SYNOPSIS
Endothelial-mesenchymal transition (EndMT) is a critical process of cardiac development and disease progression. However, little is know about the signaling mechanisms that cause endothelial cells to transform into mesenchymal cells. Here we show that transforming growth factor-beta2 (TGF-β2) stimulates EndMT through Smad, MEK, PI3K, and p38 MAPK signaling pathways. Inhibitors of these pathways prevent TGF-β2-induced EndMT. Furthermore, we show that all of these pathways are essential for increasing expression of the cell adhesion suppressing transcription factor Snail. Inhibition of Snail with siRNA prevents TGF-β2-induced EndMT. However, over-expression of Snail is not sufficient to cause EndMT. Chemical inhibition of GSK-3β allows EndMT to be induced by Snail over-expression. Expression of a mutant Snail protein that is resistant to GSK-3β-dependent inactivation also promotes EndMT. These data provide the foundation for understanding the roles of specific signaling pathways in mediating EndMT.
Keywords: EMT, TGF-beta, Snail, GSK-3beta, endothelial-mesenchymal transition
INTRODUCTION
Endothelial-mesenchymal transition (EndMT) is an essential mechanism of endocardial cushion formation during cardiac development [1–3]. EndMT also has an essential role in cancer progression by causing formation of cancer-associated fibroblasts in the tumor microenvironment [4]. Many fibroblasts formed during cardiac and renal fibrosis have been shown to be of endothelial origin [5,6]. EndMT has also been implicated in atherosclerosis [7], pulmonary hypertension [8], diabetic nephropathy [9], and wound healing [10].
EndMT is characterized by loss of cell-cell adhesion and changes in cell polarity inducing a spindle-shaped morphology. These changes are accompanied by reduced expression of the endothelial markers such as VE-cadherin and CD31, and increased expression of the mesenchymal markers like fibroblast specific protein-1 (FSP-1), alpha smooth muscle actin (α-SMA), N-cadherin, and fibronectin [11]. Loss of cell-cell adhesion is mediated by transcription factors such as Snail, Slug, ZEB-1, SIP-1, Twist, and LEF-1 that suppress transcription of genes encoding proteins involved in formation of adherens junctions and tight junctions [12–18].
Transforming growth factor-beta (TGF-β) signaling ligands are potent inducers of converting epithelial cells to mesenchymal cells [19,20]; however, EndMT appears to be stimulated primarily by the TGF-β2 isoform [12,17,21–23]. Ablation of TGF-β2 in mice prevents EndMT-mediated cardiac development. TGF-β1 or TGF-β3 knockout mice show no significant effects on EndMT and heart development [3]. TGF-β2 has been described to promote EndMT by signaling through the TGF-β type 1 receptors ALK2 and ALK5 [24,25], yet little is know about the downstream signaling events that occur to stimulate this process. Our goal was to identify essential signaling pathways that mediate TGF-β2-dependent EndMT and expression of the EndMT-inducing transcription factor Snail.
EXPERIMENTAL
Cell Culture
Human cutaneous microvascular endothelial cells (HCMEC) were provided by Dr. Bjorn R. Olsen (Harvard Medical School) and isolated as previously described [26]. Cells were previously tested for purity and found to express no markers for lymphatic endothelial cells or stromal cells (smooth muscle cells, fibroblasts, pericytes, etc.) [27]. Cells were grown in culture using EGM-2 medium (Cambrex), containing 10% FBS and 1% Penicillin/Streptomycin, followed by human endothelial serum free medium (Gibco) 24 hours prior to all experimental conditions. Recombinant TGF-β2 (R&D Systems) was added to the serum free culture medium for all relevant experiments at a concentration of 10ng/mL. Cells were treated for 15 minutes to assess ERK1/2, AKT, p38, or GSK-3β phosphorylation, 1 hour to measure Smad activity, or 48 hours to induce EndMT. DN-Smad4 adenoviral construct (provided by Dr. Diane Simeone, University of Michigan) was produced as previously described [28] and used at a 1:100 dilution in serum free medium, then added to cells for 24 hours prior to treatment with TGF-β2. Small molecule inhibitors were added to cultures 1 hour prior to treatment with TGF-β2. The p38 MAPK inhibitor SB202190 (Tocris Bioscience) was used at a concentration of 25μM, the PI3K inhibitor LY294002 (Cell Signaling Technology) was used at a concentration of 50μM, and the MEK1/2 inhibitor U0126 (Cell Signaling Technology) was used at a concentration of 10μM. Cells were transfected with 1 μg of pcDNA3-Snail (provided by Dr. M. Angela Nieto, Instituto de Neurociencias de Alicante, CSIC-UMH, Spain), pcDNA3 empty vector (Invitrogen), Snail-WT or Snail-6SA (provided by Dr. Mien-Chie Hung, M. D. Anderson Cancer Center) using Lipofectin and Plus reagents (Invitrogen). Lithium chloride was added at a concentration of 20mM to cultures 24 hours after transfection with expression plasmids. All experiments for this study were performed at minimum in triplicate.
RNA interference
siRNA gene expression knockdown studies were performed using the TriFECTa RNAi kit (Integrated DNA Technologies) and corresponding protocol. Each 27mer siRNA duplex was transfected into cells using X-tremeGene siRNA transfection reagent (Roche) following the manufacturer’s guidelines. siRNA was synthesized (Integrated DNA Technologies) with the following sequences: Snail: 5′-CCACAGAAAUGGCCAUGGGAAGGCCAC-3′; negative control: 5′-UCACAAGGGAGAGAAAGAGAGGAAGGA-3′.
Luciferase reporter gene assays
Luciferase reporter gene assays were conducted using the Luciferase Assay System (Promega) and its corresponding protocol. All plasmids (500ng) were transfected into cells using Lipofectin and Plus reagents (Invitrogen) according to the manufacturer’s guidelines. Light units were measured with a Luminometer TD-20/20 (Turner Designs). Assays were normalized for transfection efficiency by cotransfecting cells with a β-gal control plasmid and were detected with the Luminescent β-galactosidase control assay kit (Clontech). Experimental (Luciferase) results were divided by the β-gal results to provide normalized data. The p3TP-Lux reporter plasmid was provided by Dr. Joan Massague (Memorial Sloan-Kettering Cancer Center).
Immunoblotting
Western blots were performed with the following antibodies using dilutions and protocols recommended by the respective manufacturers: phospho-ERK1/2, phospho-p38 (Millipore), phospho-GSK-3β, VE-cadherin, Snail (Santa Cruz Biotechnology), phospho-AKT, AKT, ERK1/2, p38, GSK-3β (Cell Signaling Technology), CD31 (Dako), α-SMA, β-actin (Sigma-Aldrich), and FSP-1 (Abnova). Samples were run with Criterion precast SDS-PAGE Gels (Bio-Rad). HRP-conjugated IgG TrueBlot reagents (eBioscience) were used at a dilution of 1:1000.
Real-time Quantitative PCR
RNA extractions were performed using the RNeasy Mini kit (Qiagen) and protocol. Real-time PCR experiments were conducted using the Syber Green PCR system (Applied Biosystems) on an ABI 7500 cycler, with 40 cycles per sample. Cycling temperatures were as follows: denaturing 95°C; annealing and extension, 60°C. The following primers were used: Snail: Forward: 5′-ACCACTATGCCGCGCTCTT-3′; Reverse: 5′-GGTCGTAGGGCTGCTGGAA-3′; GAPDH: Forward: 5′-ACCACAGTCCATGCCATCAC-3′; Reverse: 5′-TCCACCCTGTTGCTGTA-3′.
Statistical Analyses
One-way analysis of variance (ANOVA) was performed and confirmed with two-tailed paired student’s t test using GraphPad Prism 4 software. P values less than 0.05 were considered significant.
RESULTS
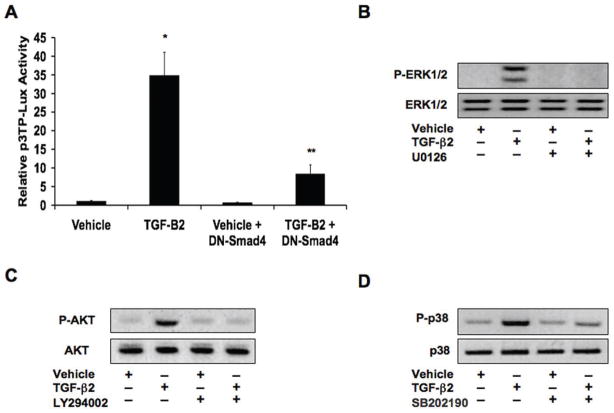
We transfected human cutaneous microvascular endothelial cells (HCMEC) with the p3TP-Lux reporter plasmid in order to determine Smad transcription factor activity. We then treated the cells with recombinant TGF-β2 for 1 hour and found that it greatly increased reporter activity. However, in cells that were also infected with an adenoviral dominant negative Smad4 (DN-Smad4) expression construct, luciferase activity was dramatically reduced (Figure 1A). To assess Smad independent signaling, we performed immunoblotting to detect phosphorylation of ERK1/2, AKT, and p38 MAPK. Upon treatment of HCMECs with TGF-β2 for 15 minutes, we found that phosphorylation levels of these kinases were all increased. Chemical inhibitors of MEK1/2 (U0126), PI3K (LY294002), and p38 (SB202190) prevented TGF-β2-induced phosphorylation of ERK1/2, AKT, and p38 MAPK, respectively (Figures 1B-1D).
Figure 1. TGF-β2 activates Smad, MEK, PI3K, and p38 MAPK signaling pathways.
(A) p3TP-Lux reporter gene assay showing increased Smad activity upon treatment of HCMECs with TGF-β2. Expression of a dominant negative Smad4 (DN-Smad4) inhibited this increased activity. Data represent mean (n=3) ± SD; *P<0.01 for TGF-β2 compared to vehicle; **P<0.05 for TGF-β2 + DN-Smad4 compared to TGF-β2. (B–D) Immunoblotting for phosphorylation levels of ERK1/2 (B), AKT (C), and p38 MAPK (D) showing that TGF-β2 increases phosphorylation of these kinases. Chemical inhibitors against MEK1/2 (U0126; 10μM), PI3K (LY294002; 50μM), and p38 (SB202190; 25μM) inhibit the increases in ERK1/2, AKT, and p38 MAPK phosphorylation, respectively.
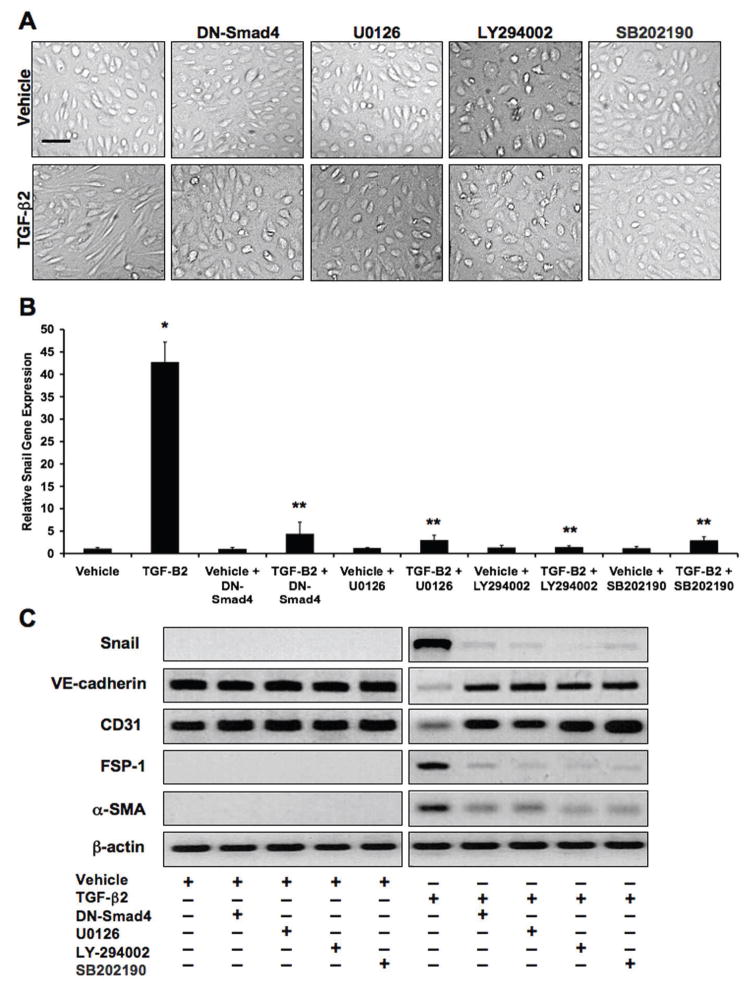
Treatment of HCMECs with TGF-β2 for 48 hours caused a dramatic change in cell morphology from the endothelial cobblestone-like form to an elongated spindle-shaped form that is characteristic of EndMT. However, pretreatment of the cells with DN-Smad4, MEK1/2 inhibitor, PI3K inhibitor, or p38 MAPK inhibitor all prevented this change in cell morphology (Figure 2A). Real-time quantitative PCR was performed to assess gene expression of the EndMT-inducing transcription factor Snail. TGF-β2 dramatically increased Snail mRNA levels, which was inhibited by the presence of inhibitors to Smad4, MEK1/2, PI3K, or p38 (Figure 2B). We performed immunoblotting to assess protein expression of Snail, endothelial markers VE-cadherin and CD31, and mesenchymal markers FSP-1 and alpha-smooth muscle actin (α-SMA). TGF-β2 treatment caused decreased expression of VE-cadherin and CD31, and increased levels of FSP-1, α-SMA, and Snail. Exposure of the cells to DN-Smad4, MEK1/2 inhibitor, PI3K inhibitor, or p38 MAPK inhibitor prevented these expression changes (Figure 2C).
Figure 2. TGF-β2 promotes EndMT through Smad-dependent and Smad-independent signaling.
(A) DIC imaging showing a change in cell morphology consistent with EndMT in HCMEC cultures treated with TGF-β2. Inhibitors against Smad4 (DN-Smad4), MEK1/2 (U0126; 10μM), PI3K (LY294002; 50μM), or p38 MAPK (SB202190; 25μM) prevented the TGF-β2-induced change in morphology. Scale bar, 20μm. (B) Real-time quantitative PCR analysis showing that TGF-β2 increases Snail gene expression, which is prevented by inhibitors of Smad4, MEK1/2, PI3K, or p38 MAPK. Data represent mean (n=3) ± SD; *P<0.01 for TGF-β2 compared to vehicle; **P<0.01 for all TGF-β2 + inhibitors compared to TGF-β2. (C) Immunoblotting showing that TGF-β2 decreases expression of VE-cadherin and CD31, and increases expression of FSP-1, α-SMA, and Snail. Inhibitors of Smad4, MEK1/2, PI3K, or p38 MAPK prevent these expression changes.
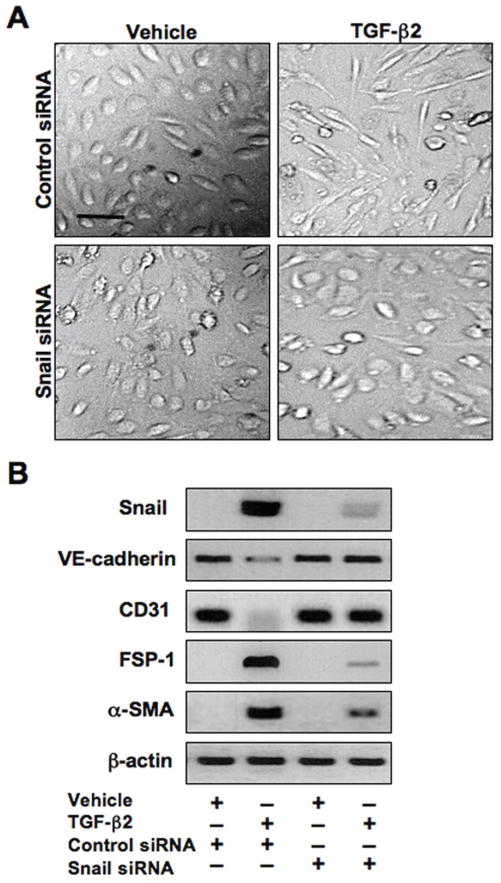
Since all four signaling pathways are necessary for increasing expression of Snail, we assessed the importance of Snail in EndMT by inhibiting its expression with siRNA. HCMECs were transfected with negative control siRNA or Snail siRNA, then treated with TGF-β2 for 48 hours. TGF-β2 induced the mesenchymal morphology in cells transfected with control siRNA, but not in those tranfected with Snail siRNA (Figure 3A). Immunoblotting showed that inhibition of Snail expression was sufficient to prevent TGF-β2-induced decreases in VE-cadherin and CD31 and increases in FSP-1 and α-SMA suggesting that Snail expression is necessary for EndMT (Figure 3B).
Figure 3. Snail activity is essential for TGF-β2-induced EndMT.
(A) DIC imaging showing change in cell morphology in cultures transfected with control siRNA treated with TGF-β2. No EndMT was observed in cultures transfected with Snail siRNA. Scale bar, 20μm. (B) Immunoblotting showing that Snail siRNA inhibits TGF-β2-induced expression changes in VE-cadherin, CD31, FSP-1, and α-SMA.
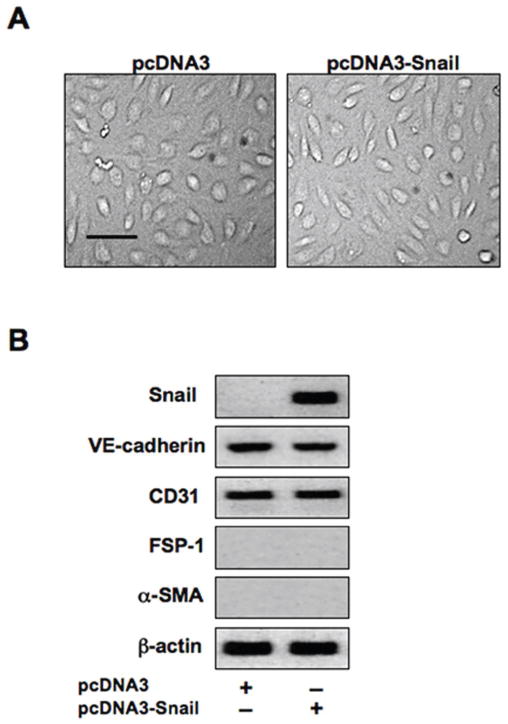
We next sought to determine whether Snail expression was sufficient to induce EndMT. We transfected HCMECs with a Snail expression plasmid for 48 hours. However, we observed no change in cell morphology (Figure 4A). Immunoblotting confirmed a dramatic increase in Snail expression in cells transfected with the Snail expression plasmid, but no significant changes were observed in expression levels of VE-cadherin, CD31, FSP-1, and α-SMA (Figure 4B), suggesting that Snail expression alone is not sufficient to induce EndMT.
Figure 4. Snail expression is not sufficient to induce EndMT.
(A) DIC imaging showing no effect of snail over-expression on cell morphology. Scale bar, 20μm. (B) Immunoblotting confirming a dramatic increase in Snail gene expression in cells transfected with the Snail expression construct. No significant changes in expression of the endothelial markers VE-cadherin and CD31 or the mesenchymal markers FSP-1 and α-SMA were observed.
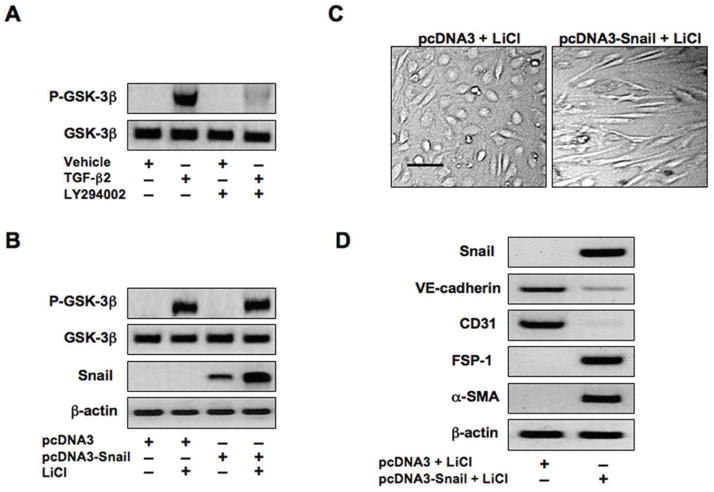
The signaling kinase GSK-3β has been shown to control Snail activity by impairing its function, and inhibitory phosphorylation of GSK-3β promotes Snail transcriptional activity [29]. GSK-3β can be phosphorylated by other kinases such as AKT [30]. We showed in Figure 1 that AKT is activated by TGF-β2 through the PI3K pathway. Immunoblotting using lysates from our cultures showed that TGF-β2 treated cells have a large increase in GSK-3β phosphorylation. This increase in phosphorylation is blocked in cells exposed to PI3K inhibitor (Figure 5A). Cells transfected with the Snail expression plasmid showed no phosphorylation of GSK-3β. Lithium chloride (LiCl), a chemical inhibitor of GSK-3β, increased GSK-3β phosphorylation in our cultures (Figure 5B). Snail protein levels were increased in cells transfected with the Snail expression plasmid and treated with LiCl, compared to cells transfected with the Snail expression plasmid in the absence of LiCl (Figure 5B).
Figure 5. Inhibition of GSK-3β allows Snail-induced EndMT.
(A) Immunoblotting showing increased phosphorylation of GSK-3β in endothelial cells treated with TGF-β2. Inhibition of PI3K with LY294002 (50μM) is sufficient to block GSK-3β phosphorylation induced by TGF-β1. (B) Immunoblotting demonstrating no phosphorylation of GSK-3β when over-expressing Snail. Lithium chloride (LiCl) is sufficient to induce phosphorylation of GSK-3β in cells transfected with pcDNA3 or pcDNA3-Snail plasmids. Snail expression is increased in cells transfected with pcDNA3-Snail and treated with LiCl. (C) DIC imaging showing that the GSK-3β inhibitor lithium chloride (LiCl) is sufficient to transform endothelial cells transfected with pcDNA3-Snail to mesenchyme. Scale bar, 20μm. (D) Immunoblotting confirming expression of Snail, decreased expression of endothelial markers VE-cadherin and CD31, and increased expression of mesenchymal markers FSP-1 and α-SMA in cells containing pcDNA3-Snail and treated with LiCl.
Next, we attempted to determine whether inhibition of GSK-3β with LiCl could allow EndMT to be induced by Snail over-expression. Cells transfected with the pcDNA3-Snail expression plasmid, then treated with LiCl, did take on the mesenchymal morphology (Figure 5C). Snail over-expression was confirmed by immunoblotting (Figure 5D). LiCl caused pcDNA3-Snail transfected cells to have reduced expression of VE-cadherin and CD31, and increased expression of FSP-1 and α-SMA (Figures 5D). These data suggest that inhibition of GSK-3β allows Snail to induce EndMT.
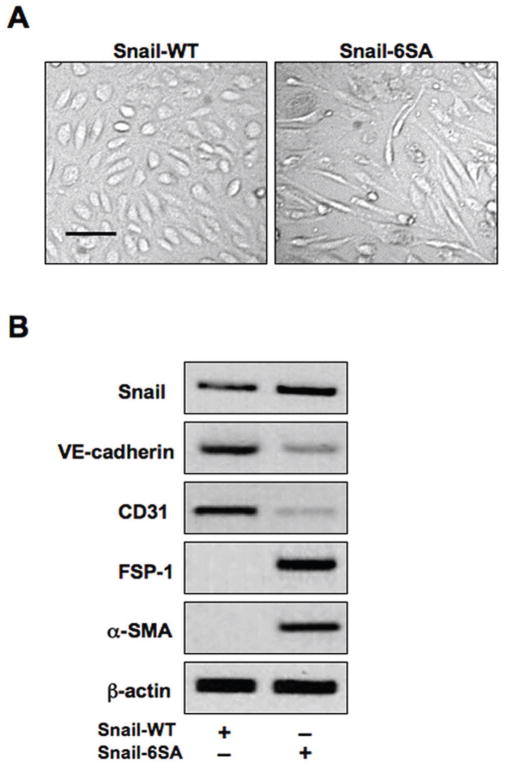
For further confirmation of GSK-3β-dependent regulation of Snail activity we transfected HCMECs with expression plasmids encoding either wild-type Snail (Snail-WT) or a mutant Snail (Snail-6SA) that is resistant to GSK-3β-dependent inhibition. The mutant Snail construct caused acquisition of the mesenchymal morphology (Figure 6A), as well as decreased expression of the endothelial markers VE-cadherin and CD31 and increased expression of the mesenchymal markers FSP-1 and α-SMA (Figure 6B), further suggesting a critical role for GSK-3β in regulating Snail-induced EndMT.
Figure 6. Induction of EndMT by a GSK-3β-resistant mutant form of Snail.
(A) DIC imaging demonstrating EndMT of cells transfected with a mutant GSK-3β-resistant Snail (Snail-6SA) construct. Scale bar, 20μm. (B) Immunoblotting showing decreased expression of endothelial markers (VE-cadherin, CD31) and increased expression of mesenchymal markers (FSP-1, α-SMA) in cells expressing mutant Snail, but not wild-type Snail (Snail-WT).
DISCUSSION
Our data provide novel insight into the signaling mechanisms that mediate EndMT. We found that TGF-β2 signals through the Smad, MEK, PI3K, and p38 MAPK pathways, and all of these pathways are essential for inducing EndMT. Furthermore, all of these pathways are necessary for promoting increased expression of the EndMT-inducing transcription factor Snail, which suppresses cell adhesion and promotes EndMT [13]. Smad proteins have been shown to bind directly to the promoter of the Snail gene to regulate its transcription [31–34]. Transcription factors induced by Smad-independent signaling that regulate Snail transcription remain unclear, but we show that these pathways have a critical role in controlling Snail gene expression.
In epithelial and cancer systems, studies have shown that tranfection of cells with a Snail expression plasmid is sufficient to induce epithelial-mesenchymal transition (EMT) [35,36]. Surprisingly, we found that expression of Snail alone is insufficient to induce endothelial-mesenchymal transition. These data suggest that other mechanisms are necessary to mediate this change in endothelial cell morphology. One such mechanism is inhibition of GSK-3β.
Snail protein stability and nuclear transport capability are inhibited through phosphorylation by GSK-3β [29]. Therefore, inhibitory phosphorylation of GSK-3β by kinases such as AKT, a downstream signaling molecule from PI3K [30], is sufficient to prevent GSK-3β-dependent inhibition of Snail. Our data show that inhibition of GSK-3β by TGF-β2-induced PI3K signaling or by direct inhibition with LiCl allows Snail to function to induce EndMT. PI3K is also necessary for controlling Snail gene expression, demonstrating a dual role for this pathway in mediating EndMT.
While other transcription factors and signal transduction pathways are likely to play a critical role in EndMT, we have established a foundation for understanding this cellular transformation by identifying four major signaling pathways that cooperate to control a known regulator of cell-cell adhesion and cellular plasticity. Identifying signaling pathways that control EndMT is necessary for translational applications in clinical medicine. Such knowledge may prove beneficial for designing therapeutic strategies for EndMT-associated diseases such as fibrosis, cancer, diabetes, and atherosclerosis [11]. Targeting the Smad, MEK, PI3K, or p38 MAPK pathways, or their downstream target Snail, with small molecule inhibitors should perturb this detrimental mechanism of disease progression.
Acknowledgments
FUNDING
This work was supported by grants CA125550, CA155370, CA151925, DK55001, DK81576 (to R. Kalluri) and F30HL095319 (to S. Potenta) from the National Institutes of Health and a grant from the Champalimaud Foundation (to R. Kalluri).
We thank Dr. Bjorn Olsen (Harvard Medical School) for providing cells and antibodies necessary for this study. We also thank Dr. Mien-Chie Hung (M. D. Anderson Cancer Center) for providing Snail plasmids.
Abbreviations used
- EndMT
endothelial-mesenchymal transition
- TGF-β
transforming growth factor-beta
- FSP-1
fibroblast specific protein-1
- α-SMA
alpha-smooth muscle actin
- MAPK
mitogen activated protein kinase
- DN
dominant negative
- PI3K
phosphinositide-3-kinase
- GSK-3β
glycogen synthase kinase-3 beta
- LiCl
lithium chloride
References
- 1.Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB. TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol. 1999;208:530–545. doi: 10.1006/dbio.1999.9211. [DOI] [PubMed] [Google Scholar]
- 2.Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol. 2002;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- 3.Azhar M, Runyan RB, Gard C, Sanford LP, Miller ML, Andringa A, Pawlowski S, Rajan S, Doetschman T. Ligand-specific function of transforming growth factor beta in epithelial-mesenchymal transition in heart development. Dev Dyn. 2009;238:431–442. doi: 10.1002/dvdy.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 5.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 6.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mironov V, Hill C, Starcher B, Shih J, Hoffman S, Markwald R. Endothelial-mesenchymal transformation in atherosclerosis: a recapitulation of embryonic heart tissue morphogenesis. Ann Biomed Eng. 1995;23:S29A. [Google Scholar]
- 8.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 9.Kizu A, Medici D, Kalluri R. Endothelial-mesenchymal transition as a novel mechanism for generating myofibroblasts during diabetic nephropathy. Am J Pathol. 2009;175:1371–1373. doi: 10.2353/ajpath.2009.090698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JG, Kay EP. FGF-2-mediated signal transduction during endothelial mesenchymal transformation in corneal endothelial cells. Exp Eye Res. 2006;83:1309–1316. doi: 10.1016/j.exer.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375–1379. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, Dejana E. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J Cell Biol. 2004;166:359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004;48:365–375. doi: 10.1387/ijdb.041794hp. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Nawhsad A, Medici D, Liu CC, Hay ED. TGFbeta3 inhibits E-cadherin gene expression in palate medial-edge epithelial cells through a Smad2-Smad4-LEF-1 transcription complex. J Cell Sci. 2006;120:1646–1653. doi: 10.1242/jcs.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medici D, Hay ED, Goodenough DA. Cooperation between snail and LEF-1 transcription factors is essential for TGF-beta1-induced epithelial-mesenchymal transition. Mol Biol Cell. 2006;17:1871–1879. doi: 10.1091/mbc.E05-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokudo T, Suzuki Y, Yoshimatsu Y, Yamazaki T, Watabe T, Miyazono K. Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci. 2008;121:3317–3324. doi: 10.1242/jcs.028282. [DOI] [PubMed] [Google Scholar]
- 18.Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through β-catenin-TCF-4-dependent expression of TGF-β3. Mol Biol Cell. 2008;19:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akhurst RJ, Derynck R. TGF-beta signaling in cancer - a double-edged sword. Trends Cell Biol. 2001;11:S44–S51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 20.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Romano LA, Runyan RB. Slug is an essential target of TGFbeta2 signaling in the developing chicken heart. Dev Biol. 2000;223:91–102. doi: 10.1006/dbio.2000.9750. [DOI] [PubMed] [Google Scholar]
- 22.Deissler H, Deissler H, Lang GK, Lang GE. TGFbeta induces transdifferentiation of iBREC to alphaSMA-expressing cells. Int J Mol Med. 2006;18:577–582. [PubMed] [Google Scholar]
- 23.Tavares AL, Mercado-Pimentel ME, Runyan RB, Kitten GT. TGF beta-mediated RhoA expression is necessary for epithelial-mesenchymal transition in the embryonic chick heart. Dev Dyn. 2006;235:1589–1598. doi: 10.1002/dvdy.20771. [DOI] [PubMed] [Google Scholar]
- 24.Mercado-Pimentel ME, Runyan RB. Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs. 2007;185:146–156. doi: 10.1159/000101315. [DOI] [PubMed] [Google Scholar]
- 25.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boye E, Yu Y, Paranya G, Mulliken JB, Olsen BR, Bischoff J. Clonality and altered behavior of endothelial cells from hemangiomas. J Clin Invest. 2001;107:745–752. doi: 10.1172/JCI11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, Bischoff J, Vikkula M, Boye E, Olsen BR. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14:1236–1246. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Graziano K, Pham T, Logsdon CD, Simeone DM. Adenovirus mediated gene transfer of dominant negative Smad4 blocks TGF-beta signaling in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1247–G1253. doi: 10.1152/ajpgi.2001.280.6.G1247. [DOI] [PubMed] [Google Scholar]
- 29.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 30.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 31.Jamora C, Lee P, Kocieniewski P, Azhar M, Hosokawa R, Chai Y, Fuchs E. A signaling pathway involving TGF-beta2 and Snail in hair follicle morphogenesis. PLOS Biol. 2005;3:131–142. doi: 10.1371/journal.pbio.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoot KE, Lighthall J, Han G, Lu SL, Li A, Ju W, Kulesz-Martin M, Bottinger E, Wang XJ. Keratinocyte-specific Smad2 ablation results in increased epithelial-mesenchymal transition during skin cancer formation and progression. J Clin Invest. 2008;118:2722–2732. doi: 10.1172/JCI33713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AP, Verrecchia A, Faga G, Doni M, Perna D, Martinato F, Guccione E, Amati B. A positive role for Myc in TGFbeta-induced Snail transcription and epithelial-to-mesenchymal transition. Oncogene. 2009;28:422–430. doi: 10.1038/onc.2008.395. [DOI] [PubMed] [Google Scholar]
- 34.Brandl M, Seidler B, Haller F, Adamski J, Schmid RM, Saur D, Schneider G. IKKalpha controls canonical TGFbeta-SMAD signaling to regulate genes expressing SNAIL and SLUG during EMT in Panc1 cells. J Cell Sci. 2010;123:4231–4239. doi: 10.1242/jcs.071100. [DOI] [PubMed] [Google Scholar]
- 35.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Bianco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transition by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 36.Vetter G, Saumet A, Moes M, Vallar L, Le Bechec A, Laurini C, Sabbah M, Arar K, Theillet C, Lecellier CH, Friederich E. miR-661 expression in SNAI1-induced epithelial to mesenchymal transition contributes to breast cancer cell invasion by targeting Nectin-1 and StarD10 messengers. Oncogene. 2010;29:4436–4448. doi: 10.1038/onc.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]