Abstract
Adolescence is a period of development in which peer relationships become especially important. A computer-based game (Cyberball) has been used to explore the effects of social exclusion in adolescents and adults. The current functional magnetic resonance imaging (fMRI) study used Cyberball to extend prior work to the cross-sectional study of younger children and adolescents (7 to 17 years), identifying age-related changes in the neural correlates of social exclusion across the important transition from middle childhood into adolescence. Additionally, a control task illustrated the specificity of these age-related changes for social exclusion as distinct from expectancy violation more generally. During exclusion, activation in and functional connectivity between ventrolateral prefrontal cortex and ventral anterior cingulate cortex increased with age. These effects were specific to social exclusion and did not exist for expectancy violation. Our results illustrate developmental changes from middle childhood through adolescence in both affective and regulatory brain regions during social exclusion.
Introduction
Adolescence represents a period of significant neural, behavioral, and social development (Blakemore, 2008; Coleman & Hendry, 1999; Giedd, Blumenthal, Jeffries, Castellanos, Liu, Zijdenbos, Paus, Evans & Rapoport, 1999; Steinberg, 2005). During this period, as relationships of primary importance shift from parents to peers, interactions with peers become especially salient (Spear, 2000; Steinberg & Morris, 2001). Coupled with this rise in salience of peer relationships is an increased sensitivity to peer exclusion (Rudolph & Hammen, 1999; Steinberg & Morris, 2001). Social exclusion has been shown to elicit a host of negative psychological effects, including increases in self-deflating behavior and externalizing behavior problems (Laird, Jordan, Dodge, Pettit & Bates, 2001; Twenge, Catanese & Baumeister, 2002), decreases in prosocial behavior and general mental health (Twenge, Baumeister, DeWall, Ciarocco & Bartels, 2007; Rigby, 2000), and increases in social avoidance and depression (Gazelle & Ladd, 2003; Gazelle & Rudolph, 2004). Thus, it follows that the nature of peer relationships in adolescence would make this phase of development one of heightened sensitivity to the negative effects of exclusion.
As it stands, adolescence is a period of mental health vulnerability, with many psychological disorders showing their initial symptoms during this stage of development (Kessler, Amminger, Aguilar-Gaxiola, Alonso, Lee & Ustun, 2007). As a result, the association between adolescent mental health and environmental factors such as social exclusion is important to explore. In addition to the aforementioned psychological effects of exclusion, past work has shown that neural responses to social exclusion were predictive of subsequent depressive symptoms in a group of early adolescents (Masten, Eisenberger, Borofsky, McNealy, Pfeifer & Dapretto, 2011). To expand previous work focused exclusively on adolescents, a developmental approach to these questions is essential.
In exploring the nature of age-related changes in the experience of social exclusion from childhood through adolescence, the investigation of brain function is of specific interest in the context of evidence indicating striking structural brain changes that occur during this period (see Giedd & Rapoport, 2010, for review). Nonlinear structural changes have been demonstrated in frontal cortex from childhood through adolescence, with gray matter volume peaking at 11–12 years (Giedd et al., 1999). In addition, distinct patterns of gray matter maturation have been shown to exist for different brain regions (Gogtay, Giedd, Lusk, Hayashi, Greenstein, Vaituzis, Nugent, Herman, Clasen, Toga, Rapoport & Thompson, 2004), with total gray and white matter volumes showing sexually dimorphic developmental patterns through childhood and adolescence (Lenroot, Gogtay, Greenstein, Wells, Wallace, Clasen, Blumenthal, Lerch, Zijdenbos, Evans, Thompson & Giedd, 2007). Further work has shown decreases in frontal cortex gray matter volume from childhood to adolescence, coupled with increases in white matter volume in the same region (Sowell, Trauner, Gamst & Jernigan, 2002). These structural findings in frontal cortex are of relevance to the present study, as medial and lateral frontal cortices have repeatedly been implicated in the neural processing of social exclusion. Though some previous work has found that age-related changes in brain function could not be fully described by structural maturation (Dumontheil, Hassan, Gilbert & Blakemore, 2010), other work has shown that developmental changes in white matter integrity from childhood to adolescence correlate with specific cognitive functions (Nagy, Westerberg & Klingberg, 2004). Based on the aforementioned evidence of structural and functional brain development occurring through childhood and adolescence, we hypothesize that psychological and neural correlates of sensitivity to social exclusion will be less evident in children, and increase with age.
A growing number of behavioral and neuroimaging studies have used a virtual interactive game called Cyberball, where participants are periodically included and excluded from a three-person computer ball-toss, to investigate social exclusion and related processes (Williams, Cheung & Choi, 2000). To date, Cyberball has been used to examine the neural correlates of social exclusion in adolescents and adults (Eisenberger, Lieberman & Williams, 2003; Krill & Platek, 2009; Masten, Eisenberger, Borofsky, Pfeifer, McNealy, Mazziotta & Dapretto, 2009; Onoda, Okamoto, Nakashima, Nittono, Ura & Yamawaki, 2009; Sebastian, Tan, Roiser, Viding, Dumontheil & Blakemore, in press). This research has identified regions involved in the experience of social exclusion including ventrolateral prefrontal cortex (vlPFC), insula, and anterior cingulate cortex (ACC). Self-reported distress during social exclusion has been shown to correlate with activation in different sub-regions of the ACC including dorsal ACC (Eisenberger et al., 2003) and subgenual ACC (Masten et al., 2009), and thus ACC has a hypothesized role in the negative emotional experience of exclusion. In contrast, right ventrolateral prefrontal cortex activation has been shown to correlate negatively with distress, pointing to a role for this region in emotion regulation (Eisenberger et al., 2003; Masten et al., 2009).
Studies comparing brain responses to social exclusion in adolescents and adults have consistently noted age-related changes in neural processing of exclusion. One recent fMRI study explored the neural correlates of Cyberball-elicited social exclusion in adolescence and demonstrated positive correlations between activity in subgenual ACC and right insula and self-reported distress, and negative correlations between distress and activity in ventrolateral prefrontal cortex and ventral striatum (Masten et al., 2009). Though this study did not directly compare adolescents to adults, the authors noted that in an adult study using the same paradigm, subgenual ACC was not significantly active. Behavioral studies demonstrate that adolescents (and not adults) report decreases in overall mood following Cyberball-elicited social exclusion (Sebastian, Viding, Williams & Blakemore, 2010b), and adolescents show greater physiological stress responses to peer rejection than do children (Stroud, Foster, Papandonatos, Handwerger, Granger, Kivlighan & Niaura, 2009), supporting the hypothesis that age-related differences in ACC activation to exclusion may relate to a heightened emotional response to social exclusion in adolescence. Two fMRI studies that directly compared adolescent and adult responses to rejection-themed stimuli and Cyberball exclusion found that adults showed activation in right ventrolateral prefrontal cortex, but adolescents did not (Sebastian et al., in press; Sebastian, Roiser, Tan, Viding, Wood & Blakemore, 2010a). These findings provide evidence that decreased emotion regulation during social exclusion may be coupled with the heightened emotional response to rejection in adolescence. Further, an fMRI study using a social feedback paradigm demonstrated developmental effects on processing social feedback in medial and lateral prefrontal regions from childhood into early adulthood (Moor, van Leijenhorst, Rombouts, Crone & van der Molen, 2010). Despite this breadth of work, the specificity of these observed age effects to social exclusion (separate from other distressing social interactions not involving rejection) remains unexplored. Additionally, while changes in processing exclusion from adolescence to adulthood are interesting given the importance of peer relationships in adolescence, investigating the earlier transition into adolescence is equally important to elucidate characteristics of brain activity in response to social exclusion that may be unique to adolescence and/or characterize the transition from middle childhood to adolescence.
As developmental investigations of social cognition advance, so does the need for more specific contrasts to elucidate the precise contributions of brain regions in the neural systems recruited during dynamic social exchanges, such as Cyberball. One such contrast lies in the distinction between ostracism and the violation of expectations. Specifically, in Cyberball, exclusion is distressing, but also violates an implicit expectation of being included (Somerville, Heatherton & Kelley, 2006). Thus, as a contrast in the present study, we used a similar game called Cybershape to elicit a social expectancy violation without excluding the participant. In this way, we attempted to establish the specificity of developmental effects to social exclusion as distinct from social expectancy violation, thus isolating the specific experience of rejection from a more general experience of social distress. In addition, our design allowed us to evaluate the presence of a functional dissociation between processing social exclusion and rule violation in children as has previously been demonstrated in adults (Bolling, Pitskel, Deen, Crowley, McPartland, Mayes & Pelphrey, 2011; Somerville et al., 2006).
The present study extends previous work with an examination of the neural response to social exclusion in a sample spanning middle childhood and adolescence. To investigate the development of brain mechanisms involved in processing social exclusion, we conducted an fMRI experiment using a version of Cyberball to elicit feelings of social exclusion in children and adolescents. We hypothesized that we would observe activation increasing with age from childhood into adolescence in key brain regions previously implicated in the processing of social exclusion, including ventral ACC and right insula. We also expected to find age-related changes in activation of ventrolateral prefrontal cortex based on previous evidence of this region's involvement in the processing of social exclusion in adults but not adolescents. We hypothesized that differences in activation of brain regions implicated in the processing of social exclusion may be echoed by age-related changes in functional connectivity between these regions. To this end, we explored task-related functional connectivity to ventral ACC, a region repeatedly implicated in the emotional processing of social exclusion, in order to try and identify a network of brain regions that may support a hypersensitivity to exclusion in adolescence. The investigation of early developmental changes in the neural processing of social exclusion is essential in determining the specificity of the adolescent neural profile of responses to peer rejection. In addition, exploring the emergence of this neural profile has the potential to inform efforts to identify periods of highest vulnerability to the negative psychological effects of social exclusion.
Experimental procedures
Participants
Participants consisted of a group of 26 typically developing children and adolescents (19 male, mean age = 12.55 years ± 2.64, 7.75 to 17.58 years). Twenty-five of the participants played Cyberball and Cybershape consecutively in the same scanning session in a counterbalanced order (one participant played Cybershape only). Following the scan all participants verbally confirmed feeling excluded during Cyberball, and noticing rules being broken in Cybershape. Four participants were excluded from Cyberball analyses, two for excessive head motion (> 2.5 mm between consecutive volume acquisitions or > 4 mm total drift from starting point), one for having previously played a version of Cyberball (and being debriefed on the fictitious nature of the players), and one for poor coregistration due to head drift during the scan. Seven participants were excluded from Cybershape analysis, three for excessive errors while playing (> 3 errors), three for head movement (> 2.5 mm between consecutive volume acquisitions or > 4 mm total drift from starting point), and one for poor coregistration due to head drift during the scan. One participant was only analyzed on the first eight (of 10) blocks of Cyberball due to excessive movement in the final minute of the scan. In sum, 21 participants (15 male, 12.90 years ± 2.59, 7.75 to 17.58 years) were included in the Cyberball study and 19 participants (14 male, 13.19 years ± 2.24, 9.42 to 17.58 years) were included in the Cybershape study. Of the 21 Cyberball participants, 11 played Cyberball first. Of the 19 Cybershape participants, nine played Cyberball first. Sixteen of these participants overlapped, having usable data from both games (12 male). Six participants included in the analysis admitted to believing that they had been playing against real people (mean age 11.61 years, 9.5 to 14.5 years). The remainder of participants suspected that the online players were not real, though behavioral evidence from adults has shown that even when participants know they are playing with computerized opponents exclusion still causes significant distress (Zadro, Williams & Richardson, 2004). The average number of participant errors (shape thrown to the wrong player by the participant) in Cybershape was 0.95 (± 0.97; maximum: 3), with seven of the participants making no errors. Written informed consent was obtained from each participant's parent(s) according to a protocol approved by the Yale University Human Investigation Committee. Verbal and written assent was obtained from each participant as well.
Experimental design
Cyberball
A game of Cyberball began with a mock Google® search engine screen followed by a ‘loading’ screen where the participant was told that he or she was being logged into the game by the experimenter. As the game loaded, the experimenter informed participants that they were being connected through the Internet so they could play with other children online. The participants were given two button boxes, one in each hand, which allowed them to throw the virtual ball to either the right or left. Prior to starting game play, instructions were delivered visually and auditorily, and the participants practiced playing the game for 16 throws. Once understanding of the game was confirmed, the fMRI session began and participants continued to play the Cyberball game for 5 minutes in 10 continuous, alternating blocks of fair play and exclusion. As in prior studies (Bolling et al., 2011; Bolling, Pitskel, Deen, Crowley, McPartland, Kaiser, Wyk, Wu, Mayes & Pelphrey, in press), an alternating block design was utilized over the original Cyberball design of inclusion followed by exclusion to reduce noise due to order effects and scanner drift, and to prevent disengagement from the task in a prolonged period of exclusion. Because the current study targeted young participants, increased movement and decreased attention at the end of a scan would disproportionately affect exclusion in a non-alternating design. Further, children might lose interest if excluded from the game for long periods of time, which made shorter, transient periods of exclusion more desirable. Each block consisted of 12 throws, lasting a total of 30 seconds. In fair play, participants received the ball for one-third of the throws; in exclusion, participants never received the ball. If the participant did not throw the ball within 3 seconds of receiving it, the ball was thrown automatically (to a randomly determined player). Participant failure to throw happened rarely, on average 0.47 times in Cyberball and 0.37 times in Cybershape. The computer players' pictures were matched on gender and ethnicity to each participant to intensify feelings of exclusion (Wirth & Williams, 2009). The players pictured appeared to be in late childhood and early adolescence. Immediately following the game, while the participant was still in the scanner, a 10-item questionnaire was given to 20 of the 21 participants analyzed in Cyberball. No fMRI data were acquired during the questionnaire administration, and questions appeared visually and auditorily, during which participants could communicate with experimenter if any questions were unclear. The questionnaire was an abbreviated version of the Needs Threat Scale (van Beest & Williams, 2006), used to assess distress following Cyberball exclusion (Eisenberger et al., 2003; Williams, 2007), and has been shown to be reliable and valid in previous work (Bolling et al., 2011). Participants were asked to rate statements about feelings of control, meaningful existence, belongingness, and self-esteem on a Likert scale from 1 = ‘not at all’ to 5 = ‘extremely’.
Cybershape
As a comparison, a game called Cybershape (Bolling et al., 2011, in press; Crowley, Bolling, Wu, Pelphrey & Mayes, 2011) was used to explore the specificity of results from Cyberball to the experience of social exclusion, in contrast to social expectancy violation more generally. Cybershape began in the same manner as Cyberball, once again with the participant being told that they were being logged into the game in order to play with other online players. Computer players varied across the two Cyber games. Instructions on how to play the game were again delivered visually and auditorily. An explanation of the shape-matching rule in which participants were asked to throw the shape in their glove to the player with the matching shape next to their picture also appeared on the screen. The participants practiced playing the game for 16 throws. Once their understanding of the game was confirmed, the fMRI session began. They played Cybershape for 5 minutes in 10 continuous, alternating blocks of fair play (rule consistent) and rule violation. Again, each block was 30 seconds long and consisted of 12 throws. In fair play, participants received the shape one-third of the time, and the shape rule was never broken. In rule violation, participants still received the shape one-third of the time, but one of the virtual players consistently threw the shape to the wrong person. The rule violations occurred both in favor of the participant (getting the ball when it was not his or her shape) and in disfavor of the participant (not getting the ball when it was his or her shape). The player who broke the shape rule alternated between rule violation blocks. Only one rule violator per violation block was employed to avoid participants feeling excluded by being the only player to follow the rules. As with previous work (Bolling et al., 2011), a 10-item questionnaire to assess rule violation-related distress was given to 17 of the 19 participants analyzed in Cybershape immediately following the completion of the game, while the participant was still in the scanner. Questions were administered visually and auditorily, and as in Cyberball, participants could communicate with the experimenter if questions were unclear.
Imaging protocol
Images were collected on a Siemens 3T Tim Trio scanner located in the Yale Magnetic Resonance Research Center. Whole-brain T1-weighted anatomical images were acquired using an MPRAGE sequence (TR = 1900 ms; TE = 2.96 ms; flip angle = 9°; FOV = 256 mm; image matrix 256 mm2; voxel size =1 × 1 × 1 mm; 160 slices; NEX =1). Whole-brain functional images were acquired using a single-shot, gradient-recalled echo planar pulse sequence (TR = 2000 ms; TE = 25 ms; flip angle = 60°; FOV = 220 mm; image matrix = 64 mm2; voxel size = 3.4 × 3.4 × 4 mm; 34 slices) sensitive to blood oxygen level dependent (BOLD) contrast.
Data analysis
Imaging data were preprocessed and analyzed using the BrainVoyager QX 2.0.08 software package (Brain Innovation, Maastricht, The Netherlands). Preprocessing of the functional data included slice time correction (using sinc interpolation), 3-dimensional rigid-body motion correction (using trilinear-sinc interpolation), spatial smoothing with a FWHM 4-mm Gaussian kernel, and temporal high-pass filtering (GLM with Fourier basis set, using 2 cycles/time course). Functional datasets were coregistered to within-session anatomical images, which were in turn normalized to Talairach space. Estimated motion plots and cine loops were examined for each participant in order to identify movement and eliminate runs with head motion greater than 2.5 mm between consecutive volumes in any direction or 4 mm total drift from the start of the experiment.
Prior to multi-participant analyses, activation from events in which participants were throwing the ball was removed from the data set for each participant in a regression analysis. Regressors for this analysis were defined as boxcar functions with values of 1 during ball throw events (defined as the period from when the participant received the ball until the participant's throw response) and zero otherwise, and convolved with a double-gamma hemodynamic response function (HRF); subsequent analyses were performed on residuals from this regression. This regression was performed to eliminate the potential confound of the lack of decision-making and motor response in the exclusion blocks. For analytic consistency, the same regression was done in Cybershape though the potential confound did not exist in this game because participants threw the ball with equal frequency in fair play and rule violation blocks.
To identify brain regions modulated by exclusion in Cyberball and rule violation in Cybershape, a random-effects multi-participant general linear model (GLM)-based analysis was performed for each game. Regressors were defined as boxcar functions with values of 1 during each condition and zero otherwise, convolved with a double-gamma HRF. To additionally account for motion during each scan, functions of all of the three directions and three translations of movement from each participant were included in each single-subject GLM-based analysis as predictors of no interest. All whole-brain analyses were corrected for multiple comparisons using a Brain Voyager QX Cluster-level Statistical Threshold Estimator plugin (Xiong, Gao, Lancaster & Fox, 1995). After 1000 iterations of a Monte-Carlo simulation, an alpha value is assigned to each cluster size based on its relative frequency. An α < .05 corresponds to a cluster size with an occurrence probability of less than 5%.
For Cyberball, a random-effects multi-participant analysis was performed and brain activation in the contrast social exclusion > fair play was assessed at an uncorrected statistical threshold of p < .05. To correct for multiple comparisons, we used a cluster threshold of 34 contiguous functional (3 mm3) voxels (Xiong et al., 1995). For Cybershape, a second random-effects multi-participant GLM analysis was performed, and the contrast of rule violation > fair play was assessed at the same statistical threshold of p < .05, with an estimated cluster threshold of 34 functional voxels.
To explore the effects of age on brain activation to social exclusion (and rule violation in comparison), we performed a whole-brain voxel-wise analysis of social exclusion > fair play (and rule violation > fair play) with chronological age as a covariate. We assessed the results at a threshold of p < .01 with a cluster threshold of 34 contiguous functional voxels. This higher threshold was used to discern meaningfully distinct regions of activation, as the results from the covariate analysis in Cyberball were especially robust. The covariate analysis in Cybershape was assessed at the same threshold for consistency.
Following our whole-brain analyses, we performed more specific, structural region of interest (ROI) analyses to explore the effects of age on brain activation. The three ROIs chosen were ventral ACC, right ventrolateral PFC, and right insula, three regions for which we had a priori hypotheses based on past research indicating their role in processing social exclusion. The ventral ACC and right ventrolateral PFC regions were modified from ACC and right middle/inferior frontal gyrus regions, respectively, as defined by the Talairach database (Lancaster, Rainey, Summerlin, Freitas, Fox, Evans, Toga & Mazziotta, 1997; Lancaster, Woldorff, Parsons, Liotti, Freitas, Rainey, Kotchunov, Nickerson, Mikiten & Fox, 2000). The modification included restricting the region to the extent of the MNI brain, and excluding all voxels above the plane z = 9, corresponding to the tip of the cingulate genu. The right insula region was defined using anatomical landmarks on the MNI brain. For each structurally defined region, we calculated average beta values in each participant for the contrasts (social exclusion–fair play) and (rule violation–fair play). These contrast beta values were correlated with age in each game, using Pearson correlations. These correlations were considered significant at an uncorrected threshold of p < .05.
To investigate functional connectivity in each game, we performed a psychophysiological interaction (PPI) analysis with a region of vACC functionally defined in the Cyberball contrast as a seed. The PPI analyses allowed us to identify regions that showed greater connectivity with vACC during exclusion (or rule violation) versus fair play. Prior to the connectivity analysis, the global mean (average signal across voxels) was removed from each volume, as a surrogate method for physiological artifact removal (Fox, Snyder, Vincent, Corbetta, Van Essen & Raichle, 2005). Using a 4 mm3 cube around the peak voxel of average activation in vACC during social exclusion > fair play (Talairach coordinates: −12, 41, 7), PPI regressors for each game were created by multiplying the difference of the two task regressors (convolved with a double-gamma HRF) by the preprocessed, normalized vACC time course for each participant. This PPI function along with the task regressors and vACC time course were used as regressors in two multi-participant random-effects GLM analyses. The results were assessed at a statistical threshold of p < .05, corrected with a cluster threshold of 34 functional voxels. To explore potential effects of age on PPI strength, whole-brain voxel-wise covariate analyses were performed on each PPI GLM analysis, with age as the covariate. The results of the covariate analyses were assessed at a statistical threshold of p < .05, corrected with a cluster threshold of 64 functional voxels.
Following analyses in each game individually, we performed paired samples t-tests on whole-brain contrast maps from each game derived from each participant, thereby constituting a random effects analysis. We compared social exclusion > fair play versus rule violation > fair play in each participant with functional data from both games (16 participants). In this way, we identified regions showing differential activation between social exclusion and rule violation when compared to fair play. The results of this whole-brain analysis were assessed at a statistical threshold of p < .05. A cluster size of 34 contiguous functional voxels was used.
Results
Self-report measures
Scores on the post-game questionnaires confirmed that participants felt distress following the experiences of social exclusion and rule violation. The average total score on the social exclusion distress questionnaire administered following a game of Cyberball was 25.70 (± 7.68, n = 20; a score of 10 would indicate no distress, a score of 50 would indicate maximum distress). The average total score on the questionnaire assessing rule violation distress (which consisted of 10 different questions) was 22.17 (± 4.76, n = 17). Scores on the rule violation questionnaire correlated significantly with age, such that older participants reported higher distress to rule violations in Cybershape (r = .53, p = .03). No such correlation existed between age and self-reported distress following social exclusion (p > .05).
Brain responses to social exclusion
To investigate brain regions modulated by the experience of social exclusion, a random-effects multi-participant GLM analysis comparing social exclusion > fair play was performed including all children and adolescents regardless of age (Figure 1). Peak coordinates, statistical values, size, and anatomical labels for the regions of differential activation in Cyberball are displayed in Table 1. Replicating a past study of social exclusion in adolescents (Masten et al., 2009), we found vACC to be more active in exclusion compared to fair play. Additional regions that were active in children and adolescents during exclusion included bilateral insula, bilateral posterior cingulate cortex (PCC), left ventrolateral prefrontal cortex (vlPFC), left hippocampus, left middle and inferior temporal gyrus (MTG, ITG), and left superior temporal sulcus (STS). In contrast, regions that were less active in social exclusion compared to fair play included left cerebellum, right precentral gyrus, parietal cortex, and bilateral superior frontal gyrus (SFG).
Figure 1.
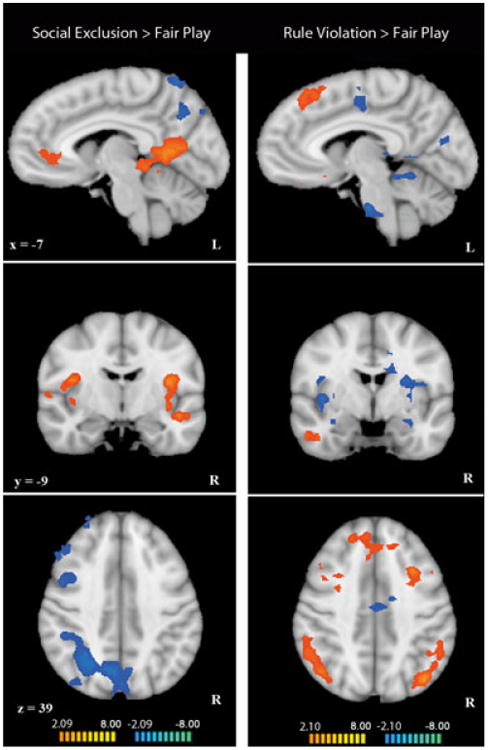
Left column: whole-brain comparison of social exclusion and fair play in all participants (n = 21). Data in these figures have been interpolated from 3 mm3 space to 1 mm3 space for visualization. Regions in orange showed greater activation in social exclusion compared to fair play. Regions in blue showed greater activation in fair play compared to social exclusion (p < .05, k = 34). Right column: whole-brain comparison of rule violation and fair play in all participants (n = 19). Regions in orange showed greater activation in rule violation compared to fair play. Regions in blue showed greater activation in fair play compared to rule violation (p < .05, k = 34).
Table 1. Areas of activation emerging from the comparison of social exclusion and fair play during Cyberball.
| Brain region | X | Y | Z | Size (mm3) | t (avg) | p (avg) |
|---|---|---|---|---|---|---|
| Social Exclusion > Fair Play | ||||||
| Right insula | 42 | −13 | 10 | 8846 | 2.60 | 0.02 |
| Left insula | −45 | −22 | 13 | 8243 | 2.70 | 0.02 |
| vACC | −12 | 41 | 7 | 1841 | 2.40 | 0.02 |
| Left vlPFC | −51 | 17 | −8 | 2106 | 2.42 | 0.03 |
| Left ITG | −54 | −28 | −23 | 1572 | 2.63 | 0.02 |
| Left hippocampus | −6 | −37 | −5 | 1795 | 2.51 | 0.02 |
| Right PCC | 9 | −52 | 7 | 2201 | 2.60 | 0.02 |
| Left PCC | −9 | −58 | 7 | 6733 | 2.84 | 0.02 |
| Left STS | −51 | −34 | 4 | 582 | 2.33 | 0.03 |
| Left MTG | −45 | −7 | −8 | 2249 | 2.50 | 0.03 |
| Fair Play > Social Exclusion | ||||||
| Left cerebellum | −42 | −40 | −38 | 1950 | −2.39 | 0.03 |
| Left SFG | −33 | 53 | 28 | 2346 | −2.46 | 0.03 |
| Right SFG | 27 | 59 | 22 | 2555 | −2.39 | 0.03 |
| Right precentral gyrus | 39 | −1 | 61 | 5755 | −2.43 | 0.03 |
| Parietal cortex | 27 | −58 | 58 | 26838 | −2.67 | 0.02 |
Note: Activation in Cyberball. Regions identified in a full brain contrast of social exclusion to fair play. Talairach coordinates refer to the voxel with the maximum signal change in each region of interest. Statistics reported are for the average of all voxels in the region of significance (avg). Region sizes are reported in structural voxels (1 × 1 × 1 mm). Abbreviations: posterior cingulate cortex (PCC), ventral anterior cingulate cortex (vACC), ventrolateral prefrontal cortex (vlPFC), medial temporal gyrus (MTG), inferior temporal gyrus (ITG), superior frontal gyrus (SFG), superior temporal sulcus (STS).
Brain responses to rule violation
In rule violation > fair play, results were strikingly different from those found in Cyberball (Figure 1). Regions active in rule violation included right STS, right MTG, bilateral dorsolateral and dorsomedial prefrontal cortex (dlPFC, dmPFC), bilateral inferior parietal lobule (IPL), and orbitofrontal cortex. Regions that were less active in rule violation compared to fair play included bilateral insula, bilateral paracentral lobule, bilateral hippocampus, and right PCC. Peak coordinates, statistical values, size, and anatomical labels for the regions of differential activation in Cybershape are displayed in Table 2.
Table 2. Areas of activation emerging from the comparison of rule violation and fair play during Cybershape.
| Brain region | x | y | z | Size (mm3) | t (avg) | p (avg) |
|---|---|---|---|---|---|---|
| Rule Violation > Fair Play | ||||||
| Right STS | 60 | −46 | −2 | 4003 | 2.63 | 0.02 |
| OFC | −18 | 59 | 1 | 27488 | 2.68 | 0.02 |
| Right MTG | 48 | −1 | −26 | 1831 | 2.44 | 0.03 |
| Right dlPFC | 33 | 11 | 46 | 2243 | 2.43 | 0.03 |
| Left dlPFC | −30 | 14 | 40 | 3540 | 2.57 | 0.02 |
| dmPFC | 3 | 29 | 46 | 7168 | 2.53 | 0.03 |
| Right IPL | 39 | −46 | 52 | 6650 | 2.64 | 0.02 |
| Left IPL | −39 | −70 | 40 | 3972 | 2.62 | 0.02 |
| Fair Play > Rule Violation | ||||||
| Right posterior insula | 33 | −16 | 25 | 1633 | −2.52 | 0.03 |
| Right insula | 39 | −4 | 4 | 3306 | −2.56 | 0.02 |
| Left insula | −27 | −4 | 19 | 5147 | −2.51 | 0.03 |
| Right cerebellum | 21 | −22 | −23 | 1784 | −2.61 | 0.02 |
| Cerebellar vermis | 3 | −49 | −11 | 3926 | −2.72 | 0.02 |
| Left MOC | −15 | −76 | 13 | 1790 | −2.44 | 0.03 |
| Left precentral gyrus | −36 | −25 | 52 | 2596 | −2.41 | 0.03 |
| Right paracentral lobule | 18 | −46 | 52 | 1307 | −2.55 | 0.02 |
| Dorsal ACC/Left Paracentral lobule | −15 | −16 | 28 | 2965 | −2.42 | 0.03 |
| Right PCC | 21 | −46 | 13 | 3148 | −2.52 | 0.03 |
| Right hippocampus | 30 | −31 | 10 | 3833 | −2.46 | 0.03 |
| Left hippocampus | −33 | −34 | −5 | 4974 | −2.74 | 0.02 |
| Left restrosplenial cortex | −15 | −43 | 4 | 3933 | −2.64 | 0.02 |
| Pons | −9 | −16 | −35 | 2428 | −2.59 | 0.02 |
Note: Activation in Cybershape. Regions identified in a full brain contrast of rule violation to fair play. Talairach coordinates refer to the voxel with the maximum signal change in each region of interest. Statistics reported are for the average of all voxels in the region of significance (avg). Region sizes are reported in structural voxels (1 × 1 × 1 mm). Abbreviations: posterior cingulate cortex (PCC), anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (dlPFC), dorsomedial prefrontal cortex (dmPFC), middle temporal gyrus (MTG), inferior parietal lobule (IPL), orbitofrontal cortex (OFC), superior temporal sulcus (STS), middle occipital cortex (MOC).
Comparison of brain responses to social exclusion and rule violation
A paired sample t-test comparing social exclusion > fair play versus rule violation > fair play within participants with usable data from both games (n = 16) confirmed regions that were differentially modulated by exclusion and rule violation (Table S1). Regions that were more active during social exclusion included bilateral insula, postcentral gyrus, hippocampus, and PCC. Regions that were more active during rule violation included bilateral vlPFC, IPL, inferior occipital gyrus (IOG), right caudate, right dlPFC and dmPFC, and left STS.
Age correlations in social exclusion
In our whole-brain voxel-wise covariate analysis, we found several regions that increased in activation to social exclusion (compared to fair play) with chronological age (Table 3, Figure 2). These regions included bilateral vlPFC, extensive regions of dorsal and ventral medial PFC, PCC and retrosplenial cortex, left anterior STS, and left posterior MTG. These regions largely overlap with the areas identified in our analysis of the main effects of social exclusion in these participants. At the same threshold, none of these regions significantly correlated with age during rule violation compared to fair play. Only two regions showed increased activation correlated with age to rule violations, middle cingulate cortex, and right precentral gyrus. No regions showed a negative correlation with age in either game.
Table 3. Activations identified via a whole-brain voxel-wise covariate analysis with chronological age in social exclusion > fair play and rule violation > fair play.
| Brain region | x | y | z | Size (mm3) | r (avg) | p (avg) |
|---|---|---|---|---|---|---|
| Social Exclusion > Fair Play | ||||||
| Right vlPFC | 39 | 38 | −5 | 1839 | 0.58 | 0.007 |
| mPFC/vlPFC | −6 | −16 | 22 | 41690 | 0.61 | 0.004 |
| Retrosplenial cortex | 9 | −28 | 25 | 3333 | 0.59 | 0.005 |
| PCC | −9 | −67 | 19 | 3149 | 0.60 | 0.005 |
| Left anterior STS | −54 | −4 | −11 | 4763 | 0.60 | 0.005 |
| Left posterior MTG | −45 | −79 | 28 | 1419 | 0.58 | 0.007 |
| Rule Violation > Fair Play | ||||||
| Right precentral gyrus | 48 | −16 | 31 | 1319 | 0.64 | 0.004 |
| mCC | 3 | −22 | 43 | 975 | 0.61 | 0.006 |
Note: Whole-brain correlation of chronological age and brain activation to social exclusion > fair play. A whole-brain covariate analysis with age was performed in the contrast of social exclusion – fair play and assessed at a statistical threshold of p < .01, k = 34. Talairach coordinates refer to the voxel with the maximum signal change in each region of interest. Statistics reported are for the average of all voxels in the region of significance (avg). Region sizes are reported in structural voxels (1 × 1 × 1 mm). Abbreviations: ventrolateral prefrontal cortex (vlPFC), medial prefrontal cortex (mPFC), superior temporal sulcus (STS), posterior cingulate cortex (PCC), middle temporal gyrus (MTG), middle cingulate cortex (mCC).
Figure 2.
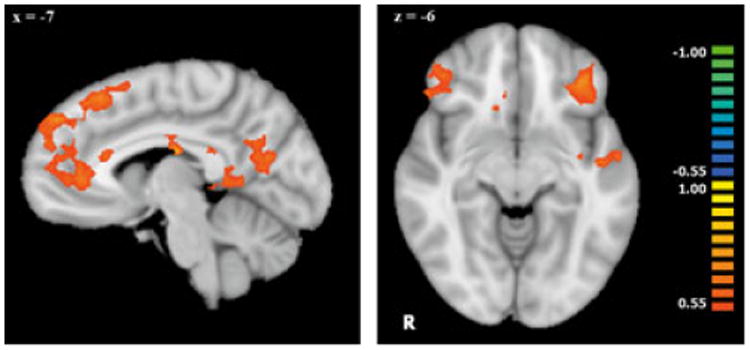
Whole-brain voxel-wise covariate analysis of activation to social exclusion > fair play that correlated with chronological age (n = 21). Data in these figures have been interpolated from 3 mm3 space to 1 mm3 space for visualization. All regions depicted showed a positive correlation between activation during exclusion and age (p < .01, k = 34). Regions of significant correlations are displayed on a Talairach-transformed template brain in radiological orientation.
Region of interest analyses
We then explored the effects of development on activation in specific regions previously implicated in the neural processing of social exclusion in adults. ROI analyses were performed in structurally defined regions of ventral ACC, right ventrolateral PFC, and right insula to determine if average levels of activation in these areas covaried with age. Activation to social exclusion (compared to fair play) in the structurally defined regions of the vACC and right ventrolateral PFC correlated positively with age, such that older participants showed greater activation (vACC: r = .46, p = .03, right vlPFC: r = .52, p = .02, Figure 3). During rule violation this effect of age did not exist for vACC (r = .17, p = .49) or right ventrolateral PFC activation (r = .28, p = .25), or for right insula activation in either game (p > .05).
Figure 3.
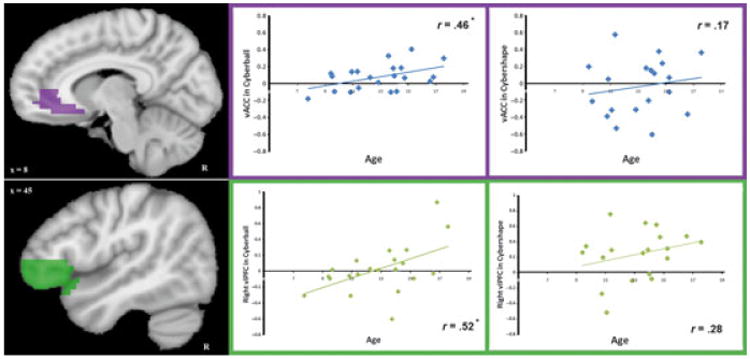
Structural region of interest (ROI) analysis of age-related activation in the vACC (top) and right ventrolateral PFC (bottom). Data in these figures have been interpolated from 3 mm3 space to 1 mm3 space for visualization. Structural ROIs (shown in purple and green) were used to correlate age with vACC and right ventrolateral PFC activity in social exclusion > fair play (left scatter plots) and in rule violation > fair play (right scatter plots). Average contrast beta values for this ROI in each game were calculated for each participant using (social exclusion – fair play) and (rule violation – fair play). These average beta values per participant are plotted against age in each scatter plot, along with a best fit line indicating the direction of the correlation. Only correlations with activation to social exclusion > fair play in vACC and right ventrolateral PFC reached significance (denoted with asterisks; p = .03 and p = .02, respectively). There was not a significant difference between Cyberball and Cybershape age correlations in vACC (z = .95, p = .34) or right vlPFC (z = .84, p = .40).
Functional connectivity
Following the discovery of a selective influence of development on vACC activation in social exclusion but not rule violation, we explored a possible dissociation in the functional connectivity of this region between games. A PPI analysis exploring differential connectivity with vACC revealed increased connectivity with medial prefrontal cortex during social exclusion compared to fair play. This connectivity pattern in vACC was specific to exclusion. In contrast, dorsal anterior cingulate cortex (dACC) and left vlPFC showed increased connectivity with vACC during rule violation compared to fair play (Table 4, Figure 4).
Table 4. Summary of activations and age correlations identified in the PPI analysis of functional connectivity.
| Brain region | x | y | z | size (mm3) | t (avg) | p (avg) |
|---|---|---|---|---|---|---|
| PPI | ||||||
| Social Exclusion > Fair Play | ||||||
| mPFC | −21 | 59 | 16 | 2110 | 2.52 | 0.03 |
| Rule Violation > Fair Play | ||||||
| dACC | −27 | 17 | 40 | 3997 | 2.51 | 0.03 |
| Left vlPFC | −24 | 50 | 13 | 1563 | 2.54 | 0.03 |
|
| ||||||
| PPI age correlation | r (avg) | p (avg) | ||||
| Social Exclusion > Fair Play | ||||||
| Right anterior insula/vlPFC | 21 | 14 | 10 | 2166 | 0.48 | 0.03 |
| Rule Violation > Fair Play | ||||||
| Right cuneus | 21 | −97 | 19 | 1994 | 0.52 | 0.03 |
| Paracentral lobule | 3 | −58 | 43 | 8088 | 0.54 | 0.02 |
Note: Psychophysiological interaction (PPI) analyses. Two PPI analyses, one for each game, identified regions of statistically reliable functional connectivity. Regions of functional connectivity in social exclusion > fair play (Cyberball) and rule violation > fair play (Cybershape) were identified (p < .05, k = 34). Regions of greater functional connectivity in the experimental conditions of social exclusion or rule violation are reported. Results from the PPI analyses in each game were correlated with age in each contrast, social exclusion > fair play and rule violation > fair play (p < .05, k = 64). Regions where PPI strength correlated positively with age in the contrasts of social exclusion > fair play or rule violation > fair play are reported. Talairach coordinates refer to the voxel with the maximum signal change in each region of interest. Statistics reported are for the average of all voxels in the region of significance (avg). Region sizes are reported in structural voxels (1 × 1 × 1 mm). Abbreviations: ventrolateral prefrontal cortex (vlPFC), medial prefrontal cortex (mPFC), dorsal anterior cingulate cortex (dACC).
Figure 4.
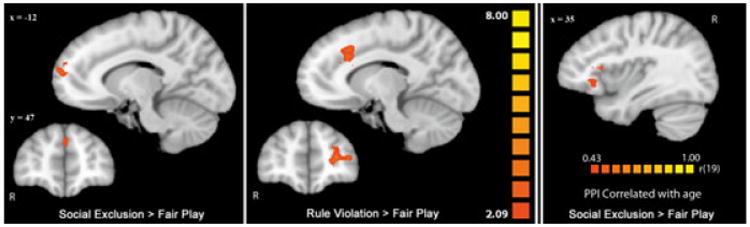
PPI analysis of differential connectivity to vACC in Cyberball (left) and Cybershape (center). Data in these figures have been interpolated from 3 mm3 space to 1 mm3 space for visualization. In both connectivity analyses the seed region used was a 4 mm cube around the peak voxel of average activation in vACC during social exclusion > fair play (Talairach coordinates: −12, 41, 7). In Cyberball (left), areas of activation in orange are regions that showed more functional connectivity to the seed region in social exclusion compared to fair play (p < .05, k = 34). In Cybershape (center), areas of activation in orange are regions that showed more functional connectivity to the seed region in rule violation compared to fair play (p < .05, k = 34). Regions where age correlated positively with PPI strength in social excluion > fair play are shown on the far right (p < .05, k = 64). Activations are displayed on a Talairach-transformed template brain in radiological orientation.
Though vACC activation did not differ significantly between games, an effect of age on vACC activation existed only in social exclusion. To explore further developmental effects in the processing of social exclusion, we included age as a covariate in our PPI analyses of functional connectivity (Table 4, Figure 4). An area of right vlPFC extending into anterior insula was the only region to show a positive correlation between age and task-related functional connectivity during social exclusion. With increasing age, participants showed increasing condition-dependent connectivity between right vlPFC and vACC identified by our PPI analysis. This connectivity pattern did not exist in rule violation, suggesting that the demonstrated age effects on PPI strength between right vlPFC and vACC are specific to the experience of social exclusion and are not a result of development more generally.
Discussion
The present study demonstrated developmental effects on the neural processing of social exclusion from childhood through adolescence. We also explored the specificity of these age effects to social exclusion (versus social distress more generally). To this end, we contrasted social exclusion with an experience of rule violation where an expectancy (to follow the rules) was violated in a social situation, creating social distress in the absence of rejection. While we did not specifically investigate a task by age interaction, we did identify age-related changes in neural processing of social exclusion that were not present in comparable analyses of neural responses to rule violation. Thus, we concluded that the neural correlates of processing social exclusion develop from childhood through adolescence, and this development cannot be attributed to developmental effects of processing social expectancy violation more generally.
First, we identified brain regions active during social exclusion in children and adolescents, extending previous research on social exclusion to a younger and wider age group. Regions active during social exclusion relative to fair play included vACC, bilateral insula, hippocampus, MTG, vlPFC, and PCC. These regions converge with those reported in previous Cyberball fMRI studies of adults and adolescents (Eisenberger et al., 2003; Onoda et al., 2009; Krill & Platek, 2009; Sebastian et al., in press).
To accomplish our developmental aims, we conducted whole-brain voxel-wise covariate analyses investigating the effects of chronological age on activation to social exclusion. We found that many of the regions that showed a main effect of social exclusion also positively correlated with age, including left vlPFC, mPFC, PCC, left STS and left MTG. In contrast to these findings, none of these regions covaried with age during rule violation. Interestingly, right vlPFC activation also correlated with age, though this region did not show a main effect of exclusion. The lack of a main effect of right vlPFC during exclusion mirrors prior results showing that this region is active during exclusion in adults but not adolescents (Sebastian et al., 2010a, in press). The present findings suggest that this decreased vlPFC activation is also seen in childhood, and thus is not specific to adolescence. In the context of this past research, the finding that right vlPFC covaried with age suggests that the region may gradually emerge during adolescence as an element of the network of brain regions responsive to exclusion.
A more specific structural ROI analysis of age effects on activation in both games corroborated the whole-brain covariate results. Structurally defined regions of vACC and right ventrolateral PFC showed positive correlations between age and activation during social exclusion, correlations that were not found in rule violation, nor in right insula in either game. Neural activation in vACC has been associated with experiencing sadness (Levesque, Eugene, Joanette, Paquette, Mensour, Beaudoin, Leroux, Bourgouin & Beauregard, 2003; Liotti, Mayberg, Brannan, McGinnis, Jerabek & Fox, 2000; Phan, Wager, Taylor & Liberzon, 2002; Shafritz, Collins & Blumberg, 2006), and with greater activation and altered connectivity among depressed patients (Drevets, 1998; Beauregard, Leroux, Bergman, Arzoumanian, Beaudoin, Bourgouin & Stip, 1998; Wu, Gillin, Buchsbaum, Hershey, Johnson & Bunney, 1992; Yoshimura, Okamoto, Onoda, Matsunaga, Ueda, Suzuki & Yamawaki, 2010; Greicious, Flores, Menon, Glover, Solvason, Kenna, Reiss & Schatzberg, 2007; Matthews, Strigo, Simmons, Yang & Paulus, 2008). In adolescents experiencing social exclusion, vACC activation has also been shown to correlate positively with distress and subsequent depressive symptoms (Masten et al., 2009, 2011) and negatively with resistance to peer influence (Sebastian et al., in press). Regions of ACC have also been shown to increase activation from childhood to early adulthood following social rejection (Moor et al., 2010). This empirical evidence, along with our finding that activation in vACC region increased with age, suggests that heightened activation in this region is part of the neural profile of adolescent sensitivity to social exclusion.
In contrast, activation in right vlPFC has been shown to negatively correlate with distress during social exclusion, and has been attributed to emotion regulation during ostracism (Eisenberger et al., 2003; Masten et al., 2009). More generally, ventrolateral PFC regions have been implicated in the cognitive control of negative emotions in adults (Goldin, McRae, Ramel & Gross, 2008; Johnstone, van Reekum, Urry, Kalin & Davidson, 2007; Kober, Mende-Siedlecki, Kross, Weber, Mischel, Hart & Ochsner, 2010; Ochsner, Bunge, Gross & Gabrieli, 2002; Ochsner, Ray, Cooper, Robertson, Chopra, Gabrieli & Gross, 2004; Wager, Davidson, Hughs, Lindquist & Ochsner, 2008). Thus, effects of age on right vlPFC activation during exclusion in the present study suggest that the function of this region in emotion regulation develops through adolescence. Further, the lack of significant group activation in this region suggests that, in addition to increased vACC activation, reduced vlPFC recruitment during social exclusion is part of the neural profile of adolescent sensitivity to peer rejection. The lack of age correlation in this region during Cybershape might be a result of a decreased emotional response to rule violation, decreasing the necessity for regulation, as vACC was not significantly active in this condition.
In exploring the functional connectivity of vACC during social exclusion, we identified regions that were functionally connected to vACC during social exclusion versus fair play. Here, we found that activation in vACC significantly covaried with activation in mPFC during social exclusion, and not in rule violation. This is in accordance with connectivity differences previously demonstrated in adults (Bolling et al., 2011). These differences in task-related functional connectivity suggest functionally distinct roles for vACC in social exclusion and rule violation.
Subsequently, we explored an effect of development on the identified psychophysiological interactions in social exclusion. Here, we demonstrated that task-related functional connectivity increased with age between vACC and a region of right vlPFC extending into anterior insula. This correlation did not exist in rule violation, suggesting contextual specificity of this effect. In relation to our finding that right vlPFC activation to exclusion positively correlated with age, this pattern of functional connectivity further supports our hypothesis that right vlPFC gradually emerges over late childhood and into adolescence as a region involved in the network of brain regions implicated in processing social exclusion.
Past findings have implicated a selective role for vACC in processing rejection (and not expectancy violation more generally; Somerville et al., 2006). Though this region was active during social exclusion in the present study, we did not see a significant difference in vACC activation between the two games, in contrast to adult findings (Bolling et al., 2011). However, vACC was not significantly activated during rule violation, and the lack of dissociation between games was likely caused by a high variability between participants in vACC activation during rule violation (average beta value in vACC during rule violation = −.03 ± .33).
The current study demonstrates developmental changes in activation and connectivity of regions of key importance in emotion processing and regulation. This conclusion holds particular importance in the consideration of adolescence, and particularly the transition from childhood to adolescence, as a period of increased mental health vulnerability. Adolescence marks an increased prevalence and sensitivity to long-term effects of depression (Costello, Pine, Hammen, March, Plotsky, Weissman, Biederman, Goldsmith, Kaufman, Lewinsohn, Hellander, Hoagwood, Koretz, Nelson & Leckman, 2002; Pine, Cohen, Gurley, Brook & Ma, 1998), and also represents a rise in peer-influenced negative behaviors, including smoking, alcohol, and cannabis consumption (Agrawal, Lynskey, Bucholz, Madden & Heath, 2007; Dishion & Owen, 2002; Mercken, Snijders, Steglich, Vartiainen & de Vries, 2010; Monahan, Steinberg & Cauffman, 2009). Relating the negative psychological profile of adolescence to peer rejection, past research has demonstrated associations between social exclusion and negative behavioral outcomes, such that exclusion results in increases in self-deflating behavior, externalizing behavior problems and social isolation (Laird et al., 2001; Gazelle & Ladd, 2003; Gazelle & Rudolph, 2004; Twenge et al., 2002), as well as decreases in prosocial behavior and general mental health (Twenge et al., 2007; Rigby, 2000). Negative effects of exclusion are also more pronounced in adults with depression and low self-esteem (Nezlek, Kowalski, Leary, Blevins & Holgate, 1997) and in children with critical self-referent attributions (Prinstein, Cheah & Guyer, 2005).
The current study's findings of age-related changes in vACC and right ventrolateral PFC describe a neural profile of adolescent processing of social exclusion that may help to explain theories addressing adolescent sensitivity to peer rejection, and further, adolescent mental health vulnerability. Specifically, significant activation in vACC and not vlPFC during exclusion in the present study may characterize a neural profile of adolescence where a rise in affective responses to peer rejection occurs in the absence of regulatory vlPFC recruitment. Past evidence has shown that activation in subgenual ACC during social exclusion correlated with subsequent increases in parent-reported depressive symptoms in a group of 13-year-old participants (Masten et al., 2011). Further work specifically evaluating the functional interaction between affective and regulatory brain functioning during exclusion could ascertain if the correlation identified by Masten and colleagues is specific to a neural profile of adolescent development.
The current study, while essential in its contribution to the understanding of the full developmental trajectory of brain mechanisms for processing social exclusion, sustained some limitations. First, the participant group in the present study consisted mainly of males. Past work on social exclusion in adolescents has focused on females (Sebastian et al., 2010b, in press), hypothesizing that females show stronger responses to exclusion than males. Thus, our investigation of predominantly males may have biased the study towards null results, and also makes the findings generalize to male populations specifically. Work on social feedback in adolescence has identified gender differences in neural processing (Guyer, McClure-Tone, Shiffrin, Pine & Nelson, 2009), and so future work on social exclusion will benefit from investigating gender differences. Second, Cyberball was analyzed completely separately from our control task, Cybershape. While not ideal, this was necessary because the ‘fair play’ periods in each game are fundamentally different, and thus require within-task comparisons to elucidate neural responses to each game manipulation. Third, while our distress measures administered after each game confirmed that participants were distressed by the experimental manipulations, no measure of baseline distress could be obtained because of the nature of the paradigm (alternating block design). This limited us in our ability to identify brain activation positively correlating with distress in either game. Because of this null result, we were cautious about interpreting the positive correlation with age identified in rule violation distress scores. Future studies would clarify our work with more direct investigations of the effects of age on responses to social rule violations. Last, while we identified positive correlations between age and activation in vACC and vlPFC during social exclusion and not rule violation, a formal comparison of these correlations between games in each region yielded insignificant differences (vACC: z = .95, p = .34; vlPFC: z = .84, p = .40). Thus, we cannot conclude that the relationship between brain activation and age significantly differed between games in either region of interest, only that there were significant effects of age in brain responses to social exclusion that were not replicated in rule violation.
The current study extends past work to a younger age group to fully characterize differences in neural processing of exclusion in adolescence. The ability of all participants to play both cyber games and report noticing and experiencing distress in response to the experimental manipulations demonstrated the effectiveness of extending Cyberball work to a lower age group. Combining the current study with previous work, it seems that while emotional brain regions (vACC) become increasingly active to exclusion from childhood to adolescence, this activation may not be sustained into adulthood (Masten et al., 2009). In contrast, regulatory brain regions (right ventrolateral PFC) not active to exclusion in adolescence show even less activation in younger children. Taken together, these results suggest that affective brain responses to exclusion in early adolescence may not be coupled with regulatory brain function until later in development. Additional work has the potential to elucidate the psychological effects of neural sensitivity to social exclusion in the adolescent neural profile we have described.
Supplementary Material
Figure S1 Average beta values for a structurally defined region of ventral anterior cingulate cortex from each experimental condition in Cyberball and Cybershape. ‘Control’ conditions refer to fair play in Cyberball and Cybershape. ‘Experimental’ conditions refer to social exclusion and rule violation in Cyberball and Cybershape, respectively. The subtraction of experimental – control conditions was done for each participant individually, and then difference values were averaged for each game. Bars represent standard error. Activation in ventral anterior cingulate cortex was significantly greater than zero in fair play, social exclusion, and in the contrast of social exclusion – fair play (p < .05), but not in any other conditions.
Table S1 Activations identified via a whole-brain paired samples t-test comparing the Cyberball and Cybershape contrasts (social exclusion > fair play and rule violation > fair play). Between game comparison of differential activation in Cyberball and Cybershape. A paired samples t-test compared social exclusion – fair play in Cyberball to rule violation – fair play in Cybershape. Talairach coordinates refer to the voxel with the maximum signal change in each region of interest. Statistics reported are for the average of all voxels in the region of significance (avg). Region sizes are reported in structural voxels (1 × 1 × 1 mm). Abbreviations: dorsomedial prefrontal cortex (dmPFC), dorsolateral prefrontal cortex (dlPFC), middle occipital cortex (MOC), posterior cingulate cortex (PCC), middle temporal gyrus (MTG), ventrolateral prefrontal cortex (vlPFC), inferior parietal lobule (IPL), inferior occipital gyrus (IOG), superior temporal sulcus (STS).
Acknowledgments
The research presented herein was supported by grants from the National Institute of Mental Health, the John Merck Scholars Fund, the Bial Foundation (MJC), and the Simons Foundation. Kevin Pelphrey was supported by a Career Development Award from the National Institutes of Health (NIMH Grant MH071284). Linda Mayes was also supported by a Career Development Award (NIDA K05 DA020091). Naomi Pitskel was supported by a grant from the Doris Duke Charitable Foundation to Yale University.
Footnotes
Supporting Information: Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Agrawal A, Lynskey MT, Bucholz KK, Madden PAF, Heath AC. Correlates of cannabis initiation in a longitudinal sample of young women: the importance of peer influences. Preventive Medicine. 2007;45:31–34. doi: 10.1016/j.ypmed.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Leroux JM, Bergman S, Arzoumanian Y, Beaudoin G, Bourgouin P, Stip E. The functional neuroanatomy of major depression: an fMRI study using an emotional activation paradigm. NeuroReport. 1998;9:3253–3258. doi: 10.1097/00001756-199810050-00022. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Mayes LC, Pelphrey KA. Dissociable brain mechanisms for processing social exclusion and rule violation. NeuroImage. 2011;54:2462–2471. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Kaiser MD, Wyk BCV, Wu J, Mayes LC, Pelphrey KA. Enhanced neural responses to rule violation in children with autism: a comparison to social exclusion. Developmental Cognitive Neuroscience. doi: 10.1016/j.dcn.2011.02.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J, Hendry LB. The nature of adolescence. London: Routledge; 1999. [Google Scholar]
- Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM, Biederman J, Goldsmith HH, Kaufman J, Lewinsohn PM, Hellander M, Hoagwood K, Koretz DS, Nelson CA, Leckman JF. Development and natural history of mood disorders. Biological Psychiatry. 2002;52:529–542. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- Crowley MJ, Bolling DZ, Wu J, Pelphrey KA, Mayes LC. Cybershape: an experimental paradigm to assess neural response to rule violation. Social Affective Neuroscience and Development Laboratory (SANDL). Yale Child Study Center; 2011. [Google Scholar]
- Dishion TJ, Owen LD. A longitudinal analysis of friendships and substance use: bidirectional influence from adolescence to adulthood. Developmental Psychology. 2002;38:480–491. doi: 10.1037//0012-1649.38.4.480. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annual Review of Medicine. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Hassan B, Gilbert SJ, Blakemore SJ. Development of the selection and manipulation of self-generated thoughts in adolescence. Journal of Neuroscience. 2010;30:7664–7671. doi: 10.1523/JNEUROSCI.1375-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazelle H, Ladd G. Anxious solitude and peer exclusion: a diathesis-stress model of internalizing trajectories in childhood. Child Development. 2003;74:257–278. doi: 10.1111/1467-8624.00534. [DOI] [PubMed] [Google Scholar]
- Gazelle H, Rudolpf KD. Moving toward and away from the world: social approach and avoidance trajectories in anxious solitary youth. Child Development. 2004;75:829–849. doi: 10.1111/j.1467-8624.2004.00709.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries JO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80:1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Amminger P, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: a review of recent literature. Current Opinions in Psychiatry. 2007;20:359–364. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences, USA. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krill A, Platek SM. In-group and out-group membership mediates anterior cingulated activation to social exclusion. Frontiers in Evolutionary Neuroscience. 2009;1:1–7. doi: 10.3389/neuro.18.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird RD, Jordan KY, Dodge KA, Pettit GS, Bates JE. Peer rejection in childhood, involvement with antisocial peers in early adolescence, and the development of externalizing behavior problems. Development and Psychopathology. 2001;13:337–354. doi: 10.1017/s0954579401002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapping. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kotchunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux J, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic–cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biological Psychiatry. 2000;30:30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents' risk for depression. Development and Psychopathology. 2011;23:283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. Journal of Affective Disorders. 2008;111:13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Mercken L, Snijders TAB, Steglich C, Vartiainen E, de Vries H. Dynamics of adolescent friendship networks and smoking behavior. Social Networks. 2010;32:72–81. doi: 10.1016/j.socscimed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Monahan KC, Steinberg L, Cauffman E. Affiliation with antisocial peers, susceptibility to peer influence, and antisocial behavior during the transition to adulthood. Developmental Psychology. 2009;45:1520–1530. doi: 10.1037/a0017417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor BG, van Leijenhorst L, Rombouts SARB, Crone EA, van der Molen MW. Do you like me? Neural correlates of social evaluation and developmental trajectories. Social Neuroscience. 2010;5:461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nezlek JB, Kowalski RM, Leary MR, Blevins T, Holgate S. Personality moderators of reactions to interpersonal rejection: depression and trait self-esteem. Personality and Social Psychology Bulletin. 1997;23:1235–1244. [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Social Neuroscience. 2009;4:443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Cheah CSL, Guyer AE. Peer victimization, cue interpretation, and internalizing symptoms: preliminary concurrent and longitudinal findings for children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34:11–24. doi: 10.1207/s15374424jccp3401_2. [DOI] [PubMed] [Google Scholar]
- Rigby K. Effects of peer victimization in schools and perceived social support on adolescent well-being. Journal of Adolescence. 2000;23:57–68. doi: 10.1006/jado.1999.0289. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Development. 1999;70:660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Roiser JP, Tan GCY, Viding E, Wood NW, Blakemore SJ. Effects of age and MAOA genotype on the neural processing of social rejection. Genes, Brain and Behavior. 2010a;9:628–637. doi: 10.1111/j.1601-183X.2010.00596.x. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Tan GCY, Roiser JP, Viding E, Dumontheil I, Blakemore SJ. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. NeuroImage. doi: 10.1016/j.neuroimage.2010.09.063. in press. [DOI] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition. 2010b;72:134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. NeuroImage. 2006;31:468–475. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine & Child Neurology. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge JM, Baumeister RF, DeWall CN, Ciarocco NJ, Bartels JM. Social exclusion decreases prosocial behavior. Journal of Personality and Social Psychology. 2007;92:56–66. doi: 10.1037/0022-3514.92.1.56. [DOI] [PubMed] [Google Scholar]
- Twenge JM, Catanese KR, Baumeister RF. Social exclusion causes self-defeating behavior. Journal of Personality and Social Psychology. 2002;83:606–615. doi: 10.1037//0022-3514.83.3.606. [DOI] [PubMed] [Google Scholar]
- van Beest I, Williams KD. When inclusion costs and ostracism pays, ostracism still hurts. Journal of Personality and Social Psychology. 2006;91:918–928. doi: 10.1037/0022-3514.91.5.918. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD. Ostracism. Annual Review of Psychology. 2007;58:425–452. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]
- Williams KD, Cheung CKT, Choi W. Cyberostracism: effects of being ignored over the internet. Journal of Personality and Social Psychology. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Wirth JH, Williams KD. ‘They don't like our kind’: consequences of being ostracized while possessing a group membership. Group Processes and Intergroup Relations. 2009;12:111–127. [Google Scholar]
- Wu JC, Gillin JC, Buchsbaum MS, Hershey T, Johnson JC, Bunney WE. Effect of sleep deprivation on brain metabolism in depressed patients. American Journal of Psychiatry. 1992;149:538–543. doi: 10.1176/ajp.149.4.538. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao JH, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Human Brain Mapping. 1995;3:287–301. [Google Scholar]
- Yoshimura S, Okamoto Y, Onoda K, Matsunaga M, Ueda K, Suzuki S, Yamawaki S. Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. Journal of Affective Disorders. 2010;122:76–85. doi: 10.1016/j.jad.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. Journal of Experimental Social Psychology. 2004;40:560–567. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Average beta values for a structurally defined region of ventral anterior cingulate cortex from each experimental condition in Cyberball and Cybershape. ‘Control’ conditions refer to fair play in Cyberball and Cybershape. ‘Experimental’ conditions refer to social exclusion and rule violation in Cyberball and Cybershape, respectively. The subtraction of experimental – control conditions was done for each participant individually, and then difference values were averaged for each game. Bars represent standard error. Activation in ventral anterior cingulate cortex was significantly greater than zero in fair play, social exclusion, and in the contrast of social exclusion – fair play (p < .05), but not in any other conditions.
Table S1 Activations identified via a whole-brain paired samples t-test comparing the Cyberball and Cybershape contrasts (social exclusion > fair play and rule violation > fair play). Between game comparison of differential activation in Cyberball and Cybershape. A paired samples t-test compared social exclusion – fair play in Cyberball to rule violation – fair play in Cybershape. Talairach coordinates refer to the voxel with the maximum signal change in each region of interest. Statistics reported are for the average of all voxels in the region of significance (avg). Region sizes are reported in structural voxels (1 × 1 × 1 mm). Abbreviations: dorsomedial prefrontal cortex (dmPFC), dorsolateral prefrontal cortex (dlPFC), middle occipital cortex (MOC), posterior cingulate cortex (PCC), middle temporal gyrus (MTG), ventrolateral prefrontal cortex (vlPFC), inferior parietal lobule (IPL), inferior occipital gyrus (IOG), superior temporal sulcus (STS).


