Abstract
In end-stage arthritis patients, total joint replacement is a very effective surgical procedure. Nevertheless, the high revision rate after surgery remains a major concern. The wear particles generated from biomaterial-induced tissue responses may lead to chronic inflammation and local bone destruction (periprosthetic osteolysis). Several important signaling pathways are involved in wear particles induced inflammatory reactions, including the transcription factor NF-κB. We recently reported that RAW264.7 macrophage cell exposure to ultra-high molecular weight polyethylene (UHMWPE) particles significantly increased the NF-κB activity in a generated NF-κB responsive luciferase reporter cell clone. The NF-κB activity induced by UHMWPE particles in a mouse RAW264.7 macrophage cell line, bone marrow derived macrophages, and human THP1 macrophage cell line, were suppressed by double strand decoy oligodeoxynucleotide (ODN) containing an NF-κB binding element. Macrophages exposure to UHMWPE particles with or without endotoxin induced pro-inflammatory cytokine and chemokine expression including TNF-α, MCP1, MIP1α, and others. Finally, the decoy ODN significantly suppressed the induced cytokine and chemokine expression in both murine and human macrophages, consequently reducing macrophage recruitment by cellular conditioned medium exposed to wear particles. These findings suggest that local suppression of inflammatory cytokine production via inhibition of NF-κB activity with decoy ODN in total joint replacement patients could potentially be an effective strategy to alleviate wear particle-induced chronic inflammation.
Keywords: wear particles, macrophage, NF-κB decoy oligodeoxynucleotide, periprosthetic osteolysis
In end-stage arthritis patients, total joint replacement is a highly successful surgical procedure. The revision rate (i.e. the rate of needing another operation for prosthesis failure) after TJR is around 10%, and the revision procedure is more complicated with more bone loss, and a poorer prognosis than the first surgery. Reducing the revision rate and limiting bone loss become prominent issues, especially as TJR has been extended to younger patients.
The generation of wear particles from implanted device for joint replacement is inevitable. Wear particles can be recognized by infiltrated immune cells including macrophages and secrete pro-inflammatory cytokines [1]. Blocking of individual cytokines via neutralizing antibody did not mitigate osteolysis in patients [2]. Nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) is a key transcription factor that plays an essential role in the inflammatory response [3]. Targeting NF-κB activity via competitive binding with double strand decoy oligodeoxynucleotide (ODN) containing NF-κB binding element has been applied to many inflammatory disorders[4]. In our recent report “Suppression of wear particle induced pro-inflammatory cytokine and chemokine production in macrophages via NF-κB decoy oligodeoxynucleotide: A preliminary report”, we investigated how NF-κB signaling play a role in the wear particle-mediated inflammatory response [5].
Wear particles induced NF-κB activation and pro-inflammatory cytokines expression in macrophages
Ultra-High Molecular-Weight PolyEthylene (UHMWPE) wear particles (1.0 ± 0.1 μm) were used in this study. Mouse macrophage RAW264.7 NF-κB reporter cell clone was generated by stably transfecting the luciferase expression vector controlled by NF-κB response elements. Macrophages exposed to UHMWPE particles directly enhanced NF-κB activity by 50%. Mouse bone marrow derived macrophages and human macrophage THP1 cells exposed to UHMWPE particles with or without endotoxin (1 μg/ml lipopolysaccharide) showed significant induction of multiple chemokine and cytokine expression including MCP1, MIP1α (macrophage attractant chemokines), IL-8, CXCL1 (neutrophil attractant chemokines), TNFα, and IL-1β (pro-inflammatory cytokines). Induction of MCP1 enhanced additional macrophage migration at later time points (cells were exposed to the particles for 24 and 48 hrs).
NF-κB decoy oligodeoxynucleotide suppressed wear particles induced cytokine expression and cellular migration in macrophages
NF-κB decoy ODN (0.5 μM) was used to suppress the NF-κB activation in macrophages induced by UHMWPE particles or endotoxin. Interestingly, naked decoy ODN demonstrated the most efficient suppression of TNFα expression induced by endotoxin, and NF-κB activation induced by wear particles in RAW264.7 cells. Next, NF-κB decoy ODN suppressed the expression of multiple cytokines and chemokines including MCP1, MIP1α, MIP1β, IL-8, CXCL1, TNFα, IL-1β, and IL-6 at various levels, which were demonstrated in mouse bone marrow derived macrophages and human macrophage THP1 cells exposed to UHMWPE particles with or without endotoxin. Finally, induction of macrophage migration by the conditioned media exposed to wear particles with or without endotoxin was also reduced in NF-κB decoy ODN-treated cells.
Roles of NF-κB activation in wear particle-induced osteolysis
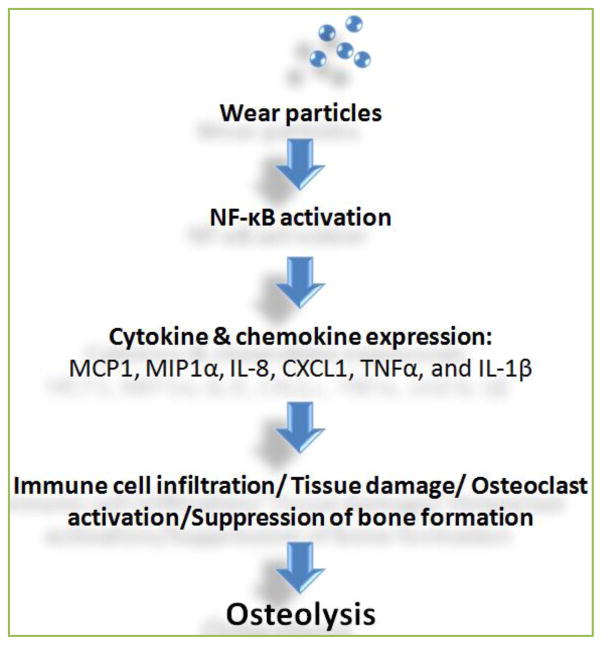
Figure 1 summarizes the current findings about the roles of NF-κB activation in UHMWPE particles induced peri-prosthetic osteolysis. Wear particles directly activate NF-κB signaling, which may be recognized through Toll-Like receptors (TLRs). Downstream cytokine and chemokine induction can recruit more immune cell infiltration, enhance tissue damage responses, and induce osteoclast maturation. Suppression of NF-κB activity through decoy ODN may simultaneously block multiple cytokine expression, and may efficiently mitigate wear particle-induced osteolysis in TJR patients.
Figure 1. Roles of NF-κB in wear particles induced inflammation and osteolytic tissue response.
Immune cells such as macrophages exposed to the wear particles enhance NF-κB activation, and turn on the downstream cytokine and chemokine gene expression including MCP1, MIP1α, IL-8, CXCL1, TNFα, and IL-1β. The resulted tissue response including immune cell infiltration, tissue damage, and osteoclast activation may eventually cause osteolysis. Bone formation is also suppressed. Suppression of NF-κB activity via decoy ODN can block multiple cytokine and chemokine expression, and may efficiently mitigate wear particle induced osteolysis.
Future directions
The therapeutic effects of NF-κB decoy ODN are currently being investigated by our group using in vivo models. Application of NF-κB targeting therapy should consider 1) the complexity of tissue microenvironment, and 2) the stability of NF-κB decoy ODN. Osteoprogenitor cells and mesenchymal cells can be recruited into the local regions with wear particles, and play essential roles in osteogenesis (new bone formation). The role of NF-κB signaling in these cells is still contradictory [6–9], therefore, the cell viability and osteogenic ability in response to NF-κB decoy ODN remains to be clarified. Local delivery of the decoy ODN may reduce the toxicity concerns compared to systemic administration. Advanced delivery strategy such as surface coating techniques or slow releasing drug delivery model could also enhance the efficiency of ODN delivery [10, 11].
Acknowledgments
This work was supported by NIH grants 2R01AR055650, 1R01AR063717 and the Ellenburg Chair in Surgery at Stanford University.
References
- 1.Johnston R. Current concepts: immunology. Monocytes and macrophages. New Engl J Med. 1988;318:747–52. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- 2.Schwarz E, Campbell D, Totterman S, Boyd A, O’Keefe R, Looney R. Use of volumetric computerized tomography as a primary outcome measure to evaluate drug efficacy in the prevention of peri-prosthetic osteolysis: a 1-year clinical pilot of etanercept vs. placebo. J Orthopaed Res. 2003;21:1049–55. doi: 10.1016/S0736-0266(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 3.Lin TH, Tamaki Y, Pajarinen J, Waters HA, Woo DK, Yao Z, et al. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-kappaB as a therapeutic target. Acta Biomater. 2014;10:1–10. doi: 10.1016/j.actbio.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osako M, Nakagami H, Morishita R. Modification of decoy oligodeoxynucleotides to achieve the stability and therapeutic efficacy. Current topics in medicinal chemistry. 2012;12:1603–7. doi: 10.2174/156802612803531397. [DOI] [PubMed] [Google Scholar]
- 5.Lin TH, Yao Z, Sato T, Keeney M, Li C, Pajarinen J, et al. Suppression of wear-particle-induced pro-inflammatory cytokine and chemokine production in macrophages via NF-kappaB decoy oligodeoxynucleotide: A preliminary report. Acta Biomater. 2014 doi: 10.1016/j.actbio.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang J, Liu F, Lee M, Wu B, Ting K, Zara JN, et al. NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc Natl Acad Sci U S A. 2013;110:9469–74. doi: 10.1073/pnas.1300532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–9. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Hu C, Wang G, Li L, Kong X, Ding Y, et al. Nuclear factor-kappaB modulates osteogenesis of periodontal ligament stem cells through competition with beta-catenin signaling in inflammatory microenvironments. Cell Death Dis. 2013;4:e510. doi: 10.1038/cddis.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho HH, Shin KK, Kim YJ, Song JS, Kim JM, Bae YC, et al. NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J Cell Physiol. 2010;223:168–77. doi: 10.1002/jcp.22024. [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, Wang H-f, Sun W-q, Xie C-s, Wei W-n, Zheng J-e, et al. Regulation of tissue factor expression in brain microvascular endothelial cells by PLA nanoparticles coating NF-kappaB decoy oligonucleotides. Zhonghua xue ye xue za zhi. 2005;26:534–8. [PubMed] [Google Scholar]
- 11.Kalinowski M, Viehofer K, Hamann C, Barry J, Kleb B, Klose K, et al. Local administration of NF-kappa B decoy oligonucleotides to prevent restenosis after balloon angioplasty: an experimental study in New Zealand white rabbits. Cardiovas Inter Rad. 2005;28:331–7. doi: 10.1007/s00270-003-0239-y. [DOI] [PubMed] [Google Scholar]