Abstract
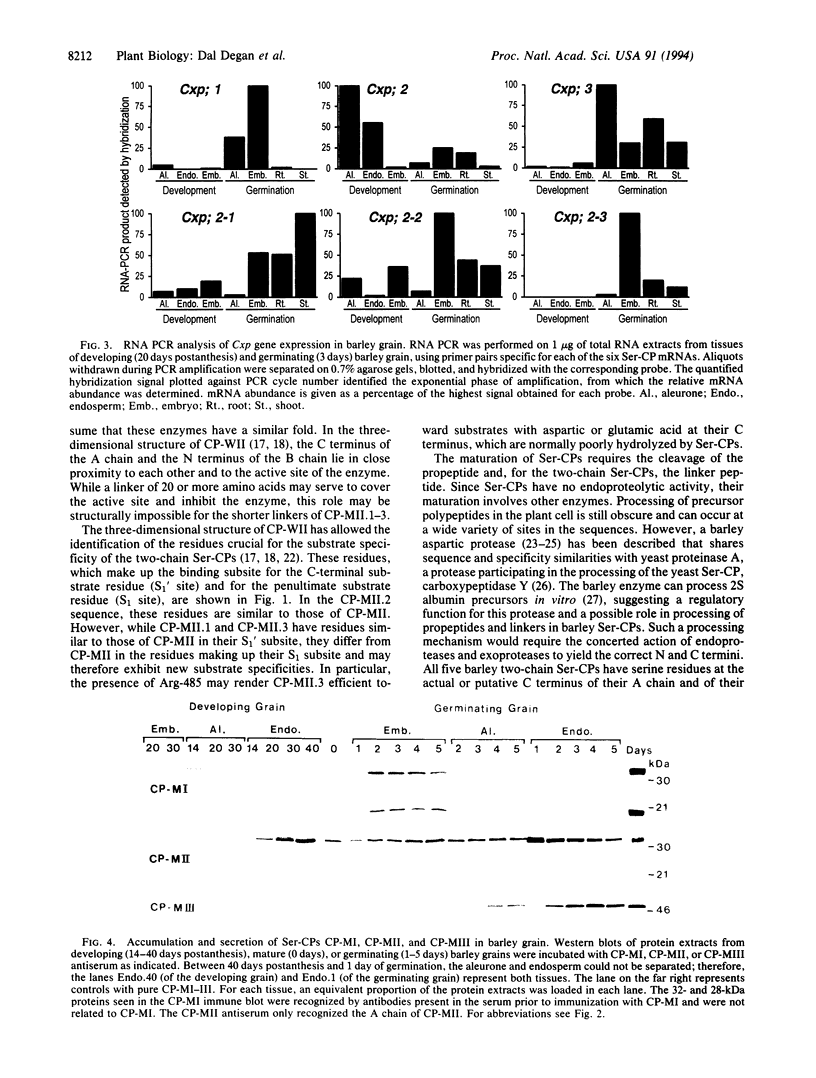
cDNA clones encoding three additional serine carboxypeptidases (Ser-CPs) have been isolated from a gibberellic acid-induced barley aleurone cDNA library. The three deduced Ser-CPs belong to the two-chain subfamily of Ser-CPs; they are synthesized as precursors with a putative signal peptide, propeptide, and linker peptide between the A and B chains. Their identification provides the proof for the existence of more than three Ser-CPs in cereal grains, and, based on their sequences, they may exhibit new substrate specificities. The expression of these and of the three previously isolated Ser-CPs from barley grains (CP-MI, CP-MII, and CP-MIII) has been investigated by Northern and Western analysis and RNA PCR. CP-MII is the only Ser-CP to be expressed and accumulate in the developing grain and is stored in its active form in the mature grain. All six Ser-CPs are expressed de novo in the germinating grain, in the scutellum, and/or in the aleurone. Furthermore, at least CP-MI, CP-MII, and CP-MIII are secreted into the endosperm. In addition, all Ser-CPs (except CP-MI) are also expressed in the roots and shoots of the growing seedling. This enzyme family thus appears to be ubiquitous in the barley plant, which suggests that Ser-CPs play additional roles besides their participation in the mobilization of storage proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulcombe D. C., Barker R. F., Jarvis M. G. A gibberellin responsive wheat gene has homology to yeast carboxypeptidase Y. J Biol Chem. 1987 Oct 5;262(28):13726–13735. [PubMed] [Google Scholar]
- D'Hondt K., Bosch D., Van Damme J., Goethals M., Vandekerckhove J., Krebbers E. An aspartic proteinase present in seeds cleaves Arabidopsis 2 S albumin precursors in vitro. J Biol Chem. 1993 Oct 5;268(28):20884–20891. [PubMed] [Google Scholar]
- Doan N. P., Fincher G. B. The A- and B-chains of carboxypeptidase I from germinated barley originate from a single precursor polypeptide. J Biol Chem. 1988 Aug 15;263(23):11106–11110. [PubMed] [Google Scholar]
- Hammerton R. W., Ho T. H. Hormonal regulation of the development of protease and carboxypeptidase activities in barley aleurone layers. Plant Physiol. 1986 Mar;80(3):692–697. doi: 10.1104/pp.80.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck G. R., Chamberlain A. K., Ho T. H. Barley embryo globulin 1 gene, Beg1: characterization of cDNA, chromosome mapping and regulation of expression. Mol Gen Genet. 1993 May;239(1-2):209–218. doi: 10.1007/BF00281620. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Payne J. W. The Peptide pools of germinating barley grains: relation to hydrolysis and transport of storage proteins. Plant Physiol. 1981 Apr;67(4):785–792. doi: 10.1104/pp.67.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kervinen J., Sarkkinen P., Kalkkinen N., Mikola L., Saarma M. Hydrolytic specificity of the barley grain aspartic proteinase. Phytochemistry. 1993 Mar;32(4):799–803. doi: 10.1016/0031-9422(93)85208-9. [DOI] [PubMed] [Google Scholar]
- Leah R., Tommerup H., Svendsen I., Mundy J. Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J Biol Chem. 1991 Jan 25;266(3):1564–1573. [PubMed] [Google Scholar]
- Liao D. I., Breddam K., Sweet R. M., Bullock T., Remington S. J. Refined atomic model of wheat serine carboxypeptidase II at 2.2-A resolution. Biochemistry. 1992 Oct 13;31(40):9796–9812. doi: 10.1021/bi00155a037. [DOI] [PubMed] [Google Scholar]
- Liao D. I., Remington S. J. Structure of wheat serine carboxypeptidase II at 3.5-A resolution. A new class of serine proteinase. J Biol Chem. 1990 Apr 25;265(12):6528–6531. doi: 10.2210/pdb2sc2/pdb. [DOI] [PubMed] [Google Scholar]
- Mechler B., Müller H., Wolf D. H. Maturation of vacuolar (lysosomal) enzymes in yeast: proteinase yscA and proteinase yscB are catalysts of the processing and activation event of carboxypeptidase yscY. EMBO J. 1987 Jul;6(7):2157–2163. doi: 10.1002/j.1460-2075.1987.tb02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikola L. Acid Carboxypeptidases in Grains and Leaves of Wheat, Triticum aestivum L. Plant Physiol. 1986 Jul;81(3):823–829. doi: 10.1104/pp.81.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen K., Kielland-Brandt M. C. Altering substrate preference of carboxypeptidase Y by a novel strategy of mutagenesis eliminating wild type background. Protein Eng. 1993 Jun;6(4):409–415. doi: 10.1093/protein/6.4.409. [DOI] [PubMed] [Google Scholar]
- Ranki H., Sopanen T., Voutilainen R. Localization of carboxypeptidase I in germinating barley grain. Plant Physiol. 1990 Aug;93(4):1449–1452. doi: 10.1104/pp.93.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S. K., Welinder K. G., Hejgaard J. cDNA cloning, characterization and expression of an endosperm-specific barley peroxidase. Plant Mol Biol. 1991 Feb;16(2):317–327. doi: 10.1007/BF00020562. [DOI] [PubMed] [Google Scholar]
- Rastogi V., Oaks A. Hydrolysis of storage proteins in barley endosperms : analysis of soluble products. Plant Physiol. 1986 Jul;81(3):901–906. doi: 10.1104/pp.81.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runeberg-Roos P., Törmäkangas K., Ostman A. Primary structure of a barley-grain aspartic proteinase. A plant aspartic proteinase resembling mammalian cathepsin D. Eur J Biochem. 1991 Dec 18;202(3):1021–1027. doi: 10.1111/j.1432-1033.1991.tb16465.x. [DOI] [PubMed] [Google Scholar]
- Schneiderbauer A., Sandermann H., Jr, Ernst D. Isolation of functional RNA from plant tissues rich in phenolic compounds. Anal Biochem. 1991 Aug 15;197(1):91–95. doi: 10.1016/0003-2697(91)90360-6. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Smeekens S. P., Ohagi S., Chan S. J. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992 Nov 25;267(33):23435–23438. [PubMed] [Google Scholar]
- Svendsen I., Hofmann T., Endrizzi J., Remington S. J., Breddam K. The primary structure of carboxypeptidase S1 from Penicillium janthinellum. FEBS Lett. 1993 Oct 25;333(1-2):39–43. doi: 10.1016/0014-5793(93)80371-z. [DOI] [PubMed] [Google Scholar]
- Søgaard M., Olsen F. L., Svensson B. C-terminal processing of barley alpha-amylase 1 in malt, aleurone protoplasts, and yeast. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8140–8144. doi: 10.1073/pnas.88.18.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen S. B., Svendsen I., Breddam K. Primary structure of carboxypeptidase III from malted barley. Carlsberg Res Commun. 1989;54(5):193–202. doi: 10.1007/BF02904473. [DOI] [PubMed] [Google Scholar]
- Valls L. A., Hunter C. P., Rothman J. H., Stevens T. H. Protein sorting in yeast: the localization determinant of yeast vacuolar carboxypeptidase Y resides in the propeptide. Cell. 1987 Mar 13;48(5):887–897. doi: 10.1016/0092-8674(87)90085-7. [DOI] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washio K., Ishikawa K. Structure and expression during the germination of rice seeds of the gene for a carboxypeptidase. Plant Mol Biol. 1992 Jul;19(4):631–640. doi: 10.1007/BF00026789. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]