Abstract
The stress response is essential to the survival of all species as it maintains internal equilibrium and allows organisms to respond to threats in the environment. Most stress research has focused on the detrimental impacts of stress on cognition and behavior. Reversal learning, which requires a change in response strategy based on one dimension of the stimuli, is one type of behavioral flexibility that is facilitated following some brief stress procedures. The current study investigated a potential mechanism underlying this facilitation by blocking glucocorticoid receptors (GRs) during stress. Thirty-seven male Long Evans rats learned to discriminate between two images on a touchscreen, one of which was rewarded. Once a criterion was reached, rats received stress (30 min of restraint stress or no stress) and drug (GR antagonist RU38486 or vehicle) administration prior to each of the first 3 days of reversal learning. We expected that stress would facilitate reversal learning and RU38486 (10 mg/kg) would prevent this facilitation in both early (<50% correct in one session) and late (>50% correct in one session) stages of reversal learning. Results showed that stressed rats performed better than unstressed rats (fewer days for late reversal, fewer correction trials, and fewer errors) in the late but not early stage of reversal learning. RU38486 did not block the facilitation of RL by stress, although it dramatically increased response, but not reward, latencies. These results confirm the facilitation of late reversal by stress in a touchscreen-based operant task in rats and further our understanding of how stress affects higher level cognitive functioning and behavior.
Keywords: Behavioral flexibility, Corticosterone, Glucocorticoid receptor, RU38486, Frontal cortex
1. Introduction
The stress response is essential to the survival of all species, as it maintains internal equilibrium and allows organisms to respond to threats in the environment. The effects of acute stress on cognition are complex and determined by a number of factors including the specific stressor, task, sex of the subject, and timing of the stress relative to the task [1–5]. Acute stress has been associated with detrimental effects on cognition, as has been demonstrated for the retrieval of declarative-like forms of memory such as spatial and recognition memory mediated by structures of the temporal lobe [2,3]. However, the influence of acute stress on forms of cognition dependent on prefrontal cortex (PFC) is less well defined [1]. Research regarding the influence of acute stress on PFC-dependent executive processing and cognitive/behavioral flexibility have been inconsistent, with some studies showing impaired performance [6–9] while others show facilitated performance [10–14], depending on the specific task and stressor.
Behavioral flexibility refers to the ability of an organism to change strategies to optimize survival or adapt to the environment [15]. Reversal learning, a type of behavioral flexibility that requires a change in strategy based on one dimension of the stimuli, is partly mediated by the orbitofrontal cortex [11,16]. Reversal learning assayed using operant-based tasks is facilitated following an acute (1 day) or subchronic (3 days) stressor in most [10–12] but not all studies [7]. Previous studies have used two operant paradigms for assessing the effects of acute stress on reversal learning. In two studies [11,12], a touchscreen-based discrimination task [17–19] that took several weeks to complete was used in mice. After being trained on a discrimination task for food reward and before initiating a reversal of the discrimination, mice were subjected to 3 days of swim stress. No immediate effects of stress were found on the early phase of reversal learning during which perseveration is high; however, late reversal learning (>50% correct) was facilitated in C57BL/6J mice subjected to the stressor [11] but not those from the DBA/2J strain [12]. The facilitation of reversal learning was prevented by BDNF infusions into the ventromedial PFC and mimicked by lesions of the same region [11]. In contrast, Thai and colleagues used an operant-based spatial reversal learning task in rats that took one session to complete [10]. A single 30 min period of acute restraint stress significantly improved performance of the rats in this task, an effect independent of mineralocorticoid or glucocorticoid receptor (GR) activation [10]. A second study using a task design similar to Thai and colleagues [10] found no effect of an in-context tail pinch stressor on reversal learning [7]. In addition, Barkus and colleagues assessed the effects of acute swim stress on a single reversal learning session of a touchscreen-based visual discrimination in mice [20]. They found no effect of acute swim stress on early reversal learning in either GluA1 knockout mice or their wild type controls (>75% C57BL/6J background).
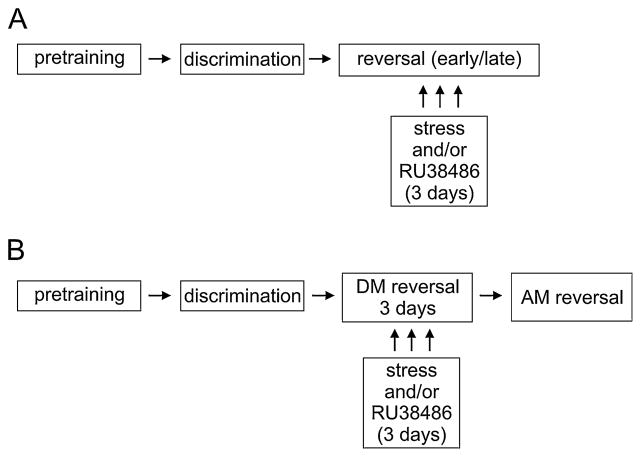
Given these previous findings, the present study was initiated with two main objectives: (1) to test whether restraint stress would also facilitate reversal learning in rats tested in the touchscreen-based discrimination task; and (2) to investigate the potential role of GRs in the stress-induced facilitation of reversal learning using the GR antagonist RU38486. In the present experiment, we introduced the stress and drug manipulation immediately prior to each of the first 3 days of reversal learning (Fig. 1). This design enabled assessment of the immediate effects of restraint stress and GR activation on early reversal learning [10], as well as the long-term effects on late reversal learning in a manner similar to that reported previously [11,12]. We hypothesized that blocking GRs would prevent the immediate influence of stress on reversal learning as GRs become saturated by elevated CORT levels following stress and blocking GRs reverses the effect of acute stress on cognitive functioning in previous studies [6,13].
Fig. 1.
Schematic of the experimental design along with the two analyses methods for reversal learning. Rats were pretrained to touch the screens for food reward (see Methods). Immediately following pretraining, discrimination was conducted (mean duration 4–5 days, Table 1). Following discrimination training, rats were split into treatment groups and RU38486 treatment (60 min before training) and/or restraint stress (30 min before training) were given on the three subsequent days. The rats were tested for reversal learning immediately following these three treatment sessions. In the early/late analysis (panel A), reversal learning was analyzed in relationship to a given rat’s performance on the task irrespective of treatment (see Section 2 for details on early vs. late distinction). While the majority of rats were in the early phase of reversal learning during treatment, six of them progressed to the late phase for days 2 or 3 of reversal testing. For the ‘during manipulation’ (DM) and ‘after manipulation’ (AM) analysis (panel B), performance of the rats was analyzed separately for sessions during the manipulation (i.e., the first 3 days of reversal) vs. after the manipulation (all subsequent days). All rats required at least one AM session to complete reversal learning.
2. Materials and methods
2.1. Subjects
Thirty-seven male Long Evans rats (initial weight: 275–310 g, Charles River, Quebec, Canada) were used in the current experiment. Rats were housed in a temperature controlled room (21 °C) on a light–dark cycle of 12 h (lights on at 0700). Upon arrival, rats were housed in pairs and given free access to food (Purina rat chow) for at least 7 days. The rats were then individually caged and food restricted to 85% of their free-feeding weights. Water was available ad libitum throughout the experiment. All rats were food restricted for 3 days prior to training and handled for a minimum of 5 min on those days. Experiments were conducted in accordance with the standards of the Canadian Council on Animal Care and were approved by the University of Saskatchewan Animal Research Ethics Board.
2.2. Apparatus
Preliminary training and behavioral testing were carried out in four automated touchscreen testing chambers for rats (Lafayette Instrument Company, Lafayette, IN). The apparatus consisted of a trapezoidal testing chamber housed within a sound-attenuating box. The box contained a 28-volt DC fan for ventilation and to mask extraneous noise. The inner operant chamber (30.5 × 24.1 × 8.25 cm) consisted of black Perspex walls, clear Perspex removable lid and a stainless steel grid floor. Each chamber contained a 45 mg food receptacle where food reward pellets (Dustless Precision Pellets, 45 mg, Rodent Purified Diet; BioServ, Frenchtown, NJ) were delivered via an automated pellet dispenser. A 3 W house light and tone generator were fitted to the top of the chamber. The magazine tray was illuminated by a 3 W light bulb and fitted with laser entry detectors to detect the rat’s presence. At the end of the box opposite the magazine was a flat-screen monitor equipped with an infrared touchscreen controlled by touchscreen software (ABET II Software, Lafayette Instrument Company). Touch-screens that use infrared photocells can detect a nose-poke without the rat having to actually touch the screen; sniffing behavior will elicit a “touch.” A Perspex mask covered the screen with two response windows that the rat could touch. Attached to the mask was a shelf positioned in front of the touchscreen that extended 7 cm from the surface of the mask supported by springs. The two response windows that displayed the stimulus images could be accessed above the mask ~15 cm from the floor of the chamber. The shelf facilitated the rat’s attention by forcing the rat to face the stimuli head-on, stop, rear up, and stretch toward the stimuli.
2.3. Habituation and pre-training
The visual discrimination and reversal learning procedures closely followed those recommended by the manufacturer and optimized in Professor Timothy Bussey’s laboratory [18,19,21]. Following the third day of food restriction, rats were put in the touchscreen operant chambers and habituated for 30 min. Food pellets were placed in the food tray and two stimulus images were displayed on the two response windows. The rats were not required to touch the screen. The images included, but were not limited to, the two images that were used for testing. All images displayed on the touchscreen were white objects on a black background supplied by the manufacturer. The house light was not illuminated in the touchscreen boxes during training and testing stages. The only light present came from the tray illumination and light from the images on the screen.
Pre-training commenced the day following habituation. The first stage of training involved rats receiving one food pellet every 30 s regardless of its behavior. Throughout training and testing, a simultaneous tone and tray light illumination indicated to the rat that there was a reward in the food tray. During this stage, one image was displayed for 30 s on one of the two response windows. If the rat touched this image, it would receive three food pellets. Each training session lasted for 60 min. In order to move to the next training stage, the rat had to achieve 100 trials in the 60 min, which usually took only one session.
During the second stage of pre-training rats had to touch the screen with the image displayed on it in order to receive one food pellet. Again, only one image was displayed on one of the response windows and it was left on until the rat touched it. The position of the image on the right or left screen was semi-random (the image could not be displayed on the same side more than three times in a row). Once the rat had chosen the correct image and received a reward pellet, it would start an inter-trial interval (ITI) of 20 s where there were no images on the screen. After the ITI, a new trial would start with an image displayed on one of the two response windows. The criterion for completion of this stage was 100 trials in 60 min, which took an average of 2 days.
The third stage of pre-training was similar to the last except now the rat had to initiate the trial. This meant that, following the ITI, the rat was required to nose poke into the food tray that was illuminated in order to start the next trial. As soon as the rat nose poked into the food tray, the image was displayed on the screen behind the rat. Similar to the other stages, the rat received a food pellet when it touched the response window with the image displayed. After a 20 s ITI the food tray would again illuminate, the rat would nose-poke into the tray, and the image would be displayed on the screen behind it. Rats moved to the next stage when they completed 100 trials in 60 min. This took an average of 3 days.
In the final stage of pre-training rats were punished for an incorrect choice. If the rats chose the blank screen a correction trial would be initiated. Correction trials result in the house light being illuminated for a time-out period of 5 s followed by an ITI of 20 s. Following the ITI, the previously incorrect trial was restarted. Correction trials persisted until the rat chose the correct image which was rewarded with a food pellet. Following a 20 s ITI, a new trial was then presented. Rats moved to discrimination training when they achieved 85% or greater correct 2 days in a row. This stage took an average of 2 days.
2.4. Discrimination training
The day following pre-training, rats were trained to discriminate between two different images displayed on the two response windows. Again, the stimuli were semi-randomly positioned on the right or left side (not more than three trials with the image on the same side). There were two stimulus images used for discrimination training and reversal learning. These images were counterbalanced so that one image was the rewarded stimulus for half of the rats (S1+) whereas the other image was the rewarded stimulus for the other half (S2+).
If the rat chose the correct image (S+), it would result in a tone sounding, tray light illumination, and delivery of a food pellet. Choosing the wrong image (S−) would result in a correction trial with the house light illuminated for 5 s and a 20 s ITI followed by the same trial. Correction trials were repeated until the rat chose the correct image. Discrimination training continued for 60 min per daily session until the rat reached 100 trials with 85% or greater correct for 2 consecutive days.
2.5. Reversal learning
Reversal learning commenced the day after successful completion of discrimination training. During reversal learning, the previously unrewarded stimulus image (S−) was the rewarded stimulus (S+) and the previously rewarded stimulus (S+) was the unrewarded stimulus (S−). Reversal learning was identical to discrimination training in that correct touches to the rewarded stimuli would result in tray illumination, a tone and a food reward. Incorrect touches to the unrewarded stimuli would result in a correction trial and the house light would turn on for a 5 s time out followed by the ITI of 20 s and then a retry of the previous trial. Correction trials would persist until the correct image (S+) was touched. The criterion for completion was 100 trials in 60 min at 85% correct or greater 2 consecutive days.
2.6. Stress and drug treatment
Eighteen rats were exposed to restraint stress using standard Plexiglas rat restraint tubes (Model 544-RR, Fisher Scientific) for 30 min in a novel room immediately prior to the first three days of reversal learning. In our laboratory, restraint stress significantly increases circulating levels of the stress hormone corticosterone [22]. The other half were left in their cages until reversal learning commenced. In addition, all rats were injected (i.p.) 60 min prior to reversal learning. Eighteen rats were injected with the GR antagonist RU38486 (10 mg/kg; Sigma–Aldrich, Oakville, Ontario) while the other 19 were injected with vehicle (50:50 DMSO:95% ethanol; 2 ml/kg). The dose of RU38486 was determined from previous studies [10,13,22].
2.7. Design and data analysis
The study was a 2 × 2 between-subjects design. Rats were separated into 4 groups for reversal learning based on the drug and stress manipulations (stressed versus unstressed). The groups were: vehicle + no stress (n = 8), vehicle + stress (n = 10), RU38486 + no stress (n = 11), and RU38486 + stress (n = 8). Three rats were excluded from analysis based on late reversal performance that was greater than two standard deviations from the mean: one rat from each of the vehicle + stress, RU38486 + no stress, and RU38486 + stress groups. Final analysis was conducted on a total of 34 rats: vehicle + no stress (n = 8), vehicle + stress (n = 9), RU38486 + no stress (n = 10), and RU38486 + stress (n = 7). Performance on the reversal learning portion of the task was split into early and late phases. The early phase of reversal learning was before rats achieved 50% correct in a session (60 min), whereas the late phase of reversal learning was after the rats achieved 50% or greater correct in one session. In a secondary analysis, the “during manipulation” phase (the 3 days during drug/stress manipulation) was compared to the “after manipulation” phase (all subsequent days). The dependent variables for both analyses of reversal learning were number of trials to criterion, number of correction trials, errors, response latency, and reward latency. Data are presented as means ± standard error of the mean and were analyzed in SPSS (Version 19.0) using a 2 × 2 ANOVA with stress and drug manipulation as the independent variables. Post hoc analyses (Tukey’s test) were conducted where appropriate. P values of <0.05 were deemed significant.
3. Results
3.1. Effects of stress and RU38486 on early and late reversal learning
There was no significant interaction between stress and drug for any of the dependent measures; thus, these statistics are not presented. Rats in the 4 treatment groups showed similar rates of discrimination learning before treatments were initiated (Table 1). No differences were observed among the groups for the number of days of discrimination training (main effect of stress: F(1,30) = 0.13, p = 0.73; main effect of drug: F(1,30) = 0.04, p = 0.85). When the performance on reversal learning was considered, a number of differences were observed (Table 1). While neither stress nor drug treatment significantly altered the total number of days to complete reversal training (main effect of stress: F(1,30) = 0.86, p = 0.36; main effect of drug: F(1,30) = 1.84, p = 0.19), a significant main effect of stress was found for days to complete late reversal (F(1,30) = 4.55, p = 0.041) but not early reversal (F(1,30) = 3.21, p = 0.083). Inspection of the data revealed that rats subjected to stress before each of the first 3 days of reversal learning took significantly fewer days for late reversal (3.19 ± 0.21 days) than rats that were not subjected to stress (3.89 ± 0.25 days). No significant main effects of drug treatment was observed (early reversal: F(1,30) = 2.86, p = 0.10; late reversal: F(1,30) = 0.60, p = 0.44).
Table 1.
Days required for discrimination and reversal learning. The reversal learning data is broken down into early and late phases. Data is mean days ± standard error of the mean.
| Group | Days
|
|||
|---|---|---|---|---|
| Discrimination | Reversal | Early reversal | Late reversal | |
| V-NS | 4.50 ± 1.3 | 8.13 ± 2.4 | 4.13 ± 2.8 | 4.00 ± 1.1 |
| V-S | 4.44 ± 0.7 | 8.22 ± 3.2 | 4.89 ± 2.9 | 3.33 ± 0.9* |
| RU-NS | 4.20 ± 1.2 | 8.60 ± 2.1 | 4.80 ± 2.0 | 3.80 ± 1.1 |
| RU-S | 4.57 ± 1.8 | 10.14 ± 2.4 | 7.14 ± 2.2 | 3.00 ± 0.8* |
V, vehicle; RU, RU38486; NS, no stress; S, stress.
Indicates a significant main effect of stress on days to complete late reversal.
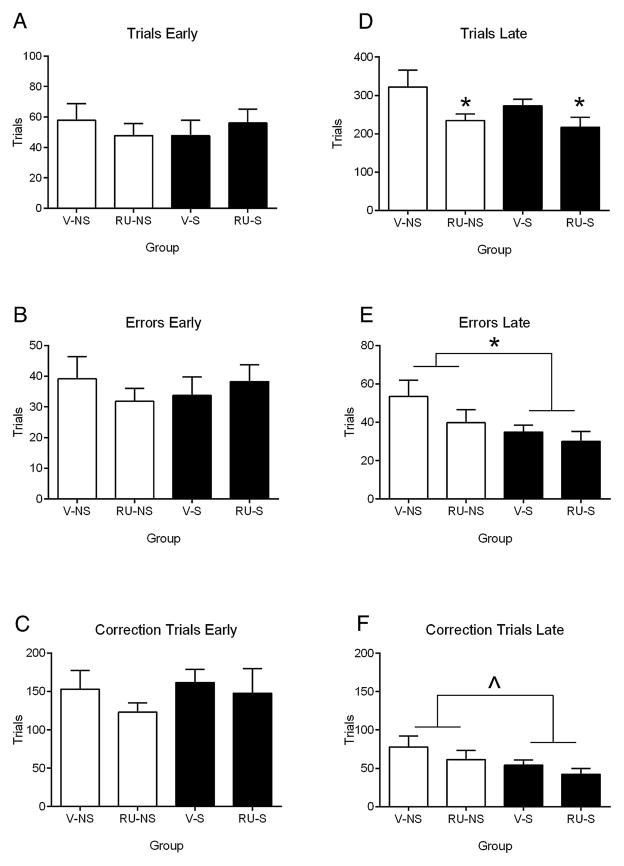
Fig. 2 depicts the effects of stress and RU38486 treatment on the early and late stages of reversal learning. Stress improved late reversal learning as demonstrated by a significant reduction in late errors (Fig. 2E; F(1,30) = 4.93, p = 0.034) and a nearly significant main effect of stress on late correction trials (Fig. 2F; F(1,30) = 3.75, p = 0.062), although the number of trials in the late period of reversal learning were not reliably affected by stress (Fig. 2D; F(1,30) = 1.50, p = 0.23). Stress had no significant effect on measures of early reversal learning (Fig. 2A–C; trials: F(1,30) = 0.01, p = 0.93; errors: F(1,30) = 0.01, p = 0.92; correction trials: F(1,30) = 0.63, p = 0.43) or latency for response (F(1,30) = 0.01, p = 0.93) or reward (Table 2; F(1,30) = 0.49, p = 0.49). Thus, stress does not appear to have significantly affected motivation to perform the task.
Fig. 2.
Average number of trials (A), errors (B), and correction trials (C) for early reversal learning (<50% correct in one session) and average number of trials (D), errors (E), and correction trials (F) for late reversal learning (all sessions after early reversal). Error bars indicate standard error of the mean. * indicates main effect of RU38486 (panel D) or stress (panel E), p < 0.05. m̂ain effect of stress, p = 0.062 (panel F). V, vehicle; RU, RU38486; NS, no stress; S, stress.
Table 2.
Response and reward latency during reversal learning. Average response and reward latencies are noted in seconds.
| Analysis type | Group | Dependent variables
|
|||
|---|---|---|---|---|---|
| Response latency
|
Reward latency
|
||||
| Mean | SE | Mean | SE | ||
| Early | V-NS | 29.92 | 9.8 | 3.09 | 0.5 |
| V-S | 38.86 | 11.0 | 2.33 | 0.3 | |
| RU-NS | 97.35* | 25.1 | 2.29 | 0.1 | |
| RU-S | 85.00* | 22.9 | 2.60 | 0.2 | |
| Late | V-NS | 6.33 | 1.4 | 2.14 | 0.1 |
| V-S | 5.52 | 1.1 | 2.25 | 0.3 | |
| RU-NS | 11.89* | 2.3 | 2.62 | 0.1 | |
| RU-S | 11.74* | 2.4 | 2.45 | 0.3 | |
| DM | V-NS | 94.70 | 64.1 | 2.99 | 0.5 |
| V-S | 37.80 | 14.2 | 2.60 | 0.6 | |
| RU-NS | 118.42 | 46.5 | 2.54 | 0.2 | |
| RU-S | 118.52 | 40.8 | 2.14 | 0.2 | |
| AM | V-NS | 11.94 | 5.0 | 2.15 | 0.1 |
| V-S | 18.66 | 6.3 | 2.20 | 0.2 | |
| RU-NS | 29.16* | 8.2 | 2.55 | 0.1 | |
| RU-S | 39.67* | 12.9 | 2.69 | 0.2 | |
AM, after manipulation; DM, during manipulation; V, vehicle; RU, RU38486; NS, no stress; S, stress; SE, standard error of the mean.
Indicates a significant main effect of RU treatment on response latency.
Administration of RU38486 did not significantly affect the number of errors (early: F(1,30) = 0.06, p = 0.81; late: F(1,30) = 2.11, p = 0.16) or correction trials (early: F(1,30) = 1.08, p = 0.31; late: F(1,30) = 1.60, p = 0.22) during the early or late periods of reversal learning (Fig. 2). The drug manipulation significantly reduced the number of trials completed in the late period of reversal learning (F(1,30) = 7.03, p = 0.013) and dramatically increased response latency during both early (Table 2; F(1,30) = 8.73, p = 0.006) and late reversal learning (F(1,30) = 9.66, p = 0.004). Interestingly, reward latency was not affected by drug administration during either period (early: F(1,30) = 0.64, p = 0.43; late: F(1,30) = 2.23, p = 0.15).
3.2. Effects of stress and RU38486 administration on reversal learning during and after manipulation
Stress and RU38486 treatments were given during the first 3 days of reversal learning regardless of whether a given animal was in the early or late reversal phase (Fig. 1). Therefore, in a secondary analysis we compared behavior during the sessions immediately after treatment (i.e., the first 3 daily sessions) to all sessions after treatment. We have defined these sessions as during manipulation (DM) and after manipulation (AM), respectively. As for the early–late reversal analysis summarized in Section 3.1, there were no significant interactions between stress and treatment and these statistical analyses are not presented.
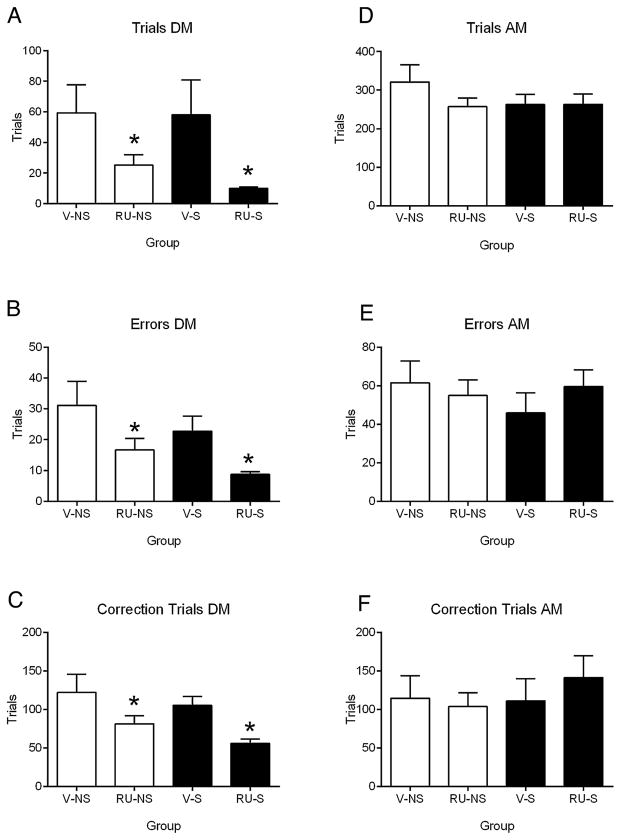
Fig. 3 and Table 2 summarize the results of the DM vs. AM analysis. No significant effect of stress on any dependent measure was found for either DM or AM sessions (statistics not presented). During manipulation errors (Fig. 3B) and correction trials (Fig. 3C) were reduced in rats subjected to stress consistent with facilitated performance, although these effects failed to reach significance (errors: (F(1,30) = 2.56, p = 0.12; correction trials: F(1,30 = 2.14; p = 0.15). A striking finding from this analysis is that all rats, regardless of vehicle or drug treatment, had long response latencies for DM sessions (Table 2). However, given the high variability, there was no significant effect of drug treatment (F(1,30) = 1.34, p = 0.26). A significant main effect of drug treatment was noted for response latency during the AM sessions (F(1,30) = 5.29, p = 0.029). Inspection of the data showed that rats treated with RU38486 had significantly longer response latencies. It should be noted that no changes were found for the reward latencies. Thus, it is unlikely that the long response latencies were due to a gross motor impairment.
Fig. 3.
Average number of trials (A), errors (B), and correction trials (C) for ‘during manipulation’ (DM) days (3 days during which treatments were administered) and average number of trials (D), errors (E), and correction trials (F) for ‘after manipulation’ (AM) sessions (all sessions following DM days). Error bars indicate standard error of the mean. * indicates main effect of RU38486 (panels A, B, C), p < 0.05. V, vehicle; RU, RU38486; NS, no stress; S, stress.
In general, rats treated with RU38486 performed poorly in sessions conducted immediately following drug treatment. The number of trials completed was significantly lower for drug treated rats for DM sessions (F(1,30) = 6.92, p = 0.013) but not AM sessions (F(1,30) = 1.04, p = 0.317). Similar results were observed for errors and correction trials with drug-treated rats completing significantly fewer of each trial type for DM sessions (main effect of drug, errors: F(1,30) = 7.87, p = 0.009; correction trials: F(1,30) = 9.72, p = 0.004) but not AM sessions (main effect of drug, errors: F(1,30) = 0.13, p = 0.72; correction trials: F(1,30) = 0.14, p = 0.71).
4. Discussion
The present experiment assessed the effects of stress and GR antagonism on reversal learning in male Long Evans rats. Stress administered immediately before each of the first three reversal learning sessions did not affect performance during early reversal learning (Table 1 and Fig. 2). However, previous stress facilitated performance of late reversal learning as evidenced by significantly fewer days required for late reversal (Table 1) and errors during the late reversal period (Fig. 2e). A trend of significantly fewer correction trials during the late period was also noted (p = 0.062; Fig. 2f). Effects of the GR antagonist RU38486 on task performance were striking. When data were analyzed using the early vs. late phase distinction, RU38486 reduced the number of trials to criterion during the late period (Fig. 2d) and dramatically increased response, but not reward, latencies (Table 2). Using the ‘during manipulation’ vs. ‘after manipulation’ analysis, RU38486 reduced the number of trials, errors, and correction trials committed ‘during manipulation’ (Fig. 3). Response, but not reward, latencies were also significantly increased during the ‘after manipulation’ period (Table 2).
To our knowledge, this is the first report to show facilitated late reversal learning in rats following stress. Previous studies in mice have shown that stress facilitates late reversal learning in C57BL/6J mice [11] but not those from the DBA/2J strain [12] using a touchscreen-based task. The design used in these experiments is similar, although it should be noted that we administered restraint stress to the rats immediately before testing in the first 3 days of reversal learning (Fig. 1) whereas the other studies gave three daily sessions of stress before initiating reversal learning [11,12]. One study also found that a single bout of swim stress had no effect on the first session of reversal learning in wild-type and GluA1 knockout mice using a touchscreen-based task [20], which is consistent with our findings. Together, these results suggest that stress has a delayed impact on reversal learning in rats and mice. While stress did not affect early reversal learning when inhibition of an old strategy was necessary, previously stressed rodents made fewer errors while learning a new strategy after high rates of perseveration had ceased (late reversal). These effects of stress were observed without changes in response or reward latency (Table 2) suggesting that neither motivation nor motor functions were altered in the rats subjected to stress.
Other studies [7,10] have assessed the effects of acute stress on reversal learning using a lever-based operant task that is typically completed by rats in a single day [16,23]. In one study, a single restraint stress session before reversal learning facilitated reversal learning but not set-shifting [10]. In contrast, an in-context tail pinch stressor impaired set-shifting but not reversal learning in a similar task [7]. It is unclear why different results were observed in the two studies; however, the environment in which the rats were subjected to stress (out of the task context vs. in the task context) may be a factor [7]. While the length of training for completion of the operant reversal task is significantly shorter than the touchscreen-based approach used here, errors are still commonly analyzed into perseverative and regressive subtypes (roughly corresponding to behavior during the periods of early and late reversal, respectively). No significant differences between perseverative and reversal error rates were observed following acute stress; rather, a significant reduction in total errors was reported (Fig. 3 of [10]). Therefore, it appears that the effects of stress on reversal learning may be determined, at least in part, by the task duration.
The second purpose of the present study was to assess whether GR activation is involved in the effects of stress on late reversal learning. To address this question, rats were injected with the GR antagonist RU38486 on the first 3 days of reversal learning (Fig. 1). When the data was analyzed using early vs. late criteria, drug treatment did not significantly affect trials, errors, or correction trials to complete the early phase of reversal learning (Fig. 2). However, drug treatment increased response latencies during the early and late phases of reversal learning without affecting reward latency (Table 2). During the late period, drug treatment significantly reduced the number of trials (Fig. 2d) without effects on errors and correction trials. These alterations in performance led to a re-examination of the reversal learning data ‘during manipulation’ vs. ‘after manipulation’ (Fig. 1). As can be seen in Table 1, there was considerable variability in the days taken to complete early reversal. Thus, for some rats, this secondary analysis produced a greater change in the organization of data than for others. During the stress and drug manipulations, rats treated with RU38486 completed significantly fewer trials and had fewer errors and correction trials (Fig. 3a–c), effects that coincided with a highly variable increase in response latency (Table 2). In contrast, reward latency was not affected by drug or vehicle treatment. In drug treated rats, response latency was also significantly increased after the manipulation. Thus, motivation to perform the instrumental response may have been reduced for a period of several days by three treatments with RU38486, although others have noted that chronic exposure to RU38486 does not alter responding during a progressive ratio for food reward in mice [24]. Motivation to collect the reward pellet and motor behavior was not dramatically affected as reward collection latencies were not altered during any phase of the task.
Support was not provided for the hypothesis that blocking GRs would prevent stress from facilitating reversal learning. In a previous study that used a lever-based operant task to test reversal learning in one session, no significant effect of blocking GRs on a stress-induced facilitation of reversal learning was found [10]. In the present design, 3 days of stress and RU38486 treatment were conducted as the touchscreen-based task requires multiple days to complete (Table 1). The effects of RU38486 are confounded by the effects of the drug manipulation on response latency during the period of administration and the subsequent several days. Previous reports also show that repeated administration of RU38486 dys-regulates the hypothalamic-pituitary-adrenal axis leading to a significant increase in circulating corticosterone/cortisol in rats [25] and humans [26]. As a result, the complex effects of increased circulating corticosterone on cognition while GRs are blocked make interpretation of the effects of systemically administered RU38486 on reversal learning difficult. It should be noted that a single systemic [13] or local intracerebral administration of RU38486 [6] is effective for assessing the mechanisms underlying stress-induced changes in cognition. Future studies using targeted local intracranial infusions of RU38486 may clarify the role of GRs in the stress-induced facilitation of reversal learning without confounds related to response latencies or alterations in levels of circulating corticosterone.
Previous research has identified potential mechanisms that may be involved in the mediation of stress-induced facilitation of reversal learning. Graybeal et al. [11] found that injection of brain-derived neurotrophic factor (BDNF) directly into the vmPFC following stress is sufficient to prevent facilitation of reversal learning. In addition, a high dose of corticotropin-releasing factor (CRF) injected into the locus coeruleus facilitates reversal learning in rats [27]. Stress-induced changes in neurotransmitters such as glutamate, dopamine, and serotonin may also play a role in reversal learning facilitation [14,15,28]. Future research integrating these and other mechanisms that cause the facilitation of late reversal learning following stress will increase the understanding of the diverse stress effects of stress on behavioral flexibility.
HIGHLIGHTS.
The effects of stress on reversal learning in rats were assessed using touchscreen-equipped operant chambers.
Stress facilitated rates of late reversal learning after perseveration ceased.
Glucocorticoid receptor antagonism failed to block the effects of stress.
Glucocorticoid receptor antagonism significantly increased response but not reward latencies in the task.
Acknowledgments
This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada to JGH. JGH is a National Alliance for Research on Schizophrenia and Depression Young Investigator and a Canadian Institutes of Health Research New Investigator. CAB was supported by an Undergraduate Student Research Award from the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–83. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cazakoff BN, Johnson KJ, Howland JG. Converging effects of acute stress on spatial and recognition memory in rodents: a review of recent behavioural and pharmacological findings. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:733–41. doi: 10.1016/j.pnpbp.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–62. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 4.Schwabe L, Joels M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: an update and integration. Neurosci Biobehav Rev. 2012;36:1740–9. doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Campeau S, Liberzon I, Morilak D, Ressler K. Stress modulation of cognitive and affective processes. Stress. 2011;14:503–19. doi: 10.3109/10253890.2011.596864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butts KA, Weinberg J, Young AH, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc Natl Acad Sci USA. 2011;108:18459–64. doi: 10.1073/pnas.1111746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butts KA, Floresco SB, Phillips AG. Acute stress impairs set-shifting but not reversal learning. Behav Brain Res. 2013;252:222–9. doi: 10.1016/j.bbr.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Devilbiss DM, Jenison RL, Berridge CW. Stress-induced impairment of a working memory task: role of spiking rate and spiking history predicted discharge. PLoS Comput Biol. 2012;8:e1002681. doi: 10.1371/journal.pcbi.1002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis DD, Zaharia MD, Shanks N, Anisman H. Stress-induced disturbances in Morris water-maze performance: interstrain variability. Physiol Behav. 1995;58:57–65. doi: 10.1016/0031-9384(95)00009-8. [DOI] [PubMed] [Google Scholar]
- 10.Thai CA, Zhang Y, Howland JG. Effects of acute restraint stress on set-shifting and reversal learning in male rats. Cogn Affect Behav Neurosci. 2013;13:164–73. doi: 10.3758/s13415-012-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, et al. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat Neurosci. 2011;14:1507–9. doi: 10.1038/nn.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graybeal C, Bachu M, Mozhui K, Saksida LM, Bussey TJ, Sagalyn E, et al. Strains and stressors: an analysis of touchscreen learning in genetically diverse mouse strains. PLoS ONE. 2014;9:e87745. doi: 10.1371/journal.pone.0087745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA. 2009;106:14075–9. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong Z, Bai Y, Wu X, Li H, Gong B, Howland JG, et al. Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem. 2008;89:567–73. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, et al. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology. 2012;62:1191–203. doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horner AE, Heath CJ, Hvoslef-Eide M, Kent BA, Kim CH, Nilsson SR, et al. The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc. 2013;8:1961–84. doi: 10.1038/nprot.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mar AC, Horner AE, Nilsson SR, Alsio J, Kent BA, Kim CH, et al. The touchscreen operant platform for assessing executive function in rats and mice. Nat Protoc. 2013;8:1985–2005. doi: 10.1038/nprot.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barkus C, Feyder M, Graybeal C, Wright T, Wiedholz L, Izquierdo A, et al. Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symptoms of schizophrenia and schizoaffective disorder? Neuropharmacology. 2012;62:1263–72. doi: 10.1016/j.neuropharm.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winters BD, Bartko SJ, Saksida LM, Bussey TJ. Muscimol, AP5, or scopolamine infused into perirhinal cortex impairs two-choice visual discrimination learning in rats. Neurobiol Learn Mem. 2010;93:221–8. doi: 10.1016/j.nlm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 22.MacDougall MJ, Howland JG. Acute stress, but not corticosterone, disrupts short- and long-term synaptic plasticity in rat dorsal subiculum via glucocorticoid receptor activation. Cereb Cortex. 2013;23:2611–9. doi: 10.1093/cercor/bhs247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Gourley SL, Swanson AM, Jacobs AM, Howell JL, Mo M, Dileone RJ, et al. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc Natl Acad Sci USA. 2012;109:20714–9. doi: 10.1073/pnas.1208342109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Revsin Y, Rekers NV, Louwe MC, Saravia FE, De Nicola AF, de Kloet ER, et al. Glucocorticoid receptor blockade normalizes hippocampal alterations and cognitive impairment in streptozotocin-induced type 1 diabetes mice. Neuropsychopharmacology. 2009;34:747–58. doi: 10.1038/npp.2008.136. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher P, Watson S, Smith MS, Ferrier IN, Young AH. Effects of adjunctive mifepristone (RU-486) administration on neurocognitive function and symptoms in schizophrenia. Biol Psychiatry. 2005;57:155–61. doi: 10.1016/j.biopsych.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Snyder K, Wang WW, Han R, McFadden K, Valentino RJ. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology. 2012;37:520–30. doi: 10.1038/npp.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2 C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;30:930–8. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]