Abstract
Age-related macular degeneration (AMD) is the leading cause of blindness in industrial countries. Vision loss caused by AMD results from geographic atrophy (dry AMD) and/or choroidal neovascularization (wet AMD). Presently, the etiology and pathogenesis of AMD is not fully understood and there is no effective treatment. Oxidative stress in retinal pigment epithelial (RPE) cells is considered to be one of the major factors contributing to the pathogenesis of AMD. Also retinal glia, as scavengers, are deeply related with diseases and could play a role. Therefore, therapeutic approaches for microglia and Müller glia, as well as RPE, may lead to new strategies for AMD treatment. This review summarizes the pathological findings observed in RPE cells, microglia and Müller glia of AMD murine models.
Keywords: Age-related macular degeneration, retinal pigment epithelium, microglia, Müller glia
Introduction
Age-related macular degeneration (AMD) is the principal cause of blindness in the elderly population of developed countries, including the US. An early clinical sign of AMD is the accumulation of drusen, yellowish deposits in fundus image (Fig. 1A). Vision loss is either due to retinal pigment epithelium (RPE) and photoreceptor cell death, called geographic atrophy (dry AMD) or from the secondary effects of choroidal neovascularization (CNV, wet AMD). Currently, the pathogenesis of AMD is not fully understood, however advanced age, ocular pigmentation, dietary factors, family history, high blood pressure and smoking are well-established risk factors [1–3]. The purpose of this review is to discuss the contribution of RPE, microglia and Müller glia to AMD pathogenesis, especially based on the findings in murine models.
Figure 1. Eye Structure.
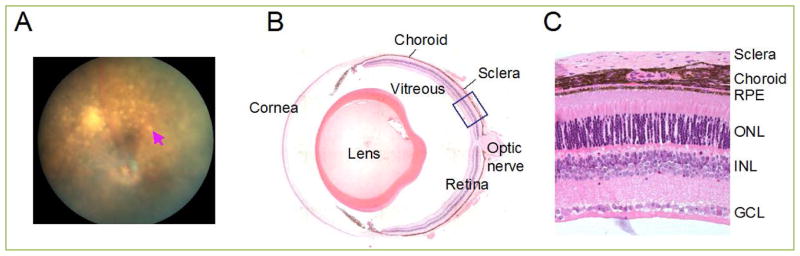
(A) Fundus image of aryl hydrocarbon receptor (AhR) knockout, one of AMD model mice showing yellowish-white spots (arrow). (B) Cross-section of the adult mouse eye. (C) Retinal histology section zoomed from boxed area in B. Abbreviations: GCL, ganglion cell layer; INL, Inner nuclear layer; OD, optic disc; ONL, outer nuclear layer; RPE, retinal pigment epithelium.
Eye and light hazard
The eye receives light input from the environment and transfers it to the brain, allowing it to be converted into visual information. Light enters the cornea, passes through the pupil and aqueous humor, then lens and vitreous, and finally arrives to the retina at the back of eye (Fig. 1B). Light-sensitive cells called photoreceptors, located in the outerlayer of the retina, next to the RPE (Fig. 1C), transfer light energy into electrochemical signals for transmission to the brain. Photoreceptors are classified into two groups, rods and cones. Rods are responsible for night vision and cones are responsible for day and color vision. Light signals are sent from photoreceptors, through the bipolar cells, into the ganglion neurons whose axons form the optic nerve and travel into the thalamic region of brain. Adjacent to the retinal tissue is the RPE, a monolayer of cuboidal cells. RPE cells have apical microvilli, which are associated with the outer segments of photoreceptor cells. The basal membrane of the RPE is in contact with Bruch’s membrane, which separates the RPE from the vascular choroid.
Mice are nocturnal and use their noses and whiskers largely, instead of eyes. Some researchers even believe that mice are blind and therefore studying vision in mice is not informative, but the basic anatomical eye structures between human and mouse are same. The main differences between human and mouse eyes is that the mouse has no maculae and a dominant number of rod photoreceptors (97%), while the human macula (around 6 mm in diameter) has predominantly cone photoreceptors, and an especially tiny area of macula called the fovea (0.8 mm) contains only cones. Despite these differences, mouse AMD models have certain pathological, physiological and biochemical features of human AMD such as A2E accumulation, abnormal ERGs, RPE and photoreceptor degeneration, and even neovascularization [4].
Light damage is one of the prominent methods to trigger AMD-like phenotypes in mice. Although light initiates the phototransduction cascade in photoreceptors and relays visual information, at the same time, light can also damage the eye if it is exposed to too much. Experimental work in AMD animal models have shown that light can lead to, or augment, retinal damage to the RPE [5] and photoreceptors [5, 6]. Although most ultraviolet radiation is absorbed by the cornea and lens, a fraction with wavelengths shorter than 400 nm does reach the retina [7]. Ocular light transmission is especially high in young eyes, while the transmission decreases with increasing age. Further, clinical reports indicate that cataract surgery may increase AMD development or progression [8]. Therefore, forming habits to protect the eyes at earlier ages, such as wearing sunglasses, might be more important than we think.
Drusen
Early AMD is characterized by the thickening of Bruch’s membrane, lipofuscin accumulation in RPE and drusen formation between the basal RPE and Bruch’s membrane. Drusen are generally found between the RPE and Bruch’s membrane, but they also form in the subretinal space between RPE and photoreceptors as subretinal drusen or drusenoid deposits [9–12]. Drusen are clinically described as hard or soft. Hard drusen have well defined borders and are relatively common in the elderly population with or without AMD, whereas soft drusen are irregular with fuzzy edges [13]. The presence of many drusen, or drusen of large size, especially soft ones, are significant risk factors for late stage AMD development [14].
The precise mechanisms of drusen formation are still unclear, however the focal deposits include proteins such as vironectin [15], apolipoproteins [16, 17], immune mediated markers [18–20], lipids [21, 22] and trace elements [23, 24]. The presence of inflammation-related molecules in drusen suggests the possible involvement of the immune system in AMD pathogenesis, and a study in age-related changes demonstrates recruitment of leukocytes and activation of the complement cascade in mouse RPE and choroid [25]. Therefore, I will review the pathological roles of retinal phagocytes including RPE, microglia and Müller glia in AMD mouse models. Other AMD pathological aspects such as molecular and genetic findings have been previously reviewed [13, 26].
RPE
RPE is a monolayer of largely hexagonal cells located subjacent to the neural retina (Fig 2A). As an epithelium, RPE cells have apical microvilli and a basal surface. The apical microvilli envelop the outer segments of photoreceptors. One RPE cell supports 30–50 photoreceptors [27]. The basal surface of RPE is separated from the vascular choroid by Bruch’s membrane.
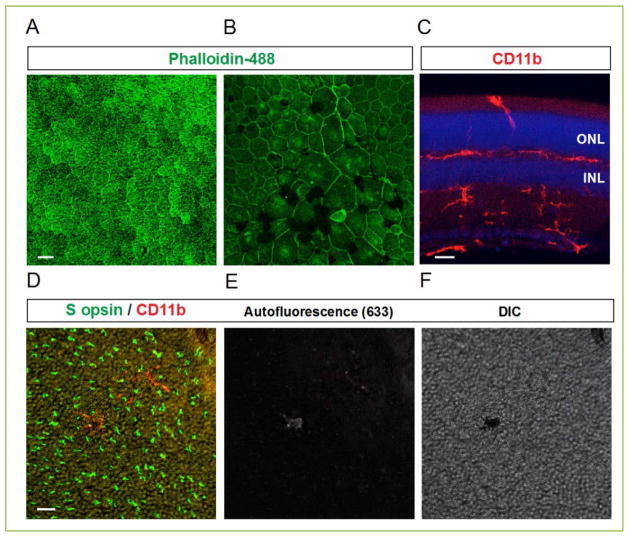
Figure 2. RPE and microglia.
(A–B) Horizontal RPE whole mounts of 12-month-old wild type (A) and AhR−/− (B) mice, a model of AMD, stained by phalloidin-488. (C) Vertical image of a 12-month-old wild type retina stained with CD11b microglia marker (red). (D–F) Horizontal retina whole mounts of 12-month-old AhR−/− mouse stained with S opsin and CD11b antibodies showing the presence of autofluorescent microglia in subretinal space. The images of autofluorescence (633 channel) and DIC are provided in E and F, respectively. Abbreviations: INL, inner nuclear layer; ONL, outer nuclear layer. Scale bars: 25 μm in A, B, 20 μm in C–F.
RPE is essential for the normal function and survival of photoreceptors. It regulates transport of ions, nutrients, and water to and from the blood supply to the subretinal space, and also is one of the components of the outer blood-retinal barrier that blocks nonspecific diffusion and transport from the choroid. RPE apical microvilli extend long processes that wrap around the tips of photoreceptor outer segment (POS) and engulf outer segment shed from photoreceptors during their regenerating process. The most important function of RPE cells is to maintain the retinoid visual cycle by regenerating and moving 11-cis retinal back to photoreceptors. Failure of phagocytosis, renewal of POS and/or visual cycling induces retina degeneration with AMD like phenotypes, as observed in knockout mice such as alphavbeta5 integrin, ATP-binding cassette transporter 4 (ABCA4) and all-trans-retinol dehydrogenase 8 (RDH8) [28–31]. Lack of alphavbeta5 integrin affects RPE phagocytic function [28], and the loss of ABCA4 and RDH8 accumulates all-trans-retinal and N-retinyl-N-retinlyidene-ethanolamine (A2E) lipofuscin, which induces NADPH-oxidase-mediated overproduction of intracellular reactive oxygen species [30–32].
In AMD mouse models such as DICER1 [33], SOD1 [34], Nrf2 [35] and AhR [36, 37] knockouts, or with age, lipofuscin and/or the debris derived from RPE are extruded to the inner collagenous layer of Bruch’s membrane, and complement activation and immune complex deposition occurs in RPE, choroid and subretinal space, inducing an infiltration of microglia to aid in the clearance of drusen, and eventually leads to RPE atrophy. Lipofuscin accumulation and oxidative stress in RPE is the major reason for the induction of geographic atrophy. Vascular endothelial growth factor (VEGF), the major angiogenic factor in the pathology of wet AMD, is secreted by the RPE [38, 39]. In vitro RPE cells under oxidative stress conditions were reported to secrete VEGF as an autocrine survival factor [40], and VEGF could be one of factors to recruit microglia [41]. RPE degeneration and/or CNV lead to the breakdown of the outer blood retina barrier that RPE maintains. Through these processes, complement activation and immune complex deposition would be accelerated around the RPE atrophy and CNV areas.
Microglia
Microglia are the resident immune cells in central nervous system (CNS), and members of the mononuclear phagocyte system with other macrophages, monocytes and dendritic cells. They have specific differences in density, phenotypes and responsiveness throughout the CNS, including brain, spinal cord and retina. As a member of mononuclear phagocytic cells, microglia survey the environment, and detect subtle cellular damage, then engulf debris and dying cells. However, even in healthy brain, microglia are highly active and dynamic [42, 43], controlling the neural circuit wiring of the nervous system by regulating cell death, pruning and synapse maturation and plasticity [44–46].
Retinal microglia are observed in the outer and inner plexiform (retinal synaptic) layers (Fig. 2C), starting from postnatal day 12 (P12), when synaptogenesis begins to happen in retina. The location and timing of retinal microglia appearance in the plexiform layers suggest that they play a role in synaptic maturation and maintenance as well, like brain microglia. Also, retrograde labeling of retinal ganglion cell (RGC) suggested microglia phagocytosis of dying RGC around P5, the time point when ganglion cell death peaks in the retina [47].
In the aging retina, microglia located in OPL tend to translocate to the subretinal space, and display engulfed melanin or autofluorescent particles (Fig 2D–F). Microglia phagocytosis performance over time is diminished, losing motility, branching complexity and process length with age, although the number of microglia increases slightly [48]. Transcriptome analysis showed increased levels of innate immune and complement genes compared to young mice [49], and isolated microglial cells showed proinflammatory markers [50].
It is unclear how retinal microglia contribute to AMD pathogenesis. However, mice with impaired macrophage recruitment and/or function, such as monocyte chemoattractant protein-1 (Ccl-2), its receptor cognate C-C chemokine receptor-2 (Ccr-2) or CX3CR1 knockouts exhibit AMD phenotypes [51, 52]. AMD mouse models have revealed activated microglia in the subretinal space, with engulfed particles of photoreceptor [52], pigmented and/or autofluorescence [37]. Microglial activation emerges concurrently with, or prior to, the onset of overt retina degeneration and angiogenesis [37, 52–56]. One postulated mechanism of activation is that secreted molecules from stressed RPE recruit and activate microglia, which accelerates severe tissue damage and CNV formation.
Microglia play an important role in tissue maintenance by phagocytosing unhealthy, malfunctional neuron debris and secreting neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF). Thus, maintenance of microglia health in the retina might slow down the onset of age-related degeneration and its progression.
Müllerglia
Müller glial cells are a special type of glia only found in retina, which span the entire neural retina from the inner limiting membrane of vitreous surface to the outer limiting membrane of subretinal space (Fig. 3A). Most importantly, they support all kinds of retinal neurons. Nutrients, waste products, ions, water, and other molecules are transported through Müller glia from retinal blood vessels to retinal cells [57]. Müller glia form the inner retinal blood barrier by surrounding capillary endothelial cells and the pericytes [58]. Also, Müller glia regulate synaptic activity by uptake of released glutamate and GABA neurotransmitters, and then supply glutamine, the precursor of neurotransmitters, to neurons [59]. The uptake of glutamate by Müller glia contributes to the rapid termination of synaptic action in the inner retina, providing spatial resolution of synaptic activity, and is also considered a protective mechanism from glutamate toxicity [60]. Müller glial cells are especially critical to the viability of cone photoreceptors (Fig. 3B and C). They phagocytize the outer segment discs shed from cone photoreceptors [61], and supply 11-cis-retinol to cone photoreceptors at a 20-fold faster speed than the RPE mediated cycle [62].
Figure 3. Müller glia.
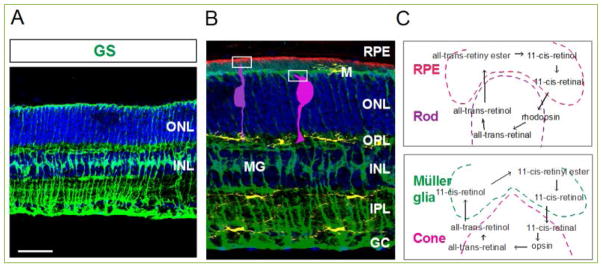
(A) Vertical image of a 6 month-old wild type retina stained with Müller glia marker, glutamine synthetase antibody (green). (B–C) Schematic illustration of microglia (M, yellow) and Müller glia (MG, green) in the vertical view of a murine retina. RPE epical microvilli are descriptive with color of red, and rod and cone photoreceptors are indicated with dark and bright purple colors, respectively (B). Visual cycles between RPE and rods, and between Müller glia and cones (C). GC, ganglion cell layer, INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; RPE, retinal pigment epithelium. Scale bar: 50 μm in A.
In stress conditions, Müller glia produce antioxidants including glutathione [63], lactate [64], alanine [65], α-ketoglutaratet [64], metallothionein [66]and cerulplasmin [67]. However, Müller glia undergo reactive gliosis upon exposure to chronic stresses, injuries, or pathogenic conditions. Gliosis, or glia activation, is protective and helpful to recover homeostasis of neurons, but may be harmful to the retina and contributes to retinal degeneration in chronic situations.
AMD mouse models exhibit Müller glia activation by showing GFAP reactivity, but the involvement of gliosis in the AMD pathogenesis is not clear. On the other hand, activated Müller glia under hypoxic or diabetic conditions secrete pro-angiogenic factors, such as VEGF and basic fibroblast growth factor, having endothelial angiogenic activity [68–70]. Therefore, further study of Müller glia during AMD pathogenesis would be valuable, and Müller glia could be one therapy target that contributes to the maintenance or recovery of healthy retinal function in the future.
Perspective
RPE and Müller glia are essential for normal retina function and the survival of photoreceptors and other retinal neurons by regulating transport of ions, nutrients, and water to and from the blood supply, and constitute outer/inner blood-retinal barriers. Currently, overloaded oxidative stress occurring in RPE is considered to be one of the main causes of AMD phenotypes. However, Müller glia activation is observed in AMD patients [71] and mouse models [37], suggesting contribution to some aspects of AMD pathogenesis, and a possible interplay with microglia migration and activation.
Müller glia activation and the recruited microglia are considered to play protective roles, but in chronic conditions, they can be harmful, accelerate atrophy, and stimulate CNV. RPE is a major target for AMD treatment, but the manipulation of microglia and Müller glia will provide additional benefits.
Acknowledgments
I would like to thank Dr. Anand Swaroop for supporting me on AMD-related research, and Dr. Gail Seabold for a critical reading of the manuscript and helpful comments. This research was supported by the Intramural Research Program of the National Eye Institute, National Institutes of Health.
List of abbreviations
- AMD
Age-related macular degeneration
- RPE
retinal pigment epithelium
- POS
photoreceptor outer segment
- VEGF
Vascular endothelial growth factor
- CNS
central nervous system
- RGC
retinal ganglion cell
References
- 1.Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Velilla S, Garcia-Medina JJ, Garcia-Layana A, Dolz-Marco R, Pons-Vazquez S, Pinazo-Duran MD, et al. Smoking and age-related macular degeneration: review and update. J Ophthalmol. 2013;2013:895147. doi: 10.1155/2013/895147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bok D. Evidence for an inflammatory process in age-related macular degeneration gains new support. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(20):7053–7054. doi: 10.1073/pnas.0502819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elizabeth Rakoczy P, Yu MJ, Nusinowitz S, Chang B, Heckenlively JR. Mouse models of age-related macular degeneration. Experimental eye research. 2006;82(5):741–752. doi: 10.1016/j.exer.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Organisciak DT, Vaughan DK. Retinal light damage: mechanisms and protection. Progress in retinal and eye research. 2010;29(2):113–134. doi: 10.1016/j.preteyeres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Progress in retinal and eye research. 2005;24(2):275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand. 2006;84(1):4–15. doi: 10.1111/j.1600-0420.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 8.Qian CX, Young LH. The impact of cataract surgery on AMD development and progression. Semin Ophthalmol. 2014;29(5–6):301–311. doi: 10.3109/08820538.2014.962166. [DOI] [PubMed] [Google Scholar]
- 9.Rudolf M, Malek G, Messinger JD, Clark ME, Wang L, Curcio CA. Sub-retinal drusenoid deposits in human retina: organization and composition. Experimental eye research. 2008;87(5):402–408. doi: 10.1016/j.exer.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117(2):303–312. e301. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Zweifel SA, Imamura Y, Spaide TC, Fujiwara T, Spaide RF. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology. 2010;117(9):1775–1781. doi: 10.1016/j.ophtha.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2010;30(9):1441–1454. doi: 10.1097/IAE.0b013e3181ee5ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Progress in retinal and eye research. 2009;28(1):1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH. Ten-year incidence and progression of age-related maculopathy: The Beaver Dam eye study. Ophthalmology. 2002;109(10):1767–1779. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 15.Hageman GS, Mullins RF, Russell SR, Johnson LV, Anderson DH. Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FASEB J. 1999;13(3):477–484. doi: 10.1096/fasebj.13.3.477. [DOI] [PubMed] [Google Scholar]
- 16.Li CM, Clark ME, Chimento MF, Curcio CA. Apolipoprotein localization in isolated drusen and retinal apolipoprotein gene expression. Investigative ophthalmology & visual science. 2006;47(7):3119–3128. doi: 10.1167/iovs.05-1446. [DOI] [PubMed] [Google Scholar]
- 17.Anderson DH, Ozaki S, Nealon M, Neitz J, Mullins RF, Hageman GS, et al. Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: implications for the process of drusen formation. Am J Ophthalmol. 2001;131(6):767–781. doi: 10.1016/s0002-9394(00)00961-2. [DOI] [PubMed] [Google Scholar]
- 18.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134(3):411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 19.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Progress in retinal and eye research. 2001;20(6):705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 20.Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Progress in retinal and eye research. 2010;29(2):95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch’s membrane. Investigative ophthalmology & visual science. 2001;42(1):265–274. [PubMed] [Google Scholar]
- 22.Mullins RF, Hageman GS. Human ocular drusen possess novel core domains with a distinct carbohydrate composition. J Histochem Cytochem. 1999;47(12):1533–1540. doi: 10.1177/002215549904701205. [DOI] [PubMed] [Google Scholar]
- 23.Hahn P, Milam AH, Dunaief JL. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch’s membrane. Arch Ophthalmol. 2003;121(8):1099–1105. doi: 10.1001/archopht.121.8.1099. [DOI] [PubMed] [Google Scholar]
- 24.Olin KL, Morse LS, Murphy C, Paul-Murphy J, Line S, Bellhorn RW, et al. Trace element status and free radical defense in elderly rhesus macaques (Macaca mulatta) with macular drusen. Proc Soc Exp Biol Med. 1995;208(4):370–377. doi: 10.3181/00379727-208-43864. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Liu B, Lukas TJ, Neufeld AH. The aged retinal pigment epithelium/choroid: a potential substratum for the pathogenesis of age-related macular degeneration. PloS one. 2008;3(6):e2339. doi: 10.1371/journal.pone.0002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A. Age-Related Macular Degeneration: Genetics and Biology Coming Together. Annu Rev Genomics Hum Genet. 2014 doi: 10.1146/annurev-genom-090413-025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonilha VL. Age and disease-related structural changes in the retinal pigment epithelium. Clinical ophthalmology. 2008;2(2):413–424. doi: 10.2147/opth.s2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. The Journal of experimental medicine. 2004;200(12):1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan JL, LaVail MM, Yasumura D, Matthes MT, Yang H, Trautmann N, et al. An RCS-like retinal dystrophy phenotype in mer knockout mice. Investigative ophthalmology & visual science. 2003;44(2):826–838. doi: 10.1167/iovs.02-0438. [DOI] [PubMed] [Google Scholar]
- 30.Radu RA, Mata NL, Bagla A, Travis GH. Light exposure stimulates formation of A2E oxiranes in a mouse model of Stargardt’s macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(16):5928–5933. doi: 10.1073/pnas.0308302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Okano K, Maeda T, Chauhan V, Golczak M, Maeda A, et al. Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration. The Journal of biological chemistry. 2012;287(7):5059–5069. doi: 10.1074/jbc.M111.315432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeda A, Maeda T, Golczak M, Chou S, Desai A, Hoppel CL, et al. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. The Journal of biological chemistry. 2009;284(22):15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaneko H, Dridi S, Tarallo V, Gelfand BD, Fowler BJ, Cho WG, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471(7338):325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imamura Y, Noda S, Hashizume K, Shinoda K, Yamaguchi M, Uchiyama S, et al. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(30):11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Z, Chen Y, Wang J, Sternberg P, Freeman ML, Grossniklaus HE, et al. Age-related retinopathy in NRF2-deficient mice. PloS one. 2011;6(4):e19456. doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu P, Herrmann R, Bednar A, Saloupis P, Dwyer MA, Yang P, et al. Aryl hydrocarbon receptor deficiency causes dysregulated cellular matrix metabolism and age-related macular degeneration-like pathology. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(43):E4069–4078. doi: 10.1073/pnas.1307574110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SY, Yang HJ, Chang YS, Kim JW, Brooks M, Chew EY, et al. Deletion of aryl hydrocarbon receptor AHR in mice leads to subretinal accumulation of microglia and RPE atrophy. Investigative ophthalmology & visual science. 2014;55(9):6031–6040. doi: 10.1167/iovs.14-15091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adamis AP, Shima DT, Yeo KT, Yeo TK, Brown LF, Berse B, et al. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochemical and biophysical research communications. 1993;193(2):631–638. doi: 10.1006/bbrc.1993.1671. [DOI] [PubMed] [Google Scholar]
- 39.Terasaki H, Shirasawa M, Otsuka H, Yamashita T, Uchino E, Hisatomi T, et al. Different Effects of Thrombin on VEGF Secretion, Proliferation, and Permeability in Polarized and Non-polarized Retinal Pigment Epithelial Cells. Current eye research. 2014:1–10. doi: 10.3109/02713683.2014.964417. [DOI] [PubMed] [Google Scholar]
- 40.Byeon SH, Lee SC, Choi SH, Lee HK, Lee JH, Chu YK, et al. Vascular endothelial growth factor as an autocrine survival factor for retinal pigment epithelial cells under oxidative stress via the VEGF-R2/PI3K/Akt. Investigative ophthalmology & visual science. 2010;51(2):1190–1197. doi: 10.1167/iovs.09-4144. [DOI] [PubMed] [Google Scholar]
- 41.Couturier A, Bousquet E, Zhao M, Naud MC, Klein C, Jonet L, et al. Anti-vascular endothelial growth factor acts on retinal microglia/macrophage activation in a rat model of ocular inflammation. Molecular vision. 2014;20:908–920. [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JE, Liang KJ, Fariss RN, Wong WT. Ex vivo dynamic imaging of retinal microglia using time-lapse confocal microscopy. Investigative ophthalmology & visual science. 2008;49(9):4169–4176. doi: 10.1167/iovs.08-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 44.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 45.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roumier A, Bechade C, Poncer JC, Smalla KH, Tomasello E, Vivier E, et al. Impaired synaptic function in the microglial KARAP/DAP12-deficient mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(50):11421–11428. doi: 10.1523/JNEUROSCI.2251-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bodeutsch N, Thanos S. Migration of phagocytotic cells and development of the murine intraretinal microglial network: an in vivo study using fluorescent dyes. Glia. 2000;32(1):91–101. doi: 10.1002/1098-1136(200010)32:1<91::aid-glia90>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 48.Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10(2):263–276. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen M, Muckersie E, Forrester JV, Xu H. Immune activation in retinal aging: a gene expression study. Investigative ophthalmology & visual science. 2010;51(11):5888–5896. doi: 10.1167/iovs.09-5103. [DOI] [PubMed] [Google Scholar]
- 50.Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55(4):412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- 51.Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9(11):1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 52.Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117(10):2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T. Retinal microglia: Just bystander or target for therapy? Progress in retinal and eye research. 2014 doi: 10.1016/j.preteyeres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 54.Langmann T. Microglia activation in retinal degeneration. Journal of leukocyte biology. 2007;81(6):1345–1351. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- 55.Ma W, Zhao L, Fontainhas AM, Fariss RN, Wong WT. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PloS one. 2009;4(11):e7945. doi: 10.1371/journal.pone.0007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang H, Parlier R, Shen JK, Lutty GA, Vinores SA. VEGF receptor blockade markedly reduces retinal microglia/macrophage infiltration into laser-induced CNV. PloS one. 2013;8(8):e71808. doi: 10.1371/journal.pone.0071808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reichenbach A, Bringmann A. New functions of Muller cells. Glia. 2013;61(5):651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- 58.Hosoya K, Tachikawa M. Inner blood-retinal barrier transporters: role of retinal drug delivery. Pharmaceutical research. 2009;26(9):2055–2065. doi: 10.1007/s11095-009-9930-2. [DOI] [PubMed] [Google Scholar]
- 59.Bringmann A, Pannicke T, Biedermann B, Francke M, Iandiev I, Grosche J, et al. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochemistry international. 2009;54(3–4):143–160. doi: 10.1016/j.neuint.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 60.Matsui K, Hosoi N, Tachibana M. Active role of glutamate uptake in the synaptic transmission from retinal nonspiking neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(16):6755–6766. doi: 10.1523/JNEUROSCI.19-16-06755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Long KO, Fisher SK, Fariss RN, Anderson DH. Disc shedding and autophagy in the cone-dominant ground squirrel retina. Experimental eye research. 1986;43(2):193–205. doi: 10.1016/s0014-4835(86)80087-2. [DOI] [PubMed] [Google Scholar]
- 62.Arshavsky V. Like night and day: rods and cones have different pigment regeneration pathways. Neuron. 2002;36(1):1–3. doi: 10.1016/s0896-6273(02)00937-6. [DOI] [PubMed] [Google Scholar]
- 63.Reichelt W, Stabel-Burow J, Pannicke T, Weichert H, Heinemann U. The glutathione level of retinal Muller glial cells is dependent on the high-affinity sodium-dependent uptake of glutamate. Neuroscience. 1997;77(4):1213–1224. doi: 10.1016/s0306-4522(96)00509-x. [DOI] [PubMed] [Google Scholar]
- 64.Tsacopoulos M. Metabolic signaling between neurons and glial cells: a short review. Journal of physiology, Paris. 2002;96(3–4):283–288. doi: 10.1016/s0928-4257(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 65.Poitry S, Poitry-Yamate C, Ueberfeld J, MacLeish PR, Tsacopoulos M. Mechanisms of glutamate metabolic signaling in retinal glial (Muller) cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(5):1809–1821. doi: 10.1523/JNEUROSCI.20-05-01809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wunderlich KA, Leveillard T, Penkowa M, Zrenner E, Perez MT. Altered expression of metallothionein-I and -II and their receptor megalin in inherited photoreceptor degeneration. Investigative ophthalmology & visual science. 2010;51(9):4809–4820. doi: 10.1167/iovs.09-5073. [DOI] [PubMed] [Google Scholar]
- 67.Chen L, Dentchev T, Wong R, Hahn P, Wen R, Bennett J, et al. Increased expression of ceruloplasmin in the retina following photic injury. Molecular vision. 2003;9:151–158. [PubMed] [Google Scholar]
- 68.Amin RH, Frank RN, Kennedy A, Eliott D, Puklin JE, Abrams GW. Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Investigative ophthalmology & visual science. 1997;38(1):36–47. [PubMed] [Google Scholar]
- 69.Eichler W, Yafai Y, Wiedemann P, Reichenbach A. Angiogenesis-related factors derived from retinal glial (Muller) cells in hypoxia. Neuroreport. 2004;15(10):1633–1637. doi: 10.1097/01.wnr.0000133071.00786.a4. [DOI] [PubMed] [Google Scholar]
- 70.Yafai Y, Iandiev I, Lange J, Yang XM, Wiedemann P, Bringmann A, et al. Basic fibroblast growth factor contributes to a shift in the angioregulatory activity of retinal glial (Muller) cells. PloS one. 2013;8(7):e68773. doi: 10.1371/journal.pone.0068773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu KH, Madigan MC, Billson FA, Penfold PL. Differential expression of GFAP in early v late AMD: a quantitative analysis. The British journal of ophthalmology. 2003;87(9):1159–1166. doi: 10.1136/bjo.87.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]