Abstract
We studied time-based neural activity with event-related potentials (ERPs) in young adults during a computer-simulated ball-toss game. Experiencing fair play initially, participants were ultimately excluded by other players. Dense-array ERPs showed time-dependent associations between slow-wave activity (580–900 ms) in left prefrontal/medial frontal cortical regions for exclusion events and self-reported distress. More subtle ‘micro-rejections’ during fair play showed a similar distress to ERP association (420–580 ms). In both cases, greater positive amplitude neural activity was associated with less post-exclusion distress. Findings suggest that rapidly occurring neural responses to social exclusion events are linked to individual differences in ostracism-related distress. Relations emerged even during fair play, providing a window into the neural basis of more subtle social-cognitive perceptual processes.
Keywords: event-related potential, social exclusion, social neuroscience
Introduction
Most people have experienced a sense of being ‘left out’ of a social situation. We may quickly perceive a preferential gaze, body orientation, or differential engagement among others in a group. Being able to detect social exclusion quickly is advantageous because it can guide coping responses and effective social bids for inclusion [1], but over sensitivity to rejection may adversely impact social relationships, and is associated with mental health problems [2]. A major hurdle for the social neurosciences is to illuminate how people continuously experience and respond to their social world as it rapidly unfolds.
In a simple interactive game called cyberball, a participant makes and receives throws from two other cyber players. Ultimately, the participant is excluded while the others continue to play. This experience is mildly distressing to people [1]. Findings using functional magnetic resonance imaging (fMRI) with cyberball suggest that the experience of social pain shares some common neural circuitry with physical pain, particularly the dorsal anterior cingulate cortex [3]. Nonetheless, the neural system that subserves social interaction in an exclusion context is likely to include monitoring and coping processes in addition to ‘social pain’ processes. For instance, the anterior cingulate cortex is also thought to monitor conflict [4], to detect behavioral errors [5], and to detect when outcomes are worse than expected [6]. Coping processes of emotion regulation involve lateral and medial prefrontal activation [7,8], which might also be active during social exclusion. In addition, brain responses to rejection need not be identical across individuals. For instance, while viewing rejection themes during fMRI, low rejection-sensitive individuals have shown greater activity in left inferior frontal and right dorsal frontal regions, which correlated negatively with self-reported distress, a pattern not observed in high rejection-sensitive individuals [9].
Although neuroimaging research speaks to brain regions relevant for the overall social exclusion experience, this work remains reticent concerning the question of ‘when’ exclusion events are processed at the level of neural activity. This question, central to unpacking the neural and psychological events of social cognition, is precisely one well suited to the real-time temporal resolution of event-related brain potentials (ERPs). Here, we report on the measurement of frontal neurophysiological activity for social exclusion events during cyberball, as these relate to perceived distress after exclusion. In addition, we examined participants’ differential sensitivity to not receiving the ball intermittently during ongoing fair play, which we termed ‘micro-rejection’.
Methods
Participants
Twenty-eight undergraduate participants (13 female) QJ;18-26 years of age (mean age = 20.56) participated for course credit. They played cyberball while an electroencephalogram (EEG) was recorded. The Edinburgh Handedness Inventory [10] identified one left-handed participant. The Human Investigation Committee of the Yale University School of Medicine approved this study. Written informed consent was provided by each participant. One male participant was excluded from the analysis because of excessive drowsiness.
Procedure
Each participant sat 60 cm before a 17-inch CRT monitor in a dimly lit (60 W bulb) sound-attenuated room. They sat for an electroencephalogram while they played an interactive computer game with two other hypothetical players.
The cyberball social exclusion task
Cyberball is a virtual ball-toss game in which a participant plays with two other players on a computer. Abruptly, the others exclude the participant, only throwing to one another. This exclusionary experience is distressing to participants, as per their self-reports of distress on a Need Threat Scale (described below) [1,3].
When the game began, the participant's glove was at the bottom center of the screen; the gloves of the other two players, chosen by the computer, were to the left and right of the screen center. Pictures of the other ‘players’ appeared above their names and respective gloves. Participants used their left and right index fingers on a response pad to throw left or right to the other players. To enhance realism of the game from throw to throw, the ball traveled randomly along different paths (straight line, arc or sine wave); life-like sound effects occurred as the ball traveled (swoosh) and landed in a glove. Following Zadro et al. [11], participants were told the game was hypothetical, and they should pretend it was an actual game with other people.
Our ERP version of cyberball consisted 137 trials across two blocks, a fair play block (90 trials) and then an exclusion block (47 trials). During the 90-trial fair play block, the cyber players threw to the participant 30 times (inclusion events). Whether a ball was thrown to the participant during any one trial was pseudorandom and predetermined within a list such that the participant waited for either 0, 1, or 2 throws by the other players before receiving the ball again (frequency 8, 14, and 8, respectively). Cyber players threw to one another and not to the participant 30 times (micro-rejection events). The participant threw back to the other ‘players’ for the remaining 30 trials. Seamlessly, fair play folded into a 47-trial exclusion block. This block represented 96% exclusion. Of the 47 exclusion trials, the ball only came to the participant twice to maintain attention, once on trial 16 and again on trial 32. Only 30 exclusion events from this block were used in ERP analyses. Seventeen trials (47–30) were not used. These included the first five trials of the exclusion block, the two throws to the participant during this block, and the five trials that directly followed each of these two throws.
Immediately after the game, participants completed the Need Threat Scale, a reliable and valid 20-item ostracism distress measure [12], which has been related to fMRI BOLD signal in an earlier research [3]. The Need Threat Scale gauges feelings of distress along four dimensions: belonging (‘I felt rejected’), self-esteem (‘I felt liked’), meaningful existence (‘I felt invisible’), control (‘I felt powerful’), on a 5-point choice, from ‘Not at all’ to ‘Extremely’.
Electrophysiological methods
Using standard procedures, a high-density EEG was recorded from 128 Ag/AgCL electrodes [Electrical Geodesics Inc., (EGI), Eugene, Oregon, USA] with Netstation v.4.2 software (EGI) and EGI high-impedance amplifiers, sampled at 250 hz (0.1 Hz high pass, 100 Hz, low pass). All electrodes were referenced to Cz for recording. All impedances remained at or below 40 kΩ. The E-prime v.1.2 (Psychology Software Tools, Pittsburgh, Pennsylvania, USA) software package controlled the stimulus presentation.
Before segmentation, EEG data were low-pass filtered at 30 Hz. ERPs were derived only when the ball reappeared after leaving the glove of the cyber players, but before traveling on the screen (100 ms baseline, 900 ms poststimulus onset, see Fig. 1). The EEG for each trial was corrected for blinks and eye movements [13]. Artifact rejection was carried out to eliminate ERPs contaminated by movement and eye artifacts. Rejection rates were comparable across stimulus conditions. Data from electrodes identified with poor signal quality (50% or more trials) were replaced using spherical spline interpolation. For data to be included in the analyses, a total of no more than 20 channels could be interpolated. Averaged data were baseline-corrected by subtracting the average microvolt value across the 100-ms prestimulus interval from the poststimulus segment. After artifact rejection, the single trial data were re-referenced from the vertex (Cz) to an average reference of all electrodes. The trial by trial data were then averaged separately for each of the 128 electrode sites and each of three stimulus conditions (inclusion, rejection, micro-ejection).
Fig. 1.
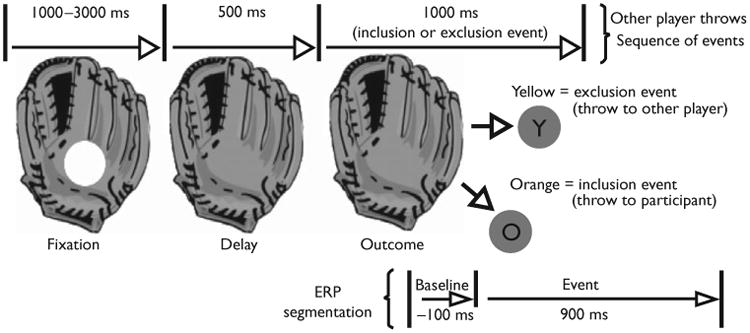
Schematic diagram of a cyber-player's glove and events. The ball arrives at one of the cyber-player's gloves, remains for a fixation period, disappears (delay), and reappears as an outcome event (yellow ball for exclusion, orange ball for inclusion). Event-related potentials (ERPs) segmented on the outcome event.
Results
Event-related potential analysis
We used temporal principal component analysis (PCA) following Dien et al. [14] to identify time windows of correlated neural activity in the frontal cortex during exclusion and micro-rejection events. ERPs from 30 frontal electrodes were clustered into two regions by averaging the data for electrodes within the frontal region in each hemisphere (for electrodes used, see Fig. 2a or Fig. 3a). Next, the mean voltage values resulting from the ERP windows in the frontal region were examined with Pearson's product moment correlations for exclusion events and then for micro-rejection events with regard to the ostracism distress measure. All analyses were performed using the SPSS v.16 software (SPSS Inc., Chicago, Illinois, USA).
Fig. 2.
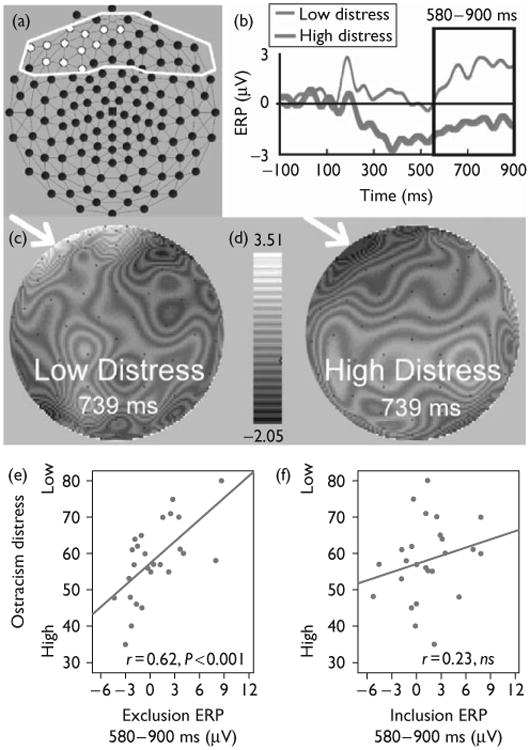
Event-related potential (ERP) waveforms, and scalp topography of social exclusion and relations with distress. (a) Frontal electrodes correlated with ostracism distress during exclusion events for an ERP slow wave, highlighted in white (principal component analysis-derived slow wave, 580–900 ms, false discovery rate controlled). (b) ERPs for the average of electrodes in (a), 10 least versus 10 most distressed participants. (c) Interpolated voltage map for low distress group. (d) Interpolated voltage map for high distress group. (e) Scatter plot of mean slow wave data for exclusion events and ostracism distress scores (n =27). (f) Scatter plot of mean slow wave data plotted for inclusion events during fair play and ostracism distress scores (n =27).
Fig. 3.
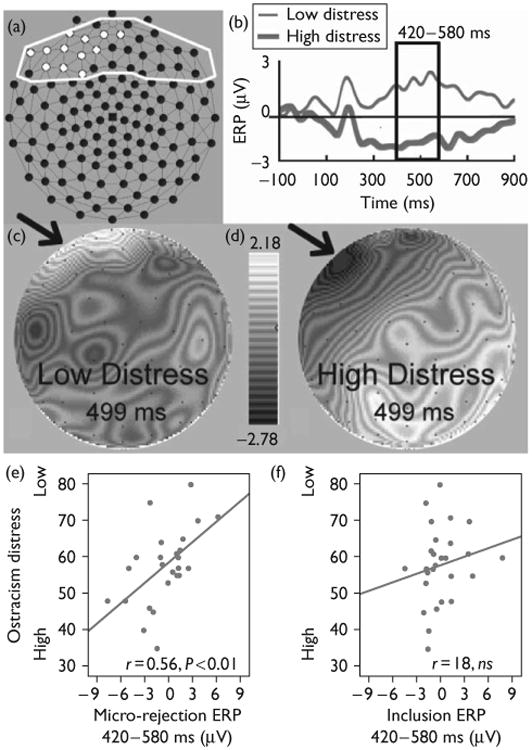
Event-related potential (ERP) waveforms, and scalp topography of micro-rejection and relations with distress. (a) Frontal electrodes correlated with ostracism distress during micro-rejection events, highlighted in white (principal component analysis-derived window, 420–580 ms, false discovery rate controlled). (b) ERPs for the average of electrodes in (a), 10 least versus 10 most distressed participants. (c) Interpolated voltage map for low distress group. (d) Interpolated voltage map for high distress group. (e) Scatter plot of mean voltage data 420–580 ms for micro-rejection events and ostracism distress scores (n=27). (f) Scatter plot of mean ERP voltage data plotted for inclusion events during fair play (n= 27).
The temporal PCA yielded four components accounting for 88% of the variance in the ERP signal. Factor 1 accounted for 33.19%, of the variance and consisted of a slow wave apparent in time interval 580–900 ms (peak time 844 ms). Factor 2 accounted for 24.41% of the variance and appeared as a 184–420-ms time interval (peak time 340 ms). Factor 3 accounted for 15.90% and appeared as a 420–580-ms time interval (peak time 528 ms). Factor 4 accounted for 14.58% of the variance and appeared as a 0–184-ms time interval (peak time 72 ms).
The correlation between the mean voltage for each of the four factors and ostracism distress was computed for each of the electrodes in the frontal region. False discovery rate (FDR) [15] was used to handle multiple comparisons (overall P<0.05), separately for each component window. FDR controls for the expected proportion of incorrectly rejected null hypotheses or type I errors among a list of rejected hypotheses. Figure 2a displays the 12 frontal scalp electrodes (white outline) significantly correlated with ostracism distress for a slow wave occurring in a window from 580 to 900 ms during exclusion events. The other components and frontal electrodes were not significantly related to ostracism distress. As an overall estimate of the left-prefrontal/medial frontal ostracism effect, we averaged the significant electrodes.
For illustrative purposes, ERPs for the 10 participants who reported the lowest levels of ostracism distress (low-distress group) and the 10 participants reporting the highest levels of ostracism distress (high-distress group) are presented in Fig. 2b. The positive polarity ERP, beginning at about 580 ms after a rejection event, was more pronounced for the low-ostracism group compared with the high-ostracism group. Figure 2c and d display whole-head interpolated voltage scalp maps for the low-distress group (left) and those 10 participants scoring highest on ostracism distress (right) at the midpoint of the PCA window (739 ms). Differences in scalp voltages can be observed broadly in the left prefrontal/medial frontal region. A scatter plot in Fig. 2e depicts the association between ostracism distress and the late positive slow wave (left frontal–medial frontal electrodes) for the total sample, (r = 0.62, P < 0.001). Providing discriminant validity for this effect, ostracism distress was unrelated to neural activity in the same event-related time window and electrodes for inclusion events across the total sample (scatter plot, Fig. 2f, r = 0.23, NS). Using Fisher's r-to-z transformation, we compared the magnitude of the difference in these correlations, which were significantly different, z= 1.70, P < 0.05, one-tailed.
We adopted an analogous approach for examining micro-rejection events with temporal PCA, correlation analysis with ostracism distress and FDR, for the frontal brain region. Figure 3a displays the 12 frontal scalp electrodes (white outline), event-locked to micro-rejection, which were most strongly related to ostracism distress, assessed postexclusion, for a component in a window of 420–580 ms. Although not identical, this cluster of electrodes was comparable with that for explicit exclusion (see Fig. 3a vs. Fig. 2a). The other PCA components for the frontal electrodes were not significantly related to ostracism distress. Again comparing the high-distress and low-distress groups, the positive polarity ERP (Fig. 3b), beginning at about 420 ms after a micro-rejection event, was more pronounced for the low-ostracism group compared with the high-ostracism group. Figure 3c and d display interpolated voltage scalp maps for the low-distress group (left) and the high-distress group (right), respectively, at the midpoint of the PCA window (499 ms). Differences in scalp voltages across the two groups can be observed broadly in the left prefrontal/medial frontal region. The scatter plot in Fig. 3e depicts the association between ostracism distress and micro-rejection for the mean voltage of the 420–580 ms time window across the whole sample, (r=0.56, P<0.01). Ostracism distress was unrelated to neural activity in the same event-related time window for inclusion events (scatter plot, Fig. 3f, r = 0.18, ns). Using Fisher's r-to-z transformation, we compared the magnitude of the difference in these correlations, which approached significance, z = 1.56, P < 0.06, one-tailed.
Discussion
Our data suggest that neural responses to rejection events relate to individual differences in perceived distress after an exclusion experience. A late positive potential (LPP) (580–900 ms, left frontal–medial frontal region) observed during explicit rejection was associated with self-reported exclusion distress during the cyberball game. In an earlier time window (420–580 ms), micro-rejection events were associated with the same self-reported distress instrument. Providing discriminant validity for our results, ERP inclusion events for either of these time windows were unrelated to self-reported ostracism distress.
We observed that greater late positive slow wave neural activity in the anterior left frontal–medial frontal region for rejection events was more pronounced for those who experienced less distress. This effect resembles the work of Kross et al. [9], who observed greater left inferior frontal effects with fMRI among low rejection-sensitive individuals. As coping processes of emotion regulation have been associated with lateral and medial prefrontal activation [7,8] and we observed that greater neural response for rejection events predicted less exclusion distress post-rejection, we believe we have tapped into neural processes that may mitigate the experience of social exclusion. ERP studies examining LPPs have focused on posterior midline cortical regions, finding that this ERP differs for emotionally salient versus neutral stimuli [16] and is reduced under conditions of voluntary suppression of negative emotion [17]. We find that a clearly frontal ERP, similar in form to LPP, has relevance for studying social cognitive events.
We believe we have also probed more fine-grained aspects of social cognition. The micro-rejection events, embedded in ongoing fair play, tracked perceived distress at the end of the game in a manner similar to obvious exclusion. The importance of this finding lies in that the fair play portion of the task presents the participant with an ambiguous situation. Social cognitive theorists [18] would agree that individuals bring cognitive perceptual biases, embodied in schemata, to social situations. These schemata can guide perception of ambiguous social events. We show that ERPs for micro-rejection events during the fair play portion of the cyberball task can serve as time-dependent neural markers, possibly reflecting differential activity in these cognitive schemata.
Conclusion
Our findings suggest that rapidly occurring neural responses to social exclusion events are linked to individual differences in ostracism-related distress. Relations emerged even during fair play, providing a window into the neural basis of more subtle social-cognitive perceptual processes. In both cases, greater positive amplitude neural activity was associated with less post-exclusion distress.
Acknowledgments
This study was supported by the NARSAD Young Investigator Award (M.J.C.), the Bial Foundation (M.J.C.); NIDA grants RO1-DA-06025 (L.C.M.), DA-017863 (L.C.M.) and KO5 (L.C.M.), and a grant from the Gustavus and Louise Pfeiffer Research Foundation (L.C.M.). The authors thank David Reiss and Mikle South, for their thoughtful comments on this manuscript.
References
- 1.Williams KD. Ostracism. Annu Rev Psychol. 2007;58:425–452. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]
- 2.Ayduk O, Zayas V, Downey G, Cole AB, Shoda Y, Mischel W. Rejection sensitivity and executive control: joint predictors of borderline personality features. J Res Pers. 2008;42:151–168. doi: 10.1016/j.jrp.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 4.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 5.Falkenstein M. Errors, conflicts, and the brain: a review of the contributions to the error conference, Dortmund 2003. J Psychophysiol. 2004;18:153–163. [Google Scholar]
- 6.Holroyd CB, Coles MGH. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 7.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. J Cogn Neurosci. 2007;19:945–956. doi: 10.1162/jocn.2007.19.6.945. [DOI] [PubMed] [Google Scholar]
- 10.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 11.Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. J Exp Soc Psychol. 2004;40:560–567. [Google Scholar]
- 12.van Beest I, Williams KD. When inclusion costs and ostracism pays, ostracism still hurts. J Pers Soc Psychol. 2006;91:918–928. doi: 10.1037/0022-3514.91.5.918. [DOI] [PubMed] [Google Scholar]
- 13.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 14.Dien J, Tucker DM, Potts G, Hartry-Speiser A. Localization of auditory evoked potentials related to selective intermodal attention. J Cogn Neurosci. 1997;9:799–823. doi: 10.1162/jocn.1997.9.6.799. [DOI] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300. [Google Scholar]
- 16.Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- 17.Moser JS, Hajcak G, Bukay E, Simons RF. Intentional modulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology. 2006;43:292–296. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 18.Crick NR, Dodge KA. A review and reformulation of social information-processing mechanisms in children's social adjustment. Psychol Bull. 1994;115:74–101. [Google Scholar]


