Abstract
Despite decades of research, acute respiratory distress syndrome (ARDS) remains an important clinical challenge due to an incomplete understanding of the pathophysiological mechanisms. No FDA-approved drug therapy currently exists for treatment of humans with ARDS. There is accumulating evidence in rodents and humans suggesting that extracellular histones are strong drivers of inflammation and tissue damage. We recently described an important role for extracellular histones during acute lung injury (ALI) in mice (Bosmann et al., FASEB J. 27:5010–5021 (2013)). Extracellular histones were detected in bronchoalveolar lavage fluids (BALF) from patients with ARDS but not in BALF from non-ARDS patients in intensive care units. Extracellular histones were also detected in BALF from mice during experimental ALI. The presence of extracellular histones was dependent on the two C5a receptors (C5aR and C5L2) and availability of neutrophils. Extracellular histones were highly pro-inflammatory, and caused severe damage to respiratory function. Intratracheal instillation of histones resulted in pro-inflammatory mediator production, epithelial cell damage, disturbances in alveolar-capillary gas exchange, lung consolidation, activation of the coagulation cascade, and in some cases, death. Antibody-mediated neutralization of extracellular histones attenuated C5a-induced ALI. Together, these data suggested a prominent role for extracellular histones in the pathophysiology of ALI. The predominant source of histones in ALI may be neutrophils that have been activated by C5a to form neutrophil extracellular traps (NETs). Therapeutic targeting of extracellular histones may provide a novel approach to combat ARDS in humans.
Keywords: Acute lung injury, acute respiratory distress syndrome, neutrophils, histones, inflammation, complement, C5a
Acute respiratory distress syndrome (ARDS) remain significant clinical problems involving >200,000 cases in the United States annually, with mortality rates from 25–60% [1, 2]. Acute lung injury (ALI) and ARDS are characterized by the production of pro-inflammatory mediators such as cytokines and chemokines, and complement activation products, and the accumulation of neutrophils in the lung. Disruption of the epithelial/endothelial barrier, along with reduced ability to clear alveolar fluid, results in persistent pulmonary edema and lung consolidation, intrapulmonary hemorrhage, and severely impaired gas exchange (reviewed, [3]). However, the molecular mechanisms of ALI/ARDS pathology remain unclear, and there is no specific FDA-approved drug therapy to improve clinical outcomes.
Evidence has accumulated suggesting that extracellular histones contribute to the pathogenesis of inflammatory diseases. Plasma levels of extracellular histones are elevated during trauma and/or sepsis in humans, baboons, and mice [4–6]. Antibody-mediated neutralization of histones provides protection during experimental sepsis in mice and baboons [4]. In our recent report, “Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury” [7], we investigated the role of extracellular histones during ALI.
Presence of extracellular histones during ARDS/ALI in humans and mice
We detected the presence of extracellular histones in bronchoalveolar lavage fluid (BALF) samples from humans with ARDS. The presence of histones in BALF appeared to peak early (up to 10 days after initial diagnosis of ARDS), and reduced levels were observed at later time points. No extracellular histones were observed in BALF samples from non-ARDS intensive care patients. In mice, extracellular histone levels were elevated in BALF from three different models of ALI (LPS, IgG immune complex, and C5a-induced). Extracellular histone presence was dependent on an intact neutrophil population as well as the two C5a receptors (C5aR and C5L2). Even though neutrophils were required for full histone release during ALI, the exact cellular source of the extracellular histones was not clear. Histones are known to be released by neutrophils associated with neutrophil extracellular traps (NETs), and NETs have been shown to be present during ALI [8]. Conversely, neutrophils may contribute to lung tissue damage and parenchymal cell death, and histones are also known to be released from apoptotic/necrotic cells [9].
Pro-inflammatory properties of extracellular histones during ALI
Antibody-mediated neutralization of histones provided protection during C5a-induced ALI. Specifically, instillation (Intratracheal (i.t.)) of neutralizing anti-histone H2A/H4 antibody [10] reduced ALI severity by 50%, determined by the level of albumin leakage measured in BALF. Histone neutralization significantly reduced the presence of inflammatory cytokines (e.g., TNF, IL-1β, IL-6, IL-12(p40), G-CSF) and chemokines (e.g., MCP-1, MIP-1α, MIP-1β) found in the BALF during C5a-induced ALI, compared to an isotype control antibody. Instillation of exogenous histones (100 μg) into the airway resulted in the production of high levels of pro-inflammatory cytokines and chemokines, the accumulation of neutrophils in the lung, and severe ALI. The exact mechanism of histone-induced cytokine/chemokine production was not clear, although there are indications that histones bind to Toll-like receptors (TLRs) -2, -4, and -9 [11–13].
Extracellular histone-induced tissue damage and ALI
The treatment of alveolar type II epithelial cell lines in vitro with purified histones induced elevations of intracellular calcium and cell death. When exogenous histones (20 μg/g body weight) were administered to mice i.t., BALF levels of lactate dehydrogenase (LDH) were elevated after 1 hour indicating extensive tissue damage in lung. Histologic and electron microscopic analysis revealed classical signs of ALI including intra-alveolar hemorrhage, fibrin deposits, and epithelial cell injury after i.t. histone instillation. Exogenous histone instillation into rats (i.t., 50 μg/g body weight) resulted in very rapid disturbances in lung air-gas exchange, cyanosis, and occasional death. Whole-body plethysmography revealed increased respiratory rate, while analysis of blood showed arterial acidification, elevated pCO2, and reduced oxyhemoglobin saturation as early as 15 minutes after histone instillation. Together, these data indicate that extracellular histones resulted in lung tissue damage, and severe and immediate ALI.
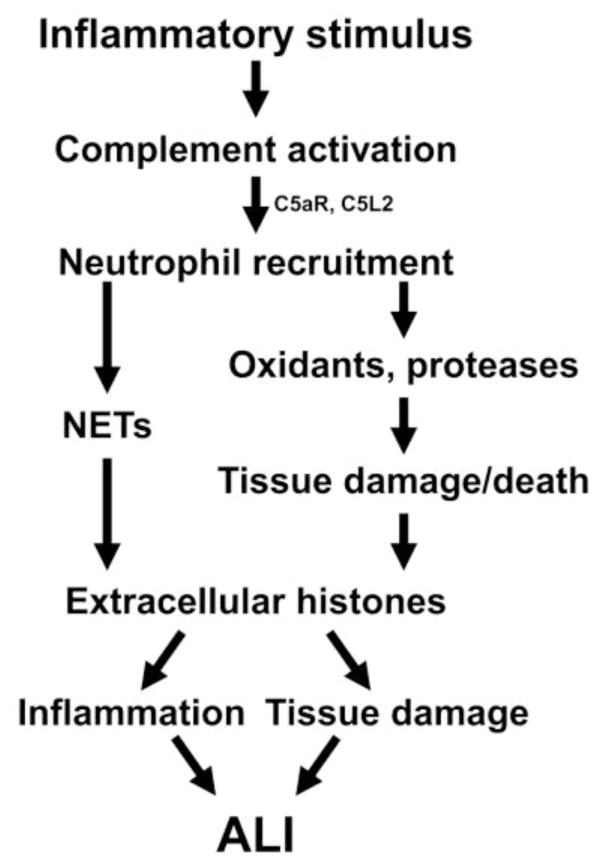
Role of extracellular histones in ALI
Figure 1 outlines our current thoughts about the role of extracellular histones during ALI. Histone presence was dependent on both C5a receptors, indicating a significant role for complement activation. Histone presence was also dependent on neutrophils, either through the release of histones associated with NETs, or through neutrophil-mediated cell damage and death. Once present, extracellular histones were highly inflammatory and damaging in the lung, and led to intense ALI.
Figure 1.
Role of extracellular histones during ALI. Extracellular histone presence during ALI was dependent on C5a receptors and neutrophils. Extracellular histones are known to be released by activated neutrophils (left pathway) or by dead/dying cells (right pathway). Once formed, extracellular histones lead to inflammation and tissue damage, resulting in ALI.
Future directions
Targeting of histone-mediated inflammation and cell damage may be a promising avenue for the development of novel therapeutics for the treatment of ARDS. We have shown that 1) extracellular histones are important for tissue damage and inflammation during rodent ALI; and 2) extracellular histones are present during human ARDS. However, several important questions remain. More investigation is needed to determine the relative contributions of individual histones (H1, H2A, H2B, H3, and H4) during ARDS. In addition, whether all of the individual histones are present in the extracellular space during human ARDS is not known. These are active areas of investigation in our group. Clarification of these important questions is required prior to the development of histone-targeted therapeutics.
Acknowledgments
The authors thank Sue Scott for excellent support in the preparation of this manuscript. This work was supported by grants from the National Institutes of Health, GM-29507 and GM-61656 (PAW) and NHLBI-T32-HL007517-29 (JJG).
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Goss CH, Brower RG, Hudson LD, Rubenfeld GD. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31:1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. http://dx.doi.org/10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. http://dx.doi.org/10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. http://dx.doi.org/10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. http://dx.doi.org/10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrowski SR, Berg RM, Windelov NA, Meyer MA, Plovsing RR, Moller K, et al. Coagulopathy, catecholamines, and biomarkers of endothelial damage in experimental human endotoxemia and in patients with severe sepsis: a prospective study. J Crit Care. 2013;28:586–596. doi: 10.1016/j.jcrc.2013.04.010. http://dx.doi.org/10.1016/j.jcrc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Crit Care Med. 2013;187:160–169. doi: 10.1164/rccm.201206-1037OC. http://dx.doi.org/10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosmann M, Grailer JJ, Russkamp NF, Ruemmler R, Monestier M, Zetoune FS, et al. Extracellular histones are essential effectors of C5aR and C5L2-dependent tissue damage and inflammation in acute lung injury. FASEB J. 2013;27:5010–5021. doi: 10.1096/fj.13-236380. http://dx.doi.org/10.1096/fj.13-236380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. http://dx.doi.org/10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu D, Ingram A, Lahti JH, Mazza B, Grenet J, Kapoor A, et al. Apoptotic release of histones from nucleosomes. J Biol Chem. 2002;277:12001–12008. doi: 10.1074/jbc.M109219200. http://dx.doi.org/10.1074/jbc.M109219200. [DOI] [PubMed] [Google Scholar]
- 10.Monestier M, Fasy TM, Losman MJ, Novick KE, Muller S. Structure and binding properties of monoclonal antibodies to core histones from autoimmune mice. Mol Immunol. 1993;30:1069–1075. doi: 10.1016/0161-5890(93)90153-3. http://dx.doi.org/10.1016/0161-5890(93)90153-3. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187:2626–2631. doi: 10.4049/jimmunol.1003930. http://dx.doi.org/10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hagele H, Lichtnekert J, et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol. 2012;23:1375–1388. doi: 10.1681/ASN.2011111077. http://dx.doi.org/10.1681/ASN.2011111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M, et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology. 2011;54:999–1008. doi: 10.1002/hep.24501. http://dx.doi.org/10.1002/hep.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]