
CCR2
Ly6C+ monocytes express high levels of the chemokine receptor CCR2 (9). The major mechanism involved in the rapid mobilization of Ly6C+ monocytes from their site of production, i.e., the bone-marrow, to the blood involves the chemokine receptor CCR2, and its ligands, mostly CCL2, CCL7 and CCL8 (46). Induced by various inflammatory signals, MCP-1/CCL2, is quickly secreted by bone marrow mesenchymal stem cells and their progeny, including CXC chemokine ligand (CXCL)12-abundant reticular cells (47), and this enables for massive release of Ly6C+ monocytes to the blood, a necessary step for these cells to access injured tissues. Hematopoietic cells can also participate to CCL2 production through TLR and type I interferon-dependent cytosolic pathways (48). Triggering of cytosolic Nod2 via bacterialderived MDP can also induce the secretion of type I IFN and CCL2, and the mobilization of Ly6C+ monocytes from the bone-marrow (19, 49).
Original studies from the Luster, Karpus and Ransohoff labs suggested that CCR2−/− and CCL2−/− (the major ligand for CCR2) mice were resistant to the development of experimental autoimmune encephalitis (EAE) induced upon injection of the myelin oligodendrocyte glycoprotein (MOG) derived peptide 35–55 (50, 51). Protection against disease progression correlated to the lack of mononuclear cell infiltrates in the central nervous system (CNS) of MOG-injected mice, altogether suggesting that CCR2+ monocytes, at least in part via CCL2, contributed to pathogenesis. This interpretation was further confirmed in recent work using combinations of irradiated WT or CCR2−/− recipient or donor bone-marrow chimeras and elegant parabiont experiments (52). A report by the Malat group documented that Ly6C+ monocytes could also be recruited to the cardiac endothelium via B cell-derived CCL7 (MCP-3), another ligand of CCR2, which contributes to tissue injury and acute myocardial infarction (53).
In murine models of acute viral infections of the CNS, using either lymphochorionmeningitis virus or West Niles virus, studies from the McGavern and the King labs, supported the importance of the CCR2/CCL2 axis for Ly6C+ monocyte-mediated brain damages (54, 55). Even though these reports provided strong evidence that CCR2 may also regulate Ly6C+ monocytes access to inflamed tissues, its 'dominant' function is likely to regulate CCR2+ monocyte egress from bone-marrow to the blood. Thus, in summary, whether retention of monocytes inside the bone-marrow is mostly accounting for increased resistance of CCR2−/− or CCL2−/− mice to EAE or viral CNS infections, was not formally assessed.
CX3CR1
In the blood, CX3CR1 in particular contributes to the access of Ly6C+ monocytes to the spleen during Lm infection where they undergo differentiation into inflammatory monocytes/Tip-DCs (56). Consistent with these observations, the CX3CR1 ligand, e.g., the fractalkin CX3CL1, is found expressed in marginal/T cell zones of the spleen. CX3CR1 was also reported by the Randolph group to be involved in Ly6C+ monocytes accumulation from blood to atherosclerotic plaques found in ApoE−/− mice, a model of increased plasma cholesterol levels (57). Of note, the patrolling Ly6Clow monocytes express high levels of CX3CR1 compared to Ly6C+ monocytes, and this is required for their endothelium scanning behavior (6).
CCR1 and CCR5
Early studies found low constitutive expression of CCR1 and CCR5 on mouse and human monocytes (58, 59). While we and others have found evidence of CCR1 and CCR5 (42, 43, 57, 60) cell-surface upregulation during activation, the precise contribution of these receptors in promoting or preventing pathology during antimicrobial responses requires further studies. In this context, an elegant study by the Cerf-Bensoussan group in mice orally infected with cysts of the parasite T. gondii showed that the chemokine CCL3 produced by a subset of IL-18 activated NK cells, promoted intestinal recruitment of Ly6C+ monocytes via CCR1, which contributed to early parasite killing but subsequently induced devastating intestinal ileitis (61). However, most evidence of the involvement of CCR1 and CCR5 on monocytes/macrophages are related to atherosclerosis/cardiovascular diseases and autoimmune pathologies such as rheumatoid arthritis, colitis, multiple sclerosis and transplant rejection (45, 60). Because levels of CCL3 and CCL5 in particular, two major ligands of both chemotactic receptors, increase in the course of distinct microbial infection, it seems likely that they participate to Ly6C+ monocyte recruitment to infected tissues/foci (42, 43).
Adhesion molecules
Ly6C+ monocytes utilize adhesion mechanisms to access infected tissues from peripheral blood. During Lm infection, Ly6C+ monocytes, use the integrins CD11b and CD44 as well as ICAM-1 to access liver infected foci (62). This process appears largely independent of G-protein mediated chemotaxis since pertussis toxin treatment of purified monocytes -which inhibits G-protein receptor signal transduction- prior to transfer did not prevent them to access infected livers with comparable efficiency as untreated monocytes. Of note, these results do not rule out the implication of other mechanisms in the context of different infections, in the liver as well as in other organs (see above). For instance PLGS-1 or L-selectin/CD62L expressed by Ly6C+ monocytes respectively contribute to adhesion to dermal venules during Leishmania major infection and to endothelia during atherosclerosis or thioglycolate-induced peritonitis (45). Interestingly, in West Nile Virus induced encephalitis, Ly6C+ monocyte accumulation in the CNS is mediated through the integrin/ligand pair VLA-4/VCAM-1, with antibodies against ICAM-1 or LFA-1 having little impact on this process (63). Thus altogether the cited studies suggest the existence of multiple adhesion mechanisms utlized by activated Ly6C+ monocytes to access infected tissues.
1.3. Microbicidal machineries
Monocytes are exquisite phagocytes exhibiting high phagocytic capacity, though the patrolling subset in humans (CD14dimCD16+) was shown to have lower phagocytic ability than the functional equivalents of the inflammatory subsets (7). However the formal comparison in mice still needs to be done. Ly6C+ monocytes are also equipped with the machinery to produce high levels of reactive oxygen and nitrogen species during bacterial (9, 42), fungal (64), parasitic (16, 65) and viral infections (66), some of which being essential for microbial killing. Of note, we have further linked the production of reactive oxygen species to antimicrobial autophagy during Lm infection (67), yet this did not appear essential during candidiasis (68). Still relying on mice immunized with Lm as a model, we recently reported potent expression by Ly6C+ monocytes of a set of genes encoding the guanylate binding proteins (Gbp), Gbp1-11 (43), that belong to the IFN-γ-inducible GTPases superfamily. These were implicated in cell-autonomous host defense against intracellular bacteria through the activation of the phagocyte oxidase, antimicrobial peptides and autophagy effectors (69, 70).
2. Orchestrating host antimicrobial protective responses
As discussed in the first part of this review, Ly6C+ monocytes can express sets of functions that make them highly fit as essential responders against microbial infections. Many studies have established the importance of these cells in mice models of bacterial, viral, parasitic and fungal infections, and this has been largely reviewed elsewhere (45, 71). Thus we decided to focus below on published evidence that support a role for inflammatory monocytes as key orchestrators of innate and adaptive responses. We propose to subdivide their roles according to their requirement to present cognate antigen to T cells or alternately, to provide other functions such as inflammatory cytokines and transportation as Trojan horses.
2.1. Mechanisms that involve cognate antigen presentation
Ly6C+ monocytes upregulate surface MHC molecules as well as costimulatory receptors in the course of activation with microbial pathogens or pathogen-derived molecules such as LPS. While this capacity to present antigen does not seem to impact the course of infection in most infection models, in some instances, it has been shown that activated Ly6C+ monocytes are able to contribute to T cell priming. The Webster group provided strong evidence along these lines by showing that Ly6C+ monocytes contributed to antigen-dependent activation of influenza-specific CD8+ T cells in mice airways, although, while required for ultimate control of viral infection, the immediate consequences of such CD8+ T cell activation were largely deleterious to the host (66). The Ardavin lab provided indirect evidence that Ly6C+ monocyte-derived DCs may present peptide-MHC complexes to prime protective Th1 CD4+ T cells during Leishmania major infection in resistant C57BL/6 mice, yet these results were conflicting with later analyses showing that L. major-derived peptide-MHC complexes could only be found on DCs but not on activated Ly6C+ monocytes {Leon, 2007 #1560;Muraille, 2010 #1561}. Another example of the importance of Ly6C+ monocytes for antigen-specific T cell priming was reported in studies from the Pamer lab using a mouse model of invasive aspergillosis, with the mold Aspergillus fumigatus. A. fumigatus can cause lethal infections in immunocompromised humans, and priming of Aspergillus-specific CD4+ T cells requires the presence of Ly6C+ monocytes and their progeny, by a process that involves transportation of fungal spores to dLNs (73). Whether Ly6C+ monocytes directly prime T cells in this model still needs to be investigated. A report from the Iwasaki lab in the HSV2 infection model also suggested that Ly6C+ monocytes derived cells in the vaginal mucosa could contribute to protective Th1 CD4+ T cell-activation, yet the requirement for cognate T cell antigen stimulation was not determined (74). Overall, the cited studies suggest that pathogen-activated Ly6C+ monocytes certainly acquire the ability to present pathogen-derived antigenic peptides to further activate or restimulate effector T cells, yet they are unlikely to initiate naive T cell priming in vivo.
2.2. Mechanisms that involve cytokines and/or transportation
A body of work from independent laboratories has established that Ly6C+ monocytes and their activated progeny contribute to host immune defenses against microbial pathogens independently of antigen-mediated activation of lymphocytes. In the first part of this review, we have listed many intrinsic functional features that are or can be expressed by activated Ly6C+ monocytes, and which represent the basis of their functional plasticity. Production of inflammatory cytokines and chemokines, expression of microbicidal molecules and transport of pathogens are amongst some of these most prominent roles.
Cytokine-dependent mechanisms: Inflammatory mediators
Monocytes express multiple sensors of microbial-derived products as extensively discussed in the first part of this review. Triggering of these pathways by microbial-derived products leads to different outcomes that largely depend on the pathogen. One of the first consequences of their ability to sense pathogens and activate innate pathways is the secretion of proinflammatory cytokines (in particular TNF-α, IL-1, IL-18, IL-12) and chemokines (CCL3, CXCL9) that directly contribute to the rapid amplification of the immune response and pathogen clearance. Since the production of these mediators may require complex multi-step processes and does not necessarily occur directly downstream innate sensing, we discuss the literature in light of these evidences.
Importance of cytokine-driven functional maturation of Ly6C+ monocytes
In the well-studied model of infection by Lm, both TNF-α and production of nitric oxid (NO) are important for resistance to the primary infection, and Ly6C+ monocytes represent by far the major producers of these mediators, which collectively supports their essential role in Lm killing through these mechanisms. Neutrophils and tissue-macrophages however can also produce these mediators and possibly could contribute to host protection in this model (75), although only to limited extent. In fact, retention of Ly6C+ monocytes in the bone-marrow of CCR2−/− mice and therefore lack of these cells -but not of neutrophils or macrophages- impairs mice resistance to Lm infection (76). While the formal demonstration that TNF-α and/or NO production by Ly6C+ monocytes indeed represents the mechanism of Lm clearance, the current body of work does provide conclusive evidence for this interpretation. Investigating further the mechanism of production of these key mediators, the recent data from the Sparwasser group have suggested that Ly6C+ monocytes can differentiate into Tip-DCs with no cell-intrinsic requirement for MyD88 (17). Results from the Locksley lab have proposed that NK-cell derived IFN-γ - themselves activated in response to cDCs-derived IL-12- is essential for Ly6C+ monocyte maturation into Tip-DCs (41), a result in agreement with our own data establishing the key function of IFN-γ in promoting Ly6C+ monocyte differentiation during recall infection with Lm (43). In this setting though, MyD88 was required for TNF-α secretion while expression of iNOS and many other functional markers -costimulation, chemokines and cytokine and their receptors-only required IFN-γ that was derived from the memory T cells. Discrepancy with the Sparwasser study could be that requirements are different during the primary and the recall infection. During mouse Brucella mellitensis infection, Muraille and colleagues also suggested a comparable mechanism in which iNOS expression by activated Ly6C+ monocytes, an essential mechanism of host protection, appears to depend on IFN-γ, with possible implication of MyD88 - though the study did not assess the monocyte-intrinsic requirements (15).
As an interesting and complex counter regulation mechanism involved in the inhibition of bone-marrow derived Ly6C+ monocytes differentiation into Tip-DCs in the spleen, LNs and liver of mice infected with the protozoan parasite Trypanosoma brucei brucei -the cause of sleeping sickness in humans-, the Beschin lab established the importance of IL-10 signals received by Ly6C+ monocytes through elegant gain and loss of function experiments (65, 77). While Tip-DCs production in this model was dependent on cell-intrinsic MyD88 and IFN-γ signals, IL-10 could limit this process and associated tissue damages, providing further evidence of the importance of cytokines in regulating their possible fates.
Work from the Sher group in mice inoculated with Toxoplasma gondii cysts, again supported the idea that Ly6C+ monocytes functional maturation is primarily driven by cytokines, by showing that NK-cell derived IFN-γ promoted the functional differentiation of Ly6C+ monocytes at the sites of infection into IL-12 producing cells and in a MyD88-independent manner. IL-12 is a critical cytokine in driving Th1 T cell responses and host resistance to this infection, yet this remained largely independent of iNOS expression (78). In another model of parasitic infection, the blood stage non-lethal murine Plasmodium chabaudi (Pc) that exhibits features reminiscent of the chronic human infection with P. falciparum, the Langhorne group has reported that Ly6C+ monocytes contributed to more efficient elimination of blood parasites, mostly during the later stage of the disease during which rebounds of parasitemia are observed over several weeks (79). Consistent with such result, patients with acute uncomplicated malaria exhibited higher numbers of CD14+CCR2+CX3CR1+ monocytes in the peripheral blood compared to malaria-exposed uninfected controls (80). While conclusions of the mouse study were based on the use of CCR2−/− mice in which Ly6C+ monocytes are retained in the bone-marrow of mice, further analysis suggested robust functional differentiation of these cells with increased expression of reactive oxygen and nitrogen species, proinflammatory cytokines and capacity to phagocytose parasites, overall consistent with the impaired parasite clearance observed in CCR2−/− mice. Which innate and/or cytokines sensing pathways control these processes in the monocytes will require further studies, though it is known that IFN-γ is protective in this parasitic infection and comparable mechanisms as described for Lm or T. gondii may apply (81).
As another example related to a different cytokine and infection, Sher and colleagues reported the differentiation of a population of Ly6C+ mononuclear cells that is likely to be the progeny of Ly6C+ monocytes, and which produces important amounts of IL-1α and β during murine Mtb lung infection, two essential cytokines for controlling this infection (44). In this model, the production of IL-1 unexpectedly did not require a functional inflammasome cytosolic sensing pathway (82), yet it was negatively regulated by type I interferon, and to some extent CD4+ T cell-derived IFN-γ.
In a very recent study utilizing the chronic Th2-polarizing helminth parasite Schistosoma mansoni and adoptive transfers of Ly6Chi monocytes, Loke and colleagues provided good evidence that these cells may differentiate inside infected livers into cells expressing markers (YM1, PD-L2) reminiscent of alternatively activated macrophages (83). The data further suggested this process to be dependent on CD4+ T cells, most likely involving the Th2 cytokines such as IL-4, IL-5 and IL-13 secreted by parasite-specific CD4+ T cells. Together, these results revealed another potential cytokine-driven functional fate of activated Ly6C+ monocytes during a parasitic worm infection.
Thus, altogether these reports underline the importance of cytokine-mediated functional differentiation of Ly6C+ monocytes that is taking place during infections, and which may occur independently from their ability to sense pathogen-derived molecules.
Importance of innate sensing-driven functional maturation of Ly6C+ monocytes
During Lm infection for instance, monocyte-intrinsic sensing occurs and this involves the cytosolic DDX41/STING pathway which largely accounts for type I IFN production (33–35). We found that type I IFN can act in a paracrine manner to promote trans-presentation of bioactive IL-15 by activated Ly6C+ monocytes (37). Concomitant stimulation of the inflammasome pathway via multiple cytosolic sensors (see section 1), leads to secretion of bioactive IL-18. Both IL-15 and IL-18 from activated monocytes drive rapid NK and memory CD8+ T cell activation and differentiation into cytolytic and IFN-γ-secreting effector cells with no need of cognate antigen recognition (37). While type IFN is acting as a secondary messenger, the initiating pathways are intrinsic to Ly6C+ monocytes. Overall such mechanism of non-cognate cytokine-mediated activation of memory CD8+ T cells can contribute to modest but measurable levels of protection against heterologous, non-related microbial pathogens, adding to existing host innate immune defense mechanisms. A study from the Iwasaki group documented the importance of Ly6C+ monocytes in eliciting protective IFN-γ-dependent effector T cell mucosal responses during primary infection with herpes simplex virus 2, possibly via a similar mechanism (74).
Along similar lines, work from the Kuchler lab in a model of murine Candida albicans infection suggested the importance of type I IFN signals in the functional maturation of Ly6C+ monocytes, ultimately promoting lethal, sepsis-like outcomes. However these studies neither addressed the cellular source of type I IFN, nor whether the observed defects were intrinsic to monocytes and involved type I IFN signals to these cells in particular (84).
Several groups utilizing models of viral infections such as Vaccinia, Influenza or herpes simplex viruses, also suggested that triggering of cell-intrinsic innate sensing pathways leads to rapid production of antiviral type I IFN or polarizing cytokines such as IL-12. The Barton group provided strong yet unexpected evidence that Ly6C+ monocytes could produce important amounts of type I IFN via a TLR2/MyD88 mechanism in vitro, though formal demonstration of the cellular origin of type I IFN produced during the viral infection in vivo was lacking (24). Reports by the Iwasaki and by the Gunn labs highlighted Ly6C+ monocytes as key producers of Th1 polarizing cytokines during HSV2 and Influenza infections, in particular IL-12, but the cell-intrinsic pathways were not defined (23, 74). A further study suggested the importance of concomitant expression of TLR7 and MAVS innate sensors in hematopoietic cells for IL-12 and type I IFN production during influenza infection, however cell-intrinsic requirements would need to be determined (85).
Transport-dependent mechanisms: the Trojan horses
While often essential for direct host protective immune defenses, Ly6C+ monocytes have also been implicated in the transport of live pathogens to tissues and evidence for such a role are discussed below. In a mouse model of invasive fungal infection with Aspergillus fumigatus, an important cause of invasive disease in immunocompromised patients, Pamer and colleagues elegantly demonstrated that Ly6C+ monocytes transported fungal spores (conidia) from infected lungs to draining lymph nodes, which was essential for subsequent clearance of fungi from infected lungs (73, 86, 87). The same group recently documented that Ly6C+ monocytes major role during pulmonary Mtb infection was indeed to carry Mtb bacteria to infected dLNs and this was an essential step in efficient priming of Mtb-specific CD4+ T cells by resident cDCs (88). During murine leishmaniasis too, Muraille and colleagues reported the presence of live L. major parasites inside Ly6C+ monocytes present in the lesions and the dLNs, also suggesting a possible role as a vehicle (16). In humans, CD14+CD16+ inflammatory monocytes were found to harbor HIV virus and can be infected in vitro, and some studies suggest that they may transport the virus to the central nervous system (89, 90).
Conclusions and Perspectives
Inflammatory monocytes, Ly6C+ in mice or CD14+/CD16int/low in humans, can express a variety of functional molecules and pathways. Their roles during microbial infections and inflammatory pathologies have been the subject of many studies over the past decade, largely in mouse models but also, to some extent in humans. These studies have revealed a plethora of functions exerted by these cells, witnessing their tremendous functional plasticity. However, the precise cues and most importantly the combinations of these cues especially those that determine their functional fates remain unclear. While defining their origin during homeostasis and whether master transcription factors regulate their development are subjects of intense investigations, providing a comprehensive understanding of their possible fates in inflammatory conditions and the potential roles of transcriptional regulators will require much further work. This is an essential task, both in terms of basic scientific knowledge and for future medical applications. Studies using siRNA approaches as well as immune modifying nanoparticles that specificaly target inflammatory monocytes are helpful in examining monocyte biology (91, 92). Systematic analyses harnessing micro-RNA and long non-coding RNA to examine transcriptional regulatory mechanisms associated with monocyte differentiation will likely further inform not only the complexity surrounding these cells, but potential therapeutic targets for future examination.
Highlights.
Ly6Chi ‘inflammatory’ monocytes use the chemokine receptor CCR2 to egress from bone marrow.
Ly6Chi monocytes possess multiple functional pathogen sensing mechanisms and innate activation pathways.
The fate of activated Ly6Chi monocytes largely depends on T and NK lymphocyte derived cytokines and chemokines.
Ly6Chi monocytes can differentiate into effector cells, tissue-macrophages and monocyte-derived dendritic cells.
Acknowledgments
Funding acknowledgements: Work in the Lauvau lab is supported by the National Institute of Health (Grants AI095835, AI103338 to GL). Core resources for FACS were supported by the Einstein Cancer Center (NCI cancer center support grant 2P30CA013330).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46:845–852. [PMC free article] [PubMed] [Google Scholar]
- 2.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 3.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- 5.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 6.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 7.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D'Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 10.Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19:891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 11.Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, Koh H, Rodriguez A, Idoyaga J, Pack M, Velinzon K, Park CG, Steinman RM. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langlet C, Tamoutounour S, Henri S, Luche H, Ardouin L, Gregoire C, Malissen B, Guilliams M. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J Immunol. 2012;188:1751–1760. doi: 10.4049/jimmunol.1102744. [DOI] [PubMed] [Google Scholar]
- 13.Mildner A, Yona S, Jung S. A close encounter of the third kind: monocyte-derived cells. Adv Immunol. 2013;120:69–103. doi: 10.1016/B978-0-12-417028-5.00003-X. [DOI] [PubMed] [Google Scholar]
- 14.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–e19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copin R, De Baetselier P, Carlier Y, Letesson JJ, Muraille E. MyD88-dependent activation of B220-CD11b+LY-6C+ dendritic cells during Brucella melitensis infection. J Immunol. 2007;178:5182–5191. doi: 10.4049/jimmunol.178.8.5182. [DOI] [PubMed] [Google Scholar]
- 16.De Trez C, Magez S, Akira S, Ryffel B, Carlier Y, Muraille E. iNOS-producing inflammatory dendritic cells constitute the major infected cell type during the chronic Leishmania major infection phase of C57BL/6 resistant mice. PLoS Pathog. 2009;5:e1000494. doi: 10.1371/journal.ppat.1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold-Schrauf C, Dudek M, Dielmann A, Pace L, Swallow M, Kruse F, Kuhl AA, Holzmann B, Berod L, Sparwasser T. Dendritic cells coordinate innate immunity via MyD88 signaling to control Listeria monocytogenes infection. Cell Rep. 2014;6:698–708. doi: 10.1016/j.celrep.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Lee SH, Carrero JA, Uppaluri R, White JM, Archambault JM, Lai KS, Chan SR, Sheehan KC, Unanue ER, Schreiber RD. Identifying the initiating events of anti-Listeria responses using mice with conditional loss of IFN-gamma receptor subunit 1 (IFNGR1) J Immunol. 2013;191:4223–4234. doi: 10.4049/jimmunol.1300910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nunez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber HA, Loschko J, Karssemeijer RA, Escolano A, Meredith MM, Mucida D, Guermonprez P, Nussenzweig MC. Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium. J Exp Med. 2013;210:2025–2039. doi: 10.1084/jem.20130903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Goldszmid RS, Caspar P, Rivollier A, White S, Dzutsev A, Hieny S, Kelsall B, Trinchieri G, Sher A. NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity. 2012;36:1047–1059. doi: 10.1016/j.immuni.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci U S A. 2006;103:17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, Duprex WP, Wagner H, Kirschning CJ, Ter Meulen V, Schneider-Schaulies S. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, Mack M, Shpigel N, Boneca IG, Murphy KM, Shakhar G, Halpern Z, Jung S. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 28.Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, Futatsugi-Yumikura S, Takeuchi O, Hoshino K, Akira S, Fujimoto J, Nakanishi K. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 29.Dresing P, Borkens S, Kocur M, Kropp S, Scheu S. A fluorescence reporter model defines "Tip-DCs" as the cellular source of interferon beta in murine listeriosis. PLoS One. 2010;5:e15567. doi: 10.1371/journal.pone.0015567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solodova E, Jablonska J, Weiss S, Lienenklaus S. Production of IFN-beta during Listeria monocytogenes infection is restricted to monocyte/macrophage lineage. PLoS One. 2011;6:e18543. doi: 10.1371/journal.pone.0018543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc Natl Acad Sci U S A. 2002;99:13861–13866. doi: 10.1073/pnas.202476699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS, Zaver SA, Schenk M, Zeng S, Zhong W, Liu ZJ, Modlin RL, Liu YJ, Cheng G. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol. 2012;13:1155–1161. doi: 10.1038/ni.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu YJ. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011;12:959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Archer KA, Durack J, Portnoy DA. STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes. PLoS Pathog. 2014;10:e1003861. doi: 10.1371/journal.ppat.1003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory Monocytes Activate Memory CD8(+) T and Innate NK Lymphocytes Independent of Cognate Antigen during Microbial Pathogen Invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, van der Meer JW, Dinarello CA. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14:9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 41.Kang SJ, Liang HE, Reizis B, Locksley RM. Regulation of hierarchical clustering and activation of innate immune cells by dendritic cells. Immunity. 2008;29:819–833. doi: 10.1016/j.immuni.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narni-Mancinelli E, Campisi L, Bassand D, Cazareth J, Gounon P, Glaichenhaus N, Lauvau G. Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes. J Exp Med. 2007;204:2075–2087. doi: 10.1084/jem.20070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soudja SM, Chandrabos C, Yakob E, Veenstra M, Palliser D, Lauvau G. Memory-T-Cell-Derived Interferon-gamma Instructs Potent Innate Cell Activation for Protective Immunity. Immunity. 2014;40:974–988. doi: 10.1016/j.immuni.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, Oland S, Gordon S, Sher A. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–1034. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 47.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jia T, Leiner I, Dorothee G, Brandl K, Pamer EG. MyD88 and Type I interferon receptor-mediated chemokine induction and monocyte recruitment during Listeria monocytogenes infection. J Immunol. 2009;183:1271–1278. doi: 10.4049/jimmunol.0900460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coulombe F, Fiola S, Akira S, Cormier Y, Gosselin J. Muramyl dipeptide induces NOD2-dependent Ly6C(high) monocyte recruitment to the lungs and protects against influenza virus infection. PLoS One. 2012;7:e36734. doi: 10.1371/journal.pone.0036734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 53.Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guerin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre JS, Mallat Z. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19:1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, King NJ. Ly6c+ "inflammatory monocytes" are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med. 2008;205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JV, Kang SS, Dustin ML, McGavern DB. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, Molina T, Charo I, Hume DA, Cumano A, Lauvau G, Geissmann F. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mack M, Cihak J, Simonis C, Luckow B, Proudfoot AE, Plachy J, Bruhl H, Frink M, Anders HJ, Vielhauer V, Pfirstinger J, Stangassinger M, Schlondorff D. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol. 2001;166:4697–4704. doi: 10.4049/jimmunol.166.7.4697. [DOI] [PubMed] [Google Scholar]
- 59.Kaufmann A, Salentin R, Gemsa D, Sprenger H. Increase of CCR1 and CCR5 expression and enhanced functional response to MIP-1 alpha during differentiation of human monocytes to macrophages. J Leukoc Biol. 2001;69:248–252. [PubMed] [Google Scholar]
- 60.Zhao Q. Dual targeting of CCR2 and CCR5: therapeutic potential for immunologic and cardiovascular diseases. J Leukoc Biol. 2010;88:41–55. doi: 10.1189/jlb.1009671. [DOI] [PubMed] [Google Scholar]
- 61.Schulthess J, Meresse B, Ramiro-Puig E, Montcuquet N, Darche S, Begue B, Ruemmele F, Combadiere C, Di Santo JP, Buzoni-Gatel D, Cerf-Bensussan N. Interleukin-15-dependent NKp46+ innate lymphoid cells control intestinal inflammation by recruiting inflammatory monocytes. Immunity. 2012;37:108–121. doi: 10.1016/j.immuni.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 62.Shi C, Velazquez P, Hohl TM, Leiner I, Dustin ML, Pamer EG. Monocyte trafficking to hepatic sites of bacterial infection is chemokine independent and directed by focal intercellular adhesion molecule-1 expression. J Immunol. 2010;184:6266–6274. doi: 10.4049/jimmunol.0904160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Getts DR, Terry RL, Getts MT, Muller M, Rana S, Deffrasnes C, Ashhurst TM, Radford J, Hofer M, Thomas S, Campbell IL, King NJ. Targeted blockade in lethal West Nile virus encephalitis indicates a crucial role for very late antigen (VLA)-4-dependent recruitment of nitric oxide-producing macrophages. J Neuroinflammation. 2012;9:246. doi: 10.1186/1742-2094-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jhingran A, Mar KB, Kumasaka DK, Knoblaugh SE, Ngo LY, Segal BH, Iwakura Y, Lowell CA, Hamerman JA, Lin X, Hohl TM. Tracing conidial fate and measuring host cell antifungal activity using a reporter of microbial viability in the lung. Cell Rep. 2012;2:1762–1773. doi: 10.1016/j.celrep.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bosschaerts T, Guilliams M, Stijlemans B, Morias Y, Engel D, Tacke F, Herin M, De Baetselier P, Beschin A. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS Pathog. 2010;6:e1001045. doi: 10.1371/journal.ppat.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aldridge JR, Jr, Moseley CE, Boltz DA, Negovetich NJ, Reynolds C, Franks J, Brown SA, Doherty PC, Webster RG, Thomas PG. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci U S A. 2009;106:5306–5311. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Narni-Mancinelli E, Soudja SM, Crozat K, Dalod M, Gounon P, Geissmann F, Lauvau G. Inflammatory Monocytes and Neutrophils Are Licensed to Kill During Memory Responses In Vivo. PLoS Pathog. 2011;29 doi: 10.1371/journal.ppat.1002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smeekens SP, Malireddi RK, Plantinga TS, Buffen K, Oosting M, Joosten LA, Kullberg BJ, Perfect JR, Scott WK, van de Veerdonk FL, Xavier RJ, van de Vosse E, Kanneganti TD, Johnson MD, Netea MG. Autophagy is redundant for the host defense against systemic Candida albicans infections. Eur J Clin Microbiol Infect Dis. 2014;33:711–722. doi: 10.1007/s10096-013-2002-x. [DOI] [PubMed] [Google Scholar]
- 69.Kim BH, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD. A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332:717–721. doi: 10.1126/science.1201711. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto M, Okuyama M, Ma JS, Kimura T, Kamiyama N, Saiga H, Ohshima J, Sasai M, Kayama H, Okamoto T, Huang DC, Soldati-Favre D, Horie K, Takeda J, Takeda K. A cluster of interferon-gamma-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity. 2012;37:302–313. doi: 10.1016/j.immuni.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 71.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muraille E, Gounon P, Cazareth J, Hoebeke J, Lippuner C, Davalos-Misslitz A, Aebischer T, Muller S, Glaichenhaus N, Mougneau E. Direct visualization of peptide/MHC complexes at the surface and in the intracellular compartments of cells infected in vivo by Leishmania major. PLoS Pathog. 2010;6:e1001154. doi: 10.1371/journal.ppat.1001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iijima N, Mattei LM, Iwasaki A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc Natl Acad Sci U S A. 2011;108:284–289. doi: 10.1073/pnas.1005201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carr KD, Sieve AN, Indramohan M, Break TJ, Lee S, Berg RE. Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection. Eur J Immunol. 2011;41:2666–2676. doi: 10.1002/eji.201041363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi C, Hohl TM, Leiner I, Equinda MJ, Fan X, Pamer EG. Ly6G+ Neutrophils Are Dispensable for Defense against Systemic Listeria monocytogenes Infection. J Immunol. 2011 doi: 10.4049/jimmunol.1101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guilliams M, Movahedi K, Bosschaerts T, VandenDriessche T, Chuah MK, Herin M, Acosta-Sanchez A, Ma L, Moser M, Van Ginderachter JA, Brys L, De Baetselier P, Beschin A. IL-10 dampens TNF/inducible nitric oxide synthase-producing dendritic cell-mediated pathogenicity during parasitic infection. J Immunol. 2009;182:1107–1118. doi: 10.4049/jimmunol.182.2.1107. [DOI] [PubMed] [Google Scholar]
- 78.Scharton-Kersten T, Contursi C, Masumi A, Sher A, Ozato K. Interferon consensus sequence binding protein-deficient mice display impaired resistance to intracellular infection due to a primary defect in interleukin 12 p40 induction. J Exp Med. 1997;186:1523–1534. doi: 10.1084/jem.186.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sponaas AM, Freitas do Rosario AP, Voisine C, Mastelic B, Thompson J, Koernig S, Jarra W, Renia L, Mauduit M, Potocnik AJ, Langhorne J. Migrating monocytes recruited to the spleen play an important role in control of blood stage malaria. Blood. 2009;114:5522–5531. doi: 10.1182/blood-2009-04-217489. [DOI] [PubMed] [Google Scholar]
- 80.Chimma P, Roussilhon C, Sratongno P, Ruangveerayuth R, Pattanapanyasat K, Perignon JL, Roberts DJ, Druilhe P. A distinct peripheral blood monocyte phenotype is associated with parasite inhibitory activity in acute uncomplicated Plasmodium falciparum malaria. PLoS Pathog. 2009;5:e1000631. doi: 10.1371/journal.ppat.1000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meding SJ, Cheng SC, Simon-Haarhaus B, Langhorne J. Role of gamma interferon during infection with Plasmodium chabaudi chabaudi. Infect Immun. 1990;58:3671–3678. doi: 10.1128/iai.58.11.3671-3678.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Nunez G, Schlueter D, Flavell RA, Sutterwala FS, Sher A. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Girgis NM, Gundra UM, Ward LN, Cabrera M, Frevert U, Loke P. Ly6Chigh Monocytes Become Alternatively Activated Macrophages in Schistosome Granulomas with Help from CD4+ Cells. PLoS Pathog. 2014;10:e1004080. doi: 10.1371/journal.ppat.1004080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Majer O, Bourgeois C, Zwolanek F, Lassnig C, Kerjaschki D, Mack M, Muller M, Kuchler K. Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLoS Pathog. 2012;8:e1002811. doi: 10.1371/journal.ppat.1002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pang IK, Pillai PS, Iwasaki A. Efficient influenza A virus replication in the respiratory tract requires signals from TLR7 and RIG-I. Proc Natl Acad Sci U S A. 2013;110:13910–13915. doi: 10.1073/pnas.1303275110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rivera A, Ro G, Van Epps HL, Simpson T, Leiner I, Sant'Angelo DB, Pamer EG. Innate immune activation and CD4+ T cell priming during respiratory fungal infection. Immunity. 2006;25:665–675. doi: 10.1016/j.immuni.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 87.Espinosa V, Jhingran A, Dutta O, Kasahara S, Donnelly R, Du P, Rosenfeld J, Leiner I, Chen CC, Ron Y, Hohl TM, Rivera A. Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS Pathog. 2014;10:e1003940. doi: 10.1371/journal.ppat.1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Samstein M, Schreiber HA, Leiner IM, Susac B, Glickman MS, Pamer EG. Essential yet limited role for CCR2+ inflammatory monocytes during Mycobacterium tuberculosis-specific T cell priming. Elife. 2013;2:e01086. doi: 10.7554/eLife.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol. 2012;91:401–415. doi: 10.1189/jlb.0811394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 91.Getts DR, Terry RL, Getts MT, Deffrasnes C, Muller M, van Vreden C, Ashhurst TM, Chami B, McCarthy D, Wu H, Ma J, Martin A, Shae LD, Witting P, Kansas GS, Kuhn J, Hafezi W, Campbell IL, Reilly D, Say J, Brown L, White MY, Cordwell SJ, Chadban SJ, Thorp EB, Bao S, Miller SD, King NJ. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med. 2014;6:219ra7. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]