Abstract
Prehypertensives exhibit marked endothelial dysfunction, a risk factor for future cardiovascular morbidity and mortality. However, the ability of exercise to ameliorate endothelial dysfunction in prehypertensives is grossly underinvestigated. This prospective randomized and controlled study examined the separate effects of resistance and endurance training on conduit artery endothelial function in young prehypertensives. Forty-three unmedicated prehypertensive (systolic blood pressure [SBP]=120–139 mmHg; diastolic blood pressure [DBP]=80–89 mmHg) but otherwise healthy men and women and 15 normotensive matched time-controls (NMTC); n = 15) between 18 and 35 y of age met screening requirements and participated in the study. Prehypertensive subjects were randomly assigned to either a resistance exercise training (PHRT; n = 15), endurance exercise training (PHET; n = 13) or time-control group (PHTC; n = 15). The treatment groups performed exercise training three days per week for eight weeks. The control groups did not initiate exercise programs throughout the study. Flow mediated dilation (FMD) of the brachial artery, biomarkers of enodothelial function and peripheral blood pressure were evaluated before and after exercise intervention or time-matched control. PHRT and PHET reduced resting SBP (9.6 ± 3.6 and 11.9 ± 3.4 mmHg, respectively; P < 0.05) and DBP (8.0 ± 5.1 and 7.2 ± 3.4 mmHg, respectively; P < 0.05). Exercise training improved brachial artery FMD absolute diameter, percent dilation and normalized percent dilation by 30%, 34% and 19% for PHRT, P < 0.05; and by 54%, 63% and 75% for PHET, P < 0.05; respectively. PHRT and PHET increased plasma concentrations of 6-keto prostaglandin F1α (19% and 22%, respectively; P < 0.05), NOx (19% and 23%, respectively; P < 0.05), and reduced endothelin-1 by (16% and 24%, respectively; P < 0.01). This study provides novel evidence that resistance and endurance exercise separately have beneficial effects on resting peripheral blood pressure, brachial artery FMD and endothelial-derived vasoactive agents in young prehypertensives.
Keywords: endothelial function, endothelin, exercise, nitric-oxide, prehypertension
Introduction
It is estimated that 25% of the US population 20 y of age or older (~54 million Americans) have prehypertension. This demographic is 11 times more likely to develop essential hypertension than normotensives.1–3 Prehypertension is defined as untreated systolic blood pressure (SBP) of 120–139 mmHg or diastolic blood pressure (DBP) of 80–89 mmHg and not having been told on two occasions by a doctor or other health-care professional that one has hypertension.4 According to the report from the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7), prehypertension is not a disease category per se, and individuals with prehypertension are not candidates for drug therapy4. Rather, the JNC 7 recommends that people with prehypertension practice lifestyle modification to prevent the progressive rise in blood pressure (BP) and increased risk of cardiovascular disease.4 The cornerstone of the JNC7 recommendation is participation in regular physical activity for the treatment of prehypertension.4
In hypertensives, endurance exercise training has been shown to effectively improve endothelial function and reduce both systolic and diastolic blood pressure 8–17 mmHg and 6–13 mmHg respectively.5,6 However, the efficacy of endurance and/or resistance training in reducing blood pressure in young prehypertensives remains nearly uninvestigated. Therefore, the purpose of the present study was to conduct the first randomized, normotensive controlled investigation to assess the separate effects of resistance and endurance training on resting blood pressure, peripheral artery flow mediated dilation (FMD) and endothelial derived vasoactive agents in young prehypertensives. We hypothesized that eight weeks of resistance or endurance training would improve brachial artery FMD, reduce resting blood pressure and would elicit commensurate changes in endothelial derived vasoactive agents in young prehypertensives.
Methods
Baseline status of subjects
Forty-three consecutive young (aged 18–35 y) but otherwise healthy subjects determined to be prehypertensive through BP screening were enrolled from the University of Florida and surrounding Gainesville, FL area. Additionally, 15 consecutive normotensive (SBP < 120 mmHg and DBP < 80 mmHg) young healthy subjects were also recruited to serve as a normotensive healthy non-exercising control group (NMTC; n = 15; 9M/6F). All subjects (n = 58) were apparently healthy, non-smokers and considered to be novice exercisers who had not participated in a structured endurance and/or resistance training program in the past six months. Prehypertensive subjects were randomized to one of the following three groups; (1) resistance training (PHRT; n = 15; 11 M/4 F), (2) endurance training (PHET; n = 13; 9 M/4 F) and (3) non-exercising time control (PHTC; n = 15; 10 M/5 F). All subjects were studied before training and after eight weeks of exercise treatment or control time period. The study was approved by the Institutional Review Board of the University of Florida and written informed consent was obtained from all patients.
Resting brachial blood pressure
Prior to participation in the study, all potential subjects underwent BP screening. Blood pressure measurements were performed according to the JNC7 guidelines.4 Briefly, subjects underwent at least three blood pressure measurements per visit on three separate occasions. After 20–30 min of rest, BP measurements were spaced by 5–10 min intervals on the left arm in a supine position using an automated oscillometric blood pressure cuff (VSM MedTech, Ltd., Coquitlam, BC, Canada). After meeting screening criteria, consent was obtained and subjects were asked to report on a separate day to the Cardiovascular Laboratory in the Center for Exercise Science at the University of Florida. Prehypertensive subjects were included in the study when resting systolic blood pressure was between 120 and 139, or diastolic blood pressure between was 80 and 89 mmHg on all three visits.
Exercise and time-control
At study entry, prehypertensive subjects were randomly assigned, using a computer random number generator, to either a group that performed resistance exercise training (RT), endurance training (ET) or to a non-exercising time-control group (TC). The PHRT and PHET groups performed exercise training three days per week for one hour. Duration of the exercise program was eight weeks. The RT regimen consisted of two sets of 8–12 repetitions to volitional fatigue on seven variable resistance machines (MedX Corp., Ocala, FL, USA) chosen to exercise all major muscle groups. The ET regimen consisted of interval treadmill walking/running to maintain a heart rate that was between 65% and 85% of their predetermined maximum exercising heart rate. A three-day familiarization period for RT and ET was instituted during the first week of training and set at 50% of each subjects predetermined one-repetition maximum (1-RM) or peak oxygen consumption (Peak VO2) and maximum heart rate, respectively. Both PHTC and NMTC groups remained sedentary and refrained from initiating a structured exercise training program for eight weeks. All subjects were instructed to maintain their current nutritional concentrations.
Blood collection and biochemical assays
Venipuncture was performed before and after eight weeks of exercise or time-control period. Measurement of the stable nitric oxide metabolites, nitrate and nitrite (NOx), was used to estimate nitric oxide bioavailability. To minimize the influence of dietary nitrates, all subjects were instructed to follow the National Institutes of Health low nitrate diet guidelines a minimum of 48 h prior to each blood draw.7 In addition, 6-keto prostaglandin F1α (6-keto-PGF1α), the major metabolite of prostacyclin, was measured by commercial assays (Cayman, Ann Arbor, MI, USA). Plasma concentrations of endothelin-1 (ET-1) (Quantikine, Minneapolis, MN, USA), angiotensin-II (ANG II) (RayBiotech, Norcross, GA, USA) were also assessed using commercially available ELISA kits. The intra- and inter-assay coefficients of variance were 3.2% and 5.0% for NOx, 5.5% and 8.1% for 6-keto-PGF1α, 3.4% and 7.6% for ET-1, and 1.3% and 2.2% for ANG II. Biochemical assays were performed after completion of the study.
Brachial FMD
The FMD technique was used to determine endothelial-dependent reactivity in the brachial artery before and after eight weeks of exercise or time-control period. Due to the variations in vascular reactivity which occur throughout the phases of the menstrual cycle, female subjects were studied during their luteal phase.8 Brachial artery FMD was assessed in the right arm using a high-resolution ultrasound machine (ATL HDI 3000; Advanced Technologies Laboratories, Bothell, WA, USA) equipped with a 10.5 MHz transducer.9 Briefly, resting baseline end diastolic brachial diameters and blood velocity were obtained with the transducer placed 3–5 cm above the anticubital fossa. Reactive hyperemia was produced by inflating a BP cuff placed on the upper forearm for five minutes at 200 mmHg followed by a rapid deflation. The brachial artery was imaged and recorded for three minutes following cuff deflation. Continuous ultrasound images were recorded digitally using Pinnacle Studio Plus 10 (Pinnacle Systems, Mountain View, CA, USA). Brachial artery diameters were determined during end-diastole via Vascular Research Tools (Medical Imaging Applications LLC, Coralville, IA, USA) by measuring the distance between the near and far wall of the intima using the automated edge detection software for video analysis.
Peak brachial FMD was expressed as a percentage increase from baseline (FMD%). FMD% is influenced by baseline diameter, and therefore, absolute changes (Amm) in diameter were also determined. Because, dilation also depends on the resultant hyperemic flow stimulus, all measurements of peak FMD% were normalized to the mean shear rate (4 × mean blood velocity/mean diameter) for the first 20 s after cuff deflation. All brachial artery FMD procedures were performed in the Clinical Exercise Physiology Laboratory at the University of Florida by one experienced ultrasound technician who had undergone previous training on this technique.
Exercise tests
PHET subjects performed maximum graded exercise tests (GXTs) on a treadmill using a Bruce protocol before and after eight weeks of exercise to determine initial exercise training intensity and cardio-respiratory fitness level. Primary measurements were total exercise duration, and VO2peak. Criteria for termination of the GXT included respiratory exchange ratio (RER) <1, a heart rate equal to age predicted maximum heart rate, plateau of VO2 and volitional fatigue.
Muscle strength was assessed by determining 1-RM in all PHRT subjects, using variable resistance MedX training equipment for leg and chest press before and after eight weeks of exercise to determine initial exercise training intensity and post-training strength level.
Statistical analysis
All statistical analyses were performed using SPSS version 18.0 for Windows (SPSS, Chicago, IL, USA). Continuous variable data are presented as mean ± SD. All data were tested for normal distribution with the Shapiro-Wilk test for normality. An alpha level of P < 0.05 was required for statistical significance. Repeated measures analysis of variance (ANOVA) was used to evaluate the continuous primary dependent variables; peripheral blood pressure and brachial FMD; and the secondary dependent variables, NOx, ET-1; the plasma biomarkers, subject characteristics and all other data. When a statistically significant main effect between treatment and control was determined, within group repeated measures ANOVAs were performed for each variable to analyze the timepoint mean differences from baseline for each group and to determine within group timepoint significance. Further, Tukey’s post hoc analysis was performed utilizing the within subject timepoint effect mean square error and test of between subject effect mean square error derived from the primary repeated measures ANOVA.
When significant differences between timepoints were observed for each group, and there were no other significant differences between groups at baseline or between time-points within the control groups, the significant P values for the absolute mean changes are reported within group by timepoint to simplify the presentation of the exercise treatment effect. Bivariate Pearson’s correlation analysis was performed to determine relationships between the continuous primary dependent variables and the secondary dependent variables. Statistically significant absolute values are represented in the figures as percent changes from baseline and Tukey’s post hoc significance between group between timepoint are reported. Repeated measures ANOVA between exercise treatment and time control was used to analyze the descriptive patient characteristics, metabolic profile, cardiac intervention history, drug regimens and GXT results. These data are reported as mean ± SD.
Sample size calculation
A power analysis was performed to estimate the statistical power related to testing the following hypotheses in 45 patients: (1) Whole body resistance and endurance training will improve endothelial function in peripheral muscular conduit arteries measured via FMD of the brachial artery. (2) Whole body resistance and endurance training will elicit commensurate changes in endothelial-derived vasoactive agent NOx. Based on the data of Edwards et al.,11 for Hypothesis 1 the post-treatment means are anticipated to be 11.2% and 8.4% for exercise treatment and time control respectively. The anticipated standard deviation was about 3.3% for the exercise treatment group and 2.3% for the time control group. A study of 30 evaluable prehypertensive subjects (n = 15 resistance and n = 15 endurance) and 30 evaluable time-controls (n = 15 prehypertensive and n = 15 normotensive) will have 99% power, based on the Satterthwaite corrected t-test to have a P value below 5% two-sided. Based on the data of Edwards et al.,11 for Hypothesis 2 the post-treatment means are anticipated to be 34.7 and 30.16 μmol/L for exercise treatment and time control, respectively. The anticipated standard deviation was about 8.67 μmol/L for the exercise treatment group and 5.64 μmol/L for the time control group. A study of 30 evaluable prehypertensive subjects (n = 15 resistance and n = 15 endurance) and 30 evaluable controls (n = 15 prehypertensive and n = 15 normotensive) will have 99% power, based on the Satterthwaite corrected t-test to have a P-value below 5% two-sided.
Results
All subjects completed the entire exercise treatment or control time period without adverse events. 1-RM strength measures increased in the PHRT group (319 ± 26 to 420.6 ± 35 and 715 ± 45 to 1003 ± 58 AU for chest-press and bilateral leg-press, respectively; P < 0.05). Measures of VO2peak were increased in the PHET group (31.9 ± 1.0 to 40.3 ± 1.4 mL/kg/min; P < 0.05). Table 1 contains the resting characteristics for the exercise training and time control participants. The prehypertensive and the normotensive groups did not differ at baseline with respect to age, height, weight, body mass index or resting heart rate. Additionally, after randomized group assignment the prehypertensive groups did not differ at baseline with respect to resting SBP or DBP. By design, the prehypertensive groups had significantly higher baseline SBP and DBP compared with normotensive time-controls at study entry.
Table 1.
Resting subject characteristics before and after exercise training and time control
| PHRT (N = 15)
|
PHET (N = 13)
|
PHTC (N = 15)
|
NMTC (N = 15)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Number of males | 11 | − | 9 | − | 10 | − | 9 | − |
| Number of females | 4 | − | 4 | − | 5 | − | 6 | − |
| Age (y) | 21.1 ± 2.5 | 21.2 ± 2.5 | 20.1 ± 1.1 | 20.3 ± 1.1 | 21.6 ± 2.9 | 21.7 ± 2.9 | 21.6 ± 2.7 | 21.8 ± 2.7 |
| Height (cm) | 174.8 ± 9.4 | 175.1 ± 9.5 | 177.0 ± 7.8 | 178.6 ± 7.1 | 180.1 ± 10.5 | 180.1 ± 10.5 | 173.3 ± 9.4 | 173.3 ± 9.4 |
| Weight (kg) | 84.2 ± 18.4 | 85.0 ± 18.9 | 86.7 ± 14.1 | 84.1 ± 12.7 | 87.8 ± 16.8 | 88.5 ± 17.8 | 80.9 ± 13.1 | 81.7 ± 13.1 |
| BMI (kg/m2) | 27.4 ± 5.1 | 27.58 ± 5.2 | 28.7 ± 5.5 | 28.3 ± 5.9 | 27.0 ± 4.3 | 27.2 ± 4.6 | 24.5 ± 3.4 | 25.6 ± 2.9 |
| Resting HR (bpm) | 63 ± 13 | 61 ± 10 | 64 ± 17 | 56 ± 6.2 | 60 ± 7 | 58 ± 7 | 57 ± 8 | 58 ± 7 |
| Resting SBP (mmHg) | 130.4 ± 2.8* | 120.8 ± 6.4†* | 131.5 ± 3.5* | 119.6 ± 5.4†* | 129.9 ± 3.7* | 130.1 ± 6.8* | 110.5 ± 5.8 | 112.2 ± 5.8 |
| Resting DBP (mmHg) | 80.3 ± 5.8* | 72.3 ± 6.4†* | 81.2 ± 5.6* | 74.0 ± 3.2†* | 80.6 ± 6.8* | 80.7 ± 5.6* | 67.0 ± 3.7 | 67.7 ± 6.2 |
Values are mean ± SD. BMI, body mass index; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; NMTC, normotensive matched time-control; PHET, prehypertensive endurance training; PHRT, prehypertensive resistance training; PHTC, prehyppertensive time-control. There were no significant differences between prehypertensive groups at baseline (P > 0.05). Significance values are reported from between group and between timepoint repeated measures ANOVA and Tukey’s post hoc analysis
P< 0.05 versus normotensive control values at same timepoint
P< 0.05 versus pretreatment values
After eight weeks of either resistance or endurance training both resting SBP and DBP were significantly reduced in the prehypertensive groups but remained significantly elevated above those of the normotensive time-controls (Table 1). Analyses for possible gender differences in the primary and secondary outcome variables were performed. There was no main effect difference between genders with regard to the changes in FMD in response to eight weeks of exercise training or time control (P = 0.832).
Exercise improved brachial artery endothelial function
Brachial artery FMD results are presented in Table 2. The prehypertensive and normotensive groups did not differ at baseline or after eight weeks with respect to resting brachial diameters. In the present study, young sedentary prehypertensives exhibited reduced endothelial dependent vasodilation, as measured by FMD, by 31%, 26% and 36% for absolute dilation in millimeters, percent dilation and percent dilation normalized to shear rate, respectively; P < 0.05 (Table 2). Further, there was a significant increase in all brachial artery FMD variables in the PHRT and PHET groups following eight weeks of training. Resistance training improved brachial artery FMD change in absolute diameter, percent dilation and normalized percent dilation by 30%, 34% and 19%, respectively; P < 0.05 (Table 2). Likewise, endurance training improved change in brachial FMD absolute diameter, percent dilation and normalized percent dilation by 54%, 63% and 75%, respectively; P < 0.05 (Table 2). No appreciable differences between exercise treatments improvement in FMD were noted. There was no significant change in the FMD variables in either control group after eight weeks. Importantly, there were no significant differences in brachial artery FMD%, absolute change in diameter and FMD normalized to shear rate between the PHRT and PHET groups, and normotensive controls after exercise training.
Table 2.
Brachial artery flow-mediated dilation before and after exercise training and time control
| PHRT (N = 15)
|
PHET (N = 13)
|
PHTC (N = 15)
|
NMTC (N = 15)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Baseline diameter (mm) | 4.18 ± 0.06 | 4.29 ± 0.06 | 3.91 ± 0.06 | 4.01 ± 0.07 | 3.99 ± 0.03 | 4.05 ± 0.04 | 4.24 ± 0.05 | 4.40 ± 0.06 |
| Absolute dilation (mm) | 0.26 ± 0.12* | 0.34 ± 0.14† | 0.22 ± 0.10* | 0.34 ± 0.19† | 0.24 ± 0.11* | 0.18 ± 0.09* | 0.35 ± 0.08 | 0.36 ± 0.09 |
| FMD (%) | 6.17 ± 3.07* | 8.30 ± 4.10† | 5.92 ± 3.80* | 9.64 ± 5.13† | 6.20 ± 3.69* | 5.85 ± 3.96* | 8.23 ± 1.95 | 8.30 ± 1.57 |
| Normalized FMD (s−1) | 0.21 ± 0.12* | 0.25 ± 0.12† | 0.16 ± 0.07* | 0.28 ± 0.13† | 0.19 ± 0.10* | 0.19 ± 0.08* | 0.29 ± 0.11 | 0.29 ± 0.07 |
Values are mean ± SD. FMD, flow-mediated dilation; NMTC, normotensive matched time-control; PHET, prehypertensive endurance training; PHRT, prehypertensive resistance training; PHTC, prehyppertensive time-control. Significance values are reported from between group and between timepoint repeated measures ANOVA and Tukey’s post hoc analysis
P< 0.05 baseline versus normotensive control values
P< 0.05 versus pretreatment values
Vasoactive balance
Plasma concentrations of (NOx), (ET-1) and NOx/ET-1 ratio are presented in Figure 1. NOx was lower at baseline in the prehypertensive groups when compared with the normotensive control group (18.9 ± 3.4, 18.8 ± 3.3, 18.2 ± 4.6 and 23.5 ± 11.2μmol/L in PHRT, PHET, PHTC and NMTC, respectively; P < 0.05). There were no significant differences in plasma NOx concentrations between the prehypertensive groups at baseline. Both RT and ET resulted in statistically significant improvements in vasoactive biomarkers from baseline but the magnitude of change was not different between these groups. Plasma NOx concentrations were increased in the PHRT and PHET groups after eight weeks exercise training (18.9 ± 3.4 to 22.4 ± 1.6 and 18.8 ± 3.3 to 24.6 ± 2.1 μmol/L, respectively; P < 0.05). Additionally, there was a positive relationship between systemic plasma concentrations of NOx and peak brachial FMD% when the absolute values for all prehypertensive subjects across all timepoints were compared (r = 0.48; P < 0.001). There were no significant changes in NOx in either time-control group.
Figure 1.
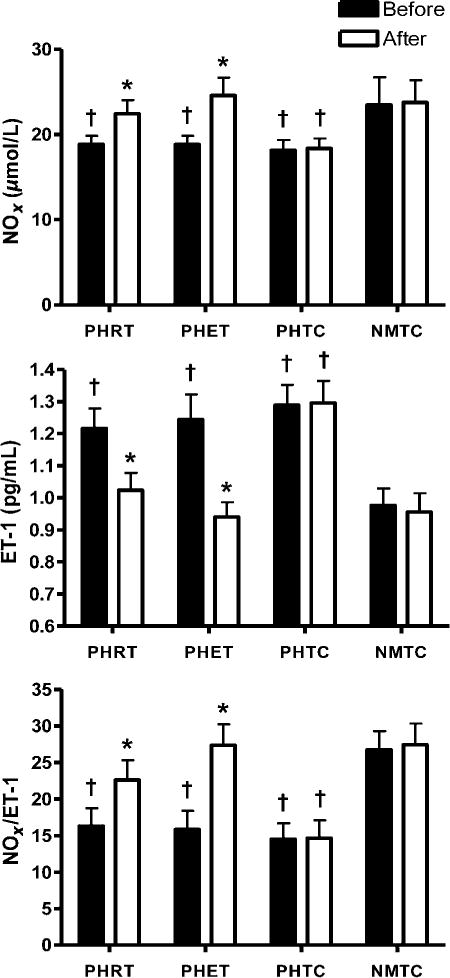
Absolute values for plasma NOx, ET-1 and NOx/ET-1 ratio are presented. *P < 0.05 versus pretreatment values within each group; †P < 0.05 PHRT, PHET and PHTC versus NMTC at the same experimental timepoint. Data are expressed as mean ± SEM. NMTC, normotensive matched time-control; PHET, prehypertensive endurance training; PHRT, prehypertensive resistance training; PHTC, prehyppertensive time-control; ET, endurance training
ET-1 was higher at baseline in the prehypertensive groups when compared with the normotensive time-control group (1.22 ± 0.23, 1.24 ± 0.27, 1.29 ± 0.24 and 0.98 ± 0.17 pg/mL in PHRT, PHET, PHTC and NMTC, respectively; P <0.05). Both RT and ET resulted in a decrease in ET-1 (1.22 ± 0.23 to 1.02 ± 0.20 and 1.24 ± 0.27 to 0.94 ± 0.16 pg/mL in PHRT and PHET, respectively; P< 0.05). There was a negative relationship between systemic plasma concentrations of ET-1 and peak brachial FMD% when the absolute values for all prehypertensive subjects across all timepoints were compared (r = −0.24; P < 0.05). There were no significant changes in ET-1 in either time-control group. Consequently, the ratio of NOx and ET-1 was substantially lower at baseline in the prehypertensive groups when compared with the normotensive control group (16.3 ± 6.0, 15.9 ± 5.9, 14.5 ± 4.7 and 26.8 ± 14.4 in PHRT, PHET, PHTC and NMTC, respectively; p < 0.05). NOx/ET-1 was increased after eight weeks of both resistance and endurance training (16.3 ± 6.0 to 22.6 ± 10.0 and 15.9 ± 5.97 to 27.4 ± 10.4 in PHRT and PHET, respectively; P < 0.05).
Vasodilation and vasoconstriction factors
Plasma concentrations of 6-keto-PGF1α and angiotensin II (ANG II) are presented in Figure 2. Plasma concentrations of 6-keto-PGF1αwere significantly lower in the prehypertensive groups when compared with the normotensive control group at baseline (223.2 ± 56.7, 235.3 ± 80.3, 217.8 ± 54.8 and 329.2 ± 128.5 pg/mL in PHRT, PHET, PHTC and NMTC, respectively; P < 0.05). Levels of 6-keto-PGF1α were increased after eight weeks of both resistance and endurance training (223.2 ± 56.7 to 276.4 ± 79.6 and 235.3 ± 80.3 to 287.7 ± 96.4 pg/mL in PHRT and PHET, respectively; P < 0.05). There were no significant changes in levels of 6-keto-PGF1α in either time-control group. No significant relationship between FMD% and 6-keto-PGF1α was observed (r < 0.001; P < 0.05). Plasma concentrations of ANG II were not significantly different in the prehypertensive groups when compared with the normotensive group at baseline or following eight weeks of exercise training or control time period (15.8 ± 6.4 to 15.9 ± 8.9, 16.5 ± 6.8 to 16.2 ± 8.5, 16.7 ± 6.9 to 16.5 ± 6.5 and 16.9 ± 6.6 to 16.8 ± 7.5 pg/mL in PHRT, PHET, PHTC and NMTC, respectively; P > 0.05).
Figure 2.
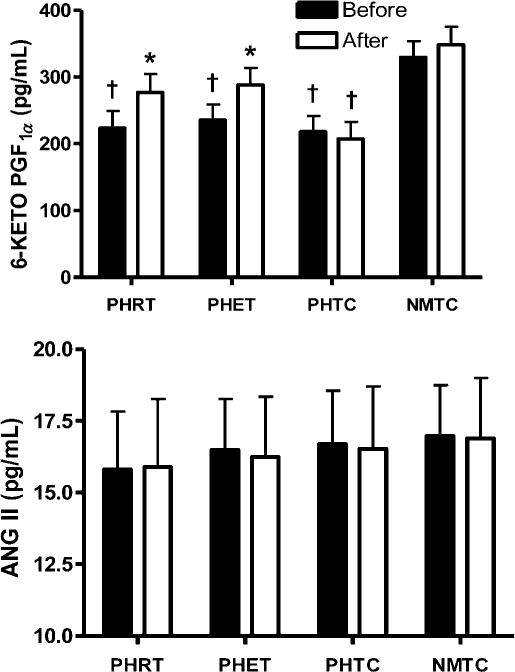
Absolute values for plasma 6-keto-PGF1α and ANG II are presented. *P < 0.05 versus pretreatment values within each group; †P < 0.05 PHRT, PHET, and PHTC versus NMTC at the same experimental timepoint. Data are expressed as mean ± SEM. NMTC, normotensive matched time-control; PHET, prehypertensive endurance training; PHRT, prehypertensive resistance training; PHTC, prehyppertensive time-control; 6-keto-PGF1α, 6-keto prostaglandin F1α; ANG II, angiotensin-II
Discussion
To the best of our knowledge, this is the first randomized, normotensive controlled study to evaluate the independent effects of eight weeks of resistance and endurance exercise training on endothelial function in young prehypertensives. The primary novel findings of the present study are that eight weeks of resistance or endurance exercise training similarly reduces peripheral blood pressures, improves endothelial function and improves vasoactive balance in young prehypertensives.
Impact of exercise training on resting peripheral systolic and diastolic blood pressure
Blood pressure reduction is the primary goal of hypertension therapy.4 The present study demonstrated that eight weeks of resistance or endurance training in previously sedentary, unmedicated prehypertensives leads to significant reductions in resting systolic and diastolic blood pressure. Resistance and endurance training separately resulted in similar significant reductions in both resting systolic and diastolic blood pressure (Table 1). The findings of the present study are in agreement with Collier et al., who found that blood pressure was reduced 3–5 mmHg following only four weeks of endurance and resistance training in pre- and stage-1 hypertensives.12 Estimates of large cohort studies and meta-analyses of randomized trials suggest that a 5 mmHg reduction in systolic blood pressure will result in a 14%, 9% and 7% reduction in risk of mortality due to stroke, coronary heart disease and overall mortality in the general US population, respectively.4,13,14 Therefore, the blood pressure reductions observed in the present study are clinically significant.
Brachial artery endothelial function and exercise training
The present study demonstrates that elevated peripheral blood pressure early in life is associated with reduced conduit artery endothelial function, as assessed by brachial artery FMD. However, short-term (8 weeks) resistance or endurance exercise training can reverse the impaired peripheral endothelial vasodilation function in young prehypertensives. Our findings suggest that the improvements in brachial artery endothelial function in both the resistance and endurance exercise trained groups may be due, in part, to increases in NOx and prostaglandin bioavailability, and/or reductions in the circulating levels of ET-1 (Figures 1 and 2). In fact, the relationships between brachial artery FMD% and basal plasma concentrations of NOx and ET-1 suggests the reduced endothelial function exhibited by our prehypertensive was, indeed, a result of a decreased bioavailability of nitric oxide (Figure 3).
Figure 3.
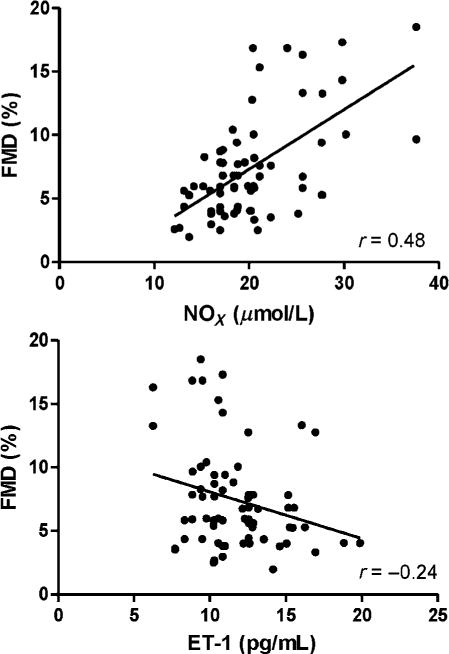
The relationship between basal plasma concentrations of nitrite/nitrate (NOx), ET-1 and peak brachial artery flow-mediated dilation (FMD%) within prehypertensive groups are presented; r = 0.48; P < 0.001 and r = −0.24; P < 0.05, respectively
Giannotti et al.15 recently evaluated endothelial function and repair capacity of early endothelial progenitor cells (EPCs) in older normotensive, prehypertensive and hypertensive men and women. They reported a decrease in radial artery endothelial function in prehypertensives when compared with matched normotensives. Moreover, nitric oxide production was decreased providing an additional possible mechanism for the observed reduction in endothelial function.15 Our study confirms these results in young prehypertensives. However, it is unknown if exercise can alter the EPC phenotype described by Giannotti et al.15
In the current study RT and ET effectively improved the impaired endothelial function observed in the young prehypertensive groups. These significant improvements in the exercise trained prehypertensive groups resulted in FMD values that were not significantly different from those exhibited by our sedentary normotensive time-controls. Moreover, when comparing both types of exercise and the improvement in FMD, we observed no significant differences between our exercise groups. In a previous study, we observed no improvement in brachial artery FMD after 12 weeks of resistance training in young normotensives16. This was likely due to the, presumably, normal endothelial function of the normotensive subjects resulting in no changes in function after training16. Indeed, the benefits of exercise on endothelial function in asymptomatic subjects are less consistent than in subjects who exhibit decreased endothelial function before exercise treatment.17
Potential mechanisms for improved brachial FMD with exercise testing
The mechanism for the improvement in resting BP and the FMD response following exercise training is likely due to improvement in vasoactive substances involved in the regulation of vascular tone and function. In the present study, we observed increased resting levels of NOx after eight weeks of exercise training (~25%), which suggests an increase in NO production and/or bioavailability (Figure 1). At study entry, the prehypertensive groups exhibited significantly lower levels of NOx when compared with normotensive time-controls. To our knowledge, this is the first study to demonstrate that plasma concentrations of NOx are attenuated in young prehypertensives. Both the resistance and endurance trained groups showed postex-ercise intervention improvements in plasma NOx concentrations of 19% and 30%, respectively (Figure 1). The improvement in endothelial function may have been due to the exercise-mediated upregulation of eNOS and the subsequent increase in the production and release of endothelial derived NO.17,18 Tinken et al. reported improvements in brachial artery FMD in normotensive subjects following six weeks of forearm resistance exercise but observed no change in the exercising forearm where acute flow during exercise was abolished. The authors concluded that exercise-induced increases in shear stress are responsible for the improved endothelial function and vascular remodeling19. It appears that the acute increase in shear stress observed with exercise training is the possible mechanism for the improved endothelial function and vasoactive balance observed in our exercise trained subjects.
Increased ET-1 expression is linked to numerous cardiovascular pathologies and hypertension.20 To our knowledge this is the first study to demonstrate elevations in resting levels of ET-1 (~28%) in young prehypertensives when compared with normotensive time-controls. Increased ET-1 expression and bioactivity contributes to age associated vascular endothelial dysfunction and is inversely related to brachial FMD.21 Additionally, ET-1A signaling has been shown to tonically suppress endothelium dependent dilation in older mice.21 In the present study, we observed reductions in levels of ET-1 (~20%) resulting in an increased NOx/ET-1 ratio (~41%) after short-term exercise training (Figure 1). These reductions may have contributed to the improvement in endothelial function observed in young prehypertensives. In fact, systemic plasma concentrations of NOx and ET-1 were related to brachial FMD percentage in our prehypertensive groups in this study (Figure 3). Similar relationships have been reported previously in young male and female normotensives and patients with coronary artery disease.22,23 Further, it has been suggested that NOx/ET-1 is a useful biomarker for predicting coronary artery disease and may play a role, when related to other cardiovascular risk factors such as hypertension, in the determination of cardiovascular risk.24,25
Prostaglandins are released from the endothelium in response to both mechanical and humoral stimuli and can profoundly affect peripheral vascular resistance. Similar to nitric oxide, shear stress is one of the major stimuli for PGI2 release. It has been suggested that 6-keto-PGF1α is acutely elevated after a single bout of exercise and chronically elevated by long-term submaximal exercise training.26,27 In the present study prehypertensives exhibited ~31% reduced plasma concentration of 6-keto-PGF1α when compared with normotensives (Figure 2). Resistance and endurance training increased levels of 6-keto-PGF1α by 19% and 22%, respectively (Figure 2). The results of the present study demonstrate that resistance and endurance training increased levels of 6-keto-PGF1α comparable to young normotensives (Figure 2).
To the best of our knowledge, this is the first study to examine the effects of endurance and resistance training on circulating ANG II concentrations in young prehypertensive humans. Our data suggest that resting plasma concentrations of ANG II do not differ between young prehypertensive and normotensive humans. Further, we demonstrated that circulating ANG II concentrations were unaltered after eight weeks of resistance or endurance training (Figure 2). Based on these results we conclude that ANG II was not likely responsible for the increased blood pressures and impaired endothelial function in our cohort of young prehypertensives. Moreover, our data suggest that the improvements in peripheral blood pressure and endothelial function following exercise training were not through ANG II-mediated mechanisms. Indeed, the peripheral blood pressure reductions observed in this study appear to be in response to the improvement in endothelial function and vasoactive factors.
In conclusion, this study demonstrates that short-term (8 weeks) resistance or endurance training independently reduce resting peripheral blood pressures, improve endothelial function, increase NO bioavailability, decrease ET-1 and increase prostaglandin in a population that is at increased risk for developing essential hypertension. Our novel data indicate that even small decreases in BP favorably influence endothelial function in young, otherwise healthy, humans. These findings are supportive of the JNC7 recommendation that exercise training may be used prophylactically to prevent development of hypertension in young prehypertensives.
Future directions
It is essential to investigate EPC phenotype in young prehypertensives. Further, if differences do exist between young prehypertensives and young normotensives, can this phenotype be altered by exercise? Moreover, it is important to explore the possible additive effect of long-term exercise therapy (16 weeks) and/or combined resistance and endurance training. Additionally, it will be necessary to investigate any possible gender or racial differences in young prehypertensives.
Experimental considerations
The interpretation of our endothelium-dependent FMD data would be strengthened with accompanying vascular smooth muscle (i.e. endothelium-independent) testing. Unfortunately, endothelium-independent vasodilation (via nitroglycerin) testing was not performed in the present study. However, endothelium-independent relaxation has been shown to be preserved in prehypertensives,15 thus suggesting that the impairment in FMD and subsequent improvements with exercise training in the present study are likely due to changes in endothelium-dependent pathways.
Indices of SNS activity were not measured in the present study and, consequently, the effects of SNS modulation in response to the exercise training cannot be ruled out. However, Ray et al.28 demonstrated that isometric handgrip training improved endothelial function and decreased resting arterial blood pressure without altering sympathetic nerve activity or resting heart rate in normotensive subjects with ‘normal’ central sympathetic outflow. There is recent evidence that ET-1 may elicit a sympathoexcitatory effect in both normotensive and hypertensive subjects via endothelin A receptors.29 Therefore, we cannot rule out the possibility that our observed ~20% reduction in ET-1 concentrations after eight weeks of exercise contributed to reduced sympathetic vasomotor tone.
Acknowledgments
The authors thank their subjects for their time and effort.
This work was supported, in part, by a National Institutes of Health predoctoral training grant (NIH 5-T32-HL083810-04) awarded by the University of Florida Hypertension Center.
Footnotes
Author contributions: All authors participated in the design and interpretation of the study and review of the manuscript. DTB and DPC conducted the experiments and analyzed the data. DTB, JSM and BDE trained the subjects, supervised the trainers and collected data for the study. DTB, RWB and JSM wrote and revised the manuscript.
References
- 1.Greenlund KJ, Croft JB, Mensah GA. Prevalence of heart disease and stroke risk factors in persons with prehypertension in the United States, 1999–2000. Arch Intern Med. 2004;164:2113–8. doi: 10.1001/archinte.164.19.2113. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV. Prehypertension revisited. Hypertension. 2006;48:812–4. doi: 10.1161/01.HYP.0000241684.29799.14. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams R, Carnethon M, De SG, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 5.Braith RW, Stewart KJ. Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation. 2006;113:2642–50. doi: 10.1161/CIRCULATIONAHA.105.584060. [DOI] [PubMed] [Google Scholar]
- 6.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–53. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 7.Pannala AS, Mani AR, Spencer JP, Skinner V, Bruckdorfer KR, Moore KP, Rice-Evans CA. The effect of dietary nitrate on salivary, plasma, and urinary nitrate metabolism in humans. Free Radic Biol Med. 2003;34:576–84. doi: 10.1016/s0891-5849(02)01353-9. [DOI] [PubMed] [Google Scholar]
- 8.Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 2010;235:111–8. doi: 10.1258/ebm.2009.009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 10.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–69. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards DG, Schofield RS, Lennon SL, Pierce GL, Nichols WW, Braith RW. Effect of exercise training on endothelial function in men with coronary artery disease. Am J Cardiol. 2004;93:617–20. doi: 10.1016/j.amjcard.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Collier SR, Kanaley JA, Carhart R, Jr, Frechette V, Tobin MM, Hall AK, Luckenbaugh AN, Fernhall B. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens. 2008;22:678–86. doi: 10.1038/jhh.2008.36. [DOI] [PubMed] [Google Scholar]
- 13.Stamler R. Implications of the INTERSALT study. Hypertension. 1991;17:I16–20. doi: 10.1161/01.hyp.17.1_suppl.i16. [DOI] [PubMed] [Google Scholar]
- 14.Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, Karimbakas J. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288:1882–8. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 15.Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, Horvath T, Jiang H, Sorrentino SA, Steenken N, Manes C, Marzilli M, Rudolph KL, Luscher TF, Drexler H, Landmesser U. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension. 2010;55:1389–97. doi: 10.1161/HYPERTENSIONAHA.109.141614. [DOI] [PubMed] [Google Scholar]
- 16.Casey DP, Beck DT, Braith RW. Progressive resistance training without volume increases does not alter arterial stiffness and aortic wave reflection. Exp Biol Med (Maywood) 2007;232:1228–35. doi: 10.3181/0703-RM-65. [DOI] [PubMed] [Google Scholar]
- 17.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–8. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 19.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55:312–8. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 20.Touyz RM, Schiffrin EL. Role of endothelin in human hypertension. Can J Physiol Pharmacol. 2003;81:533–41. doi: 10.1139/y03-009. [DOI] [PubMed] [Google Scholar]
- 21.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–H32. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casey DP, Beck DT, Braith RW. Systemic plasma levels of nitrite/nitrate (NOx) reflect brachial flow-mediated dilation responses in young men and women. Clin Exp Pharmacol Physiol. 2007;34:1291–3. doi: 10.1111/j.1440-1681.2007.04715.x. [DOI] [PubMed] [Google Scholar]
- 23.Casey DP, Nichols WW, Conti CR, Braith RW. Relationship between endogenous concentrations of vasoactive substances and measures of peripheral vasodilator function in patients with coronary artery disease. Clin Exp Pharmacol Physiol. 2010;37:24–8. doi: 10.1111/j.1440-1681.2009.05225.x. [DOI] [PubMed] [Google Scholar]
- 24.Cagnacci A, Tarquini R, Perfetto F, Arangino S, Zanni AL, Cagnacci P, Facchinetti F, Volpe A. Endothelin-1 and nitric oxide levels are related to cardiovascular risk factors but are not modified by estradiol replacement in healthy postmenopausal women. A cross-sectional and a randomized cross-over study. Maturitas. 2003;44:117–24. doi: 10.1016/s0378-5122(02)00319-5. [DOI] [PubMed] [Google Scholar]
- 25.Kurita A, Matsui T, Ishizuka T, Takase B, Satomura K. Significance of plasma nitric oxide/endothelial-1 ratio for prediction of coronary artery disease. Angiology. 2005;56:259–64. doi: 10.1177/000331970505600304. [DOI] [PubMed] [Google Scholar]
- 26.Boger RH, Bode-Boger SM, Schroder EP, Tsikas D, Frolich JC. Increased prostacyclin production during exercise in untrained and trained men: effect of low-dose aspirin. J Appl Physiol. 1995;78:1832–8. doi: 10.1152/jappl.1995.78.5.1832. [DOI] [PubMed] [Google Scholar]
- 27.Williamson S, Varma D, Brown M, Jansen S. Eicosanoid production following one bout of exercise in middle-aged African American pre- and stage 1 hypertensives. J Aging Res. 2011;302802 doi: 10.4061/2011/302802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ray CA, Carrasco DI. Isometric handgrip training reduces arterial pressure at rest without changes in sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2000;279:H245–H9. doi: 10.1152/ajpheart.2000.279.1.H245. [DOI] [PubMed] [Google Scholar]
- 29.Bruno RM, Sudano I, Ghiadoni L, Masi L, Taddei S. Interactions between sympathetic nervous system and endogenous endothelin in patients with essential hypertension. Hypertension. 2011;57:79–84. doi: 10.1161/HYPERTENSIONAHA.110.163584. [DOI] [PubMed] [Google Scholar]


