Abstract
Ipilimumab, a fully human, recombinant, monoclonal antibody to cytotoxic T-lymphocyte antigen 4 improves overall survival (OS) in previously treated and untreated metastatic melanoma. This retrospective analysis reports data gathered by a questionnaire on the demographics, outcomes, and toxicity of ipilimumab administered through an Expanded Access Program (EAP). Ipilimumab 3 mg/kg was administered intravenously every 3 weeks for four cycles to adults with metastatic melanoma. Efficacy outcomes included complete response, partial response (PR), progressive disease, stabilized disease, and OS. EAP data were collected from EAP physicians. A subgroup analysis examined efficacy in elderly patients (≥70 years) and factors predictive of survival were identified. Of 355 requests for ipilimumab, resulting in 288 treatments, completed questionnaires were received for 153 ipilimumab recipients (median age 58 years, 57.2% men). Efficacy was evaluated in 144 patients: complete response in 1.3%, PR in 9.6%, PR with previous progression 8.4%, stabilized disease in 14.5%, and progressive disease in 66.2%. The median OS was 6.5 months (199 days); 1-year survival was 32.9%. Predictive survival factors included lymphocytes over 1000/ml (P=0.0008) and lactate dehydrogenase more than 1.5×upper limit of normal (P=0.003). Cutaneous, hepatic, and gastrointestinal toxicities were mild. In 30 patients aged more than 70 years, ipilimumab efficacy and tolerability was similar to that of the overall population. In the clinical practice setting, ipilimumab is effective and well tolerated in patients with advanced melanoma, including elderly patients, when administered at the recommended dosage. Ipilimumab improves treatment options for patients who, until recently, have had little hope of an improved prognosis.
Keywords: Expanded Access Program, ipilimumab, melanoma, questionnaire, retrospective study
Introduction
The incidence of melanoma continues to increase more rapidly than any other malignancy, except for lung cancer in women 1. In 2008, estimates of annual age-standardized incidence were 2.8/100 000 population worldwide and 6.8/100 000 in Europe 2. In Spain, the estimated annual age-standardized melanoma incidence was 5.6/100 000 2. The prognosis for patients with advanced melanoma is poor as it is often highly resistant to chemotherapy, radiotherapy, hormonal therapy, and older immunotherapeutic approaches. As a result, the median overall survival (OS) of patients with metastatic disease is less than 9 months 3, with a 10-year observed survival rate of ∼10–15% 4.
Numerous treatment options, mostly local/regional, are available for patients presenting with metastatic disease. These include cytotoxic drugs such as dacarbazine, taxanes such as paclitaxel, nitrosoureas such as fotemustine, targeted therapy with vemurafenib for patients with the V600 BRAF gene mutation, and immunotherapy with interferon, high-dose interleukin-2, and ipilimumab 1,5. However, apart from ipilimumab and vemurafenib, most of these options have only yielded modest treatment response rates and negligible to modest improvements in survival in first-line and second-line treatment settings 3,6–9. Therefore, improving OS remains a key objective in this patient population 10.
Ipilimumab is a promising new immunotherapy 11. It is a fully human, recombinant monoclonal antibody targeted at cytotoxic T-lymphocyte antigen 4 (CTLA-4) 11. Ipilimumab blocks the inhibitory action of CTLA-4, causing T-cell activation and proliferation, thereby enhancing the immune response – specifically the cytotoxic T-cell-mediated antitumor response 11. In a phase III randomized trial, ipilimumab improved OS in patients with previously treated metastatic melanoma compared with a glycoprotein 100 peptide vaccine (gp100) 12. The median OS with ipilimumab plus gp100 was 10.0 months [hazard ratio (HR) for death vs. gp100 alone, 0.68; P<0.001] and 10.1 months with ipilimumab alone (HR for death vs. gp100 alone, 0.66; P=0.003), with no difference between the ipilimumab groups (HR, 1.04; P=0.76) 12. Ipilimumab is also effective in patients with untreated metastatic melanoma when administered in combination with dacarbazine 9. As a result of these data, ipilimumab is approved for use in Europe in adult patients with advanced (unresectable or metastatic) melanoma who have been treated previously 13. Ipilimumab is acknowledged in the European Society for Medical Oncology guidelines as being a ‘promising’ treatment for melanoma, but was not recommended as publication of these guidelines 5 preceded that of the phase III ipilimumab trial 12. Ipilimumab is approved in the USA in those with unresectable or metastatic disease 14, and is among the preferred regimens recommended by the National Comprehensive Cancer Network for patients with advanced or metastatic melanoma 1.
There is no evidence for the use of ipilimumab in Spain in a real-world setting. The manufacturer of ipilimumab made the drug available in Spain under an Expanded Access Program (EAP) following European marketing authorization but before drug launch. To date, outcomes data for patients enrolled in this program have not been published in full, although these and other retrospective studies using EAP data have been presented in abstract form at the Annual Meetings of the American Society of Clinical Oncology and the European Society for Medical Oncology 15–19.
The aim of this retrospective study carried out by the Spanish Melanoma Group [Grupo Español Multidisciplinar de Melanoma (GEM)] is to report clinical outcomes of patients treated with ipilimumab enrolled in the EAP. Data on demographics, and ipilimumab outcomes and toxicity were obtained from treating physicians involved in the EAP through questionnaires.
Materials and methods
Questionnaire design
Questionnaires were distributed to physicians with patients enrolled in the EAP. All physicians, members of the GEM, were mailed the questionnaire on three occasions 1 month apart. The period of data collection was mid-December 2011 to mid-March 2012. Physicians completed the questionnaires retrospectively for patients in their care receiving ipilimumab. Clinician participation was voluntary, without any remuneration.
The following information was requested in the questionnaire: hospital, sex, date of birth, metastasis site, starting serum lactate dehydrogenase (LDH) level, basal lymphocyte count, Eastern Cooperative Oncology Group (ECOG) performance status, previous adjuvant treatment, previous chemotherapy or novel treatments, previous treatment for metastatic disease, time from diagnosis of metastatic disease to first ipilimumab dose, the number of ipilimumab cycles received, reasons for receiving less than four cycles (i.e. reasons for discontinuation), serum LDH concentration at the end of ipilimumab treatment, lymphocyte count at the end of treatment, treatment response, overall and disease-free survival (DFS) rates, whether reinduction was administered, treatment response to reinduction, and toxicity and grade during and after reinduction. Treatment response and grading of toxicities was left to individual physician judgment according to standard practice, that is, physicians were not asked specifically to assess treatment response according to predefined criteria nor to grade toxicities according to common toxicity criteria.
Expanded Access Program
The EAP in Spain is part of a group of EAPs established by Bristol-Myers Squibb (New York, NY, USA) in European countries. This EAP was evaluated and authorized by the Spanish Ministry of Health and was not required to be submitted to an ethics committee as it was not a clinical trial.
All physicians who wanted to include patients in the EAP were required to register and complete an e-learning course on the management of drug toxicities and patterns of response before requesting the treatment by accessing the website http://www.ipilimumabcu.es. This website also provided information for other healthcare professionals and patients, and provided a list of references and help centers for management of toxicities. Once the e-learning course was completed, the physicians obtained authorization from Bristol-Myers Squibb and could then include patients in the EAP.
To participate in the EAP, as for any compassionate use of a medication, the patient had to sign a specific expanded-access informed consent form, after which the physician submitted an application through the http://www.ipilimumabcu.es website with information to enable Bristol-Myers Squibb to decide whether the patient was a suitable candidate for treatment. Once approved by Bristol-Myers Squibb, the patient was assigned a code that was used for registration with the Ministry of Health informing them of the patient’s inclusion in the EAP. Subsequently, Bristol-Myers Squibb delivered the medication to the hospital for treatment of the patient. The investigator was only obligated to report serious adverse events (AEs) and toxicity. Efficacy data did not have to be reported.
Patient inclusion criteria
EAP inclusion criteria in Spain included the following: age more than 18 years; progressive metastatic disease; no use of corticosteroids; absence of autoimmune disease, HIV, and hepatitis B or C infection; failure of treatment with at least one other systemic regimen; and no previous treatment with anti-CTLA-4 drugs.
Treatment
The ipilimumab dosage used in the EAP in Spain was 3 mg/kg administered intravenously every 21 days for four cycles. The patient was a candidate for reinduction therapy if there was significant response or stabilization lasting at least 3 months.
Response endpoints
The following efficacy outcomes were assessed retrospectively: complete response (CR); partial response (PR); disease progression, followed by posterior response; progressive disease; stable disease (SD); reinduction rate; response to reinduction; time to disease progression; and OS and DFS. Progression-free survival was not recorded.
Statistical analyses of questionnaire data
The questionnaires were submitted to the secretary of the GEM. A database on SPSS (IBM, Armonk, NY, USA) was created and the data were entered by one of the investigators without further validation of the data. Missing variables from incomplete questionnaires were coded as not available. Descriptive statistics were used for patient demographics, disease characteristics, and AEs. The median survival was estimated using the Kaplan–Meier method.
A descriptive analysis was carried out on the population who initially requested ipilimumab and on the 288 patients who received at least one dose of the drug. This analysis involved only demographic and descriptive variables related to treatment. A separate univariate analysis (using log-rank tests and Kaplan–Meier curves) was carried out with data from patients in the EAP database for whom treatment results were available.
A subgroup analysis of efficacy, tolerability, and predictive factors in patients aged at least 70 years was also carried out.
Results
Patients in the Expanded Access Program
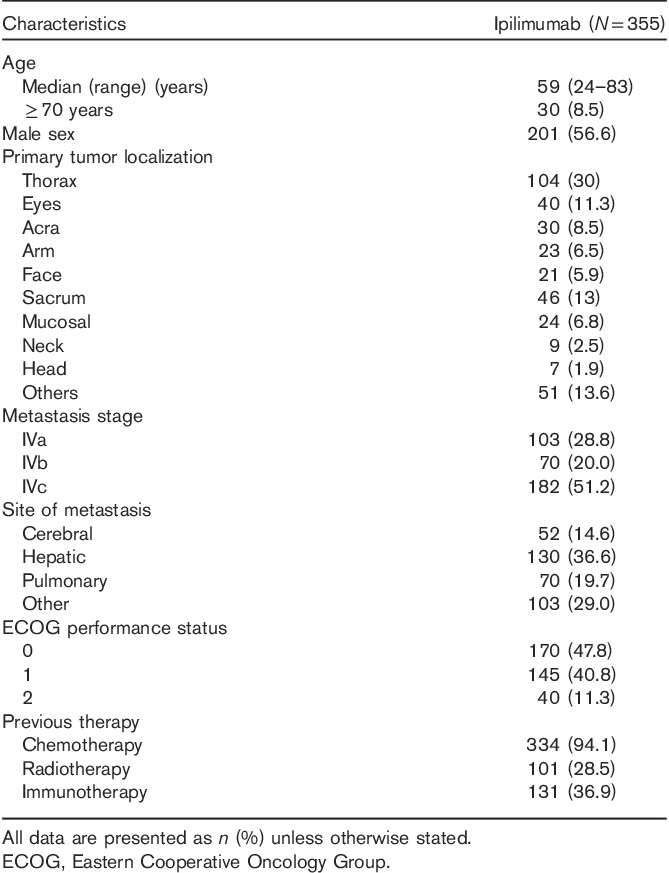
The Spanish EAP continued for ∼18 months (June 2010 to November 2011). Table 1 summarizes the patient baseline characteristics of the 355 patients for whom ipilimumab was requested under the EAP. In total, 288/355 (81%) patients received ipilimumab: 138/355 patients (39%) received the recommended four cycles of ipilimumab. The remaining 14% (50 patients), 13% (46 patients), 15% (54 patients), and 19% (67 patients) received three, two, one, and no cycles, respectively. Seventeen patients (5%) received reinduction, of whom eight received four cycles (2%).
Table 1.
Baseline characteristics of all ipilimumab-treated patients in the Spanish Expanded Access Program
Questionnaire respondents
In total, 31 of 88 physicians returned completed surveys for 153 patients. Baseline data were available for all 153 patients. Efficacy was evaluated in 144 patients as treatment response could not be evaluated in nine patients (three were still on treatment and six had just completed treatment).
Patient characteristics and treatment
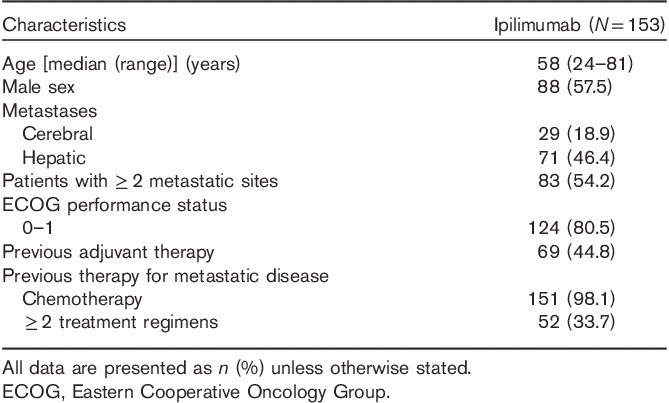
A total of 153 patients were included in the current analysis, representing ∼53.1% of the 288 patients administered ipilimumab under the EAP. Table 2 shows the patient baseline demographic and disease characteristics of the 153 patients. The median time between the diagnosis of metastasis and initiation of ipilimumab treatment was 11.2 months. Almost two-thirds of these patients received all four cycles of ipilimumab (60.9%). Of patients discontinuing before the fourth dose, the reason given was death or disease progression in 87% and toxicity in 3.7%.
Table 2.
Baseline characteristics of the ipilimumab-treated patients enrolled in the Spanish Expanded Access Program in Spain included in the physician surveys
Efficacy
Response
Responses to treatment for the 144 efficacy-evaluable patients were as follows: CR in 1.3% (two patients), PR in 9.6% (14 patients), PR with previous progression in 8.4% (12 patients), SD in 14.5% (21 patients), and disease progression in 66.2% (95 patients) (Fig. 1).
Fig. 1.
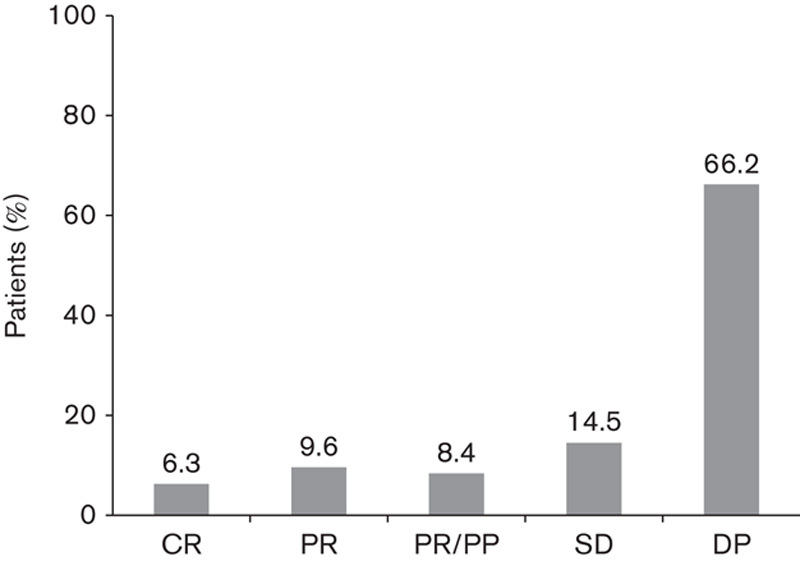
Treatment responses achieved with ipilimumab therapy in evaluable patients (n=144) according to treating physicians. CR, complete response; DP, disease progression; PR, partial response; PR/PP, partial response with previous progression; SD, stabilized disease.
Reinduction
Ipilimumab reinduction was requested in 13 cases and completed in eight; in five patients with a previous PR, two achieved a new PR and three achieved SD; and three patients with previous SD progressed.
Survival
The 1-year and 18-month OS rates were 32.9 and 28.8%, respectively, according to Kaplan–Meier survival estimates (Fig. 2). The median OS was 6.5 months (199 days) (95% confidence interval 125.3–272.6).
Fig. 2.
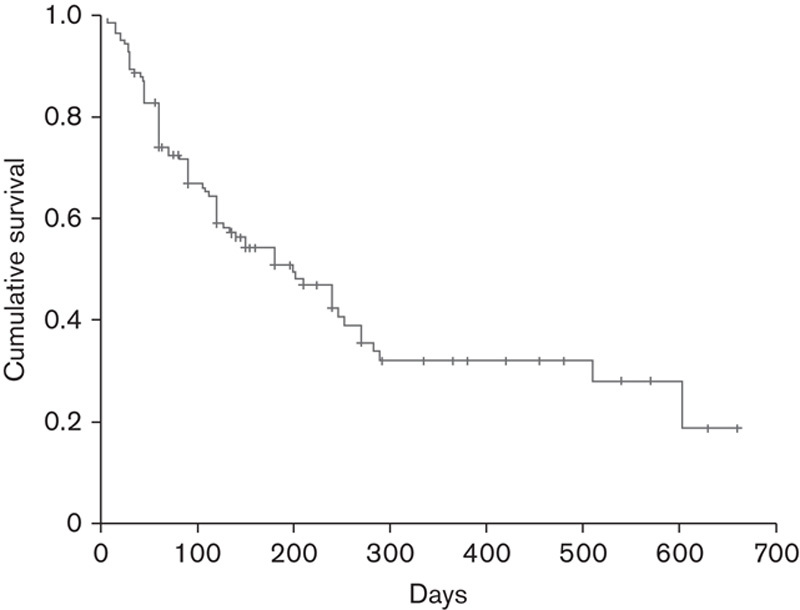
Cumulative survival achieved with ipilimumab therapy in evaluable patients (n=144) on the basis of Kaplan–Meier estimates.
Predictors of survival
Factors significantly predictive of survival included baseline lymphocyte counts over 1000/ml (P=0.0008) and LDH more than 1.5×upper limit of normal (P=0.003).
Brain metastases
Of the 29 patients with brain metastases (Table 2), only 28 were evaluable because one died after the first cycle before evaluation. The median age of these patients was 53.4 years (24–80) and they had highly advanced disease, with 3.7 (2–7) median number of metastases, median 1.7 lines of previous treatment, and a median LDH level of 511 IU/l. Of the 28 patients evaluated, 15 completed treatment, four received three cycles, four received two cycles, and five received only one cycle. The observed responses were as follows: PR in three patients (10.8%) (one conventional response and two with immunological response criteria); disease stabilization in two (7.1%); and progressive disease in 23 (82.2%). At the time of survival analysis, five patients remain alive and the median Kaplan–Meier survival was 120 days (32–208).
Tolerability
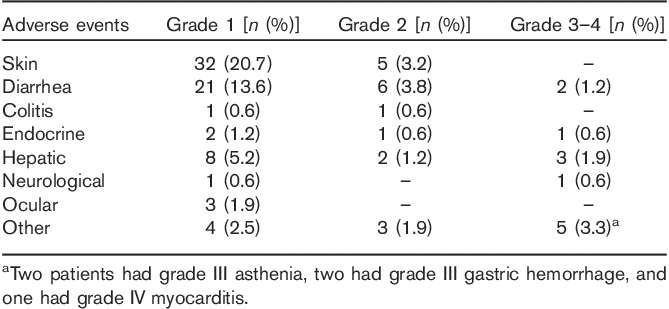
AEs reported during treatment with ipilimumab are shown in Table 3. Ipilimumab was well tolerated. Only nine patients (6.3%) had grade III or grade IV toxicity related to treatment. Two treatment-related severe AEs occurred: one episode of grade IV diarrhea and one case of immune grade IV myocarditis that led to death.
Table 3.
Adverse events during ipilimumab therapy for advanced melanoma in 153 treated patients
Subgroup analysis in the elderly
Of 34 patients aged more than 70 years, data were available for 30 patients for efficacy analysis (of the four nonevaluable patients, three had just completed the treatment and one was still on treatment). The median age was 75 years (range 70–81 years) and 53.5% were men. Approximately half had metastases in at least three locations (53.3%). Ipilimumab treatment was initiated at a median of 14.2 months after the diagnosis of metastatic disease. Most patients received all four planned cycles (76.7%). The reason for discontinuation was death because of progression or progression in all patients.
The treatment responses and survival rates achieved with ipilimumab treatment in this subgroup are reported in Table 4. Of the 30 patients, three achieved a PR, three achieved PR with previous progression (10%), and three achieved SD (10%). Disease progression was reported in 17 patients (56.7%). The median OS was 6 months (95% confidence interval 119.7–240.2) and the annual survival rate was 21%. No patients received reinduction treatment.
Table 4.
Efficacy of ipilimumab in 30 elderly patients (>70 years) evaluable for response outcomes

Significant predictors of survival were basal lymphocytes over 1000/ml (P=0.005) and LDH more than 1.5×upper limit of normal (P=0.027).
Ipilimumab was generally well tolerated in elderly patients. Toxicities in elderly patients were similar to those observed in the overall population. The most common treatment-related AEs were skin and liver toxicities and diarrhea. Only four patients experienced grade III or IV toxicities because of treatment.
Discussion
Ipilimumab administered at a dose of 3 mg/kg every 3 weeks for four cycles was well tolerated in the clinical practice setting in previously treated patients with advanced metastatic melanoma who were enrolled in an EAP in Spain. OS was 6.5 months, with one-third of patients surviving to 1 year and 29% to 18 months. DFS could not be determined because of lack of data. Although not comparable because of differences between patient populations, these efficacy data are worse than those shown in pivotal clinical trials of ipilimumab. In a phase III trial of ipilimumab in patients with unresectable stage III/IV metastatic melanoma failing on previous therapy, OS with ipilimumab 3 mg/kg monotherapy was 10.1 months, with 45.6% of patients surviving to 1 year and 33.2% to 18 months 12. In a phase II clinical trial in patients with previously treated stage III/IV melanoma, OS with ipilimumab 3 mg/kg monotherapy was 8.7 months, with 39.3% of patients surviving to 1 year and 30.2% surviving to 18 months 20. These differences are to be expected, given the poorer prognosis and ECOG status of patients in the EAP (89% ECOG 0–1 and 11% ECOG 2, data not shown) compared with those enrolled into clinical trials (all ECOG 0–1) and the more stringent selection criteria used in the clinical trial setting. In addition, in the EAP, treatment was requested for 355 patients and administered to only 288 as over 60 patients died before they could receive treatment, reflecting the poorer performance status condition of the general population compared with patient populations in clinical trials. The efficacy results in this population of patients, which was relatively unselected and had a poorer prognosis, more closely reflect those that would be expected in the real-world setting than those from a clinical trial.
Tolerability in the EAP was similar to that observed in clinical trials, with grade III/IV events observed in 6.3% in the EAP and 10–15% in the phase III clinical trial 12. As in the EAP, in the clinical trials, skin-related and gastrointestinal-related AEs (predominantly diarrhea) were the most commonly reported 12,20. Infrequent AEs have been reported in patients treated with ipilimumab 21. In our sample, we observed one patient with myocarditis, with a biopsy suggesting an autoimmune origin, and one upper digestive hemorrhage event in which toxicity or a necrosis of submucosal metastasis could not be ruled out as the cause.
This study is the first report from this EAP; although data are from physician answers to a questionnaire, rather than directly from patient case records, the findings of the study provide useful insight into the use of ipilimumab in the real-world setting. Two other studies have evaluated the ipilimumab experience in the clinical practice setting (i.e. under an expanded-access/compassionate use setting): one in Italy 22 and the other in the USA at the Memorial Sloan-Kettering Cancer Center 23. In these two studies, ipilimumab was administered at an investigational dose of 10 mg/kg every 3 weeks for four cycles, which is higher than that recommended 1,13. However, the protocol of both studies allowed the use of 3 mg/kg doses 22,23.
Our findings are only generalizable to second-line treatment using the same dose of 3 mg/kg as the Spanish EAP did not permit its use as first-line treatment. This was based on the European approval of the 3 mg/kg dose of ipilimumab in pretreated patients. Bristol-Myers Squibb has completed the recruitment of a well-controlled trial to compare the efficacy and tolerability of the 3 and 10 mg/kg dosages 10.
Predictors of survival identified in the current study are consistent with previous studies 6,24. The main factors shown in previous studies were site of metastasis and elevated serum LDH; hence, some melanoma staging systems include these factors for prognosis assessment, but several other factors have been shown to be associated with shorter survival, including neutrophil and leukocyte count 24, older age, poor performance status, male sex, and greater number of metastatic sites 6.
Few data are available on treated patients with brain metastases and most of them are from the analysis of data from EAPs. In 38 patients with brain metastases in the French EAP, a response was observed in 5.3% of patients, with a median survival of 101 days 25. Our data are similar to those in the French EAP, which suggests that patients had advanced disease at the time of treatment.
The efficacy and tolerability of ipilimumab in elderly patients was broadly similar to that of the overall patient population. There appeared to be no increase in immune-related AEs, suggesting that ipilimumab is a valuable option for older patients. On the basis of the drug’s clinical trial program, it was not certain that ipilimumab improved OS in women aged more than 50 years 10. However, our small subgroup analysis of 30 elderly patients included 14 women; a post-hoc survival analysis by sex found that there was no difference in survival between men and women (data not shown).
Approximately 40% of patients in the current study did not receive all four cycles because of early progression or death. It seems important then to improve patient selection for treatment. Patients with asymptomatic low-volume metastases might be ideal candidates as, in such patients, there may be time for an antitumor immune response to develop before the disease progresses 1.
Data indicate that patients with the BRAF V600 mutation should be treated with the BRAF inhibitor vemurafenib 1,26,27. Findings from a recently published small retrospective study comparing vemurafenib or dabrafenib and ipilimumab therapy in patients with the BRAF mutation suggest that some patients identified as rapid progressors after failing BRAF inhibitor therapy should receive ipilimumab first, followed by a BRAF inhibitor as they may not survive long enough after failing treatment with a BRAF inhibitor to then complete a course of ipilimumab 28. The study found that elevated LDH, low performance status, and the presence of brain metastases were all predictors of rapid progression on relapse with a BRAF inhibitor 28. In our study, the BRAF mutation was not evaluated because during the period of the EAP, vemurafenib was only accessible in clinical trials in Spain. Some patients participated in clinical trials for vemurafenib after ipilimumab treatment, but they were very few and their results were not collected.
This study is subject to the usual limitations and bias associated with retrospective uncontrolled analyses. In addition, as it is questionnaire-based, the findings are subject to potential variability between respondents in defining the treatment response and severity of AEs. The study may also be subject to selection bias as it included only 53% of patients included in the Spanish EAP. The data for the elderly population should be considered indicative as the number of patients was very small. It is also worth noting that although the EAP population was not strictly selected and included patients who would have been excluded from a clinical trial because of their poor overall medical condition, these results may be more representative of patients seen in the clinical setting. Nevertheless, because of this, these results are not likely to be replicable in a clinical trial.
Conclusion
In the clinical practice setting, ipilimumab is well tolerated in patients with advanced melanoma, including those aged more than 70 years, when administered at the recommended dose. Identifying patients for treatment with ipilimumab to maximize benefits in OS observed with the complete regimen of four cycles of drug is the next step in improving treatment options for a patient population who until recently have had little hope of an improved prognosis.
Acknowledgements
Medical writing assistance was provided by Tracy Harrison and Mary Hines of Springer HealthcareCommunications. This assistance was funded by Bristol-Myers Squibb. The authors thank the following physicians who were part of the EAP and provided valuable patient data: A. Ballesteros, Hospital Universitario La Princesa, Madrid; J.P. Berros, Hospital Universitario Central de Asturias, Oviedo; R. Blanco, Hospital de Terrasa, Barcelona; B. Campos, Hospital LucusAugusti, Lugo; P. Cerezuela, Hospital Santa Maria del Rosell, Cartagena; D. Cumplido, Hospital de Torrevieja, Alicante; V. Escrig, Hospital Clinico, Valencia; A. Garcia Castaño, Hospital Marques de Valdecilla, Santander; I. Gil, Hospital Reina Sofia, Navarra; M Gonzalez Cao, Hospital Dexeus, Barcelona; M Hidalgo, H de Txagorritxu, Victoria; L. Leon, Hospital Clinico Universitario de Santiago de Compostela, Santiago de Compostela; I. Marquez, Hospital Gregorio Marañon, Madrid; J.I. Mayordomo, Hospital Clinico Zaragoza, Zaragoza; J. Medina, Hospital Virgen de la Salud, Toledo; P. Mut, Hospital Son Llatzer, Mallorca; E. Ortega, Hospital Arnau de Villanova, Lerida; D. Rodriguez Abreu, Hospital Universitario Insular de Gran Canaria, Gran Canaria; P. Sancho, Hospital Reina Sofia, Sevilla; M. Sereno, Hospital Infanta Sofia, Madrid; A. Soria, Hospital Ramon y Cajal, Madrid; T. Puertolas, Hospital Miguel Servet, Zaragoza; F. Zambrana, Hospital Infanta Sofia, Madrid.
Conflicts of interest
Dr Berrocal has received consultancy fees and travel support from Bristol-Myers Squibb, and consultancy and expert testimony payment for lectures from Roche. Dr Arance has received support for travel, fees for advisory committees, and consultancy fees from Bristol-Myers Squibb, Roche, and GSK; also, she has received payment for lectures from Bristol-Myers Squibb, and Roche, and for educational presentations from Bristol-Myers Squibb. Dr López Martin has received consultancy and lecture fees, and also grants for clinical research from Bristol-Myers Squibb. Dr Espinosa has received consultancy fees in the past and payment for lectures from Bristol-Myers Squibb. Dr Valdivia has received payment for participating on several review boards for Bristol-Myers Squibb. Dr Martín-Algarra has received board membership treatments and lecture fees from several companies. For the remaining authors there are no conflicts of interest.
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Melanoma: Version 2 2013Washington:National Comprehensive Cancer Network; Available at: http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. [Accessed 28 May 2013]. [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2 Cancer incidence and mortality worldwide: IARC Cancer Base No. 10. 2010. Available at: http://globocan.iarc.fr [Accessed 28 May 2013].
- 3.Gasent Blesa JM, Grande Pulido E, Alberola Candel V, Provencio Pulla M. Melanoma: from darkness to promise. Am J Clin Oncol 2011; 34:179–187. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Melanoma skin cancer 2013New York:American Cancer Society; Available at: http://www.cancer.org/acs/groups/cid/documents/webcontent/003120-pdf.pdf. [Accessed 28 May 2013]. [Google Scholar]
- 5.Dummer R, Hauschild A, Guggenheim M, Jost L, Pentheroudakis G. ESMO Guidelines Working Group. Melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010; 21Suppl 5v194–v197. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park) 2009; 23:488–496. [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle GM. Therapy for metastatic melanoma: an overview and update. Expert Rev Anticancer Ther 2011; 11:725–737. [DOI] [PubMed] [Google Scholar]
- 8.Lee B, Mukhi N, Liu D. Current management and novel agents for malignant melanoma. J Hematol Oncol 2012; 5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517–2526. [DOI] [PubMed] [Google Scholar]
- 10.Hanaizi Z, van Zwieten-Boot B, Calvo G, Lopez AS, van Dartel M, Camarero J, et al. The European Medicines Agency review of ipilimumab (Yervoy) for the treatment of advanced (unresectable or metastatic) melanoma in adults who have received prior therapy: summary of the scientific assessment of the Committee for Medicinal Products for Human Use. Eur J Cancer 2012; 48:237–242. [DOI] [PubMed] [Google Scholar]
- 11.Sanford M. Ipilimumab: in previously treated patients with advanced melanoma. BioDrugs 2012; 26:185–193. [DOI] [PubMed] [Google Scholar]
- 12.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bristol-Myers Squibb Pharma EEIG. Yervoy 5 mg/ml concentrate for solution for infusion: summary of product characteristics 2012London:Bristol-Myers Squibb Pharma EEIG; Available at: http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002213/WC500109299.pdf. [Accessed 28 May 2013]. [Google Scholar]
- 14.Bristol-Myers Squibb Company. Yervoy (ipilimumab) prescribing information 2013New York:Bristol-Myers Squibb Company; Available at: http://packageinserts.bms.com/pi/pi_yervoy.pdf. [Accessed 28 May 2013]. [Google Scholar]
- 15.Arance A, Blanco R, LópezCriado P, Leon L, Rodriguez Abreu D, Espinosa E, et al. Experience of the Spanish Melanoma Group with the ipilimumab Expanded Access Program. Annual Meeting of the Spanish Society of Medical Oncology (SEOM). Madrid, Spain; 2012.
- 16.Berrocal A, Lopez-Martin JA, Arance AM, Soriano V, Espinosa E, Pilar Lopez Criado M, et al. Spanish experience with the ipilimumab Expanded Access Program [abstract]. J Clin Oncol 2012; 30:e19023. [Google Scholar]
- 17.Di Giacomo AM, Calabrò L, Danielli R, Pesce I, Fonsatti E, Bertocci E, et al. Long term survival and immunological correlates in metastatic melanoma treated with ipilimumab at 10 mgs within an Expanded Access Program [abstract 1117PD]. Ann Oncol 2012; 23:ix361–ix375. [Google Scholar]
- 18.Lopez Martin JA, Cao MG, Sereno M, Mayordomo J, Hidalgo M, Campos B, et al. Ipilimumab in older patients: Spanish melanoma multidisciplinary group (GEM) experience in the Expanded Access Programme (EAP) [abstract 1151]. Ann Oncol 2012; 23:ix361–ix375. [Google Scholar]
- 19.Martin Algarra S, Alonso L, Valdivia J, Garcia Castaño A, Escrig V, Mut P, et al. Spanish melanoma multidisciplinary group (GEM) experience with ipilimumab (IPI) in the Expanded Access Programme (EAP) [abstract 1150]. Ann Oncol 2012; 23:ix361–ix375. [Google Scholar]
- 20.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010; 11:155–164. [DOI] [PubMed] [Google Scholar]
- 21.Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One 2013; 8:e53745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Giacomo AM, Danielli R, Calabrò L, Bertocci E, Nannicini C, Giannarelli D, et al. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy). Cancer Immunol Immunother 2011; 60:467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer 2010; 116:1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt H, Suciu S, Punt CJ, Gore M, Kruit W, Patel P, et al. Pretreatment levels of peripheral neutrophils and leukocytes as independent predictors of overall survival in patients with American Joint Committee on Cancer Stage IV Melanoma: results of the EORTC 18951 Biochemotherapy Trial. J Clin Oncol 2007; 25:1562–1569. [DOI] [PubMed] [Google Scholar]
- 25.Konstantinou MP, Dutriaux C, Gaudy-Marqueste C, Mortier L, Bedane C, Girard C, et al. Ipilimumab in melanoma patients with brain metastasis: a retro-spective multicentre evaluation of thirty-eight patients. Acta Derm Venereol 2014; 94:45–49. [DOI] [PubMed] [Google Scholar]
- 26.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012; 366:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ascierto PA, Simeone E, Giannarelli D, Grimaldi AM, Romano A, Mozzillo N. Sequencing of BRAF inhibitors and ipilimumab in patients with metastatic melanoma: a possible algorithm for clinical use. J Transl Med 2012; 10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]


