Abstract
Epithelial cells of the lung are located at the interface between the environment and the organism and serve many important functions including barrier protection, fluid balance, clearance of particulate, initiation of immune responses, mucus and surfactant production, and repair following injury. Because of the complex structure of the lung and its cyclic deformation during the respiratory cycle, epithelial cells are exposed to continuously varying levels of mechanical stresses. While normal lung function is maintained under these conditions, changes in mechanical stresses can have profound effects on the function of epithelial cells and therefore the function of the organ. In this review, we will describe the types of stresses and strains in the lungs, how these are transmitted, and how these may vary in human disease or animal models. Many approaches have been developed to better understand how cells sense and respond to mechanical stresses, and we will discuss these approaches and how they have been used to study lung epithelial cells in culture. Understanding how cells sense and respond to changes in mechanical stresses will contribute to our understanding of the role of lung epithelial cells during normal function and development and how their function may change in diseases such as acute lung injury, asthma, emphysema, and fibrosis.
Introduction
The lung is a structurally complex and highly dynamic organ with the primary purpose of providing efficient gas exchange. This process of gas exchange requires the application of mechanical forces that distend the structures of the lung and prevent the collapse of prestressed units. While the normal physiologic functions of the lung are maintained in this dynamic mechanical environment, it has become increasingly recognized that changes in the applied mechanical forces or the mechanical properties of the tissue can contribute to or be caused by injury and disease. Located at the interface between the environment and the organism, lung epithelial cells are particularly sensitive to such changes in deforming stress or tissue properties. In this review, we will focus on how lung epithelial cells sense and respond to mechanical forces. We will first examine how stresses and strains are transmitted in the lungs, and we will discuss how this may impact epithelial cells in patients and in animal models of disease. We will then describe methods that are used to apply mechanical forces to cells in culture and how mechanical properties of cells can be measured. The scope of lung epithelial responses to mechanical stress will be described, and we will also examine how injury and repair are affected by mechanical forces.
Stress and Strain Transmission in the Lung
Lung tissues are continuously subjected to cyclic stretch owing to spontaneous breathing or mechanical ventilation. Breathing frequency and volume amplitude vary to match lung ventilation to the metabolic state of the subject (283). During normal tidal breathing at rest, lungs expand and recoil above functional residual capacity (FRC) with a rate of about 12 cycles/min and a tidal volume (VT) that approaches 10% of total lung capacity (TLC) in humans. Breathing frequency and VT increase during exercise to fulfill the rise in O2 consumption and CO2 production. Lung volume ranges from TLC at maximum inspiratory effort to ∼20% of TLC at maximal expiration (residual volume, RV) (3). Measurement of bronchial dimensions of excised dog lungs with stereoscopic radiography has shown that bronchial length and diameter are proportional to changes in the cube root of absolute lung volume (123). Assuming that linear dimensions of lung tissue scale isotropically with the cube root of lung volume, normal tidal breathing at rest has been estimated to correspond to an average tissue strain (ε = ΔL/L0, where ΔL is the change in length and L0 is the initial length) of about 4% (91). Accordingly, tissue strain increases to 12% in a deep inspiration and up to 25% in a vital capacity (VC) maneuver from TLC to RV (91). Consistently with the volume cube root scaling, Tschumperlin and Margulies (260) measured changes in epithelial basement membrane surface area in isolated rat lungs and found ∼40% increase in surface area (ΔSA) when lungs were inflated from 2 to 25 cmH2O transpulmonary pressure (PL). This change in surface area corresponds to a tissue strain of ∼18% [assuming isotropic expansion, ε = (1 + ΔSa)1/2−1]. Recently, Perlman and Bhattacharya (196) performed direct measurements of alveolar expansion by realtime confocal microscopy in isolated perfused rat lungs. The mean length of alveolar perimeter segments rose by 14% when lungs were inflated with PL = 20 cmH2O (Fig. 1). Importantly, alveolar distension was highly heterogeneous, even within the single alveolus, with some septal segments reaching 30% strain. Nonuniform alveolar expansion could be exacerbated in diseased lungs leading to large local strains in mechanically ventilated lungs, which compromises the structural integrity of lung parenchyma.
Figure 1.
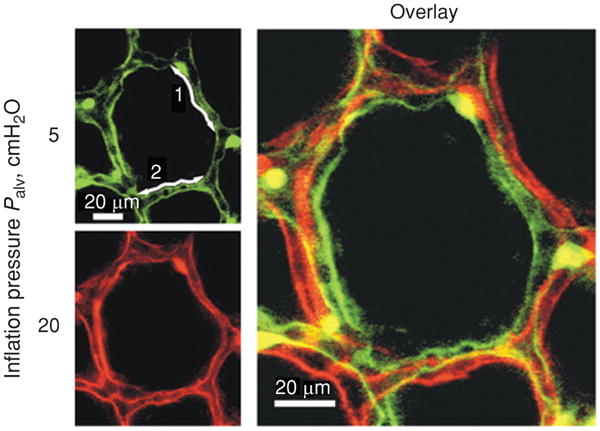
Alveolar distension in lung inflation. An alveolus was imaged at baseline (Palv = 5 cmH2O, green pseudocolor) and hyperinflation (Palv = 20 cmH2O, red pseudocolor). Numbers in baseline image label two perimeter segments. An overlay of the images demonstrates inflation-induced alveolar expansion, which increased perimeter length and alveolar diameter by 13% and 15%, respectively. Adapted (with permission) from reference (196).
If the chest wall opens to the atmosphere, lungs spontaneously collapse to their free stress volume which, in normal lungs, is lower than RV. In the physiological state, lungs remain expanded within the closed thoracic cavity by an inflation pressure corresponding to the difference between internal alveolar pressure (Palv) and external pressure applied onto the pleural surface (Ppl). Therefore, the lungs can be considered as a stress-supported structure under a preexisting state of tensile stress (prestress) (144). The stress (force per unit of area acting on a surface) applied onto the pleural surface by the combination of the forces exerted by respiratory muscles and chest wall recoil is transmitted in the lung throughout the parenchymal meshwork (156). If we consider an arbitrary region of the lung isolated from the remainder by a transecting plane of area (A), the sum of the forces exerted on the walls of the lung region must be zero (Fig. 2) (92, 168). The force acting on the lung region at the transecting plane is the sum of discrete tensile forces (Fi) exerted by the tissues and air-liquid surfaces cut by the plane and the force exerted by gas pressure (Palv). The net force applied on the lung region at this plane (ΣFi/A + Palv) must be counterbalanced by the force exerted by pleural pressure at the remaining region boundaries. Therefore, the average distending stress of the tissue elements transecting the plane equals PL = Ppl − Palv Accordantly, lung distending stress is very low (∼1 cmH2O) at RV and increases nonlinearly with lung volume, approaching ∼5 cmH2O at FRC and ∼30 cmH2O at TLC. Nonlinear behavior has also been found in lung parenchymal tissue strips subjected to uniaxial distending stresses of similar magnitude (179). Indeed, parenchymal stress rose exponentially with strain revealing a marked strain-hardening behavior. Young's modulus (increase in stress relative to strain under uniaxial stretching) increased from ∼5 kPa at distending stresses (5 cmH2O) corresponding to FRC to ∼10 kPa at distending stresses (20 cmH2O) approaching TLC.
Figure 2.

Diagram of pressures and forces acting on region of lung isolated by a plane transecting lung. A is the area of the transaction and ΣFi is the sum of all tensile forces in the tissue elements transecting the plane. Adapted (with permission) from reference (168).
Transmission of Mechanical Stresses to Epithelial Cells
Lung parenchyma stiffness is higher than the values reported for different pulmonary cell types. Direct measurements of Young' modulus in bronchial and alveolar epithelial cells with atomic force microscopy (AFM) have revealed a value of ∼0.5 kPa (4). Comparable values have been reported in fibroblast (11, 157) and airway smooth muscle cells (11, 228). The low stiffness of pulmonary cells indicates that they bear small tensile forces. This is consistent with measurements of internal tensile stress of cells with traction microscopy revealing prestress values ranging from ∼0.1 kPa for alveolar epithelial cells to ∼1 kPa for airway smooth muscle cells (238, 274). Consequently, the extracellular matrix (ECM) and air-liquid surfaces appear as the dominant force-bearing components of lung tissue (302).
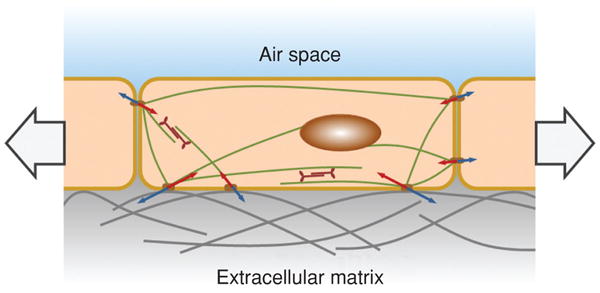
In a uniformly expanded lung the pressure applied to one side of an interior alveolus is equivalent to the pressure applied to the other side, and the air spaces are distended by the forces applied by surrounding tissue. The PL is thus transmitted from the pleural surface to the internal structures (92, 168). However, the lung consists of a complex, interconnected foam-like architecture (Fig. 3) (283), and external stresses are transmitted throughout a discrete three-dimensional (3D) meshwork which concentrates stress into the parenchymal tissues. Stress concentration is particularly important in the thin alveolar septa (thickness of a few micrometers) constituted by two monolayers of alveolar epithelial cells separated by a layer of ECM-embedding pulmonary microcapillaries. The apical surface of alveolar epithelial cells forms an air-liquid interface. Because the radius of curvature of the interface is very small, alveolar surface tension is considered to support most of the PL (168, 231, 283, 289). However, the relative contribution of epithelial cells to the tensile stress of alveolar septa remains poorly defined. Local differences in the applied stress or in the structure and composition of alveolar septa result in nonuniform epithelial cell stretching. Thus, the stress sustained by alveolar epithelial cells is determined by cell stiffness as well as mechanical properties of cell microenvironment. Accordingly, type II alveolar cells (AEII) which are usually located in the alveolar corners, could exhibit lower stretching with inflation pressure than type I cells (AEI) located on the alveolar septum (196).
Figure 3.

Model of the disposition of axial, septal, and peripheral fibers in an acinus showing the effect of surface forces (arrows). From reference (283). Reprinted with permission of the publisher. Copyright © 1984 by the President and Fellows of Harvard College.
Mechanical microenvironment of lung epithelial cells
Parenchymal ECM gives structural support to the epithelial cell monolayer and provides physical pathways for mechanical signaling between the cell cytoskeleton (CSK) and ECM proteins. In addition to this mechanical role, cell-ECM interactions regulate the composition and spatial organization of the matrix and mediate a large variety of critical cell functions including adhesion, differentiation, polarization, contraction, and migration. ECM constitutes a 3D elastic meshwork of fibrous proteins embedded into a hydrated gel-like substance composed of glycosaminoglycans (GAG) and proteoglycan molecules (170, 190, 195).
Collagens are the most abundant proteins of the ECM. The collagen family includes over 20 proteins. Some of them, such as collagen types I and III are fibrillar, or fibril-forming collagens, while others (e.g., IV, V, and VI) are nonfibrillar. A collagen molecule consists of three polypeptide chains wound around one another forming a rope-like regular triple helix 300 nm long and 1.5 nm in diameter (227). Collagen molecules are very stiff with Young's modulus on the order of gigapascal (217, 227). Type I is a fiber-forming collagen secreted by fibroblasts. In the extracellular space, type I collagen molecules assemble into long fibrils, achieving hundreds of micrometers in length and 10 to 300 nm in diameter. Collagen fibrils have a linear stress-strain behavior for small strains (< 5%) with a maximum elongation before rupture of 10% to 15% (94, 227). These fibrils in turn arrange into cable-like fibers several micrometers in diameter. Type I collagen fibers arrange randomly as 3D networks (248) that resist tensile forces and provide the ECM with most of its tensile strength.
ECM tensile resistance is also provided by elastin fibers which are intermingled with the collagen fibers. The main component of elastic fibers is elastin, a fibrous random coil protein. Elastin molecules covalently crosslink forming rubber-like fibers which are three orders of magnitude softer (94) and more stretchable before rupturing than collagen. In addition, elastin fibers exhibit fairly linear elasticity up to ∼200% stretch. Young's modulus reported for single collagen and elastin fibers contrast with the low values found in parenchymal strips, showing that ECM fiber organization is a major determinant of lung tissue strength (158). At low lung volumes, collagen fibers are wavy and floppy (94), the elastic fibers being the major ECM stress-bearing element. Collagen fibers are progressively tightened during lung inflation, becoming the dominant load-bearing element as lung volume approaches TLC (158).
Elastin and collagen fibers are integrated into a gel composed of GAGs and proteoglycans. These are highly charged molecules that attract water into the matrix. This hydrated gel gives ECM resistance to compressive stresses. ECM has differential structure and composition at the cell-matrix interface. The specialized ECM underlying the epithelia contains the glycoprotein laminins and type IV collagen that are crosslinked by the protein nidogen and the proteoglycan perlecan to form a thin flexible sheet (40-120 nm thick) referred to as basement membrane. Type IV collagen is the main stress-bearing constituent of the basement membrane. Laminin and other adhesive proteins bond with cell surface receptors, anchoring the epithelial cell monolayer to the basement membrane and providing physical pathways for force transmission between CSK and ECM.
Mechanical properties and function of ECM depend on the relative amount and distribution of the different matrix components. Interstitial cells not only secrete matrix components but also proteolytic enzymes that degrade them. Most of these proteases are Ca2+- or Zn2+-dependent matrix metalloproteases and serine proteases. Therefore, ECM structure and composition are regulated by the balance between protein secretion and degradation (166). This dynamic balance is impaired in parenchymal pulmonary diseases, altering mechanical properties of the cell microenvironment and stress and strain distribution throughout the lung (16, 235). Asthma is linked to augmented deposition of collagen and proteoglycans remodeling the ECM underlying airway epithelial cells (52). Pulmonary fibrosis is associated with excessive deposition of collagen and elastic fibers and proteoglycans (21, 61, 155, 264, 285), resulting in ECM hardening. Imbalance of protease-antiprotease activity, which increases enzymatic elastin degradation, is a major mechanism of parenchyma destruction in emphysema (15).
Force balance at the epithelial cell monolayer
Lung epithelium constitutes a selective physical, chemical, and immunological barrier that separates internal lung tissues from the external environment (52, 161, 202, 239). The mosaic of lung epithelial cells lining airways and alveoli forms a polarized monolayer with cell-to-cell lateral junctions that link their cytoskeletons. In addition, the basal surface of epithelial cells is anchored to the underlying matrix by means of transmembrane receptors that link the CSK to ECM proteins. The physical integrity of the epithelial cell monolayer is regulated by the force balance at the cell-cell and the cell-matrix attachments (Fig. 4). Under physiological conditions, cells bear a state of internal tension (prestress) which is produced by a combination of active contractile tension generated by actomyosin motors and passive elastic recoil exerted by the actin meshwork. Maintenance of the integrity of the epithelial cell monolayer requires inward tension applied at cell-cell and cell-matrix attachments to be counterbalanced by outward adhesive forces that tether the cells to one another and to the ECM (70). However, this is not a static balance of forces. Lung cells are continuously subjected to cyclic stretch. Therefore, maintenance of cell monolayer integrity requires cell adhesion to withstand the increased elastic recoil induced by stretching. Internal tension further increases if cells become stiffer or if the contractile tension is enhanced as a result of stretching or inflammatory activation. If cell adhesion can no longer support inward forces, monolayer disruption occurs with paracellular gap formation. Breakdown of the epithelial barrier results in leakage of liquid and passage of macro-molecules and inflammatory cells to the alveolar airspace. In the next section, we will examine how the micromechanical environment of the lungs changes during development or in response to injury or disease and how these changes impact epithelial cell function.
Figure 4.
Force balance in the epithelial cell monolayer subjected to in-plane stretch. Inward tension (red arrows) produced by active contractile tension generated by actomyosin motors and passive elastic recoil exerted by the actin meshwork is counterbalanced by outward adhesive forces (blue arrows) exerted by the adjacent cells and the extracellular matrix.
Epithelial Responses to Mechanical Forces In Vivo
Lung mechanics in development
Changes in lung expansion in utero are a major factor in regulating fetal development (109, 118, 142, 292). The fetal lung is fluid-filled during development, with fluid being secreted into the luminal space through the epithelial cells. While some fluid is effluxed through the upper airways, the retained fluid provides a distending pressure that opposes lung recoil. Fetal breath movements, detected during the early stages of gestation, are caused by contractions of the diaphragm and promote cyclic distention of the tissue. Wigglesworth and Desai (286) showed that when these breath movements were blocked by transecting the spinal cord above the level of the phrenic nerve in rabbits, lung growth was decreased by 70% compared with control rabbits. In addition to the decreased lung size, the terminal air sacs exhibited thick walls and poor expansion. Furthermore, the degree of lung expansion plays a major role both in the growth of the lung and in the differentiation of alveolar epithelial cells (118). Surgical obstruction of the trachea in animal models allows fluid to accumulate in the lungs, and the subsequent expansion of the lungs has been shown to stimulate lung growth in fetal sheep (6, 117, 174). When tracheal obstruction was used during the alveolar stage of lung development, there was nearly a doubling of fetal lung weight, DNA, and protein content. Nardo et al. showed that fetal lung expansion caused increased proliferation of fibroblasts, endothelial cells, and type II alveolar epithelial cells (178). An increase in lung expansion, and thus the extent of cell stretch, has also been shown to stimulate the differentiation of type II cells to type I cells through an intermediate cell type (19, 85). On the other hand, while tracheal obstruction reduced the fraction of type II cells in the lungs to less than 2%, subsequent release of the obstruction and lung deflation was followed by an increase in the proportion of type II cells (87).
Following birth the fluid within the airspaces is rapidly cleared, and several important changes in the mechanical environment occur (118). Surface tension develops at the interface between the air in the lungs and the thin liquid lining on the epithelial cells. This surface tension is reduced by surfactant but still increases the recoil of the lung. The collapse of the lungs is opposed by the negative intrapleural pressure between the lung and chest wall, but the overall level of lung expansion decreases. Flecknoe et al. have shown that these changes alter the proportion of type II and type I cells in fetal sheep (86). They found that the percentage of type I cells increased from 4.8% at 91 days gestation to 63% at 111 days, remained at this level until birth, and then decreased to 44.8% after birth. The percentage of type II cells increased from 4.3% at 111 days to 29.6% at 128 days, and then increased to 52.9% after birth. These results and others suggest that increased levels of lung expansion promote a higher proportion of type I cells, while a reduced level promotes the type II cell population. There has been support for this paradigm from studies using cultured alveolar epithelial cells from adult animals in which attachment of cells to a substrate in a more distended state promoted a type I-like phenotype (51, 224). Thus, there is substantial in vivo data suggesting that mechanical distention increases lung growth during development and alters the distribution of alveolar type I and type II cells after birth.
Partial pneumonectomy and compensatory lung growth
Just as changes in lung expansion regulate fetal lung growth and development, mechanical signals also appear to regulate compensatory lung growth following partial pneumonectomy in many species including humans, dogs, cats, rabbits, ferrets, hampsters, rats, and mice [reviewed in (206)]. Cohn first suggested that the size of the thoracic cage would limit the growth of the remaining lung after partial pneumonectomy (45), and filling the thoracic cage with inert material has been shown to restrict compensatory growth (27). Following partial pneumonectomy several changes occur [reviewed in (120, 206)]: there is overinflation of the remaining lung into the vacated portion of the thoracic cage, the entire cardiac output flows through the remaining pulmonary circulation, and there will be increased growth of the remaining lung. The magnitude of distention that occurs in different parenchymal compartments and in individual cells is not well characterized, but it is likely that the stresses and strains are nonuniform. Compensatory growth has been most extensively studied in young rats, where lung mass, volume, number of cells, and number of alveoli were recovered to the level of controls following pneumonectomy (29, 146, 207, 208, 246). Lung volume was also restored in adult mice 20 days after pneumonectomy, and new growth of alveoli was shown (78). Interestingly, Hoffman et al. compared the response to pneumonectomy in mice with either elastin insufficiency or with elastase-induced emphysema (113). They found that filling the thoracic cage with plombage significantly reduced lung regrowth compared with controls, and proliferation of Clara cells, type II cells, and progenitor cells that were positive for both Clara cell secretory protein (CCSP) and surfactant protein C (CCSP+/SP−C+) was also reduced. In the elastin-deficient mice the proliferation of type II and CCSP+/SP−C+ cells was also significantly reduced, but compensatory lung regrowth still occurred. However, lung regrowth was impaired in elastase-treated mice, but only type II cell proliferation was affected. Presumably lung compliance, and therefore lung expansion, would be decreased in elastin-deficient mice and increased in elastase-treated mice, but elastase injury can be heterogeneous.
Acute lung injury and ventilator-induced lung injury
There are prominent changes in mechanical stresses and strains in acute lung injury and its more severe form, acute respiratory distress syndrome (ARDS). Acute lung injury can be initiated by numerous causes including pneumonia, aspiration of gastric contents, inhalation injury, lung transplant, sepsis, and severe trauma (89, 275). A prominent feature of the pathogenesis of this disease is injury to the epithelial lining of the airways and alveoli and the endothelial lining of the capillaries. Loss of barrier function results in pulmonary edema and increased susceptibility to infection, and restoration of this barrier is a critical determinant of outcome. The evolution of the disease leads to substantial changes in lung mechanics including decreased compliance caused by edema, increased surface tension caused by alveolar flooding and loss of surfactant activity, compressive stresses due to the increased weight of fluid filled tissue, and stresses caused by interdependence between adjacent air-filled and water-filled units (122). In addition to these changes that occur in the course of the disease, positive pressure mechanical ventilation is used in these patients to provide breathing support and supplemental oxygen. While this supportive therapy is frequently life saving for patients, it is clear from both experimental and clinical studies that mechanical ventilation causes additional changes in lung mechanics that can, in fact, contribute to lung injury, termed ventilator-induced lung injury (VILI) (67, 89, 169, 200, 211, 252). Webb and Tierney first recognized that mechanical ventilation of healthy rats with high tidal volumes resulted in increased lung edema and injury (281). More recently, a randomized clinical trial by the ARDS network (1) demonstrated a 22% reduction in mortality of ARDS patients when the tidal volume was reduced from a conventional setting of 12 ml/kg to a lower setting of 6 ml/kg. This decrease in morbidity and mortality stimulated significant interest in the mechanisms of VILI and the development of lung protective strategies (89, 96, 108, 154, 163, 252, 266).
Two primary mechanisms have been proposed for the initiation of VILI: overdistention of air-filled regions of the lung, or volutrauma; and repeated collapse and reopening of air-ways, or atelectrauma. Because mechanically ventilated patients with severe lung injury develop high airway pressures that can lead to direct injury to alveolar units and air leaks, the term “barotrauma” has been used clinically. However, earlier studies by Dreyfuss et al. (68), and confirmed by others in several species (2, 34, 111), showed that mechanical ventilation with low volume and high pressure was not injurious, but that ventilation with high volume and low pressure did cause injury. Thus, it is the actual distention of the tissue, not the pressure, that causes the injury, and the term “volutrauma” is now used to describe injury associated with high tidal volume mechanical ventilation (66, 67). The conventional view is that the heterogeneity of regional compliance in the injured lung causes some air-filled regions to be overdistended, while other less compliant or fluid-filled regions receive little or no volume. Atelectrauma occurs when edema increases the weight of injured lungs so that dependent regions are compressed and collapse [reviewed in (65, 122)]. Additional injury to the lung occurs because large stresses are generated in the parenchyma surrounding these regions, and high shear stresses, pressure gradients, and surface tension forces develop when collapsed airways and alveoli are reopened using high pressures (24). Based upon these ideas, the use of positive end-expiratory pressure (PEEP) has been hypothesized to protect the lung by preventing the repeated opening and closing of these regions. Because there is limited direct evidence that collapse and reopening actually occurs in the injured lung in vivo, Hubmayr challenged the collapse and shear hypothesis, suggesting that injury might occur due to overinflation of aerated regions adjacent to fluid- or foam-filled regions (122, 184).
While both volutrauma and atelectrauma have been proposed to occur in vivo, there is surprisingly little direct knowledge of the location and levels of the changes in mechanical forces and the resultant tissue deformation in acute lung injury or VILI. Many studies have utilized morphometric measurements of fixed (noninjured) lung tissue to assess tissue deformation as a function of lung inflation (12, 102, 103, 187, 260), but these techniques are limited by the potential for artifacts during the fixation process and the use of isolated lungs. Nevertheless, these studies have raised questions about the homogeneity of deformation throughout the lung as well as the mechanisms by which deformation takes place. Other studies have demonstrated that the degree of epithelial stretching and unfolding and alveolar septal unfolding were highly dependent upon the lung volume history before fixation (12, 187). Tschumperlin and Margulies, using rat lungs volume cycled prior to fixation, demonstrated that epithelial basement membrane surface area changed little during low lung volume inflation, but changed significantly (∼40%) when lungs were inflated at high lung volumes (260). To avoid potential artifacts due to fixation, Carney et al. used intravital microscopy to visualize recruitment and derecruitment of alveolar structures in dog lungs (35). This technique also has limitations since only subpleural structures are visualized, and the technique is highly invasive. Nevertheless, this group found little change in the volume of individual alveoli during lung expansion from 20% to 80% TLC, in general agreement with Tschumperlin and Margulies (260). More importantly, however, other studies by the group using intravital microscopy in pigs have suggested that while some recruitment may occur during lung expansion at low volumes in normal lungs, alveolar overdistention does occur in injured lungs (167, 221, 232). This is consistent with the view that lung injury may lead to substantial changes in the levels of mechanical strain experienced by epithelial cells. As discussed above, Perlman and Bhattacharya recently demonstrated substantial heterogeneity of deformation of the septal walls of subpleural alveoli in the isolated rat lung using confocal microscopy (196). They also observed that type I cells experienced greater mechanical strain than type II cells. In a subsequent study they examined the effect of interdependence on adjacent fluid-filled and air-filled alveoli. Because the fluid-filled alveolus tended to shrink, the air-filled alveolus was distended to a greater extent (Fig. 5) (197). Further evidence for alveolar distention in vivo comes from a study utilizing confocal fluorescence microscopy to measure plasma membrane stress failure in subpleural rat alveoli following mechanical ventilation (95). Such stress failure occurred only if the cells underwent large deformations, and a significantly higher level of stress failure was observed in lungs ventilated with high tidal volume.
Figure 5.

Alveolar edema results in the expansion of the air-filled alveolus and the contraction of the fluid-filled alveolus. From reference (197). Reprinted with permission of the American Thoracic Society. Copyright © American Thoracic Society.
While overdistention of alveolar epithelial cells is thought to play a significant role in the initiation of VILI, there is limited information on the mechanotransduction pathways that lead to epithelial dysfunction. Several groups have demonstrated deformation-induced injury to alveolar epithelial cells, and mechanisms involving direct plasma membrane injury and necrotic and apoptotic pathways have been proposed (95, 105, 106, 259, 261, 268, 269). For example, Gajic et al. perfused propidium iodide into isolated rat lungs before mechanical ventilation and showed an increase in cell injury with high tidal volumes (95). Cell injury was decreased when the dye was perfused after injurious mechanical ventilation, suggesting that the plasma membrane failure that allowed the dye into the cells was reversible. Lesions of both the alveolar and bronchial epithelium have been demonstrated in ARDS patients (275) and in animal models of VILI (88, 177, 243). Muscedere et al. demonstrated that ventilation of isolated, saline-lavaged rat lungs without PEEP resulted in severe damage to the epithelium both in the alveoli and in the distal air-ways (177). Taskar et al. showed that ventilation of surfactant-deficient rabbit lungs led to both alveolar damage and bronchial epithelial necrosis (243). In a study of mechanically ventilated rats injured by instillation of acid into the lungs, rats ventilated at 12 ml/kg exhibited greater injury to the alveolar epithelium and the small airway epithelium than did rats ventilated at 6 ml/kg (88). In whole animals and cultured cells, Hubmayr and coworkers examined Vlahakis and collaborators examined plasma membrane stress failure in alveolar epithelial cells following stretch-induced injury (95, 268, 269). Margulies and coworkers also demonstrated deformation-induced injury in cultured type II cells (259, 261). However, there are still substantial gaps in our knowledge of how changes in mechanical forces contribute to epithelial injury.
It is interesting to note that a large proportion of patients that succumb to ARDS develop multiple organ dysfunction syndrome (MODS). This observation prompted the development of the biotrauma hypothesis in which it is proposed that changes in stresses and strains during mechanical ventilation stimulates increased release of proinflammatory mediators from lung tissue and changes in the processes of repair and remodeling [reviewed in (65, 251)]. Using isolated rat lungs, Tremblay and collaborators showed that cytokine levels (TNF-α, IL-1β, IL-6, IFNγ, MIP-2, and IL-10) in bronchoalveolar lavage (BAL) were increased to varying degrees in lungs mechanically ventilated with higher tidal volumes and in the absence of PEEP (249). They and others have also demonstrated that expression of c-fos and other proinflammatory genes was stimulated to a greater degree in lungs following ventilation with higher tidal volumes without PEEP (46, 250). These studies and others have utilized in situ hybridization to demonstrate differential responses to VILI in bronchioloar epithelium compared with alveolar epithelium, and it has been suggested that the alveolar epithelium may be less responsive to mechanical stretch (65, 266). However, as described above with regards to cell injury, the mechanisms responsible for sensing mechanical stress and activating pathways to alter gene expression have not been clearly defined.
Asthma
Asthma is a complex and heterogeneous disease characterized by chronic inflammation and structural alterations of the air-ways resulting in episodes of airway obstruction. Remodeling of the airway includes epithelial denudation, increased airway smooth muscle mass, subepithelial fibrosis, and changes in ECM composition (7, 171). Because the bronchial epithelium is damaged and the extent of damage correlates with airway hyper-responsiveness (41), it has been proposed that the abnormal epithelium predisposes the asthmatic toward allergen sensitization (116). Airway hyper-responsiveness is one of the key features of asthma and involves excessive narrowing of the airways, but the precise nature of changes in the mechanisms that regulate smooth muscle contractility is unclear (7, 188). Previous studies in animal models suggested that mechanical stretch of the airways during tidal breathing decreases the extent of airway responsiveness (225, 245, 276, 295, 296), and it has been proposed that airway stretch may be reduced in asthmatics and result in hyper-responsiveness (90, 104). However, there is limited information on the levels of mechanical stretch in vivo and how these might change in hyper-responsive airways. Also, it has long been recognized that there can be significant folding of airway walls during bronchoconstriction, and this may lead to substantial changes in the stresses and strains on bronchial epithelial cells at the surface and smooth muscle cells in the walls of the airways (92, 258, 287). For example, there is likely to be increased compressive stress within these folds. Furthermore, the potential regulation of airway contractility by stressed or damaged epithelial cells has not been well-characterized.
Interstitial lung diseases
Diseases affecting interstitial lung tissue can have a profound effect on the mechanical properties of the tissue, as described above. Emphysema involves breakdown and destruction of alveolar structures that markedly increases the compliance of the lung (236,237). On the other hand, pulmonary fibrosis involves tissue damage and excessive deposition of matrix that causes stiffening of the lung (149, 199). There is currently little understanding of how such changes affect epithelial cells. However, phenotypic changes have been demonstrated in other types of cells that were dependent upon the stiffness of the substrate upon which the cells were grown (13,22,134, 151, 153, 242). Furthermore, epithelial mesenchymal transition, in which epithelial cells differentiate into fibroblasts or myofibroblasts, has been shown to occur in human idiopathic pulmonary fibrosis (140,141). At this point, however, a connection between alterations of epithelial function and changes in material properties of lung tissue are speculative.
Measurements in Cells: How Are Mechanical Properties Measured; How Are Cells Stressed
While there is strong evidence that changes in mechanical stresses can lead to injury or alter epithelial function in vivo, such studies are limited because it is difficult to determine or control the levels of mechanical forces or the direct responses of epithelial cells. In addition, the responses of cells are dependent upon their intrinsic mechanical properties, and these are difficult to discern in tissue. In response to these limitations, substantial effort has been directed toward developing approaches for applying mechanical stresses to cultured cells and for measuring the mechanical properties of cells. In the section below, we will describe some of the approaches that have been developed.
Methods for probing cell mechanics
Mechanical characterization of the cell
The mechanical properties of a body are characterized by the relationship between force (F) and deformation. When a pure elastic solid of length L and constant cross section area A is subjected to a force normal to A, the linear strain (ε = ΔL/L) is related to the normal stress (σ = F/A) according to the constitutive equation σ = E ε, where E is Young's modulus. Alternatively, application of a force tangent to A results in an angular deformation (θ) related to the shear stress (τ = F/A) as τ = Gθ, where G is the shear modulus. For a homogeneous isotropic elastic body, G = E/2(1 + v), where v is Poisson's ratio of the material. As a simplification, if the material is incompressible v = 0.5 and G = E/3. Elastic solids store energy during deformation that is returned in unloading to recover its shape. On the other hand, a pure viscous body dissipates energy. When a pure Newtonian viscous liquid is subjected to a shear stress, the velocity of the liquid layers increases with a shear rate (dv/dx) normal to shear direction according to the constitutive equation τ =μ dv/dx, where μ is the coefficient of viscosity. The cell exhibits a viscoelastic behavior, and as such, it stores and dissipates energy. A straightforward and robust approach to characterizing a viscoelastic body is to measure the complex shear modulus (G*) defined as the ratio in the frequency domain between the applied stress and the resulting strain. G* can be separated into real and imaginary parts G* = G′ + iG″. The inphase component, G′, is the storage or elastic modulus and accounts for storage energy and elastic resistance to deformation. The out-of-phase component, G″, is the viscous or loss modulus accounting for energy dissipation and frictional resistance to deformation. The ratio between loss and storage moduli G″/G′, known as the tangent ratio, provides an index of the degree of liquid-like or solid-like behavior. For a pure elastic solid, G″ = 0 and the tangent ratio is 0. On the other hand, the tangent ratio is infinite for a pure viscous body (G′ = 0).
Measurement of mechanical properties of the cell
The cell is a very soft material with a heterogeneous structure exhibiting local variations in stiffness. Therefore, probing global and local mechanical properties of the cell requires tools and techniques able to manipulate cells with nanometer resolution and to measure forces in the piconewton (pN) to nanonewton (nN) range. Because of the difficulty associated with studying the mechanical properties of cells in situ, most studies have been conducted in vitro. Mechanical, optical and magnetic techniques combined with advanced microscopy have provided us with a suite of powerful tools to explore the mechanical behavior of lung epithelial cells. These include techniques that probe mechanical properties at the local subcellular (e.g., AFM indentation) or at the global cellular level (e.g., microplates), that take measurements in a single cell (e.g., AFM) or in cell populations (e.g., magnetic twisting cytometry, MTC), that permit intracellular measurements (e.g., optical tweezers) and that allow monitoring of the mechanical properties during cell stretching (e.g., MTC).
Atomic force microscopy
AFM probes the sample with a microfabricated flexible cantilever with a sharp tip placed at its free end (26, 115). The tip indents the cell surface by displacing either the sample or the cantilever with a piezoactuator (Fig. 6). The force applied by the tip during indentation is computed as F = k·d, where d is the deflection of the cantilever tip relative to its relaxed position and k is the bending spring constant of the cantilever. The deflection of the cantilever is obtained by focusing a laser beam on the cantilever end and monitoring the displacement of the reflected beam with a segmented photodiode. AFM allows 3D subnanometer cell manipulation with simultaneous measurement of the normal force applied by the cantilever in a wide dynamic range (10-106 pN) and with resolution of a few piconewtons. Using a quadrant photodetector, lateral forces can also be measured but with a resolution 1 order of magnitude smaller. Cell mechanics are usually probed with soft cantilevers (spring constant ∼0.01 nN/nm) that allow force measurements up to several nanonewtons. In addition to mechanical measurements, scanning the surface of the cell with the AFM tip provides high-resolution topographic images of the cell. AFM can be coupled to an inverted optical microscope to combine mechanical measurements with optical imaging techniques (212). Moreover, tip functionalization with ligands to membrane receptors provides a powerful approach to probing cell adhesion and, specifically, unbinding ligand-receptor forces at the single molecule level (20).
Figure 6.

Measurement of cell mechanics with atomic force microscopy.(Top) The atomic force microscope indents the surface of the cell with a flexible cantilever with a sharp tip placed at its end. The cantilever is displaced with a piezoactuator. Force is computed from the lateral displacement on a segmented photodetector of a laser beam reflected on the cantilever. (Bottom) Force-displacement (F-z) curve recorded in an alveolar epithelial cell showing the force measured while the cantilever was approached (solid line) toward the cell and retracted (dashed line) at constant velocity (6 μm/s). The curve exhibits hysteresis indicative of viscoelasticity. The retracted limb exhibits unspecific adhesion before the tip-cell contact was lost (F < 0). The arrow indicates the estimated contact point. Sinusoidal oscillations were applied at a given indentation to compute the complex shear modulus. Reprinted from reference (4) with permission from Elsevier.
It is important to note that the force applied by the cantilever on the cell surface depends on the mechanical properties of the cell as well as on tip-cell contact geometry. With commonly used AFM tips with pyramidal, conical, or spherical shapes, the area of contact between the tip and the cell increases with indentation, resulting in a nonlinear force-indentation (F-δ) relationship (Fig. 6). The mechanical parameters of the cell can be obtained from F-δ recordings by using appropriate contact models. Since tip-cell contact geometry is well defined, AFM provides reliable estimation of the magnitude of cellular mechanical parameters.
For an ideal four-sided pyramidal tip indenting a linear elastic half space of Young's modulus E, the F-δ relationship is defined by the pyramidal Hertz model as
where θ is the semi-included angle of the pyramid and v is Poisson's ratio. Assuming that the cell is incompressible, v is generally taken as 0.5 (4). E can be obtained by fitting F-<δ recordings with the pyramidal Hertz model. According to this model, the projected contact area between the surface and the tip increases with indentation as 1.58 (tan θ)2δ2 (25). Cells are usually probed by indenting the surface down to about half a micron. Therefore, measurements with a pyramidal tip provide an average estimation of mechanical parameters over a cell surface of about 0.2 μm2. Pyramidal tips permit combination of mechanical measurements with AFM cell imaging. The Hertz model assumes an infinite sample thickness. The thickness of epithelial cells is 5 to 10 μm in the nuclear region and decreases markedly at the cell periphery. Mechanical measurements in cells are generally limited to indentations lower than 10% to 20% of cell thickness to avoid the effect of the underlying rigid substrate (157, 212).
An alternative to probe the cell with well-defined contact geometry is to use a cantilever with a microsphere attached at its end. The Hertz model for a spherical tip of radius R is
The projected contact area of a spherical tip increases proportionally with indentation as πRδ. At 0.5 μm indentation the contact area of a spherical tip of R = 2 μm is 3-fold larger than that of a pyramidal tip.
The problems associated with the increasing contact area can be avoided by using flat-ended cylindrical tips (213). A cylindrical punch ensures a constant and controlled contact area during indentation with a linear F-δ relationship
where a is the radius of the tip. Cylindrical tips are ideally suited to study nonlinear mechanical responses of living cells. The same proportional dependence of force on deformation applies when the cell is pulled with a cylindrical cantilever attached to the cell surface. This allows measurement of cell mechanical properties both during indentation and pulling under controlled contact area. The linear force-deformation relationship and the constant contact area during pulling are also important advantages when probing cell adhesion with AFM (213).
The Hertz contact model assumes that the indented sample behaves as a pure elastic material. However, cells exhibit viscoelastic features. In particular, the apparent Young's modulus estimated by fitting the contact model to F-δ recordings increases with indentation velocity (152). The complex shear modulus can be measured by imposing low-amplitude sinusoidal oscillations over a wide frequency range (4).
Expressing the Hertz model in terms of the shear modulus G = E/2(1 + v) and transforming the model equation to the frequency domain through the use of the correspondence principle, G*(ω) can be computed form the frequency spectra of force and indentation oscillations. In particular, for small sinusoidal oscillations with a pyramidal tip around an operating indentation δ0
where F(ω) and δ(ω) are the Fourier transforms of F and δ, respectively. In contact dynamic experiments, the force measured with the cantilever is the sum of the force applied by the cell and the hydrodynamic drag force due to the viscous friction of the cantilever with the surrounding liquid. Measurements can be corrected for the hydrodynamic artifact by estimating the viscous drag coefficient from noncontact measurements taken at different tip-cell distances (5).
Magnetic probes
Magnetic microparticles are versatile probes for measuring cell mechanics. These microprobes can be manipulated by applying mechanical forces or torques on the particle with magnetic fields generated with electrical coils or with permanent magnets. In MTC (Fig. 7), ferromagnetic microbeads are first magnetized in one direction with a brief large magnetic pulse and then twisted with a weak magnetic field directed at a different angle. Mechanical properties of the cell are derived from measurements of the applied torque and the resulting bead rotation. MTC was first used by Crick and Hughes in 1950 (48) to study the cytoplasm viscosity of fibroblasts that had engulfed magnetic microparticles. In 1993, Wang et al. (273) improved the method by attaching functionalized magnetic microbeads to the cell surface. In this work, ferrimagnetic microbeads (∼5 μm in diameter) were coated with ligands to different cell membrane receptors. First, the beads were magnetized with a brief large magnetic pulse (∼10 μs, ∼100 mT) generated by a pair of coaxial coils. Subsequently, a rotatory torque (T) was applied to the beads with a weak (∼2 mT) twisting magnetic field (H) in a direction different from that of the magnetization axis. The angle rotated by the bead (φ) relative to the magnetization axis (φ0) was measured with an inline magnetometer.
Figure 7.
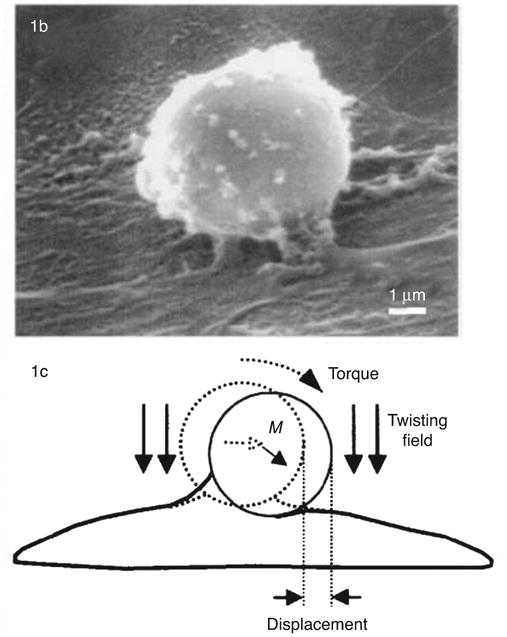
Magnetic twisting cytometry (MTC). (Top) Scanning electron microscopy of a bead bound to the surface of a human airway smooth muscle (HASM) cell. (Bottom) A magnetic field introduces a torque which causes the bead to rotate and to displace. M denotes the direction of the bead's magnetic moment. Images reproduced (with permission) from reference (76).
The twisting torque applied to the bead is
where c is a bead calibration constant. An index of cell stiffness is computed as the ratio of the applied torque and the angular rotation of the bead. It should be noted that the apparent stiffness depends on the degree of bead embedment into the cell surface, which is generally unknown (130). Special care should be taken to avoid microparticle clustering since this could cause significant artifacts in the determination of cell stiffness.
MTC was extended to oscillatory measurements to assess a dynamic modulus of the cell (159, 204). When beads are twisted with a sinusoidal magnetic field with amplitude Ha and angular frequency ω0 (ω = 2πf,f is frequency)
the oscillatory response is characterized by an effective shear modulus defined as the complex ratio in the Fourier domain between the applied torque and the induced bead rotation computed at the oscillatory frequency
The effective modulus g* can be transformed into the conventional complex shear modulus as G* = βg*, where β is a geometric scale factor that depends on the degree of bead embedding and the shape of the cell. Finite element analysis estimated β = 6.8 μm with 10% of the bead diameter (4.5 μm) embedded in a cell 5 μm high (172). However, 3D reconstruction from confocal microscopy imaging of alveolar epithelial cells revealed large embedment variability with half-angle of bead immersion ranging from 36° to 86° (150). Thus, rescaling g* can only provide a rough estimation of the actual magnitude of G*. It should be noted that the frequency dependence and relative changes in g* in response to treatments are independent of β. Interestingly, the scale factor cancels out when computing the tangent ratio (g″/g′ = G″ /G′).
MTC was further refined by Fabry et al. in 2001 (76). Instead of measuring bead rotation of the whole cell culture, lateral displacements of each bead were measured with a charge coupled device (CCD) camera. The cell culture was placed on an inverted microscope and the lateral displacement of the bead centroid was tracked by image processing algorithms with an accuracy of a few nanometers. An effective shear modulus is computed as the complex ratio in the frequency domain between the applied torque and the induced lateral displacement of the bead. As the cell sample is visualized during the measurements, clusters of microbeads can be avoided and only single microbeads appropriately placed on the cell surface are taken for analysis. By using optical detection and phase-locking techniques Fabry et al. (76) expanded the range of oscillatory measurements over 5 decades (10−2-103 Hz).
Since the magnetic beads are manipulated by a uniform magnetic field and the position of the beads are computed from CCD images, g* can be monitored in moving cells carrying beads attached to their surface. In particular, changes in g* during cell stretching can be measured by plating cells on a flexible substrate and distending it while the beads are subjected to an oscillatory twisting field (255). A useful advantage of MTC is that simultaneous parallel measurements can be taken in a large number of cells, which facilitates statistical analysis.
An alternative to probe cell mechanics with magnetic beads is to apply linear forces with a magnetic field gradient. Magnetic gradients can be easily generated by inverting the polarity of one of the magnetizing coils of the MTC device after bead magnetization. In this inverse configuration, the coils generate a local homogeneous magnetic gradient while the field in the central plane vanishes. When applied to ferrimagnetic beads of 5 μm this configuration induces forces up to ∼2 pN with fN resolution (254). Larger magnetic gradients can be obtained with one-pole microneedle permanent magnet (164) or with electromagnets with a sharpened soft iron core (17,18, 139). High forces can be achieved when the magnetic tip approaches the magnetic microbead at distances in the order of a few micrometers. However, a limitation of the technique is that the force-distance dependence is strongly nonlinear. Using microneedle magnets, forces up to ∼10 nN can be achieved with superparamagnetic microbeads and up to ∼100 nN with ferrimagnetic microbeads (18, 139).
Optical tweezers
Dielectric microparticles can be manipulated with optical forces applied with laser beams focused through a high-numerical aperture microscope objective (10, 110, 282). A microbead with high refractive index (e.g., silica or latex) placed in the focused laser beam is subjected to optical forces that pull the bead toward the focus. The force of the optical trap is proportional to the power of the laser. However, the power of the laser must be limited to a few hundreds of milliwatts to avoid sample heating and harmful effects to cells. The wavelength of the laser is usually in the near-infrared range to minimize cell damage (181). The position of the bead can be determined using video recording or measuring the deflection of forward-scattered light of a second laser beam coaxial with the optical trap axis. Optical detection allows high-speed particle tracking with time resolution on the order of microseconds. After calibration of the stiffness of the trap (k), the force applied to the bead is computed with sub-pN accuracy as F = k·d, where d is the displacement of the bead from the optical axis. F-δ measurements can be obtained with sub-pN accuracy in the two directions normal to the optical axis. However, to avoid cell damage, maximum force should be limited to ∼100 pN, which restricts measurements to shallow cell indentations. Cell rheology can be probed by trapping either endogenous intracellular organelle or microbeads attached to the cell surface (Fig. 8) (282, 299). Similar to magnetic microparticle techniques, the poor definition of bead-cell contact geometry does not allow reliable measurement of the magnitude of cell mechanical parameters.
Figure 8.
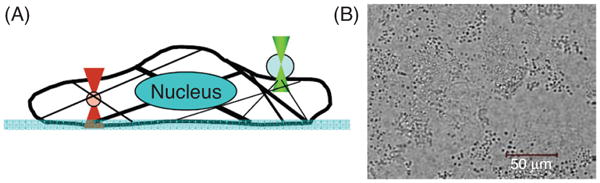
Optical tweezers. (A) A sketch of optical tweezers-based cytorheometer. Optical tweezers were used to manipulate an intracellular granular structure (lamellar body, left circle), or an extracellular antibody coated glass bead (right circle). (B) A bright-field image of lamellar bodies that abundantly exist in alveolar epithelial type II cells. Reprinted (with permission) from reference (282).
Microplates
In the microplates technique, a single cell is attached to a rigid glass microneedle and stretched with a second flexible glass microneedle (59, 82, 247) (Fig. 9). Microplates are fabricated pulling glass plates (∼1 mm wide, ∼0.1 mm thick). Rigid microplates are pulled to reach a size ∼60 μm wide and ∼30 μm thick. Flexible microplates are pulled to obtain a microneedle with an end ∼30 μm wide and ∼5 μm thick. The separation between the two microplates is controlled with a micromanipulator. Coupling a piezoactuator to micromanipulators allows fine control of microneedle separation with subnanometer resolution. Microplates are coupled to an inverted optical microscope to monitor cell manipulation and microneedle displacement. The deflection of the flexible microneedle relative to its relaxed axis (d) can be measured with video microscopy. Higher speed detection can be achieved measuring the deflection of the tip of the flexible microneedle with a position-sensitive photodetector (59). After calibration of the spring constant (k) of the flexible microplate, force is computed as F = k·d. Adhesion of the cell to the microplates allows application of compressive and tensile stresses as well as lateral shear stress. Experiments similar to microplates can be performed with AFM using the cantilever as a flexible microplate to stretch a cell attached to a rigid substrate (39,40).
Figure 9.

Mice myoblast stretched with microplates. Reprinted from reference (59) with permission from Elsevier.
Micropipette aspiration
Micropipette aspiration technique, first developed by Mitchison and Swann in 1954 (173), consists of the suction of the cell surface with a micropipette (Fig. 10). Aspiration causes the cell to extend a protrusion into the micropipette. The evolution of cell protrusion is then recorded through the acquisition of optical microscopy images as a function of time, aspiration pressure, and micropipette radius. The observed resistance of the cell protrusion to enter the micropipette is interpreted through different viscoelastic mechanical models. Micropipette aspiration has been extensively employed to probe blood cells, mostly erythrocytes (280) and leukocytes (180). Nevertheless, this technique has been less used to probe adherent cells (32, 112). The time course of the protrusion is usually interpreted in terms of liquid droplet models consisting of an elastic cortex enclosing either a viscous Newtonian liquid (180, 301) or a viscoelastic liquid (62). These models, exhibiting one or two characteristic time constants, describe the narrow experimental time window of micropipette aspiration data. In contrast to this model-dependent approach, recent AFM measurements taken in leukocytes over a wide frequency range revealed a scale-free power-law behavior that cannot be accounted for by liquid droplet models (215).
Figure 10.

Microaspiration of adhered cells. The micropipette is gently pressed against the glass slide and slid into contact with the adherent cell (A). A vacuum is applied; aspiration of the cell arrow into the pipette bore (B and C). Reprinted from reference (32) with permission from Elsevier.
Passive rheology
Rheology can be probed without applying external forces by tracking spontaneous thermal fluctuations of microparticles embedded in the medium (Fig.11). The mechanical properties of the medium surrounding the microparticle are related to the mean square displacement 〈r2(Δt)〉 of the probe by the generalized Stokes-Einstein relationship (30, 161)
Figure 11.
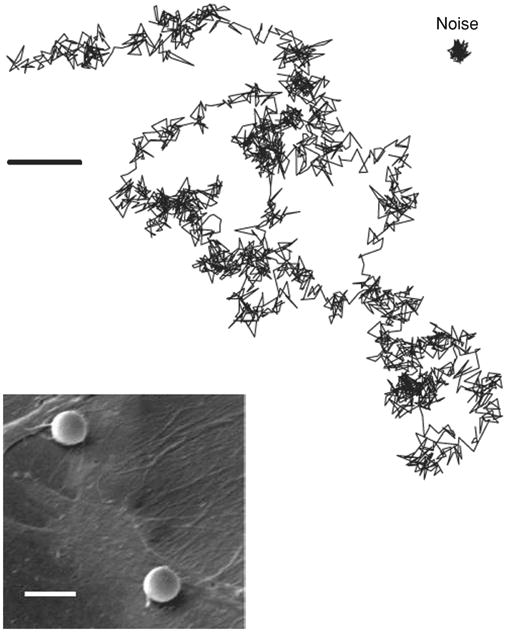
Spontaneous displacement fluctuations of a microbead attached to the surface of human airway smooth muscle cells. Scale bar = 5 μm. Reprinted (with permission) from reference (30).
where T is absolute temperature, kB is Boltzmann's constant, a is probe diameter, i2 = 1, and F−1 denotes the inverse Fourier transform. Spontaneous fluctuations of microbeads attached to the cell surface or introduced into the cell can be tracked with image-processing algorithms (128) or recorded form the lateral deviations of a forward-scattered laser beam focused on the microbead. Alternatively, cytoskeleton rheology can be studied by tracking spontaneous fluctuations of intracellular granules (299). A more reliable estimation of cell rheology is reached by tracking the spontaneous fluctuations of two microparticles embedded in the same cell (49, 114, 263). The method is based on the crosscorrelation of the thermal motion of pairs of particles. This two-point rheology does not depend on the details of the coupling between the particles and their environment or the assumed deformation geometry (49). However, there is evidence that the generalized Stokes-Einstein relationship is not applicable to cells (30, 114, 147, 238). In addition to thermal forces, microparticle fluctuations are also driven by the action of molecular motors. The difference between the frequency spectrum computed form microbead spontaneous fluctuations and active rheology provides an estimation of the contribution of cellular active forces (147).
Methods for measuring cell traction forces
Traction microscopy
A simple procedure to display traction forces exerted by the cell against its microenvironment is to plate cells on a thin flexible membrane. Wrinkling of the membrane reveals the action of the traction forces exerted by the cell. A quantitative analysis of cell-generated traction forces is obtained by plating the cells on a linear elastic gel with small fluorescent beads (∼200 nm in diameter) embedded in the gel (Fig. 12) (31, 53, 54). As the cell contracts the gel becomes deformed and, consequently, the beads are displaced. At the end of the experiment, cells are detached and an additional image is taken to determine the position of the beads in the relaxed gel. The deformation of the surface of the gel is computed with cross-correlation algorithms from the displacement of the beads placed just below the surface of the gel. Bead displacements relative to their relaxed position are obtained by comparing the image taken before and after cell detachment. Once the value of Young's modulus of the gel is known, the traction field is computed from the displacement field using different algorithms. Dembo and Wang (54) devised a mesh-based approach that requires intensive computing. Butler et al. (31) developed a fast algorithm based on the computation of the traction field in the Fourier space. Traction microscopy has been recently extended to 3D measurements combining laser scanning confocal microscopy and digital volume correlation (160). In addition, traction microscopy has been coupled to stretchable substrates to monitor the change in traction forces during cell stretching (97, 143).
Figure 12.

Measurements of cell tractions exerted to the substrate. (Top) Traction microscopy. Cell tractions exerted by a human airway smooth cell on a polyacrylamide gel coated with collagen. Colors show the magnitude and direction of the traction vectors in pascal. Adapted (with permission) from reference (31). (Bottom) Micropost array. Confocal image of immunofluorescence staining of a smooth muscle cell on posts. Position of fibronectin (red) on the tips of the posts was used to calculate force exerted by cells (white arrows). Reprinted (with permission) from reference (240). Copyright (2003) National Academy of Sciences, USA.
Micropost arrays
Traction forces can also be measured by plating cells on top of an array of flexible microposts (Fig. 12). Tan et al. (240) microfabricated an array of polydimethylsiloxane (PDMS) microneedle-like posts with soft-lithography techniques. The tips of the posts were fluorescence stained and the array was placed in an inverted microscope to measure the lateral deflection of the posts (d). When the cells lying on top of the posts contract, the posts are deflected. After calibration of the bending constant of the posts (k), tractions are computed as F = k·d. du Roure et al. (69) refined the microfabrication technique reducing post diameter to improve spatial resolution.
Methods for applying local and global stretch
Cell stretching devices
Cells sense and respond to mechanical stimuli exerted by adjacent cells and by the ECM. Adjacent cells exert mechanical forces and deformations to specific regions of the cell surface. Additionally, cells are also subjected to global stretching due to the deformation of surrounding tissues. Devices used in active rheological techniques (AFM, MTC, optical tweezers, and microplates) are well suited for applying local mechanical stresses to single cells. On the other hand, several cell-stretching tools have been devised to mimic in vivo cell mechanical stimulation transferred to the cell from the deformation of the surrounding tissue. It should be noted, however, that transmission of substrate strain to cells depends on the strength of cell-substrate adhesion and on the strain amplitude. Week adhesion or high stretch amplitude results in partial cell detachment with incomplete strain transmission from the substrate to the cultured cells (23, 255, 271). Of importance to the study of mechanosensing and mechanoresponse of lung cells is the ability to generate high amplitude cyclic loading with well-defined waveforms capable of mimicking the dynamic strain experienced by cells during breathing or mechanical ventilation. We review below methods to expose lung epithelial cells to mechanical stress.
Uniaxial stretching
Unixial stretching can be produced by pulling apart the opposite ends of an elastic rectangular strip (Fig. 13). Static and dynamic stretching are usually generated with motor-operated systems (176, 198). Compressive strains can be delivered by relaxing a prestressed elastic membrane (100). Uniaxial stretching devices can be adapted to an optical microscope for real-time imaging (198). However, stretching decreases substrate thickness, which requires refocusing of the cell culture surface. A common shortcoming of stretching devices is the lateral displacement of the cells. Gerstamir et al. (100) developed a computer-controlled stretch apparatus that compensates for the lateral displacement during stretch to maintain any selected point of the substrate at a constant position on the microscope. Stretching a rectangular strip does not actually deliver a pure uniaxial strain to the cultured cells owing to the concomitant transverse constriction determined by Poisson's effect of the elastic material. Caille et al. (33) laterally deformed a transparent silicone channel with two piezoelectric translators to impose almost purely uniaxial deformation. Micropaterning techniques have been used to align the cultured cells relative to the direction of stretch (241).
Figure 13.
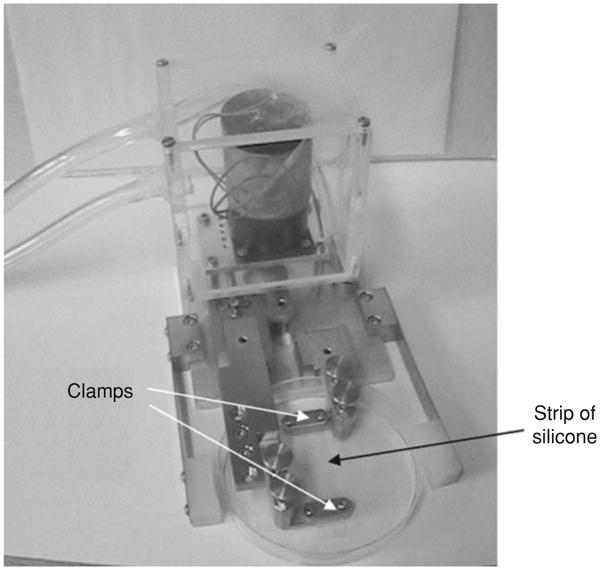
Uniaxial stretching device. A strip of silicone is held between the two clamps within the dish. Reprinted from reference (176) with kind permission from Springer Science+Business Media.
Out-of-plane biaxial bending of an elastic diaphragm
Cells cultured on top of a flexible-bottomed well can be exposed to biaxial strain by applying a pressure difference across the plate. Banes et al. (14) first subjected cultured cells to cyclic tension and compression by applying vacuum under the bottom of a circular plastic Petri dish. The bottom of a plastic dish 60 mm in diameter deflected downward up to 1.5 mm, yielding 0.13% compression to cells on the inner surface. The system was refined by Winston et al. (290) using a thinner flexible membrane to improve strain homogeneity. These authors achieved biaxial strains up to 10% by inflating a 100-μm-thick polyurethane urea circular sheet clamped at the edges with positive pressure applied below the dish. An important drawback of clamped circular flexible membranes operated by pressure is strain heterogeneity, exhibiting equibiaxial strain only at the center of the well. Radial strain increases slightly with the radial distance to the center of the diaphragm whereas circumferencial strain drops parabolically to zero at the clamped edge of the diaphragm (288). These differences are decreased by the use of thinner membranes, but the peripheral regions of the membrane experience heterogeneous strain. An additional limitation is that the vertical displacement of the membrane hampers microscopic observation of the cell culture. Inflation of an elastic diaphragm has also been used to probe rheology of cell layers. Selby and Shannon (223) subjected a human epidermal keratinocyte (NHEK) sheet attached to a thin (10 μm) PDMS circular membrane to cyclic inflation-deflation tests (Fig. 14). Because the compliance of the thin PDMS membrane was greater than that of the attached cell layer, cell rheology was inferred from the relationship between applied transdiaphragmatic pressure and displaced volume.
Figure 14.

Cell stretcher based on the inflation of a clamped elastic diaphragm. Human epidermal keratinocytes (NHEKs) plated on a thin polydimethylsiloxane (PDMS) membrane are subjected to biaxial strain when subject to transdiaphragm pressure. Reprinted from reference (223), Copyright (2007), with permission, from IOS Press.
In-plane biaxial stretching of an elastic membrane
Strain homogeneity can be improved by pushing the central region of a clamped diaphragm with an indenter. Hung and Williams (126) devised a manually operated device to stretch the central portion of a 94-μm-thick circular polyurethane elastomeric membrane with a ring indenter. The same year, Schaffer et al. (220) developed a motor-driven device to cyclically indent 76-μm-thick silicone elastomer membranes with a cylindrical platen. In these devices, the central area of the membrane atop the indenter experiences isotropic and equibiaxial strain. Moreover, the in-plane deformation of the substrate facilitates microscopic observation of the cell culture.
Different manual and motor-driven devices have been developed to improve strain homogeneity, strain ratio, frequency range, microscopy imaging, and to stretch several membranes simultaneously (9, 121, 205, 230, 234). It should be noted that cells located at the peripheral annular region outside the top of the indenter are exposed to uncontrolled strain. Also, the contact between the membrane and the indenter can result in heat generation due to friction if cyclic deformation is used. An alternative to the displacement of the indenter relative to the membrane is the application of negative pressure underneath the annular outer region of the membrane (Fig. 15) (255). The downward deformation of the annular portion of the membrane results in isotropic and biaxial strain of its central region atop the loading post (23, 162, 271). The commercially available Flexcell Strain Unit uses this approach to deform thin membranes biaxially across fixed cylinders (loading posts) placed beneath the membranes. A predominantly uniaxial strain can be obtained with a camped circular diaphragm by changing the shape of the indenter (162, 271). A different approach to applying biaxial cell strain is to stretch the four sides of a cruciform silicone rubber membrane (Fig. 16) (277). This technique can also apply anisotropic strains by imposing different extensions to the two axes of the cruciform membrane.
Figure 15.

In-plane biaxial cell membrane stretcher coupled to MTC. A flexible-bottomed well is positioned on a sample holder based on a hollow cylindrical loading post, concentric with the objective of the microscope. The application of a negative pressure underneath the annular outer region of the sample results in a homogeneous and equibiaxial strain of the central area. Two pairs of coaxial coils are coupled to the stretching device to perform MTC. The pair transverse to the sample plane was used to magnetize the beads with a short and strong magnetic pulse. The pair coaxial to the optical axis applied oscillatory twisting fields. Adapted (with permission) from reference (255).
Figure 16.
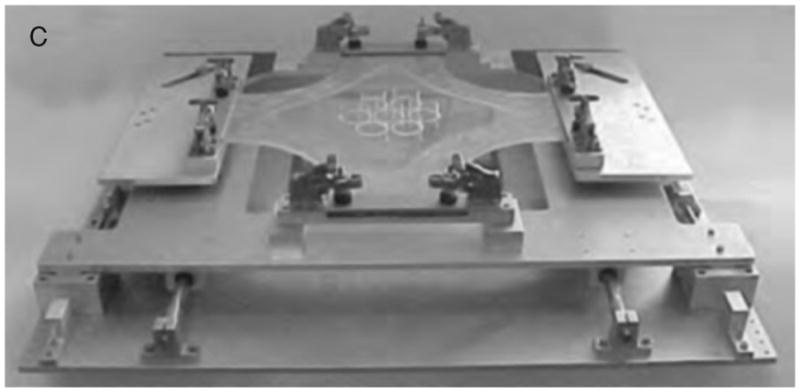
Device to produce homogenous strains in a cruciform silicone membrane. Adapted (with permission) from reference (277).
Micromechanical systems
Microfabrication techniques have been recently employed to produce Micro Electro Mechanical System (MEMS) for the application of controlled strains to single cells or small populations of cells. Arrays of microstretchers can be implemented in a single platform to perform high-throughput screening for cell-stretch response. Moraes et al. (175) microfabricated a 9 × 12 array of pressure-activated cylindrical microindenters pushing on 15-μm-thick PDMS clamped diaphragms ∼1 mm in diameter. The array was capable of simultaneously imposing cyclic isotropic and equibiaxial substrate strains ranging from 2% to 15% to small populations of cells. Scuor et al. (222) devised a MEMS composed of a large array of inter-digitated microactuactors able to provide a few micrometers biaxial displacement at the single cell level. Micropaterning techniques allow precise cell alignment relative to local strain direction. Microfabrication of microgrooves on PDMS membranes enables orientation of cells to uniaxial or biaxial strains of the substrate (241, 272). Integration of microstretchers with complementary microtechniques permits the design of lab-on-a-chip systems. A biomimetic microsystem has been recently developed to reconstitute the alvelor-capillary interface of the human lung (125) (Fig. 17). The system consisted of epithelial and endothelial cell sheets attached to the opposite sides of a thin (10 μm) porous PDMS rectangular membrane laterally attached to two closely apposed (∼500 μm) microchanels. Application of vacuum to the microchanels produces unixal strain (up to 15%) of the membrane across the channel length.
Figure 17.

Biologically inspired design of a human breathing lung-on-a-chip microdevice. Application of vacuum to the side chambers causes mechanical stretching of membrane forming the alveolar-capillary barrier. Adapted (with permission) from reference (125). Reprinted (with permission) from AAAS.
Responses to Mechanical Stress in Cultured Epithelial Cells
Due in part to the increased recognition of the importance of tissue deformation in regulating lung function and in causing injury, and also due to limitations of controlling variables in patients or animals, many studies have examined the response of lung epithelial cells to mechanical stretch in culture. Although these studies cannot define the magnitude of mechanical stretch that occurs in vivo, it is informative to examine these studies with an emphasis on the comparison of responses to different levels of deformation. Some of these studies were designed to identify the response of cells to a “physiologic” level of stretch (compared with unstretched controls), while others were designed to identify injurious levels of stretch. Most of this work has been carried out in alveolar epithelial cells whereas fewer studies are available on airway epithelial cells. Figure 18 and Table 1 show a comparison of selected studies of the response of alveolar epithelial cells to varying levels of mechanical stretch. To facilitate the comparison, we converted changes in surface area (ΔSA) reported in some studies to linear strain (ε), and the frequency of stretch was reported in cycles/min. We discuss below specifically some of the studies that have helped improve our understanding of surfactant release, inflammatory responses, remodeling and fibrosis, and cell death and injury. In addition we describe some of the studies that have examined cell mechanical properties in lung epithelial cells.
Figure 18.

Map of selected in vitro experiments utilizing alveolar epithelial cells, immortalized or primary. To distinguish the type of cells utilized in these studies, that is, AEII, A549, and AEI-like, color coding is utilized as shown in the map. The AEI-like cells are primary AEII cells that are cultured to four or more days. The reference numbers are given in the boxes and are listed in Table 1.
Table 1.
Selected Responses to Cyclic Stretch in Cultured Epithelial Cells (See Also Figure 22).
| Reference No. | Authors (year) | Description | Frequency (cycles/min) |
|---|---|---|---|
| (8) | Arold et al. (2009) | Phospatidyl choline (PC) secretion and cell death | 3 |
| (28) | Budinger et al. (2008) | Activation of 5′ AMP-activated protein kinase (AMPK) | 30 |
| (38) | Chapman et al. (2005) | Increased reactive oxygen species (ROS) production | 30 |
| (44) | Cohen et al. (2010) | Mitogen-activated protein kinase (MAPK) activation and tight junction protein expression | 15 |
| (56) | Desai et al. (2008) | Inhibition of migration | 10-30 |
| (63) | dos Santos et al. (2004) | Changes in gene expression | 30 |
| (72) | Edwards et al. (1998) | Induction of apoptosis and PC secretion | 3 |
| (79) | Felder et al. (2007) | Increased cytokeratin 8-ser431 phosphorylation and plasma membrane injury | N/A |
| (84) | Fisher and Margulies (2002) | Increased Na+-K+-ATPase activity | 15 |
| (93) | Frick et al. (2004) | Increased Ca2+ release and lactate dehydrogenase (LDH) threshold | N/A |
| (98) | Geiger et al. (2009) | Increased tubulin acetylation, cytoskeletal reorganization, and apoptosis | 15 |
| (99) | Geiger et al. (2006) | Cytoskeletal reorganization, decreased polymerized tubulin | 30 |
| (105) | Hammerschmidt et al. (2007) | Increased apoptosis | 20 |
| (107) | Hammerschmidt et al. (2005) | Increased inflammatory response | 40 |
| (127) | Jafari et al. (2004) | Decreased glutathione, increased IL-8 and IL-6 (glutathione dependent) | 20 |
| (129) | Jones et al. (2005) | MAPK activation transduced by laminin-6 | 30 |
| (145) | Lam and Dean (2008) | Increased gene traficking | 30 |
| (165) | McAdams et al. (2006) | Increased proliferation (normoxia) and decreased cell death (hyperoxia) | 30 |
| (183) | Ning and Wang (2007) | Increased IL-8 production | N/A |
| (193) | Patel et al. (2005) | Increased PC release in ATII but not ATI-like cells; increased ATP release in ATI-like cells. | N/A |
| (194) | Patel and Kwon (2009) | Increased nitric oxide production | 12 |
| (203) | Pugin et al. (2008) | Increased acidification and bacterial growth | 20 |
| (210) | Ren et al. (2009) | Increased cell death, F-actin reorganization, morphological remodeling | N/A |
| (259) | Tschumperlin and Margulies (1998) | Increased cell death | 15 |
| (261) | Tschumperlin et al. (2000) | Increased cell injury | 15-60 |
| (267) | Vlahakis et al. (1999) | Increased IL-8 release | 20 |
| (278) | Waters et al. (1999) | Increased Na+-K+-ATPase activity | 30 |
| (291) | Wirtz and Dobbs (1990) | Increased surfactant release | N/A |
| (293) | Wu et al. (2009) | Increased pentraxin 3 release | 18 |
| (300) | Yerrapureddy et al. (2010) | Changes in gene expression | 15 |
Surfactant release
While it had previously been recognized that a deep inspiration stimulated surfactant release in vivo (77,182, 294), Wirtz and Dobbs first showed that either a single stretch or a ten-cycle stretch (ε = 8.2%) of cultures of primary rat AEII cells caused an increase in both intracellular Ca2+ and surfactant secretion (as measured by phosphatidylcholine release) (291). Patel et al. later showed increased surfactant release with a 15 minute static stretch (ε = 11.8%), and they also demonstrated that coculture with AEI cells enhanced the response (193).
Other studies demonstrated stimulation of surfactant secretion by primary cultures of rat AEII exposed to relatively low-frequency (3 cycles/min) cyclic mechanical stretch (8, 72). Edwards et al. showed that 22% linear strain for 30 min increased phospatidyl choline (PC) release 1 to 4 h later (72). In contrast, Arold et al. reported that surfactant release was stimulated under some conditions and inhibited by others (8). A low level of stretch (ε = 6%) stimulated surfactant release after 30 min while moderate to high levels (11.8% and 22.5%) caused a decrease in surfactant release. Sanchez-Esteban et al. used a low level of stretch (ε = 5%) with a higher frequency (50 cycles/min) to simulate fetal lung movements and found that increases in surfactant protein synthesis by fetal lung epithelial cells were dependent upon gestational stage at which the cells were isolated from the rabbits (216). While it is clear that there is a relationship between mechanical stretch and surfactant release, these studies do not point toward a clear threshold that serves as a trigger for surfactant release. However, Patel et al. did demonstrate that ATP release occurred at a lower threshold in AEI cells (ε = 10%) compared with AEII cells (ε = 14%) and this secretion stimulated the ATII surfactant release (193).
Inflammatory response
Because of the strong inflammatory response induced in patients during mechanical ventilation and in animals in response to high tidal volume ventilation, many studies have investigated whether mechanical stretch of alveolar epithelial cells stimulated inflammatory responses. One of the potential initiating events in an inflammatory response is the generation of reactive oxygen species (ROS), and Chapman et al. (38) showed that superoxide production was significantly increased in primary rat AEII, in A549 cells, and in 16HBE14o– cells following 2 h of cyclic (30 cycles/min) stretch at 20% linear strain. Papaiahgari et al. (189) also showed an increase in ROS production in a murine AEII cell line (C10) after 6 h of either 5% or 18% linear strain, and they further demonstrated that cyclic stretch stimulated the transcription factor NrF2 through the antioxidant response element and increased expression of antioxidant enzymes. Stretch of A549 cells for 4 h (ε = 15%, 20 cycles/min) caused an initial decrease of intracellular gluthathione (GSH) followed by NF-κB and AP-1 activation and increased IL-8 and IL-6 production (127). In contrast, Vlahakis et al. showed that IL-8 was increased by exposure to linear strain of 30% and not 20% over longer periods (12, 24, and 48 h) (267). Hammerschmidt et al. suggested that long-term (24 h) cyclic stretch- (40 cycles/min) induced anti-inflammatory responses at low levels (ε = 6.3%), but this shifted to proinflammatory responses at higher levels (ε = 14%) (107).
Other studies have demonstrated stretch-induced increases in the proinflammatory peptide pentraxin 3 (293) in A549 cells, increases in inflammatory cytokines IL-1β, IL-6, and MIP-2 in fetal lung epithelial cells (47), increased IL-8 release from A549 cells (183, 267), and increases in proin- flammatory cytokine released from primary rat AEII cells (107). Ning and Wang showed an increase of IL-8 production with ε = 5% after 4 h of cyclic stretch (12 cycles/min) and also showed that the frequency of the applied stretch did not affect the IL-8 protein production (183). While these studies show that mechanical stretch induces proinflammatory responses, there is no clear indication of the level of stretch required. In fact, a recent report suggested that expression of a biomarker of asthma and chronic inflammation, the chitinase-like protein YKL-40, was increased in human bronchial epithelial cells subjected to compressive stress (191).
Remodeling and fibrotic response
As suggested from in vivo studies described above, changes in mechanical stresses may also alter pathways involving tissue remodeling and fibrosis, but the response to stress appears to vary depending upon the duration and the pathways involved. Patel and Kwon showed that low-to-moderate levels of stretch (ε = 5%-10%) increased the concentration of nitrites and matrix metalloproteinase 2 (MMP-2) in media supernatants only when A549 cells were stretched for 24 h and then incubated another 48 hours (193). However, the same study showed that MMP-2 secretion was inversely related to stretch (ε = 15% and 20%) without a poststretch incubation period. This is an interesting result, because it raises the question of whether the presence of mechanical stretch eliminated the need for cells to remodel, while the lack of stretch increased the need to remodel and to adjust to the static environment. A significant downregulation of another remodeling factor, MMP-7 gene, with ε = 11.8% for 6 h (15 cycles/min) was observed, in contrast to no effect with ε = 5.8% (300). This provides more evidence for a critical strain level between 5.8% and 11.8% for differential responses. On the other hand, Tschumperlin and collaborators have identified several profibrotic responses in bronchial epithelial cells exposed to a compressive stress. When human bronchial epithelial cells were cultured at an air-liquid interface and exposed to a 30 cmH2O transcellular pressure, the expression and release of transforming growth factor-β and endothelin were increased, and conditioned media from the cells-stimulated fibrotic protein synthesis (262). Subsequent studies demonstrated that the compressive stress caused shrinkage of the interstitial space between the cells and epidermal growth factor receptor activation (137, 257), induction of the plasminogen activator system (42), and increased goblet cell number and expression of mucin proteins (192).
Cell injury and death
As discussed above, high tidal volume mechanical ventilation has been shown to cause injury to lungs, but there is limited mechanistic information about how the injury results from overdistention (64, 201, 211). Moreover, it is not fully understood whether the localized increase in deformation causes injury due to cell plasma membrane bleb formation, basement membrane rupture, capillary failure (284), or formation of gaps between cells. However, many have hypothesized that plasma membrane failure is a key component of the lung injury due to overdistention. Vlahakis and Hubmayr (265) summarized the four potential configurations in which the plasma membrane responds to deformation (see Fig. 19):
Unfolding of the plasma membrane that is excessive,
Stretching of the plasma membrane lipids,
Lipid trafficking to plasma membrane internally from cellular stores,
Rupture or failure of the plasma membrane.
Figure 19.

Illustration indicating four ways in which the plasma membrane can respond to stretch. Adapted (with permission) from reference (265).
In support of lipid exocytosis as a potential response, mechanical deformation of human alveolar epithelial cells (A549, derived from a lung adenocarcinoma) was shown to cause increased lipid transport to the plasma membrane (268), and this response was inhibited by cholesterol depletion and by manipulation of the cytoskeleton (269). Fisher et al. also suggested that lipid trafficking was enhanced by tonic deformation of rat AEII cells, and that this may be more prominent than unfolding of the plasma membrane (83). However, deformation-induced plasma membrane failure has also been shown to occur, and this has been shown to be dependent upon both the strain amplitude and the strain rate in A549 cells (269). Recently, Oeckler et al. showed that increased media osmolarity prevented bleb formation in alveolar epithelial cells (A549 and rat AEII), and this inhibited cell injury due to a mechanical stimulus caused by a bubble traveling over the epithelial layer (186). The injury was thought to arise from the inability of the plasma membrane to withstand the deformations when there is a laxity in the connection between the cortical cytoskeleton and plasma membrane; the plasma membrane forms a bleb in front of the bubble and ruptures with excessive stress. A review by Oeckler and Hubmayr summarizes cellular stress failure and plasma membrane repair mechanisms (185).
Other groups have investigated stretch-induced damage, necrosis, and apoptosis in cultured cells. Using fluorescent dye incorporation as markers of intact membranes versus damaged membranes, there is clear evidence that stretch as low as ε = 5.8% for 1 h caused an increased percentage of dead AEII cells when compared to static controls (8, 259). However, a major increase in the percentage of dead cells was evident when cells were stretched at 11.8% linear strain. When primary cells were cultured to day 5, at which time the cells have more AEI-like characteristics, the ratio of dead-to-live cells decreased compared with AEII cells at the same level of stretch (259). For AEI-like cells, the number of dead cells increased significantly at a stretch level falling between 17% and 22.5%, whereas for AEII cells this shift in cell death occurred for stretch levels falling between 5.8% and 11.8%. Initiation of apoptosis has also been demonstrated in primary AEII cells in response to 14% or 22% linear strain, but these studies did not examine differential responses with stretch level. It is possible that this increased resistance to injury resulting from deformation may be because AEI-like cells are inherently stronger than the AEII cells or because primary cells are initially weak in culture and have a lower resistance to mechanical stretch (72, 105).
It is interesting to note that studies using A549 cells suggest a higher tolerance for mechanical stretch compared with primary cells, as stretch between 10% and 16% for 24 h failed to induce changes in cell viability or apoptosis (99, 165). However, Stroetz et al. found that the occurrence of reversible plasma membrane breaks were dependent on the level of stretch in A549 cells (234). Using the binding of fluorescently tagged ouabain as an indication of loss of barrier integrity, Cavanaugh et al. (37) showed AEII barrier loss with 1 h of stretch at ε = 17% (15 cycles/min), but not with 5.8% or 11.8%. A later study by the same group found that the permeability of small tracer molecules was significantly increased by ε = 17% at both 15 and 60 cycles/min or by 11.8% at 60 cycles/min, but was unaffected by 11.8% at 15 cycles/min or 5.8% at either frequency (43). Nevertheless, it is interesting to note that the majority of studies involving primary AEII cells involve shorter duration and lower levels of stretch. This may be related to the high level of cell death induced by stretch or loss of cell attachment. In our own studies, we have found that maintaining adherence of primary AEII cells depends upon the underlying matrix, the magnitude of stretch, and the frequency of stretch (unpublished results). Based on studies to this point, we can suggest that mechanically stretched cells do not respond differently from static controls if the stretch level is below ε = 6%. Some cellular responses are stimulated by stretch in the 6% to 10% range, and some are not. While some level of injury or cell death seems to occur even at low levels of stretch, stretch levels above ε = 17% are significantly injurious to primary cultures.
As mentioned above, atelectrauma is one of the mechanisms in which the distal airways can be injured in ARDS patients during mechanical ventilation. Although it has been difficult to develop in vivo models to better understand this mechanism, technological advances allowed research groups to develop in vitro devices to study microfluidics of small airways (24, 124, 229). Using both computational and experimental resources, several groups have improved our understanding of how the repetitive opening and closing of an airway, modeled by the progression of an air bubble through a parallel plate chamber with epithelial cells cultured on one side, is injurious to epithelial cells. The injury arises due to the large magnitude (and gradients) of stresses, shear, and normal, generated by the progression of the bubble and due to the changes in surface tension at air-fluid interfaces (see Fig. 20). The extent of cell injury following bubble progression was shown to depend upon the magnitude of the pressure gradient when a cell line of fetal rat lung epithelial cells, CCL149, was used (24), and a later study showed that injury was not dependent upon the duration of exposure to the bubble in A549 cells (133). Yalcin et al. showed that a reduction in the height of the channel, simulating the diameter of the airway, caused an increase in the pressure gradient for a given bubble velocity and resulted in increased levels of CCL149 cell injury and death (298). The same group showed that an increase in the deformability of A549 cells, controlled by the polymerization level of actin filaments, decreased cell injury (297). A recent review by Ghadiali and Gaver (2008) summarizes this line of work in detail (101).
Figure 20.
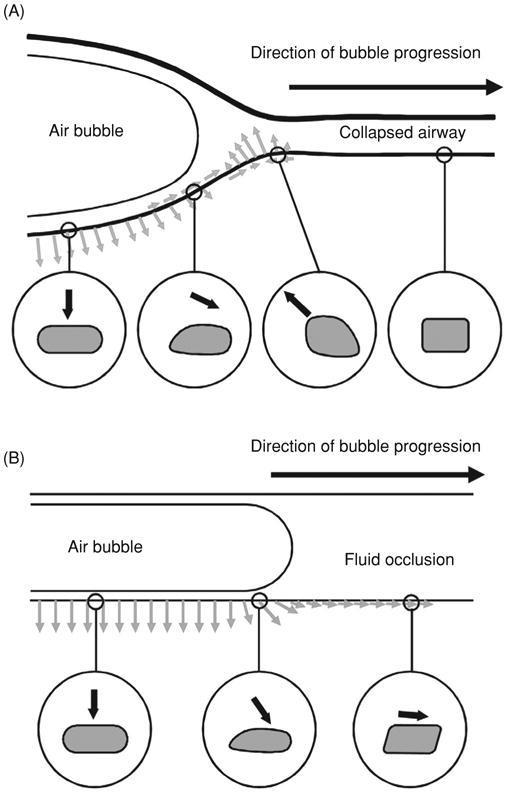
Progression of an air bubble within the collapsed small airways results in large normal and shear stresses that deform the cells significantly and result in plasma membrane injury. Adapted (with permission) from reference (24).
In addition to bubble propagation, recent work on “liquid plug” formation and bursts has provided additional insights to the microfluidic aspects of the distal airways. Using small airway epithelial cells (SAEC), Huh et al. showed that greater than 10 plug propagations resulted in cell necrosis and that rupture of the plugs was associated with the potentially relevant crackle sound (124). In a recent acellular study, Tavana et al. showed that both liquid plug generation and propagation were affected by surfactant (244). These approaches will be useful for generating a more physiological environment to aid in our understanding of how changes in local mechanics impacts cell injury.
Cell mechanical behavior
Cell rheological behavior is mainly governed by the viscoelastic properties of the cytoskeleton and by the level of prestress imposed by the contractile active tension. Lung epithelial cell viscoelasticity and contraction are substantially modulated by mechanical stress. As most cell types (76), alveolar and airway epithelial cells display a free-scale viscoelastic behavior over a broad frequency range with the elastic modulus increasing with frequency as a weak power law with exponent ∼0.2 (4). Lung epithelial cells are very soft with G′ ∼0.5 kPa at breathing frequencies and a tangent ratio ∼1/3, which is indicative of a predominantly solid-like behavior (4). Conforming to structural damping models, an increase in the tangent ratio is associated with an increase in the power-law exponent.
Cell stretch increases stiffness of lung epithelial cells. Trepat et al. (255,256) found a ∼60% increase in the storage modulus and a more solid-like behavior in A549 AEII cells subjected to ∼20% biaxial strain (Fig. 21). This stretch-induced stiffening response was inhibited by disruption of the actin cytoeskeleton (255). Enhancement of actomyosin contraction increased cell stiffness but attenuated the stretch- induced stiffening response, suggesting partial disruption of cytoskeletal structures (256). To analyze the contribution of actomyosin contraction to changes in cell stiffening induced by stretch, Gavara et al. (97) measured cell contraction during stretch with traction microscopy. Cell contraction increased with imposition of strain but dropped below baseline after stretch release (2 min duration), recovering unstretched levels in a few minutes. Inhibition of actomyosin machinery resulted in a larger relative increase in cell contraction during stretch and in a lower decrease after stretch application. The fall in cell contraction after a stretch release is consistent with sudden softening and fluidization observed in several cell types after a short transient stretch (4 s duration) (253). Taken together, these data suggest that the strain-hardening response of the CSK is modulated by a fall in prestress caused by partial disruption of the actomyosin apparatus. Therefore, the actual change in cell stiffness induced by stretch depends on the nonlinear viscoelastic behavior of cells and on the degree of actomyosin activation.
Figure 21.
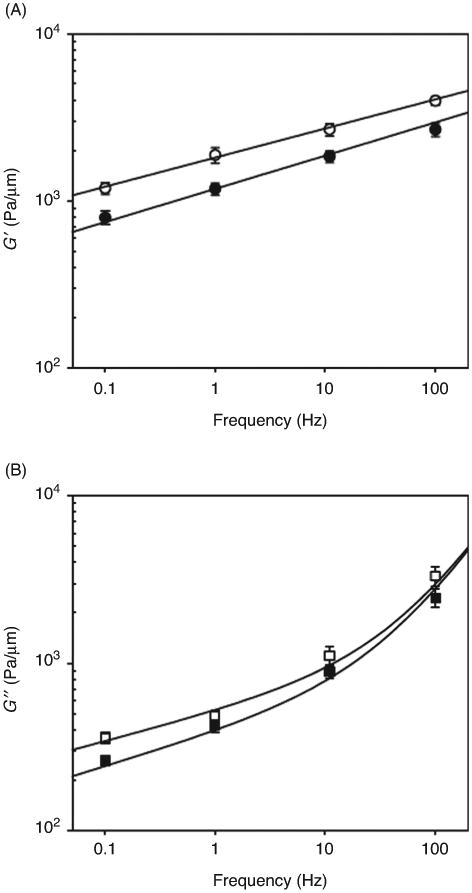
Frequency dependence of G′ (A) and G″(B) under baseline conditions (filled symbols) and during application of a single stretch of 14.1% (open symbols). Data are plotted as means ± SEM. Adapted (with permission) from reference (255).
There is some evidence of CSK remodeling of lung epithelial cells in response to stretch. DiPaolo et al. (60) exposed (for up to 1 h) primary rat AEI-like cells to biaxial cyclic (15 cycles/min) strain at different amplitudes (6%, 12%, and 17%) or to 12% tonic strain. They found formation of a perijunctional actin ring after 10 min of exposure to high levels of cyclic strain (12% and 17%), but not at 6% cyclic strain or at 12% tonic strain. Silbert et al. (226) observed CSK remodeling with actin alignment parallel to the long axis of the cell in fetal AEII cells after 6 h of exposure to 5% cyclic (60 cycles/min) strain. Further data are needed to better define the relationship between changes in cell rheology and cytoskeleton reorganization in pulmonary epithelial cells.
The increase in CSK tension exerted at the cell-cell and cell-matrix attachments during distension could exceed the strength of the adhesive forces, leading to disruption of the epithelial monolayer. Trepat et al. (256) exposed A549 AEII cells to 20% strain and found incomplete transmission from the deformable substrate to overlying cells, which is indicative of partial cell detachment. Cavanaugh et al. (36) subjected primary rat AEII cells to biaxial cyclic (15 cycles/min) strain (6%-17%) for 1 h and measured transepithelial permeability to uncharged tracers. Only the largest strain (17%) produced a significant increase in transepithelial permeability, suggesting a threshold for epithelial barrier disruption. Consistent with in vitro data, these authors found an increase in alveolar permeability at high inflation volumes in whole lung experiments (36).
How do Mechanical Stresses Affect Epithelial Repair Processes?
As described in previous sections, lung diseases such as asthma, pulmonary fibrosis, emphysema, and acute lung injury (and VILI) can result in dramatic changes in mechanical stresses on lung tissue, and many of these diseases involve injury or dysregulated repair of the epithelium. We described in the previous section how changes in mechanical forces can cause injury and stimulate apoptosis and cell death in lung epithelial cells. However, despite the importance of the maintenance and restoration of epithelial barrier, the role of physical forces in regulating repair processes is still not well understood. Epithelial injury can occur both in distal and in proximal regions of the lung, and in addition to the acute inflammatory response, the initial epithelial repair processes involve spreading and migration of nearby progenitor cells to cover the damaged surface [reviewed in (50, 202, 233)]. Circulating progenitor cells may also be recruited to the injury site, and over time the progenitor cells will proliferate and differentiate to restore the integrity and function of the epithelial barrier. Because of technical limitations it is difficult to directly examine the early processes of spreading and migration of lung epithelial cells in vivo. Several groups have used the tracheal injury model to show that spreading and migration of airway epithelial cells occurs within 12 to 24 h of injury, and that proliferation and restoration of a pseudostratified epithelium occurs over a much longer period of time (71, 73-75, 119, 136). Insights into early repair processes involving alveolar epithelial cells have been limited primarily to in vitro studies [reviewed in (50)], and these have demonstrated a similar progression of spreading, migration, and later proliferation (135).
Epithelial restitution
It is important to recognize that epithelial repair in the lungs is somewhat unique in that few other tissues undergo such large changes in mechanical stresses and deformation. To determine how changes in the mechanical environment might alter epithelial repair, we must first examine some of the key mechanisms. Epithelial repair mechanisms inherently involve biomechanical processes, for example, remodeling of the cytoskeleton, lamellipodial and filipodial extensions, formation and loss of adhesion sites, cell contraction and relaxation, and cell locomotion. The remodeling that occurs in migrating cells involves the establishment of directional polarity, and focal adhesions, Rho GTPases, integrins, and other signaling molecules are thought to be key in this process (132, 138,148, 214). As the cell spreads and migrates, ECM components provide binding sites for integrin molecules and provide traction for the cell to pull itself forward. Force is created for cell movement by contraction of actomyosin complexes, and the actin cytoskeleton is mechanically coupled to focal adhesion complexes, integrins, and other signaling complexes. Actin bundles have previously been observed in cells at the leading wound edge of both bronchial and alveolar type II cells (55, 56), and these structures have been proposed as a site of force distribution for migrating cells (81).
Mechanotransduction in repair
How then are these processes altered by mechanical forces? Using both cell lines and primary cultures of human bronchial epithelial cells grown on elastic substrates, cell-stretching devices (as described above) were used to apply either cyclic stretch or cyclic compression to scratch-wounded cell monolayers. Both cell spreading and cell migration were inhibited, and this was shown to be dependent upon the duration of strain during each cycle and not upon the actual frequency of strain (219). Keratinocyte growth factor (KGF) stimulated both cell spreading and migration of mechanically stretched cells and overcame the inhibitory effects (279). On the other hand, proliferation of airway epithelial cells, as measured by BrdU incorporation, was stimulated by cyclic stretch and cyclic compression (218). Subsequent studies demonstrated that mechanical stretch decreased the phosphorylation of focal adhesion kinase (FAK), caused dissociation of c-Jun N- terminal kinase (JNK)-interacting protein 3 (JIP-3) from FAK, and decreased phosphatidylinositol 3-kinase (PI3K) activity (57,58). Efficient cell migration was restored in mechanically stretched cells when vectors were used to coexpress FAK, JIP- 3, and a constitutively active form of PI3K (58). Cyclic stretch was also shown to inhibit wound repair of cultured rat alveolar type II cells in a dose-dependent manner, with significant inhibition occurring with 10% and 15% cyclic stretch, but no inhibition with 5% stretch (56). Lamellipodial extensions were inhibited by cyclic stretch, and this was related to decreased membrane association of Tiam-1 which resulted in decreased Rac1 activity. Another study demonstrated that alveolar type II cells isolated from rats following 2 h of high tidal volume mechanical ventilation (25 ml/kg) had decreased cell adhesion, decreased phosphorylation of FAK and paxillin, and increased RhoA activity (56). It was also shown in this study that expression of a kinase-inactive form of FAK (FRNK) inhibited wound repair of cultured alveolar type II cells, while overexpression of wild-type FAK stimulated repair.
Mechanical properties of cells and repair
The studies discussed above suggest that mechanical forces can alter signaling pathways globally and cause dysregulation of repair processes. However, coordinated cell migration requires spatial variation in the activation and localization of signaling pathways including focal adhesions, integrins, Rho GTPases, and others. The relationship between these dynamic processes and the local mechanical properties of the cells is less well understood. Little is known about the role of mechanical tension in regulating these processes. Raucher and Sheetz manipulated membrane tension by changing phospholipid composition or by osmotically swelling cells, and found that lamellipodial extension rates were decreased with increased membrane tension (209). They suggested that membrane tension may regulate the actin polymerization rate during lamellipodial extension. Cells adhered to a pliable substrate exhibit less organized actin networks, weaker focal adhesions, and generate less tension compared with cells adhered to firm substrates (80). Using force applied to the cell body using a microneedle or uniaxial substrate distention, Kaverina and colleagues demonstrated increased polymerization of microtubules toward adhesion sites under elevated stress (131). Thus, mechanical tension can clearly affect processes involved in epithelial repair. However, it is difficult to determine the spatial variation of mechanical tension that might affect cell migration. One approach that has been used to map the elastic modulus of cultured bronchial epithelial cells (16HBE14o–) involves AFM in the indentation mode (as described above). This approach demonstrated substantial variations in the elastic modulus of cells migrating at the wound edge with a significant peak approximately 10 to 15 μm from the wound edge (see Fig. 22) (270). Such variations in the localization of mechanical properties may regulate the directional polarity and signaling of migrating cells. In addition, as described above, mechanical stresses can also cause injury and initiate apoptotic processes in lung epithelial cells. There are still many unanswered questions regarding how changes in the mechanical environment alter the balance between injury and repair.
Figure 22.

Elastic modulus of cells is dependent on the distance from the wound edge in 16HBE cells. (A) Representative elastic modulus map of migrating 16HBE cells at a wound edge 2 h after wounding. Arrows indicate the wound edge as cells were migrating from right to left. Grey regions indicate plastic substrate. (B) Median elastic modulus values as a function of distance from the wound edge were summarized from four different fields. The dashed line indicates the median value from control cells far away from the wound edge (2.4 kPa), and the asterisks indicate a significant difference from this value (P < 0.05). Adapted (with permission) from reference (270).
Conclusion
Mechanical forces play a major role in lung development, in normal lung function, and in pathological changes in disease. Although we have a fundamental understanding of the types of mechanical stresses that occur during normal breathing and in pathological situations, there are still many unanswered questions about the magnitude, distribution, and heterogeneity of some of these forces in vivo. Significant challenges exist for the identification, measurement, and localization of these forces both clinically and in animal models, but such studies will improve our understanding of lung function and how it changes in disease. Epithelial cells exist at a unique interface that is subjected to continuous changes in mechanical stresses. Epithelial proliferation and differentiation are affected by fetal breath movements and by changes in mechanics following birth, but the underlying mechanisms for how the cells sense these forces and the mechanotransduction mechanisms that convert the mechanical signals into biological signals are not well understood. Similarly, changes in mechanical forces following acute lung injury or during the development of pathological conditions such as asthma, emphysema, or fibrosis can lead to injury of epithelial cells or changes in their differentiation. However, the fundamental mechanisms that lead to injury or changes in cell phenotype in response to changes in mechanical forces are still unclear. Substantial progress has been made in the development of approaches to probe how cells sense and respond to mechanical stresses, and these approaches are valuable for enhancing our understanding of lung epithelial responses in vitro. Such advances will ultimately lead to better approaches for investigating these mechanisms in intact tissue or animals. While our understanding of these mechanisms has advanced significantly over the last two decades, there is still much to learn about mechanosensing and mechanoresponses in lung epithelial cells.
Acknowledgments
Supported by NIH grant HL094366 (C.M. Waters) and Spanish Ministry of Science and Innovation grant PI081908 (D. Navajas).
References
- 1.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Adkins WK, Hernandez LA, Coker PJ, Buchanan B, Parker JC. Age effects susceptibility to pulmonary barotrauma in rabbits. Crit Care Med. 1991;19:390–393. doi: 10.1097/00003246-199103000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Agostoni E, Hyatt RE. Static behaviour of the respiratory system. In: Macklem PT, Mead J, editors. Handbook of Physiology Mechanics of Breathing. Bethesda, MD: American Physiological Society; 1986. pp. 113–130. sect. 3, pt. 1. [Google Scholar]
- 4.Alcaraz J, Buscemi L, Grabulosa M, Trepat X, Fabry B, Farre R, Navajas D. Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys J. 2003;84:2071–2079. doi: 10.1016/S0006-3495(03)75014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcaraz J, Buscemi L, Puig-de-Morales M, Colchero J, Baro A, Navajas D. Correction of microrheological measurements of soft samples with atomic force microscopy for the hydrodynamic drag on the cantilever. Langmuir. 2002;18:716–721. [Google Scholar]
- 6.Alcorn D, Adamson TM, Lambert TF, Maloney JE, Ritchie BC, Robinson PM. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J Anat. 1977;123:649–660. [PMC free article] [PubMed] [Google Scholar]
- 7.An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chitano P, Deng L, Dowell M, Eidelman DH, Fabry B, Fairbank NJ, Ford LE, Fredberg JJ, Gerthoffer WT, Gilbert SH, Gosens R, Gunst SJ, Halayko AJ, Ingram RH, Irvin CG, James AL, Janssen LJ, King GG, Knight DA, Lauzon AM, Lakser OJ, Ludwig MS, Lutchen KR, Maksym GN, Martin JG, Mauad T, McParland BE, Mijailovich SM, Mitchell HW, Mitchell RW, Mitzner W, Murphy TM, Pare PD, Pellegrino R, Sanderson MJ, Schellenberg RR, Seow CY, Silveira PS, Smith PG, Solway J, Stephens NL, Sterk PJ, Stewart AG, Tang DD, Tepper RS, Tran T, Wang L. Airway smooth muscle dynamics: A common pathway of airway obstruction in asthma. Eur Respir J. 2007;29:834–860. doi: 10.1183/09031936.00112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arold SP, Bartolák-Suki E, Suki B. Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Am J Physiol Lung Cell Mol Physiol. 2009;296:L574–L581. doi: 10.1152/ajplung.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arold SP, Wong JY, Suki B. Design of a new stretching apparatus and the effects of cyclic strain and substratum on mouse lung epithelial-12 cells. Ann Biomed Eng. 2007;35:1156–1164. doi: 10.1007/s10439-007-9262-5. [DOI] [PubMed] [Google Scholar]
- 10.Ashkin A, Dziedzic JM, Yamane T. Optical trapping and manipulation of single cells using infrared laser beams. Nature. 1987;330:769–771. doi: 10.1038/330769a0. [DOI] [PubMed] [Google Scholar]
- 11.Azeloglu EU, Bhattacharya J, Costa KD. Atomic force microscope elastography reveals phenotypic differences in alveolar cell stiffness. J Appl Physiol. 2008;105:652–661. doi: 10.1152/japplphysiol.00958.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachofen H, Schurch S, Urbinelli M, Weibel ER. Relations among alveolar surface tension, surface area, volume, and recoil pressure. J Appl Physiol. 1987;62:1878–1887. doi: 10.1152/jappl.1987.62.5.1878. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj P, Tang X, Saif TA, Bashir R. Stiffness of the substrate influences the phenotype of embryonic chicken cardiac myocytes. J Biomed Mater Res A. 2010;95:1261–1269. doi: 10.1002/jbm.a.32951. [DOI] [PubMed] [Google Scholar]
- 14.Banes AJ, Gilbert J, Taylor D, Monbureau O. A new vacuum-operated stress-providing instrument that applies static or variable duration cyclic tension or compression to cells in vitro. J Cell Sci. 1985;75:35–42. doi: 10.1242/jcs.75.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Barnes PJ. Medical progress: Chronic obstructive pulmonary disease. New Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 16.Bates JHT, Davis GS, Majumdar A, Butnor KJ, Suki B. Linking parenchymal disease progression to changes in lung mechanical function by percolation. Am J Respir Crit Care Med. 2007;176:617–623. doi: 10.1164/rccm.200611-1739OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bausch AR, Moller W, Sackmann E. Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys J. 1999;76:573–579. doi: 10.1016/S0006-3495(99)77225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bausch AR, Ziemann F, Boulbitch AA, Jacobson K, Sackmann E. Local measurements of viscoelastic parameters of adherent cell surfaces by magnetic bead microrheometry. Biophys J. 1998;75:2038–2049. doi: 10.1016/S0006-3495(98)77646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benachi A, Delezoide AL, Chailley-Heu B, Preece M, Bourbon JR, Ryder T. Ultrastructural evaluation of lung maturation in a sheep model of diaphragmatic hernia and tracheal occlusion. Am J Respir Cell Mol Biol. 1999;20:805–812. doi: 10.1165/ajrcmb.20.4.3359. [DOI] [PubMed] [Google Scholar]
- 20.Benoit M, Gabriel D, Gerisch G, Gaub HE. Discrete interactions in cell adhesion measured by single-molecule force spectroscopy. Nat Cell Biol. 2000;2:313–317. doi: 10.1038/35014000. [DOI] [PubMed] [Google Scholar]
- 21.Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154:1819–1828. doi: 10.1164/ajrccm.154.6.8970376. [DOI] [PubMed] [Google Scholar]
- 22.Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105:1148–1160. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- 23.Bieler FH, Ott CE, Thompson MS, Seidel R, Ahrens S, Epari DR, Wilkening U, Schaser KD, Mundlos S, Duda GN. Biaxial cell stimulation: A mechanical validation. J Biomech. 2009;42:1692–1696. doi: 10.1016/j.jbiomech.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Bilek AM, Dee KC, Gaver DP., 3rd Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol. 2003;94:770–783. doi: 10.1152/japplphysiol.00764.2002. [DOI] [PubMed] [Google Scholar]
- 25.Bilodeau GG. Regular pyramid punch problem. J Appl Mech. 1992;59:519–523. [Google Scholar]
- 26.Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986;56:930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- 27.Brody JS, Burki R, Kaplan N. Deoxyribonucleic acid synthesis in lung cells during compensatory lung growth after pneumonectomy. Am Rev Respir Dis. 1978;117:307–316. doi: 10.1164/arrd.1978.117.2.307. [DOI] [PubMed] [Google Scholar]
- 28.Budinger GR, Urich D, DeBiase PJ, Chiarella SE, Burgess ZO, Baker CM, Soberanes S, Mutlu GM, Jones JC. Stretch-induced activation of AMP kinase in the lung requires dystroglycan. Am J Respir Cell Mol Biol. 2008;39:666–672. doi: 10.1165/rcmb.2007-0432OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buhain WJ, Brody JS. Compensatory growth of the lung following pneumonectomy. J Appl Physiol. 1973;35:898–902. doi: 10.1152/jappl.1973.35.6.898. [DOI] [PubMed] [Google Scholar]
- 30.Bursac P, Lenormand G, Fabry B, Oliver M, Weitz DA, Viasnoff V, Butler JP, Fredberg JJ. Cytoskeletal remodelling and slow dynamics in the living cell. Nat Mater. 2005;4:557–561. doi: 10.1038/nmat1404. [DOI] [PubMed] [Google Scholar]
- 31.Butler JP, Tolic-Norrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol. 2002;282:C595–C605. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 32.Byfield FJ, Reen RK, Shentu TP, Levitan I, Gooch KJ. Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J Biomech. 2009;42:1114–1119. doi: 10.1016/j.jbiomech.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caille N, Tardy Y, Meister JJ. Assessment of strain field in endothelial cells subjected to uniaxial deformation of their substrate. Ann Biomed Eng. 1998;26:409–416. doi: 10.1114/1.132. [DOI] [PubMed] [Google Scholar]
- 34.Carlton DP, Cummings JJ, Scheerer RG, Poulain FR, Bland RD. Lung overexpansion increases pulmonary microvascular protein permeability in young lambs. J Appl Physiol. 1990;69:577–583. doi: 10.1152/jappl.1990.69.2.577. [DOI] [PubMed] [Google Scholar]
- 35.Carney DE, Bredenberg CE, Schiller HJ, Picone AL, McCann UG, Gatto LA, Bailey G, Fillinger M, Nieman GF. The mechanism of lung volume change during mechanical ventilation. Am J Respir Crit Care Med. 1999;160:1697–1702. [PubMed] [Google Scholar]
- 36.Cavanaugh KJ, Cohen TS, Margulies SS. Stretch increases alveolar epithelial permeability to uncharged micromolecules. Am J Physiol Cell Physiol. 2006;290:C1179–C1188. doi: 10.1152/ajpcell.00355.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavanaugh KJ, Jr, Oswari J, Margulies SS. Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2001;25:584–591. doi: 10.1165/ajrcmb.25.5.4486. [DOI] [PubMed] [Google Scholar]
- 38.Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L834–L841. doi: 10.1152/ajplung.00069.2005. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhuri O, Parekh SH, Fletcher DA. Reversible stress softening of actin networks. Nature. 2007;445:295–298. doi: 10.1038/nature05459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudhuri O, Parekh SH, Lam WA, Fletcher DA. Combined atomic force microscopy and side-view optical imaging for mechanical studies of cells. Nat Meth. 2009;6:383–387. doi: 10.1038/nmeth.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chetta A, Foresi A, Del Donno M, Consigli GF, Bertorelli G, Pesci A, Barbee RA, Olivieri D. Bronchial responsiveness to distilled water and methacholine and its relationship to inflammation and remodeling of the airways in asthma. Am J Respir Crit Care Med. 1996;153:910–917. doi: 10.1164/ajrccm.153.3.8630572. [DOI] [PubMed] [Google Scholar]
- 42.Chu EK, Cheng J, Foley JS, Mecham BH, Owen CA, Haley KJ, Mariani TJ, Kohane IS, Tschumperlin DJ, Drazen JM. Induction of the plasminogen activator system by mechanical stimulation of human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2006;35:628–638. doi: 10.1165/rcmb.2006-0040OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen TS, Cavanaugh KJ, Margulies SS. Frequency and peak stretch magnitude affect alveolar epithelial permeability. Eur Respir J. 2008;32:854–861. doi: 10.1183/09031936.00141007. [DOI] [PubMed] [Google Scholar]
- 44.Cohen TS, Gray Lawrence G, Khasgiwala A, Margulies SS. MAPK activation modulates permeability of isolated rat alveolar epithelial cell monolayers following cyclic stretch. PLoS One. 2010;5:e10385. doi: 10.1371/journal.pone.0010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohn R. Factors affecting the postnatal growth of the lung. Anat Rec. 1938;75:195–205. [Google Scholar]
- 46.Copland IB, Kavanagh BP, Engelberts D, McKerlie C, Belik J, Post M. Early changes in lung gene expression due to high tidal volume. Am J Respir Crit Care Med. 2003;168:1051–1059. doi: 10.1164/rccm.200208-964OC. [DOI] [PubMed] [Google Scholar]
- 47.Copland IB, Post M. Stretch-activated signaling pathways responsible for early response gene expression in fetal lung epithelial cells. J Cell Physiol. 2007;210:133–143. doi: 10.1002/jcp.20840. [DOI] [PubMed] [Google Scholar]
- 48.Crick FHC, Hughes AFW. The physical properties of cytoplasm: A study by means of the magnetic particle method. 1. Experimental. Exp Cell Res. 1950;1:37–80. [Google Scholar]
- 49.Crocker JC, Valentine MT, Weeks ER, Gisler T, Kaplan PD, Yodh AG, Weitz DA. Two-point microrheology of inhomogeneous soft materials. Phys Rev Lett. 2000;85:888–891. doi: 10.1103/PhysRevLett.85.888. [DOI] [PubMed] [Google Scholar]
- 50.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danto SI, Shannon JM, Borok Z, Zabski SM, Crandall ED. Reversible transdifferentiation of alveolar epithelial cells. Am J Respir Cell Mol Biol. 1995;12:497–502. doi: 10.1165/ajrcmb.12.5.7742013. [DOI] [PubMed] [Google Scholar]
- 52.Davies DE. The role of the epithelium in airway remodeling in asthma. Proc Am Thorac Soc. 2009;6:678–682. doi: 10.1513/pats.200907-067DP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dembo M, Oliver T, Ishihara A, Jacobson K. Imaging the traction stresses exerted by locomoting cells with the elastic substratum method. Biophys J. 1996;70:2008–2022. doi: 10.1016/S0006-3495(96)79767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desai LP, Aryal AM, Ceacareanu B, Hassid A, Waters CM. RhoA and Rac1 are both required for efficient wound closure of airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1134–L1144. doi: 10.1152/ajplung.00022.2004. [DOI] [PubMed] [Google Scholar]
- 56.Desai LP, Chapman KE, Waters CM. Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1. Am J Physiol Lung Cell Mol Physiol. 2008;295:L958–L965. doi: 10.1152/ajplung.90218.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desai LP, White SR, Waters CM. Mechanical stretch decreases FAK phosphorylation and reduces cell migration through loss of JIP3- induced JNK phosphorylation in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L520–L529. doi: 10.1152/ajplung.00076.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Desai LP, White SR, Waters CM. Cyclic mechanical stretch decreases cell migration by inhibiting phosphatidylinositol 3-kinase- and focal adhesion kinase-mediated JNK1 activation. J Biol Chem. 2010;285:4511–4519. doi: 10.1074/jbc.M109.084335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Desprat N, Richert A, Simeon J, Asnacios A. Creep function of a single living cell. Biophys J. 2005;88:2224–2233. doi: 10.1529/biophysj.104.050278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DiPaolo BC, Lenormand G, Fredberg JJ, Margulies SS. Stretch magnitude and frequency-dependent actin cytoskeleton remodeling in alveolar epithelia. Am J Physiol Cell Physiol. 2010;299:C345–C353. doi: 10.1152/ajpcell.00379.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dolhnikoff M, Mauad T, Ludwig MS. Extracellular matrix and oscillatory mechanics of rat lung parenchyma in bleomycin-induced fibrosis. Am J Respir Crit Care Med. 1999;160:1750–1757. doi: 10.1164/ajrccm.160.5.9812040. [DOI] [PubMed] [Google Scholar]
- 62.Dong C, Skalak R, Sung KLP, Schmidschonbein GW, Chien S. Passive deformation analysis of human-leukocytes. J Biomech Eng. 1988;110:27–36. doi: 10.1115/1.3108402. [DOI] [PubMed] [Google Scholar]
- 63.dos Santos CC, Han B, Andrade CF, Bai X, Uhlig S, Hubmayr R, Tsang M, Lodyga M, Keshavjee S, Slutsky AS, Liu M. DNA microarray analysis of gene expression in alveolar epithelial cells in response to TNFalpha, LPS, and cyclic stretch. Physiol Genomics. 2004;19:331–342. doi: 10.1152/physiolgenomics.00153.2004. [DOI] [PubMed] [Google Scholar]
- 64.dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator- induced lung injury: A perspective. J Appl Physiol. 2000;89:1645–1655. doi: 10.1152/jappl.2000.89.4.1645. [DOI] [PubMed] [Google Scholar]
- 65.dos Santos CC, Slutsky AS. The contribution of biophysical lung injury to the development of biotrauma. Annu Rev Physiol. 2006;68:585–618. doi: 10.1146/annurev.physiol.68.072304.113443. [DOI] [PubMed] [Google Scholar]
- 66.Dreyfuss D, Saumon G. Barotrauma is volutrauma, but which volume is the one responsible? Intensive Care Med. 1992;18:139–141. doi: 10.1007/BF01709236. [DOI] [PubMed] [Google Scholar]
- 67.Dreyfuss D, Saumon G. Ventilator-induced lung injury: Lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 68.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 69.du Roure O, Saez A, Buguin A, Austin RH, Chavrier P, Siberzan P, Ladoux B. Force mapping in epithelial cell migration. Proc Natl Acad Sci U S A. 2005;102:2390–2395. doi: 10.1073/pnas.0408482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dudek SM, Garcia JGN. Cytoskeletan regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 71.Dupuit F, Gaillard D, Hinnrasky J, Mongodin E, de Bentzmann S, Copreni E, Puchelle E. Differentiated and functional human airway epithelium regeneration in tracheal xenografts. Am J Physiol Lung Cell Mol Physiol. 2000;278:L165–L176. doi: 10.1152/ajplung.2000.278.1.L165. [DOI] [PubMed] [Google Scholar]
- 72.Edwards YS, Sutherland LM, Power JH, Nicholas TE, Murray AW. Cyclic stretch induces both apoptosis and secretion in rat alveolar type II cells. FEBS Lett. 1999;448:127–130. doi: 10.1016/s0014-5793(99)00357-9. [DOI] [PubMed] [Google Scholar]
- 73.Erjefalt JS, Erjefalt I, Sundler F, Persson CG. Microcirculation-derived factors in airway epithelial repair in vivo. Microvasc Res. 1994;48:161–178. doi: 10.1006/mvre.1994.1047. [DOI] [PubMed] [Google Scholar]
- 74.Erjefalt JS, Erjefalt I, Sundler F, Persson CG. Effects of topical budes- onide on epithelial restitution in vivo in guinea pig trachea. Thorax. 1995a;50:785–792. doi: 10.1136/thx.50.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erjefalt JS, Erjefalt I, Sundler F, Persson CG. In vivo restitution of airway epithelium. Cell Tissue Res. 1995b;281:305–316. doi: 10.1007/BF00583399. [DOI] [PubMed] [Google Scholar]
- 76.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. Scaling the microrheology of living cells. Phys Rev Lett. 2001;87:148102. doi: 10.1103/PhysRevLett.87.148102. [DOI] [PubMed] [Google Scholar]
- 77.Faridy EE, Permutt S, Riley RL. Effect of ventilation on surface forces in excised dogs' lungs. J Appl Physiol. 1966;21:1453–1462. doi: 10.1152/jappl.1966.21.5.1453. [DOI] [PubMed] [Google Scholar]
- 78.Fehrenbach H, Voswinckel R, Michl V, Mehling T, Fehrenbach A, Seeger W, Nyengaard JR. Neoalveolarisation contributes to compensatory lung growth following pneumonectomy in mice. Eur Respir J. 2008;31:515–522. doi: 10.1183/09031936.00109407. [DOI] [PubMed] [Google Scholar]
- 79.Felder E, Siebenbrunner M, Busch T, Fois G, Miklavc P, Walther P, Dietl P. Mechanical strain of alveolar type II cells in culture: Changes in the transcellular cytokeratin network and adaptations. Am J Physiol Lung Cell Mol Physiol. 2008;295:L849–L857. doi: 10.1152/ajplung.00503.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Felsenfeld DP, Schwartzberg PL, Venegas A, Tse R, Sheetz MP. Selective regulation of integrin–cytoskeleton interactions by the tyrosine kinase Src. Nat Cell Biol. 1999;1:200–206. doi: 10.1038/12021. [DOI] [PubMed] [Google Scholar]
- 81.Fenteany G, Janmey PA, Stossel TP. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr Biol. 2000;10:831–838. doi: 10.1016/s0960-9822(00)00579-0. [DOI] [PubMed] [Google Scholar]
- 82.Fernandez P, Pullarkat PA, Ott A. A master relation defines the nonlinear viscoelasticity of single fibroblasts. Biophys J. 2006;90:3796–3805. doi: 10.1529/biophysj.105.072215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fisher JL, Levitan I, Margulies SS. Plasma membrane surface increases with tonic stretch of alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31:200–208. doi: 10.1165/rcmb.2003-0224OC. [DOI] [PubMed] [Google Scholar]
- 84.Fisher JL, Margulies SS. Na(+)-K(+)-ATPase activity in alveolar epithelial cells increases with cyclic stretch. Am J Physiol Lung Cell Mol Physiol. 2002;283:L737–L746. doi: 10.1152/ajplung.00030.2001. [DOI] [PubMed] [Google Scholar]
- 85.Flecknoe S, Harding R, Maritz G, Hooper SB. Increased lung expansion alters the proportions of type I and type II alveolar epithelial cells in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1180–L1185. doi: 10.1152/ajplung.2000.278.6.L1180. [DOI] [PubMed] [Google Scholar]
- 86.Flecknoe SJ, Wallace MJ, Cock ML, Harding R, Hooper SB. Changes in alveolar epithelial cell proportions during fetal and postnatal development in sheep. Am J Physiol Lung Cell Mol Physiol. 2003;285:L664–L670. doi: 10.1152/ajplung.00306.2002. [DOI] [PubMed] [Google Scholar]
- 87.Flecknoe SJ, Wallace MJ, Harding R, Hooper SB. Determination of alveolar epithelial cell phenotypes in fetal sheep: Evidence for the involvement of basal lung expansion. J Physiol. 2002;542:245–253. doi: 10.1113/jphysiol.2001.014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frank JA, Gutierrez JA, Jones KD, Allen L, Dobbs L, Matthay MA. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med. 2002;165:242–249. doi: 10.1164/ajrccm.165.2.2108087. [DOI] [PubMed] [Google Scholar]
- 89.Frank JA, Matthay MA. Science review: Mechanisms of ventilator- induced injury. Crit Care. 2003;7:233–241. doi: 10.1186/cc1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fredberg JJ. Airway smooth muscle in asthma: Flirting with disaster. Eur Respir J. 1998;12:1252–1256. doi: 10.1183/09031936.98.12061252. [DOI] [PubMed] [Google Scholar]
- 91.Fredberg JJ, Inouye D, Miller B, Nathan M, Jafari S, Raboudi SH, Butler JP, Shore SA. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am J Respir Crit Care Med. 1997;156:1752–1759. doi: 10.1164/ajrccm.156.6.9611016. [DOI] [PubMed] [Google Scholar]
- 92.Fredberg JJ, Kamm RD. Stress Transmission in the lung: Pathways from organ to molecule. Annu Rev Physiol. 2006;68:507–541. doi: 10.1146/annurev.physiol.68.072304.114110. [DOI] [PubMed] [Google Scholar]
- 93.Frick M, Bertocchi C, Jennings P, Haller T, Mair N, Singer W, Pfaller W, Ritsch-Marte M, Dietl P. Ca2+ entry is essential for cell strain- induced lamellar body fusion in isolated rat type II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2004;286:L210–L220. doi: 10.1152/ajplung.00332.2003. [DOI] [PubMed] [Google Scholar]
- 94.Fung YC. Biomechanics Mechanical Properties of Living Tissues. 2nd. New York, NY: Springer-Verlag; 1993. [Google Scholar]
- 95.Gajic O, Lee J, Doerr CH, Berrios JC, Myers JL, Hubmayr RD. Ventilator-induced cell wounding and repair in the intact lung. Am J Respir Crit Care Med. 2003;167:1057–1063. doi: 10.1164/rccm.200208-889OC. [DOI] [PubMed] [Google Scholar]
- 96.Gattinoni L, Caironi P, Carlesso E. How to ventilate patients with acute lung injury and acute respiratory distress syndrome. Curr Opin Crit Care. 2005;11:69–76. doi: 10.1097/00075198-200502000-00011. [DOI] [PubMed] [Google Scholar]
- 97.Gavara N, Roca-Cusachs P, Sunyer R, Farre R, Navajas D. Mapping cell-matrix stresses during stretch reveals inelastic reorganization of the cytoskeleton. Biophys J. 2008;95:464–471. doi: 10.1529/biophysj.107.124180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Geiger RC, Kaufman CD, Lam AP, Budinger GR, Dean DA. Tubulin acetylation and histone deacetylase 6 activity in the lung under cyclic load. Am J Respir Cell Mol Biol. 2009;40:76–82. doi: 10.1165/rcmb.2007-0307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Geiger RC, Taylor W, Glucksberg MR, Dean DA. Cyclic stretch- induced reorganization of the cytoskeleton and its role in enhanced gene transfer. Gene Ther. 2006;13:725–731. doi: 10.1038/sj.gt.3302693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gerstmair A, Fois G, Innerbichler S, Dietl P, Felder E. A device for simultaneous live cell imaging during uni-axial mechanical strain or compression. J Appl Physiol. 2009;107:613–620. doi: 10.1152/japplphysiol.00012.2009. [DOI] [PubMed] [Google Scholar]
- 101.Ghadiali SN, Gaver DP. Biomechanics of liquid-epithelium interactions in pulmonary airways. Respir Physiol Neurobiol. 2008;163:232–243. doi: 10.1016/j.resp.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gil J, Bachofen H, Gehr P, Weibel ER. Alveolar volume-surface area relation in air- and saline-filled lungs fixed by vascular perfusion. J Appl Physiol. 1979;47:990–1001. doi: 10.1152/jappl.1979.47.5.990. [DOI] [PubMed] [Google Scholar]
- 103.Gil J, Weibel ER. Morphological study of pressure-volume hysteresis in rat lungs fixed by vascular perfusion. Respir Physiol. 1972;15:190–213. doi: 10.1016/0034-5687(72)90098-9. [DOI] [PubMed] [Google Scholar]
- 104.Gunst SJ, Tang DD. The contractile apparatus and mechanical properties of airway smooth muscle. Eur Respir J. 2000;15:600–616. doi: 10.1034/j.1399-3003.2000.15.29.x. [DOI] [PubMed] [Google Scholar]
- 105.Hammerschmidt S, Kuhn H, Gessner C, Seyfarth HJ, Wirtz H. Stretch- induced alveolar type II cell apoptosis: role of endogenous bradykinin and PI3K-Akt signaling. Am J Respir Cell Mol Biol. 2007;37:699–705. doi: 10.1165/rcmb.2006-0429OC. [DOI] [PubMed] [Google Scholar]
- 106.Hammerschmidt S, Kuhn H, Grasenack T, Gessner C, Wirtz H. Apoptosis and necrosis induced by cyclic mechanical stretching in alveolar type II cells. Am J Respir Cell Mol Biol. 2004;30:396–402. doi: 10.1165/rcmb.2003-0136OC. [DOI] [PubMed] [Google Scholar]
- 107.Hammerschmidt S, Kuhn H, Sack U, Schlenska A, Gessner C, Gillissen A, Wirtz H. Mechanical stretch alters alveolar type II cell mediator release toward a proinflammatory pattern. Am J Respir Cell Mol Biol. 2005;33:203–210. doi: 10.1165/rcmb.2005-0067OC. [DOI] [PubMed] [Google Scholar]
- 108.Han B, Lodyga M, Liu M. Ventilator-induced lung injury: Role of protein-protein interaction in mechanosensation. Proc Am Thorac Soc. 2005;2:181–187. doi: 10.1513/pats.200501-008AC. [DOI] [PubMed] [Google Scholar]
- 109.Harding R, Hooper SB. Regulation of lung expansion and lung growth before birth. J Appl Physiol. 1996;81:209–224. doi: 10.1152/jappl.1996.81.1.209. [DOI] [PubMed] [Google Scholar]
- 110.Henon S, Lenormand G, Richert A, Gallet F. A new determination of the shear modulus of the human erythrocyte membrane using optical tweezers. Biophys J. 1999;76:1145–1151. doi: 10.1016/S0006-3495(99)77279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hernandez LA, Peevy KJ, Moise AA, Parker JC. Chest wall restriction limits high airway pressure-induced lung injury in young rabbits. J Appl Physiol. 1989;66:2364–2368. doi: 10.1152/jappl.1989.66.5.2364. [DOI] [PubMed] [Google Scholar]
- 112.Hochmuth RM. Micropipette aspiration of living cells. J Biomech. 2000;33:15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- 113.Hoffman AM, Shifren A, Mazan MR, Gruntman AM, Lascola KM, Nolen-Walston RD, Kim CF, Tsai L, Pierce RA, Mecham RP, Ingenito EP. Matrix modulation of compensatory lung regrowth and progenitor cell proliferation in mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L158–L168. doi: 10.1152/ajplung.90594.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hoffman BD, Massiera G, Van Citters KM, Crocker JC. The consensus mechanics of cultured mammalian cells. Proc Natl Acad Sci U S A. 2006;103:10259–10264. doi: 10.1073/pnas.0510348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoh JH, Schoenenberger CA. Surface-morphology and mechanical- properties of Mdck monolayers by atomic-force microscopy. J Cell Sci. 1994;107:1105–1114. doi: 10.1242/jcs.107.5.1105. [DOI] [PubMed] [Google Scholar]
- 116.Holgate ST, Lackie P, Wilson S, Roche W, Davies D. Bronchial epithelium as a key regulator of airway allergen sensitization and remodeling in asthma. Am J Respir Crit Care Med. 2000;162:S113–S117. doi: 10.1164/ajrccm.162.supplement_2.ras-12. [DOI] [PubMed] [Google Scholar]
- 117.Hooper SB, Han VK, Harding R. Changes in lung expansion alter pulmonary DNA synthesis and IGF-II gene expression in fetal sheep. Am J Physiol. 1993;265:L403–L409. doi: 10.1152/ajplung.1993.265.4.L403. [DOI] [PubMed] [Google Scholar]
- 118.Hooper SB, Wallace MJ. Role of the physicochemical environment in lung development. Clin Exp Pharmacol Physiol. 2006;33:273–279. doi: 10.1111/j.1440-1681.2006.04358.x. [DOI] [PubMed] [Google Scholar]
- 119.Horiba K, Fukuda Y. Synchronous appearance of fibronectin, integrin alpha 5 beta 1, vinculin and actin in epithelial cells and fibroblasts during rat tracheal wound healing. Virchows Archiv. 1994;425:425–434. doi: 10.1007/BF00189581. [DOI] [PubMed] [Google Scholar]
- 120.Hsia CC. Signals and mechanisms of compensatory lung growth. J Appl Physiol. 2004;97:1992–1998. doi: 10.1152/japplphysiol.00530.2004. [DOI] [PubMed] [Google Scholar]
- 121.Huang L, Mathieu PS, Helmke BP. A stretching device for high- resolution live-cell imaging. Ann Biomed Eng. 2010;38:1728–1740. doi: 10.1007/s10439-010-9968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hubmayr RD. Perspective on lung injury and recruitment: A skeptical look at the opening and collapse story. Am J Respir Crit Care Med. 2002;165:1647–1653. doi: 10.1164/rccm.2001080-01CP. [DOI] [PubMed] [Google Scholar]
- 123.Hughes JM, Hoppin FG, Jr, Mead J. Effect of lung inflation on bronchial length and diameter in excised lungs. J Appl Physiology. 1972;32:25–35. doi: 10.1152/jappl.1972.32.1.25. [DOI] [PubMed] [Google Scholar]
- 124.Huh D, Fujioka H, Tung YC, Futai N, Paine R, 3rd, Grotberg JB, Takayama S. Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc Natl Acad Sci U S A. 2007;104:18886–18891. doi: 10.1073/pnas.0610868104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hung CT, Williams JL. A method for inducing equi-biaxial and uniform strains in elastomeric membranes used as cell substrates. J Biomech. 1994;27:227–232. doi: 10.1016/0021-9290(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 127.Jafari B, Ouyang B, Li LF, Hales CA, Quinn DA. Intracellular glutathione in stretch-induced cytokine release from alveolar type-2 like cells. Respirology. 2004;9:43–53. doi: 10.1111/j.1440-1843.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 128.Jonas M, Huang H, Kamm RD, So PTC. Fast fluorescence laser tracking microrheometry, I: Instrument development. Biophys J. 2008;94:1459–1469. doi: 10.1529/biophysj.106.098111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jones JC, Lane K, Hopkinson SB, Lecuona E, Geiger RC, Dean DA, Correa-Meyer E, Gonzales M, Campbell K, Sznajder JI, Budinger S. Laminin-6 assembles into multimolecular fibrillar complexes with perlecan and participates in mechanical-signal transduction via a dystroglycan-dependent, integrin-independent mechanism. J Cell Sci. 2005;118:2557–2566. doi: 10.1242/jcs.02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kamgoue A, Ohayon J, Tracqui P. Estimation of cell Young's modulus of adherent cells probed by optical and magnetic tweezers: Influence of cell thickness and bead immersion. J Biomech Eng. 2007;129:523–530. doi: 10.1115/1.2746374. [DOI] [PubMed] [Google Scholar]
- 131.Kaverina I, Krylyshkina O, Beningo K, Anderson K, Wang YL, Small JV. Tensile stress stimulates microtubule outgrowth in living cells. J Cell Sci. 2002;115:2283–2291. doi: 10.1242/jcs.115.11.2283. [DOI] [PubMed] [Google Scholar]
- 132.Kaverina I, Krylyshkina O, Small JV. Regulation of substrate adhesion dynamics during cell motility. Int J Biochem Cell Biol. 2002;34:746–761. doi: 10.1016/s1357-2725(01)00171-6. [DOI] [PubMed] [Google Scholar]
- 133.Kay SS, Bilek AM, Dee KC, Gaver DP., 3rd Pressure gradient, not exposure duration, determines the extent of epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol. 2004;97:269–276. doi: 10.1152/japplphysiol.01288.2003. [DOI] [PubMed] [Google Scholar]
- 134.Khatiwala CB, Peyton SR, Putnam AJ. Intrinsic mechanical properties of the extracellular matrix affect the behavior of pre-osteoblastic MC3T3-E1 cells. Am J Physiol Cell Physiol. 2006;290:C1640–C1650. doi: 10.1152/ajpcell.00455.2005. [DOI] [PubMed] [Google Scholar]
- 135.Kheradmand F, Folkesson HG, Shum L, Derynk R, Pytela R, Matthay MA. Transforming growth factor-alpha enhances alveolar epithelial cell repair in a new in vitro model. Am J Physiol. 1994;267:L728–L738. doi: 10.1152/ajplung.1994.267.6.L728. [DOI] [PubMed] [Google Scholar]
- 136.Kim JS, McKinnis VS, Adams K, White SR. Proliferation and repair of guinea pig tracheal epithelium after neuropeptide depletion and injury in vivo. Am J Physiol. 1997;273:L1235–L1241. doi: 10.1152/ajplung.1997.273.6.L1235. [DOI] [PubMed] [Google Scholar]
- 137.Kojic N, Chung E, Kho AT, Park JA, Huang A, So PT, Tschumperlin DJ. An EGFR autocrine loop encodes a slow-reacting but dominant mode of mechanotransduction in a polarized epithelium. FASEB J. 2010;24:1604–1615. doi: 10.1096/fj.09-145367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kole TP, Tseng Y, Jiang I, Katz JL, Wirtz D. Intracellular mechanics of migrating fibroblasts. Mol Biol Cell. 2005;16:328–338. doi: 10.1091/mbc.E04-06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kollmannsberger P, Fabry B. High-force magnetic tweezers with force feedback for biological applications. Rev Sci Instrum. 2007;78:114301. doi: 10.1063/1.2804771. [DOI] [PubMed] [Google Scholar]
- 140.Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, Eickelberg O. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One. 2008;3:e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Konigshoff M, Eickelberg O. WNT signaling in lung disease: a failure or a regeneration signal? Am J Respir Cell Mol Biol. 2010;42:21–31. doi: 10.1165/rcmb.2008-0485TR. [DOI] [PubMed] [Google Scholar]
- 142.Kotecha S. Lung growth for beginners. Paediatr Respir Rev. 2000;1:308–313. doi: 10.1053/prrv.2000.0069. [DOI] [PubMed] [Google Scholar]
- 143.Krishnan R, Park CY, Lin YC, Mead J, Jaspers RT, Trepat X, Lenormand G, Tambe D, Smolensky AV, Knoll AH, Butler JP, Fredberg JJ. Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. Plos One. 2009;4:e5486. doi: 10.1371/journal.pone.0005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Laifook SJ. Lung parenchyma described as a prestressed compressible material. J Biomech. 1977;10:357–365. doi: 10.1016/0021-9290(77)90008-2. [DOI] [PubMed] [Google Scholar]
- 145.Lam AP, Dean DA. Cyclic stretch-induced nuclear localization of transcription factors results in increased nuclear targeting of plasmids in alveolar epithelial cells. J Gene Med. 2008;10:668–678. doi: 10.1002/jgm.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Langston C, Sachdeva P, Cowan MJ, Haines J, Crystal RG, Thurlbeck WM. Alveolar multiplication in the contralateral lung after unilateral pneumonectomy in the rabbit. Am Rev Respir Dis. 1977;115:7–13. doi: 10.1164/arrd.1977.115.1.7. [DOI] [PubMed] [Google Scholar]
- 147.Lau A, Hoffman B, Davies A, Crocker J, Lubensky T. Microrheology, stress fluctuations, and active behavior of living cells. Phys Rev Lett. 2003;91:198101. doi: 10.1103/PhysRevLett.91.198101. [DOI] [PubMed] [Google Scholar]
- 148.Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 149.Laurent GJ, McAnulty RJ, Hill M, Chambers R. Escape from the matrix: Multiple mechanisms for fibroblast activation in pulmonary fibrosis. Proc Am Thorac Soc. 2008;5:311–315. doi: 10.1513/pats.200710-159DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Laurent VM, Henon S, Planus E, Fodil R, Balland M, Isabey D, Gallet F. Assessment of mechanical properties of adherent living cells by bead micromanipulation: Comparison of magnetic twisting cytometry vs optical tweezers. J Biomech Eng. 2002;124:408–421. doi: 10.1115/1.1485285. [DOI] [PubMed] [Google Scholar]
- 151.Leong WS, Tay CY, Yu H, Li A, Wu SC, Duc DH, Lim CT, Tan LP. Thickness sensing of hMSCs on collagen gel directs stem cell fate. Biochem Biophys Res Commun. 2010;401:287–292. doi: 10.1016/j.bbrc.2010.09.052. [DOI] [PubMed] [Google Scholar]
- 152.Li QS, Lee GYH, Ong CN, Lim CT. AFM indentation study of breast cancer cells. Biochem Biophys Res Commun. 2008;374:609–613. doi: 10.1016/j.bbrc.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 153.Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246–1256. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 154.Lionetti V, Recchia FA, Ranieri VM. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care. 2005;11:82–86. doi: 10.1097/00075198-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 155.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Macklem PT, Macklem DM, Detroyer A. A model of inspiratory muscle mechanics. J Appl Physiol. 1983;55:547–557. doi: 10.1152/jappl.1983.55.2.547. [DOI] [PubMed] [Google Scholar]
- 157.Mahaffy RE, Park S, Gerde E, Kas J, Shih CK. Quantitative analysis of the viscoelastic properties of thin regions of fibroblasts using atomic force microscopy. Biophys J. 2004;86:1777–1793. doi: 10.1016/S0006-3495(04)74245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maksym GN, Bates JHT. A distributed nonlinear model of lung tissue elasticity. J Appl Physiol. 1997;82:32–41. doi: 10.1152/jappl.1997.82.1.32. [DOI] [PubMed] [Google Scholar]
- 159.Maksym GN, Fabry B, Butler JP, Navajas D, Tschumperlin DJ, Laporte JD, Fredberg JJ. Mechanical properties of cultured human airway smooth muscle cells from 0.05 to 0.4 Hz. J Appl Physiol. 2000;89:1619–1632. doi: 10.1152/jappl.2000.89.4.1619. [DOI] [PubMed] [Google Scholar]
- 160.Maskarinec SA, Franck C, Tirrell DA, Ravichandran G. Quantifying cellular traction forces in three dimensions. P Natl Acad Sci U S A. 2009;106:22108–22113. doi: 10.1073/pnas.0904565106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mason TG, Weitz DA. Optical measurements of frequency-dependent linear viscoelastic moduli of complex fluids. Phys Rev Lett. 1995;74:1250–1253. doi: 10.1103/PhysRevLett.74.1250. [DOI] [PubMed] [Google Scholar]
- 162.Matheson LA, Jack Fairbank N, Maksym GN, Paul Santerre J, Labow RS. Characterization of the Flexcell(TM) Uniflex(TM) cyclic strain culture system with U937 macrophage-like cells. Biomaterials. 2006;27:226–233. doi: 10.1016/j.biomaterials.2005.05.070. [DOI] [PubMed] [Google Scholar]
- 163.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: Four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Matthews BD, Overby DR, Alenghat FJ, Karavitis J, Numaguchi Y, Allen PG, Ingber DE. Mechanical properties of individual focal adhesions probed with a magnetic microneedle. Biochem Biophys Res Commun. 2004;313:758–764. doi: 10.1016/j.bbrc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 165.McAdams RM, Mustafa SB, Shenberger JS, Dixon PS, Henson BM, DiGeronimo RJ. Cyclic stretch attenuates effects of hyperoxia on cell proliferation and viability in human alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L166–L174. doi: 10.1152/ajplung.00160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McAnulty RJ, Laurent GJ. Collagen-synthesis and degradation in vivo. Evidence for rapid rates of collagen turnover with extensive degradation of newly synthesized collagen in tissues of the adult-rat. Coll Relat Res. 1987;7:93–104. doi: 10.1016/s0174-173x(87)80001-8. [DOI] [PubMed] [Google Scholar]
- 167.McCann UG, 2nd, Schiller HJ, Carney DE, Gatto LA, Steinberg JM, Nieman GF. Visual validation of the mechanical stabilizing effects of positive end-expiratory pressure at the alveolar level. J Surg Res. 2001;99:335–342. doi: 10.1006/jsre.2001.6179. [DOI] [PubMed] [Google Scholar]
- 168.Mead J, Takishim T, Leith D. Stress distribution in lungs: A model of pulmonary elasticity. J Appl Physiol. 1970;28:596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- 169.Mendez JL, Hubmayr RD. New insights into the pathology of acute respiratory failure. Curr Opin Crit Care. 2005;11:29–36. doi: 10.1097/00075198-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 170.Mercer RR, Crapo JD. Spatial distribution of collagen and elastin fibers in the lungs. J Appl Physiol. 1990;69:756–765. doi: 10.1152/jappl.1990.69.2.756. [DOI] [PubMed] [Google Scholar]
- 171.Meurs H, Gosens R, Zaagsma J. Airway hyperresponsiveness in asthma: Lessons from in vitro model systems and animal models. Eur Respir J. 2008;32:487–502. doi: 10.1183/09031936.00023608. [DOI] [PubMed] [Google Scholar]
- 172.Mijailovich SM, Kojic M, Zivkovic M, Fabry B, Fredberg JJ. A finite element model of cell deformation during magnetic bead twisting. J Appl Physiol. 2002;93:1429–1436. doi: 10.1152/japplphysiol.00255.2002. [DOI] [PubMed] [Google Scholar]
- 173.Mitchison TJ, Swann MM. The mechanical properties of the cell surface: I. The cell elastimeter. J Exp Biol. 1954;31:443–460. [Google Scholar]
- 174.Moessinger AC, Harding R, Adamson TM, Singh M, Kiu GT. Role of lung fluid volume in growth and maturation of the fetal sheep lung. J Clin Invest. 1990;86:1270–1277. doi: 10.1172/JCI114834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Moraes C, Chen JH, Sun Y, Simmons CA. Microfabricated arrays for high-throughput screening of cellular response to cyclic substrate deformation. Lab on a Chip. 2010;10:227–234. doi: 10.1039/b914460a. [DOI] [PubMed] [Google Scholar]
- 176.Moretti M, Prina-Mello A, Reid AJ, Barron V, Prendergast PJ. Endothelial cell alignment on cyclically-stretched silicone surfaces. J Mater Sci Mater Med. 2004;15:1159–1164. doi: 10.1023/B:JMSM.0000046400.18607.72. [DOI] [PubMed] [Google Scholar]
- 177.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149:1327–1334. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 178.Nardo L, Maritz G, Harding R, Hooper SB. Changes in lung structure and cellular division induced by tracheal obstruction in fetal sheep. Exp Lung Res. 2000;26:105–119. doi: 10.1080/019021400269907. [DOI] [PubMed] [Google Scholar]
- 179.Navajas D, Maksym GN, Bates JHT. Dynamic viscoelastic nonlinearity of lung parenchymal tissue. J Appl Physiol. 1995;79:348–356. doi: 10.1152/jappl.1995.79.1.348. [DOI] [PubMed] [Google Scholar]
- 180.Needham D, Hochmuth RM. Rapid flow of passive neutrophils into a 4 Mu-M pipette and measurement of cytoplasmic viscosity. J Biomech Eng. 1990;112:269–276. doi: 10.1115/1.2891184. [DOI] [PubMed] [Google Scholar]
- 181.Neuman KC, Chadd EH, Liou GF, Bergman K, Block SM. Characterization of photodamage to Escherichia coli in optical traps. Biophys J. 1999;77:2856–2863. doi: 10.1016/S0006-3495(99)77117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nicholas TE, Barr HA. Control of release of surfactant phospholipids in the isolated perfused rat lung. J Appl Physiol. 1981;51:90–98. doi: 10.1152/jappl.1981.51.1.90. [DOI] [PubMed] [Google Scholar]
- 183.Ning QM, Wang XR. Response of alveolar type II epithelial cells to mechanical stretch and lipopolysaccharide. Respiration. 2007;74:579–585. doi: 10.1159/000101724. [DOI] [PubMed] [Google Scholar]
- 184.Oeckler RA, Hubmayr RD. Alveolar microstrain and the dark side of the lung. Crit Care. 2007;11:177. doi: 10.1186/cc6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Oeckler RA, Hubmayr RD. Cell wounding and repair in ventilator injured lungs. Respir Physiol Neurobiol. 2008;163:44–53. doi: 10.1016/j.resp.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Oeckler RA, Lee WY, Park MG, Kofler O, Rasmussen DL, Lee HB, Belete H, Walters BJ, Stroetz RW, Hubmayr RD. Determinants of plasma membrane wounding by deforming stress. Am J Physiol Lung Cell Mol Physiol. 2010;299:L826–L833. doi: 10.1152/ajplung.00217.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Oldmixon EH, Hoppin FG., Jr Alveolar septal folding and lung inflation history. J Appl Physiol. 1991;71:2369–2379. doi: 10.1152/jappl.1991.71.6.2369. [DOI] [PubMed] [Google Scholar]
- 188.Panettieri RA, Jr, Kotlikoff MI, Gerthoffer WT, Hershenson MB, Woodruff PG, Hall IP, Banks-Schlegel S. Airway smooth muscle in bronchial tone, inflammation, and remodeling: Basic knowledge to clinical relevance. Am J Respir Crit Care Med. 2008;177:248–252. doi: 10.1164/rccm.200708-1217PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Papaiahgari S, Yerrapureddy A, Hassoun PM, Garcia JG, Birukov KG, Reddy SP. EGFR-activated signaling and actin remodeling regulate cyclic stretch-induced NRF2-ARE activation. Am J Respir Cell Mol Biol. 2007;36:304–312. doi: 10.1165/rcmb.2006-0131OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Papakonstantinou E, Karakiulakis G. The ‘sweet’ and ‘bitter’ involvement of glycosaminoglycans in lung diseases: Pharmacotherapeutic relevance. Br J Pharmacol. 2009;157:1111–1127. doi: 10.1111/j.1476-5381.2009.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Park JA, Drazen JM, Tschumperlin DJ. The chitinase-like protein YKL- 40 is secreted by airway epithelial cells at base line and in response to compressive mechanical stress. J Biol Chem. 2010;285:29817–29825. doi: 10.1074/jbc.M110.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Park JA, Tschumperlin DJ. Chronic intermittent mechanical stress increases MUC5AC protein expression. Am J Respir Cell Mol Biol. 2009;41:459–466. doi: 10.1165/rcmb.2008-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Patel AS, Reigada D, Mitchell CH, Bates SR, Margulies SS, Koval M. Paracrine stimulation of surfactant secretion by extracellular ATP in response to mechanical deformation. Am J Physiol Lung Cell Mol Physiol. 2005;289:L489–L496. doi: 10.1152/ajplung.00074.2005. [DOI] [PubMed] [Google Scholar]
- 194.Patel H, Kwon S. Interplay between cytokine-inducedand cyclic equibiaxial deformation-induced nitric oxide production and metalloproteases expression in human alveolar epithelial cells. Cell Mol Bioeng. 2009;4:615–624. doi: 10.1007/s12195-009-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pelosi P, Rocco PR. Effects of mechanical ventilation on the extracellular matrix. Intensive Care Med. 2008;34:631–639. doi: 10.1007/s00134-007-0964-9. [DOI] [PubMed] [Google Scholar]
- 196.Perlman CE, Bhattacharya J. Alveolar expansion imaged by optical sectioning microscopy. J Appl Physiol. 2007;103:1037–1044. doi: 10.1152/japplphysiol.00160.2007. [DOI] [PubMed] [Google Scholar]
- 197.Perlman CE, Lederer DJ, Bhattacharya J. The micromechanics of alveolar edema. Am J Respir Cell Mol Biol. 2010;44:34–39. doi: 10.1165/rcmb.2009-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pfister BJ, Weihs TP, Betenbaugh M, Bao G. An in vitro uniaxial stretch model for axonal injury. Ann Biomed Eng. 2003;31:589–598. doi: 10.1114/1.1566445. [DOI] [PubMed] [Google Scholar]
- 199.Pinart M, Serrano-Mollar A, Llatjos R, Rocco PR, Romero PV. Single and repeated bleomycin intratracheal instillations lead to different biomechanical changes in lung tissue. Respir Physiol Neurobiol. 2009;166:41–46. doi: 10.1016/j.resp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 200.Pingleton SK. Complications of acute respiratory failure. Am Rev Respir Dis. 1988;137:1463–1493. doi: 10.1164/ajrccm/137.6.1463. [DOI] [PubMed] [Google Scholar]
- 201.Pinhu L, Whitehead T, Evans T, Griffiths M. Ventilator-associated lung injury. Lancet. 2003;361:332–340. doi: 10.1016/S0140-6736(03)12329-X. [DOI] [PubMed] [Google Scholar]
- 202.Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:726–733. doi: 10.1513/pats.200605-126SF. [DOI] [PubMed] [Google Scholar]
- 203.Pugin J, Dunn-Siegrist I, Dufour J, Tissières P, Charles PE, Comte R. Cyclic stretch of human lung cells induces an acidification and promotes bacterial growth. Am J Respir Cell Mol Biol. 2008;38:362–370. doi: 10.1165/rcmb.2007-0114OC. [DOI] [PubMed] [Google Scholar]
- 204.Puig-de-Morales M, Grabulosa M, Alcaraz J, Mullol J, Maksym GN, Fredberg JJ, Navajas D. Measurement of cell microrheology by magnetic twisting cytometry with frequency domain demodulation. J Appl Physiol. 2001;91:1152–1159. doi: 10.1152/jappl.2001.91.3.1152. [DOI] [PubMed] [Google Scholar]
- 205.Rana OR, Zobel C, Saygili E, Brixius K, Gramley F, Schimpf T, Mischke K, Frechen D, Knackstedt C, Schwinger RHG, Schauerte P, Saygili E. A simple device to apply equibiaxial strain to cells cultured on flexible membranes. Am J Physiol Heart Circ Physiol. 2008;294:H532–H540. doi: 10.1152/ajpheart.00649.2007. [DOI] [PubMed] [Google Scholar]
- 206.Rannels DE. Role of physical forces in compensatory growth of the lung. Am J Physiol. 1989;257:L179–L189. doi: 10.1152/ajplung.1989.257.4.L179. [DOI] [PubMed] [Google Scholar]
- 207.Rannels DE, Stockstill B, Mercer RR, Crapo JD. Cellular changes in the lungs of adrenalectomized rats following left pneumonectomy. Am J Respir Cell Mol Biol. 1991;5:351–362. doi: 10.1165/ajrcmb/5.4.351. [DOI] [PubMed] [Google Scholar]
- 208.Rannels DE, White DM, Watkins CA. Rapidity of compensatory lung growth following pneumonectomy in adult rats. J Appl Physiol. 1979;46:326–333. doi: 10.1152/jappl.1979.46.2.326. [DOI] [PubMed] [Google Scholar]
- 209.Raucher D, Sheetz MP. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J Cell Biol. 2000;148:127–136. doi: 10.1083/jcb.148.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ren Y, Zhan Q, Hu Q, Sun B, Yang C, Wang C. Static stretch induces active morphological remodeling and functional impairment of alveolar epithelial cells. Respiration. 2009;78:301–311. doi: 10.1159/000207632. [DOI] [PubMed] [Google Scholar]
- 211.Ricard JD, Dreyfuss D, Saumon G. Ventilator-induced lung injury. Eur Respir J Suppl. 2003;42:2s–9s. doi: 10.1183/09031936.03.00420103. [DOI] [PubMed] [Google Scholar]
- 212.RicoF, Roca-Cusachs P, Gavara N, Farre R, Rotger M, Navajas D. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys Rev E. 2005;72:021914. doi: 10.1103/PhysRevE.72.021914. [DOI] [PubMed] [Google Scholar]
- 213.Rico F, Roca-Cusachs P, Sunyer R, Farre R, Navajas D. Cell dynamic adhesion and elastic properties probed with cylindrical atomic force microscopy cantilever tips. J Mol Recognit. 2007;20:459–466. doi: 10.1002/jmr.829. [DOI] [PubMed] [Google Scholar]
- 214.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 215.Roca-Cusachs P, Almendros I, Sunyer R, Gavara N, Farre R, Navajas D. Rheology of passive and adhesion-activated neutrophils probed by atomic force microscopy. Biophys J. 2006;91:3508–3518. doi: 10.1529/biophysj.106.088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sanchez-Esteban J, Cicchiello LA, Wang Y, Tsai SW, Williams LK, Torday JS, Rubin LP. Mechanical stretch promotes alveolar epithelial type II cell differentiation. J Appl Physiol. 2001;91:589–595. doi: 10.1152/jappl.2001.91.2.589. [DOI] [PubMed] [Google Scholar]
- 217.Sasaki N, Odajima S. Stress-strain curve and young's modulus of a collagen molecule as determined by the X-ray diffraction technique. J Biomech. 1996;29:655–658. doi: 10.1016/0021-9290(95)00110-7. [DOI] [PubMed] [Google Scholar]
- 218.Savla U, Olson LE, Waters CM. Mathematical modeling of airway epithelial wound closure during cyclic mechanical strain. J Appl Physiol. 2004;96:566–574. doi: 10.1152/japplphysiol.00510.2003. [DOI] [PubMed] [Google Scholar]
- 219.Savla U, Waters CM. Mechanical strain inhibits repair of airway epithelium in vitro. Am J Physiol. 1998;274:L883–L892. doi: 10.1152/ajplung.1998.274.6.L883. [DOI] [PubMed] [Google Scholar]
- 220.Schaffer JL, Rizen M, Litalien GJ, Benbrahim A, Megerman J, Gerstenfeld LC, Gray ML. Device for the application of a dynamic biaxially uniform and isotropic strain to a flexible cell-culture membrane. J Orth Res. 1994;12:709–719. doi: 10.1002/jor.1100120514. [DOI] [PubMed] [Google Scholar]
- 221.Schiller HJ, Steinberg J, Halter J, McCann U, DaSilva M, Gatto LA, Carney D, Nieman G. Alveolar inflation during generation of a quasistatic pressure/volume curve in the acutely injured lung. Crit Care Med. 2003;31:1126–1133. doi: 10.1097/01.CCM.0000059997.90832.29. [DOI] [PubMed] [Google Scholar]
- 222.Scuor N, Gallina P, Panchawagh HV, Mahajan RL, Sbaizero O, Sergo V. Design of a novel MEMS platform for the biaxial stimulation of living cells. Biomed Microdevices. 2006;8:239–246. doi: 10.1007/s10544-006-8268-3. [DOI] [PubMed] [Google Scholar]
- 223.Selby JC, Shannon MA. Mechanical response of a living human epidermal keratinocyte sheet as measured in a composite diaphragm inflation experiment. Biorheology. 2007;44:319–348. [PubMed] [Google Scholar]
- 224.Shannon JM, Jennings SD, Nielsen LD. Modulation of alveolar type II cell differentiated function in vitro. Am J Physiol. 1992;262:L427–L436. doi: 10.1152/ajplung.1992.262.4.L427. [DOI] [PubMed] [Google Scholar]
- 225.Shen X, Gunst SJ, Tepper RS. Effect of tidal volume and frequency on airway responsiveness in mechanically ventilated rabbits. J Appl Physiol. 1997;83:1202–1208. doi: 10.1152/jappl.1997.83.4.1202. [DOI] [PubMed] [Google Scholar]
- 226.Silbert O, Wang Y, Maciejewski BS, Lee HS, Shaw SK, Sanchez-Esteban J. Roles of RhoA and Rac1 on actin remodeling and cell alignment and differentiation in fetal type II epithelial cells exposed to cyclic mechanical stretch. Exp Lung Res. 2008;34:663–680. doi: 10.1080/01902140802339615. [DOI] [PubMed] [Google Scholar]
- 227.Silver FH, Freeman JW, Seehra GP. Collagen self-assembly and the development of tendon mechanical properties. J Biomech. 2003;36:1529–1553. doi: 10.1016/s0021-9290(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 228.Smith BA, Tolloczko B, Martin JG, Grutter P. Probing the viscoelastic behavior of cultured airway smooth muscle cells with atomic force microscopy: Stiffening induced by contractile agonist. Biophys J. 2005;88:2994–3007. doi: 10.1529/biophysj.104.046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Smith BJ, Yamaguchi E, Gaver DP., 3rd A translating stage system for micro-PIV measurements surrounding the tip of a migrating semi-infinite bubble. Meas Sci Technol. 2010;21:1–13. doi: 10.1088/0957-0233/21/1/015401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sotoudeh M, Jalali S, Usami S, Shyy JYJ, Chien S. A strain device imposing dynamic and uniform equi-biaxial strain to cultured cells. Ann Biomed Eng. 1998;26:181–189. doi: 10.1114/1.88. [DOI] [PubMed] [Google Scholar]
- 231.Stamenovic D. Micromechanical foundations of pulmonary elasticity. Physiol Rev. 1990;70:1117–1134. doi: 10.1152/physrev.1990.70.4.1117. [DOI] [PubMed] [Google Scholar]
- 232.Steinberg J, Schiller HJ, Halter JM, Gatto LA, Dasilva M, Amato M, McCann UG, Nieman GF. Tidal volume increases do not affect alveolar mechanics in normal lung but cause alveolar overdistension and exacerbate alveolar instability after surfactant deactivation. Crit Care Med. 2002;30:2675–2683. doi: 10.1097/00003246-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 233.Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc. 2008;5:328–333. doi: 10.1513/pats.200711-167DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Stroetz RW, Vlahakis NE, Walters BJ, Schroeder MA, Hubmayr RD. Validation of a new live cell strain system: characterization of plasma membrane stress failure. J Appl Physiol. 2001;90:2361–2370. doi: 10.1152/jappl.2001.90.6.2361. [DOI] [PubMed] [Google Scholar]
- 235.Suki B, Bates JHT. Extracellular matrix mechanics in lung parenchymal diseases. Respir Physiol Neurobiol. 2008;163:33–43. doi: 10.1016/j.resp.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema: Roles of proteases, inflammation, and mechanical forces. Am J Respir Crit Care Med. 2003;168:516–521. doi: 10.1164/rccm.200208-908PP. [DOI] [PubMed] [Google Scholar]
- 237.Suki B, Majumdar A, Nugent MA, Bates JH. In silico modeling of interstitial lung mechanics: Implications for disease development and repair. Drug Discov Today Dis Models. 2007;4:139–145. doi: 10.1016/j.ddmod.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sunyer R, Trepat X, Fredberg JJ, Farre R, Navajas D. The temperature dependence of cell mechanics measured by atomic force microscopy. Phys Biol. 2009;6:025009–025010. doi: 10.1088/1478-3975/6/2/025009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Swindle EJ, Collins JE, Davies DE. Breakdown in epithelial barrier function in patients with asthma: Identification of novel therapeutic approaches. J Allergy Clin Immunol. 2009;124:23–34. doi: 10.1016/j.jaci.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 240.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc Natl Acad Sci USA. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tan W, Scott D, Belchenko D, Qi HJ, Xiao L. Development and evaluation of microdevices for studying anisotropic biaxial cyclic stretch on cells. Biomed Microdevices. 2008;10:869–882. doi: 10.1007/s10544-008-9201-8. [DOI] [PubMed] [Google Scholar]
- 242.Tang X, Kuhlenschmidt TB, Zhou J, Bell P, Wang F, Kuhlenschmidt MS, Saif TA. Mechanical force affects expression of an in vitro metastasis-like phenotype in HCT-8 cells. Biophys J. 2010;99:2460–2469. doi: 10.1016/j.bpj.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Taskar V, John J, Evander E, Robertson B, Jonson B. Surfactant dysfunction makes lungs vulnerable to repetitive collapse and reexpansion. Am J Respir Crit Care Med. 1997;155:313–320. doi: 10.1164/ajrccm.155.1.9001330. [DOI] [PubMed] [Google Scholar]
- 244.Tavana H, Kuo CH, Lee QY, Mosadegh B, Huh D, Christensen PJ, Grotberg JB, Takayama S. Dynamics of liquid plugs of buffer and surfactant solutions in a micro-engineered pulmonary airway model. Langmuir. 2010;26:3744–3752. doi: 10.1021/la903038a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tepper RS, Shen X, Bakan E, Gunst SJ. Maximal airway response in mature and immature rabbits during tidal ventilation. J Appl Physiol. 1995;79:1190–1198. doi: 10.1152/jappl.1995.79.4.1190. [DOI] [PubMed] [Google Scholar]
- 246.Thet LA, Law DJ. Changes in cell number and lung morphology during early postpneumonectomy lung growth. J Appl Physiol. 1984;56:975–978. doi: 10.1152/jappl.1984.56.4.975. [DOI] [PubMed] [Google Scholar]
- 247.Thoumine O, Ott A. Time scale dependent viscoelastic and contractile regimes in fibroblasts probed by microplate manipulation. J Cell Sci. 1997;110:2109–2116. doi: 10.1242/jcs.110.17.2109. [DOI] [PubMed] [Google Scholar]
- 248.Toshima M, Ohtani Y, Ohtani O. Three-dimensional architecture of elastin and collagen fiber networks in the human and rat lung. Arch Histol Cytol. 2004;67:31–40. doi: 10.1679/aohc.67.31. [DOI] [PubMed] [Google Scholar]
- 249.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Tremblay LN, Miatto D, Hamid Q, Govindarajan A, Slutsky AS. Injurious ventilation induces widespread pulmonary epithelial expression of tumor necrosis factor-alpha and interleukin-6 messenger RNA. Crit Care Med. 2002;30:1693–1700. doi: 10.1097/00003246-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 251.Tremblay LN, Slutsky AS. Ventilator-induced injury: From barotrauma to biotrauma. Proc Assoc Am Physicians. 1998;110:482–488. [PubMed] [Google Scholar]
- 252.Tremblay LN, Slutsky AS. Ventilator-induced lung injury: From the bench to the bedside. Intensive Care Med. 2006;32:24–33. doi: 10.1007/s00134-005-2817-8. [DOI] [PubMed] [Google Scholar]
- 253.Trepat X, Deng LH, An SS, Navajas D, Tschumperlin DJ, Gerthoffer WT, Butler JP, Fredberg JJ. Universal physical responses to stretch in the living cell. Nature. 2007;447:592–595. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Trepat X, Grabulosa M, Buscemi L, Rico F, Fabry B, Fredberg JJ, Farre R. Oscillatory magnetic tweezers based on ferromagnetic beads and simple coaxial coils. Rev Sci Instrum. 2003;74:4012–4020. [Google Scholar]
- 255.Trepat X, Grabulosa M, Puig F, Maksym GN, Navajas D, Farre R. Viscoelasticity of human alveolar epithelial cells subjected to stretch. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1025–L1034. doi: 10.1152/ajplung.00077.2004. [DOI] [PubMed] [Google Scholar]
- 256.Trepat X, Puig F, Gavara N, Fredberg JJ, Farre R, Navajas D. Effect of stretch on structural integrity and micromechanics of human alveolar epithelial cell monolayers exposed to thrombin. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1104–L1110. doi: 10.1152/ajplung.00436.2005. [DOI] [PubMed] [Google Scholar]
- 257.Tschumperlin DJ, Dai G, Maly IV, Kikuchi T, Laiho LH, McVittie AK, Haley KJ, Lilly CM, So PT, Lauffenburger DA, Kamm RD, Drazen JM. Mechanotransduction through growth-factor shedding into the extracellular space. Nature. 2004;429:83–86. doi: 10.1038/nature02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tschumperlin DJ, Drazen JM. Chronic effects of mechanical force on airways. Annu Rev Physiol. 2006;68:563–583. doi: 10.1146/annurev.physiol.68.072304.113102. [DOI] [PubMed] [Google Scholar]
- 259.Tschumperlin DJ, Margulies SS. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am J Physiol. 1998;275:L1173–L1183. doi: 10.1152/ajplung.1998.275.6.L1173. [DOI] [PubMed] [Google Scholar]
- 260.Tschumperlin DJ, Margulies SS. Alveolar epithelial surface area- volume relationship in isolated rat lungs. J Appl Physiol. 1999;86:2026–2033. doi: 10.1152/jappl.1999.86.6.2026. [DOI] [PubMed] [Google Scholar]
- 261.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med. 2000;162:357–362. doi: 10.1164/ajrccm.162.2.9807003. [DOI] [PubMed] [Google Scholar]
- 262.Tschumperlin DJ, Shively JD, Kikuchi T, Drazen JM. Mechanical stress triggers selective release of fibrotic mediators from bronchial epithelium. Am J Respir Cell Mol Biol. 2003;28:142–149. doi: 10.1165/rcmb.2002-0121OC. [DOI] [PubMed] [Google Scholar]
- 263.Van Citters KM, Hoffman BD, Massiera G, Crocker JC. The role of F- actin and myosin in epithelial cell rheology. Biophys J. 2006;91:3946–3956. doi: 10.1529/biophysj.106.091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Venkatesan N, Ebihara T, Roughley PJ, Ludwig MS. Alterations in large and small proteoglycans in bleomycin-induced pulmonary fibrosis in rats. Am J Respir Crit Care Med. 2000;161:2066–2073. doi: 10.1164/ajrccm.161.6.9909098. [DOI] [PubMed] [Google Scholar]
- 265.Vlahakis NE, Hubmayr RD. Invited review: Plasma membrane stress failure in alveolar epithelial cells. J Appl Physiol. 2000;89:2490–2496. doi: 10.1152/jappl.2000.89.6.2490. discussion 2497. [DOI] [PubMed] [Google Scholar]
- 266.Vlahakis NE, Hubmayr RD. Cellular stress failure in ventilator-injured lungs. Am J Respir Crit Care Med. 2005;171:1328–1342. doi: 10.1164/rccm.200408-1036SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Vlahakis NE, Schroeder MA, Limper AH, Hubmayr RD. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol. 1999;277:L167–L173. doi: 10.1152/ajplung.1999.277.1.L167. [DOI] [PubMed] [Google Scholar]
- 268.Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD. Deformation- induced lipid trafficking in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L938–L946. doi: 10.1152/ajplung.2001.280.5.L938. [DOI] [PubMed] [Google Scholar]
- 269.Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD. Role of deformation-induced lipid trafficking in the prevention of plasma membrane stress failure. Am J Respir Crit Care Med. 2002;166:1282–1289. doi: 10.1164/rccm.200203-207OC. [DOI] [PubMed] [Google Scholar]
- 270.Wagh AA, Roan E, Chapman KE, Desai LP, Rendon DA, Eckstein EC, Waters CM. Localized elasticity measured in epithelial cells migrating at a wound edge using atomic force microscopy. Am J Physiol Lung Cell Mol Physiol. 2008;295:L54–L60. doi: 10.1152/ajplung.00475.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wall ME, Weinhold PS, Siu T, Brown TD, Banes AJ. Comparison of cellular strain with applied substrate strain in vitro. J Biomech. 2007;40:173–181. doi: 10.1016/j.jbiomech.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 272.Wang JHC, Yang GG, Li ZZ. Controlling cell responses to cyclic mechanical stretching. Ann Biomed Eng. 2005;33:337–342. doi: 10.1007/s10439-005-1736-8. [DOI] [PubMed] [Google Scholar]
- 273.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell-surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 274.Wang N, Tolic-Norrelykke IM, Chen JX, Mijailovich SM, Butler JP, Fredberg JJ, Stamenovic D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol. 2002;282:C606–C616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- 275.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 276.Warner DO, Gunst SJ. Limitation of maximal bronchoconstriction in living dogs. Am Rev Respir Dis. 1992;145:553–560. doi: 10.1164/ajrccm/145.3.553. [DOI] [PubMed] [Google Scholar]
- 277.Waters CM, Glucksberg MR, Lautenschlager EP, Lee CW, Van Matre RM, Warp RJ, Savla U, Healy KE, Moran B, Castner DG, Bearinger JP. A system to impose prescribed homogenous strains on cultured cells. J Appl Physiol. 2001;91:1600–1610. doi: 10.1152/jappl.2001.91.4.1600. [DOI] [PubMed] [Google Scholar]
- 278.Waters CM, Ridge KM, Sunio G, Venetsanou K, Sznajder JI. Mechanical stretching of alveolar epithelial cells increases Na(+)-K(+)-ATPase activity. J Appl Physiol. 1999;87:715–721. doi: 10.1152/jappl.1999.87.2.715. [DOI] [PubMed] [Google Scholar]
- 279.Waters CM, Savla U. Keratinocyte growth factor accelerates wound closure in airway epithelium during cyclic mechanical strain. J Cell Physiol. 1999;181:424–432. doi: 10.1002/(SICI)1097-4652(199912)181:3<424::AID-JCP6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 280.Waugh R, Evans EA. Thermoelasticity of red blood-cell membrane. Biophys J. 1979;26:115–131. doi: 10.1016/S0006-3495(79)85239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis. 1974;110:556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 282.Wei MT, Zaorski A, Yalcin HC, Wang J, Hallow M, Ghadiali SN, Chiou A, Ou-Yang HD. A comparative study of living cell micromechanical properties by oscillatory optical tweezers. Optics Express. 2008;16:8594–8603. doi: 10.1364/oe.16.008594. [DOI] [PubMed] [Google Scholar]
- 283.Weibel E. The Pathway for Oxygen Structure and Function in the Mammalian Respiratory System. Cambridge, UK: Harvard University Press; 1984. [Google Scholar]
- 284.West JB. Invited review: Pulmonary capillary stress failure. J Appl Physiol. 2000;89:2483–2489. doi: 10.1152/jappl.2000.89.6.2483. discussion 2497. [DOI] [PubMed] [Google Scholar]
- 285.Westergrenthorsson G, Hernnas J, Sarnstrad B, Oldberg A, Heinegard D, Malmstrom A. Altered expression of small proteoglycans, collagen, and transforming growth factor-beta 1 in developing bleomycin- induced pulmonary fibrosis in rats. J Clin Invest. 1993;92:632–637. doi: 10.1172/JCI116631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wigglesworth JS, Desai R. Effect on lung growth of cervical cord section in the rabbit fetus. Early Hum Dev. 1979;3:51–65. doi: 10.1016/0378-3782(79)90020-3. [DOI] [PubMed] [Google Scholar]
- 287.Wiggs BR, Hrousis CA, Drazen JM, Kamm RD. On the mechanism of mucosal folding in normal and asthmatic airways. J Appl Physiol. 1997;83:1814–1821. doi: 10.1152/jappl.1997.83.6.1814. [DOI] [PubMed] [Google Scholar]
- 288.Williams J, Chen J, Belloli D. Strain fields on cell stressing devices employing clamped circular elastic diaphragms as substrates. J Biomech Eng. 1992;114:377–384. doi: 10.1115/1.2891398. [DOI] [PubMed] [Google Scholar]
- 289.Wilson TA, Bachofen H. A model for mechanical structure of the alveolar duct. J Appl Physiol. 1982;52:1064–1070. doi: 10.1152/jappl.1982.52.4.1064. [DOI] [PubMed] [Google Scholar]
- 290.Winston FK, Macarak EJ, Gorfien SF, Thibault LE. A system to reproduce and quantify the biomechanical environment of the cell. J Appl Physiol. 1989;67:397–405. doi: 10.1152/jappl.1989.67.1.397. [DOI] [PubMed] [Google Scholar]
- 291.Wirtz HR, Dobbs LG. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science. 1990;250:1266–1269. doi: 10.1126/science.2173861. [DOI] [PubMed] [Google Scholar]
- 292.Wirtz HR, Dobbs LG. The effects of mechanical forces on lung functions. Respir Physiol. 2000;119:1–17. doi: 10.1016/s0034-5687(99)00092-4. [DOI] [PubMed] [Google Scholar]
- 293.Wu Q, Shu H, Yao S, Xiang H. Mechanical stretch induces pentraxin 3 release by alveolar epithelial cells in vitro. Med Sci Monit. 2009;15:BR135–B140. [PubMed] [Google Scholar]
- 294.Wyszogrodski I, Taeusch HW, Jr, Kyei-Aboagye K, Avery ME. Mechanical regulation of alveolar surfactant in adult cats: the effects of hyperventilation and end-expiratory pressure in vivo. Chest. 1975;67:15S–16S. doi: 10.1378/chest.67.2.15s. [DOI] [PubMed] [Google Scholar]
- 295.Xue Z, Zhang L, Liu Y, Gunst SJ, Tepper RS. Chronic inflation of ferret lungs with CPAP reduces airway smooth muscle contractility in vivo and in vitro. J Appl Physiol. 2008;104:610–615. doi: 10.1152/japplphysiol.00241.2007. [DOI] [PubMed] [Google Scholar]
- 296.Xue Z, Zhang L, Ramchandani R, Liu Y, Antony VB, Gunst SJ, Tepper RS. Respiratory system responsiveness in rabbits in vivo is reduced by prolonged continuous positive airway pressure. J Appl Physiol. 2005;99:677–682. doi: 10.1152/japplphysiol.00165.2005. [DOI] [PubMed] [Google Scholar]
- 297.Yalcin HC, Hallow KM, Wang J, Wei MT, Ou-Yang HD, Ghadiali SN. Influence of cytoskeletal structure and mechanics on epithelial cell injury during cyclic airway reopening. Am J Physiol Lung Cell Mol Physiol. 2009;297:L881–L891. doi: 10.1152/ajplung.90562.2008. [DOI] [PubMed] [Google Scholar]
- 298.Yalcin HC, Perry SF, Ghadiali SN. Influence of airway diameter and cell confluence on epithelial cell injury in an in vitro model of airway reopening. J Appl Physiol. 2007;103:1796–1807. doi: 10.1152/japplphysiol.00164.2007. [DOI] [PubMed] [Google Scholar]
- 299.Yamada S, Wirtz D, Kuo SC. Mechanics of living cells measured by laser tracking microrheology. Biophys J. 2000;78:1736–1747. doi: 10.1016/S0006-3495(00)76725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Yerrapureddy A, Tobias J, Margulies SS. Cyclic stretch magnitude and duration affect rat alveolar epithelial gene expression. Cell Physiol Biochem. 2010;25:113–122. doi: 10.1159/000272056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Yeung A, Evans E. Cortical shell-liquid core model for passive flow of liquid-like spherical cells into micropipets. Biophys J. 1989;56:139–149. doi: 10.1016/S0006-3495(89)82659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Yuan H, Ingenito EP, Suki B. Dynamic properties of lung parenchyma: Mechanical contributions of fiber network and interstitial cells. J Appl Physiol. 1997;83:1420–1431. doi: 10.1152/jappl.1997.83.5.1420. [DOI] [PubMed] [Google Scholar]


