Abstract
Current understanding of the etiology of neurodevelopmental disorders is limited; however, recent epidemiological studies demonstrate a strong correlation between prenatal infection during pregnancy and the development of schizophrenia in adult offspring. In particular, schizophrenia patients subjected to prenatal infection exhibit impairments in executive functions greater than schizophrenia patients not exposed to an infection while in utero. Acute prenatal treatment of rodents with the viral mimetic polyinosinic-polycytidylic acid (PolyI:C) induces behavioural and neuropathological alterations in the adult offspring similar to schizophrenia. However, impairments on tasks of executive function that involve the prefrontal cortex (PFC) have been rarely examined for the prenatal infection model. Hence, we investigated the effects of acute prenatal injection of PolyI:C (4.0 mg/kg, i.v., gestational day 15) on strategy set-shifting and reversal learning in an operant-based task. Our results show male, but not female, PolyI:C-treated adult offspring require more trials to reach criterion and perseverate during set-shifting. An opposite pattern was seen on the reversal day where the PolyI:C-treated male rats made fewer regressive errors. Females took more pre-training days and were slower to respond during the trials when compared to males regardless of prenatal treatment. The present findings validate the utility of the prenatal infection model for examining alterations of executive function, one of the most prominent cognitive symptoms of schizophrenia.
Keywords: Set-shifting, Reversal learning, Operant conditioning, PolyI:C, Schizophrenia, Medial prefrontal cortex
1. Introduction
The etiology of neurodevelopmental psychiatric disorders such as schizophrenia is complicated and poorly understood. Genetic factors play a significant role; however, monozygotic concordance rates in most studies are significantly lower than 100% (approximately 50% for schizophrenia), suggesting environmental factors are also involved (Brown, 2011; Patterson, 2007). Epidemiological studies show early environmental factors including prenatal infection, season of birth, and obstetric complications increase the risk of neurodevelopmental disorders later in life (Ciaranello and Ciaranello, 1995; Weinberger, 1995; Wong and Van Tol, 2003). In particular, recent prospective studies using serum measures to confirm maternal infection provide strong support for a dramatically increased risk (three- to seven-fold) of schizophrenia in offspring of mothers who had respiratory infections (Boksa, 2008; Brown et al., 2004; Brown, 2006; Brown and Derkits, 2010; Brown and Susser, 2002; Patterson, 2007, 2009; Pearce, 2001). These findings have prompted research into the behavioural and neuropathological effects of prenatal infection in rodents as models of neuropsychiatric illness (Meyer et al., 2009; Patterson, 2009; Pearce, 2001).
Prenatal infection in rodents causes an array of behavioural changes in the offspring relevant to psychiatric disorders including alterations in prepulse inhibition, locomotor activity, latent inhibition, anxiety, social interaction, and memory (Bitanihirwe et al., 2010a, 2010b; Meyer et al., 2005; Meyer et al., 2009; Ozawa et al., 2006; Shi et al., 2003; Wolff and Bilkey, 2008, 2010; Zuckerman et al., 2003; Zuckerman and Weiner, 2003, 2005). At least some of the changes show post-pubertal emergence consistent with the typical course of schizophrenia in humans (Meyer et al., 2009; Ozawa et al., 2006; Zuckerman and Weiner, 2003). However, the prenatal infection model has not been well characterized with regard to the most prominent cognitive symptoms of schizophrenia including disruptions in executive functions mediated by the prefrontal cortex.
The importance of cognitive symptoms has been emphasized in schizophrenia because the symptoms are severe, particularly enduring, and strongly correlated with long-term patient outcomes (Elvevag and Goldberg, 2000; Floresco et al., 2005; Keefe and Fenton, 2007; Lewis and Gonzalez-Burgos, 2006, 2008). Patients with schizophrenia, like those with frontal lobe lesions, display perseverative deficits on measures related to cognitive flexibility such as attentional set-shifting (Leeson et al., 2009; Pantelis et al., 1999) and reversal learning (Leeson et al., 2009; McKirdy et al., 2009; Murray et al., 2008; Pantelis et al., 1999; Waltz and Gold, 2007). Notably, schizophrenia patients whose mothers had a confirmed infection during pregnancy made significantly more errors on the Wisconsin Card Sorting Task and Trails B test than either controls or schizophrenia patients that were not exposed to infection during fetal development (Brown et al., 2009).
Given these observations, the present study assessed the effects of prenatal infection on behavioural flexibility in rats using a well characterized task in rodents (Floresco et al., 2008, 2009). This automated operant-based procedure involves simple discriminations, strategy shifts, and reversals between visual cues and egocentric spatial response strategies to obtain food reward. We hypothesized that strategy set-shifting and reversal learning would be altered in the adult male and female offspring of pregnant rats exposed to PolyI:C in mid-late gestation (gestational day (GD) 15).
2. Materials and methods
2.1. Subjects
Timed pregnant Long-Evans dams (gestational day (GD) 7; Charles River Laboratories, Quebec, Canada) were singly housed in transparent plastic cages in a temperature controlled (21 °C) colony room on a 12/12 h light/dark cycle with food (Purina Rat Chow) and water available ad libitum. All experiments were conducted during the light phase of a 12:12 h light/dark cycle (lights on at 07:00 h). All experiments were performed in accordance with the Canadian Council on Animal Care and were approved by the University of Saskatchewan Animal Care and Use Program.
2.2. Prenatal treatment
On GD 15, dams (n = 20) were individually transported to a room where weight and rectal temperature (Homeothermic Blanket System, Harvard Instruments, MA) were measured. Dams were then anesthetized with isoflurane (5% induction and 2.5% maintenance) and injected intravenously with a single dose of either saline or PolyI:C (4.0 mg/kg, High Molecular Weight, InVitroGen, San Diego, CA) via the tail vein. This procedure took an average of 10 min per animal and care was taken to ensure the saline-treated dams were anaesthetized for the same length of time as the PolyI:C-treated dams. Weight and temperature were measured again 8, 24, and 48 h after the injection. Dams were otherwise left undisturbed until the day after parturition. Experimenters were blind to the treatment of the dams and pups during the course of all experiments.
The day of parturition was designated postnatal day (PND) 0. On PND 1, litters were weighed and culled to 10 pups per litter (6 males and 4 females where possible). Observations of maternal behaviour were initiated the next day and continued for 7 days, with two 10 min sessions twice daily (08:00–10:00 h and 15:00–17:00 h). A maternal behaviour rating scale was adapted from previous studies (Barha et al., 2007). The maternal behaviours recorded were: 1) arch-back nursing/licking and grooming (ABN/LG): mom over the pups in an arched position and licking them; 2) blanket nursing: mom lying over the pups, relaxed; 3) passive nursing: mom lying on her side nursing the pups; 4) off nest: mom outside of nest and away from the pups. Other than maternal observations and routine husbandry (including taking litter weights on PND 8 and 14), litters were left undisturbed until weaning on PND 21. Weaned pups from the same litter were housed in same-sex cages of 3 or 4 in separate colony rooms. On PND 53, 1 or 2 pups of each sex from each litter were randomly selected for the present experiment. Rats were individually housed and food restricted to 85% of their free feeding weight until PND 60 when lever training commenced.
2.3. Estrous cycle measurements
From PND 56 to the last day of experimental testing, vaginal smears were taken for each female offspring (n = 22). Every morning between 08:30 and 10:00 h, a vaginal sample was collected by inserting a plastic pipette tip loaded with 20 μl of 0.9% saline into the vagina (~5 mm deep). The saline was ejected, immediately reloaded into the pipette, and then ejected onto a glass slide. Typically, 5 samples were put on a given slide, after which Cytoprep (Fisher Scientific) was sprayed onto the slide. Wet samples were viewed using a light microscope and estrous cycle was determined using established cytology methods (Goldman et al., 2007; Marcondes et al., 2002). Four phases were identified: 1) proestrus: majority of the cells present are round, nucleated epithelial cells; 2) estrus: majority are irregular, not nucleated cells; 3) metestrus: consists of equal number of nucleated epithelial, non-nucleated cells, leukocytes in the sample; and 4) diestrus: majority of cells are leukocytes. Female rats in both treatment groups displayed normal alterations in the stage of the estrous cycle during the period prior to behavioural testing. During testing, no effort was made to control for the stage of the estrous cycle; rather, performance was correlated with the naturally occurring stage of the estrous cycle. No correlation was observed between stage of the estrous cycle and trials to criterion (TTC) for either the visual-cue or set-shifting test day in either treatment group (Pearson r’s < 0.20, p’s > 0.48 for visual-cue and set-shifting). A significant correlation was observed between stage of estrous cycle and TTC on reversal learning day in the saline-treated offspring (r = −0.677, p = 0.045) but not the PolyI:C-treated offspring (r = 0.04, NS). TTC were greater for females in metestrus and diestrus (n = 3, TTC = 107.67) than proestrus and estrus (n = 6, TTC = 80.83) in the saline-treated group. The importance of this observation is not clear; however, it is worth noting that the group means for TTC on the reversal learning day were not different between the two treatment groups (see Results, Section 3.6). In addition, the small sample size (n = 9 observations) makes drawing firm conclusions from this correlation difficult.
2.4. Behavioural testing
2.4.1. Apparatus
All training and testing were conducted in four operant chambers (32 × 25.5 × 25 cm; Med Associates Systems, St. Albans, VT, USA). Each operant chamber was located within a wooden sound-attenuating box (63.6 × 35.6 × 75.6 cm; W × H × L) equipped with a fan that provided background noise and ventilation. Each operant chamber included a food receptacle where food rewards (Dustless Precision Pellets, 45 mg, Rodent Purified Diet; BioServ, Frenchtown, NJ) were delivered via a pellet dispenser. A retractable lever and a stimulus light were positioned on either side of the receptacle. A single house light (100 mA) located near the top and center of the wall opposite the levers illuminated the chamber. The chamber floor consisted of a removable metal grid which contained a detachable tray. A personal computer and interface box controlled the presentation of the trials and recorded all experimental data.
2.4.2. Lever training
Pre-training protocols closely followed those previously published (Floresco et al., 2008, 2009). Rats were given 20 reward pellets in their home cages the day prior to the commencement of lever training. On the first day of training, ~2–3 reward pellets were crushed into a fine powder and placed on the extended lever. Rats were trained under a fixed-ratio 1 (FR1) schedule to a criterion of 45 presses in 30 min. Rats were first trained on one lever before being trained on the other (counterbalanced left/right between subjects). On subsequent days, rats were familiarized with the insertion of levers into the chamber and trained to press them within 10 s of insertion. These training sessions consisted of 90 trials and began with both levers retracted and the chamber in darkness. A trial began every 20 s with the illumination of the house light and the insertion of one of the levers into the chamber. If the rat failed to press the lever within 10 s, the lever was retracted, the house light was extinguished, and the trial was scored as an omission. If the rat responded within 10 s, the lever was retracted, a reward pellet was delivered, and the house light remained on for 4 additional seconds. The left and right levers were each presented once in every pair of trials with the order of presentation randomized within each pair. The stimulus lights above the levers were never illuminated during lever training sessions. The criterion to pass training was to make ≤5 omissions in a training session. Rats were trained in this manner for a minimum of 5 days or until the criterion was reached. Upon reaching criterion, the side preference of the rat was determined. Here, both levers were inserted and pressing either one would result in the delivery of a reward pellet. On subsequent trials, rats were required to alternate between left and right levers to obtain a reward. The session ended after 7 reward pellets were delivered. A rat’s side preference was determined using the total number of left and right lever presses. If the total right and left lever presses were comparable, then the lever chosen first was counted as the side preference. However, if a rat consistently focused on one lever during the session (≥2:1 ratio), then that side would be taken as the side preference. Testing began the day following the final training session.
2.4.3. Visual-cue discrimination
The initial discrimination required rats to press the lever with the stimulus light illuminated above it. Rats were trained to a criterion of 10 consecutive correct choices. Each daily session consisted of a minimum of 30 trials and a maximum of 150 trials. If a rat reached criterion before 30 trials were conducted, the program terminated after 30 trials. If a rat did not reach criterion within 150 trials, visual-cue discrimination testing continued on subsequent days until criterion was reached. Each session began with both levers retracted and the chamber in darkness (the inter-trial state). Every 20 s, a trial began with one of the stimulus lights being illuminated. The house light was illuminated 3 s later with both levers being inserted into the chamber. A correct response (pressing the lever underneath the illuminated stimulus light) resulted in the retraction of both levers and the delivery of a food pellet. The house light remained on for 4 s before being extinguished and the chamber was returned to the inter-trial state. An incorrect response (pressing the lever not underneath the illuminated stimulus light) resulted in the chamber immediately reverting to the inter-trial state and no food reward being delivered. Failure to respond to either lever within 10 s also resulted in the chamber returning to the inter-trial state with the trial being scored as an omission. The left and right stimulus lights were illuminated once in every pair of trials with the order within each pair randomized. The lever the rat chose and the stimulus light illuminated were recorded for each trial. Omissions were not included in the TTC.
2.4.4. Shift to response discrimination (strategy set-shift)
On the day following visual-cue discrimination testing, rats were required to ignore the visual-cue (illuminated stimulus light) and respond to a spatial-cue (the lever opposite to their side preference) to receive a reward pellet. Trial format and criterion measures were the same as the visual-cue discrimination day. Errors during this phase of testing were broken down into 3 subtypes as has been previously described (Floresco et al., 2008, 2009; Haluk and Floresco, 2009; Ragozzino, 2002, 2007). Perseverative errors were scored when a rat continued to use the previously relevant but currently irrelevant strategy. For example, if a rat pressed the lever opposite to the side they required to respond while the stimulus light above it was illuminated, a perseverative error was counted. Eight out of every 16 consecutive trials (defined as a block) allowed the rat to respond this way. For each block, perseverative errors were counted when rats pressed the incorrect lever 6 or more trials per block. Once a rat made 5 or fewer incorrect lever presses during a block, all subsequent perseverative errors were counted as regressive errors. Perseverative errors are considered as a measure of disengaging from a strategy while regressive errors are commonly considered a measure of maintaining a new strategy (Floresco et al., 2008, 2009; Haluk and Floresco, 2009; Ragozzino, 2002, 2007). Never-reinforced errors were scored when rats pressed the incorrect lever when the stimulus light was illuminated above the correct lever.
2.4.5. Response reversal
During this phase of testing, rats were required to press the lever opposite to the one required during the previous set-shifting day (e.g., press left lever on set-shift day then press right lever on reversal day). The trial format and criterion were identical to the visual cue and set-shifting test days. Errors were broken down into 2 subtypes. Perseverative and regressive errors were counted in a similar manner to the set-shifting day. However, perseverative errors were scored when a rat made 10 or more errors within a block of 16 trials, excluding the first block when errors were always scored as perseverative (Floresco et al., 2009). All subsequent errors (i.e., once less than 10 errors per block were made) were scored as regressive errors.
2.5. Data analysis
All analyses were computed using SPSS Version 18. Effects of PolyI:C treatment on the pregnant dams and litters were analyzed with repeated measures ANOVA using Treatment (saline vs. PolyI:C) as a between subjects factor and Time (0, 8, 24, 48 h post-treatment) as a within subjects factor. Data related to operant behaviour testing were analyzed with two-way ANOVA/ANCOVA with Treatment and Sex as between subject factors for each test day and average response latency as a covariate where appropriate. Simple main effects were tested with independent samples t-tests. Errors during behavioural testing were analyzed with repeated measures ANOVA using Error Type as a within subjects factor and Treatment and Sex as between subjects factors. Post-hoc tests for the repeated measures ANOVA were performed by hand using the Neumann-Keuls method. P values of <0.05 were considered significant.
3. Results
3.1. Effects of PolyI:C on dams and pups
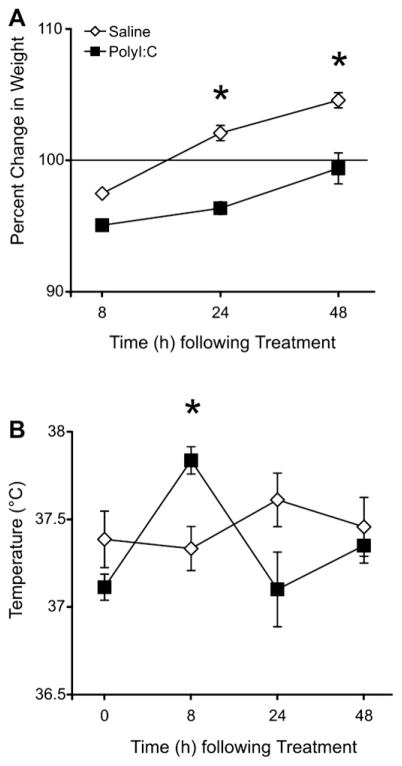
Pregnant dams were randomly assigned to receive either PolyI:C (n = 11) or saline (n = 9). Weight and rectal temperature were taken at 0, 8, 24, and 48 h after PolyI:C injection. Statistical analysis of dam weights revealed a significant main effect of Time (F(3, 54) = 37.38, p < 0.001), a significant Treatment by Time interaction (F(3, 54) = 11.56, p < 0.001), and no main effect of Treatment (F(1,18) = 2.47, NS). Post-hoc analyses revealed that dam weights differed between the saline (0 h: 361.44 ± 13 g; 8 h: 352.09 ± 12 g) and PolyI:C-treated groups (0 h: 397.02 ± 8 g; 8 h: 377.55 ± 8 g) at 0 and 8 h (p < 0.05). Further analysis of the weight data revealed that dams in both groups lost weight in response to being anesthetized, although the PolyI:C-treated animals lost more weight (Fig. 1A; saline: 2.52%; PolyI:C 4.93%). At 24 and 48 h, the PolyI:C-treated animals gained significantly less weight than the saline-treated animals, as revealed by a significant Time by Group interaction for the percentage of weight lost at 8, 24, 48 h relative to initial weight at 0 h (F(2, 36) = 5.62, p < 0.007; post-hoc for 24, 48 h, p < 0.05). Analysis of temperature data (Fig. 1B) showed a significant Treatment by Time interaction (F(3, 54) = 6.41, p = 0.001) without significant main effects of Treatment (F(1, 18) = 0.54, NS) or Time (F(3, 54) = 2.68, NS). Post-hoc analyses revealed that the temperature of the PolyI:C-treated animals was significantly higher than the saline-treated animals 8 h after injection. An average of 13.89 ± 1 and 12.55 ± 1 pups were born to the saline-treated and PolyI:C-treated dams, respectively. No significant effect was noted for prenatal treatment regarding the number (t(18) = 0.77, NS) or weight of the pups on PND 1, 8, 14, or 21 (data not shown; F(3, 54) = 0.59, NS).
Fig. 1.
A. Effects of prenatal treatment on weight of pregnant dams (gestational day 15; Saline: n = 9; PolyI:C: n = 11). Weight change is expressed as a percentage of baseline weight at 0 h immediately prior to treatment. Dams treated with saline gained significantly more weight than those treated with PolyI:C at the 24 and 48 h time points. B. Effects of treatment on rectal temperature of dams. PolyI:C significantly increased rectal temperature of the dams 8, but not 24 or 48 h after treatment. All values are means ± SEM. * denotes p < 0.05.
3.2. Maternal behaviour
Maternal behaviour was measured twice daily from PND 2 to 8 during the light cycle. Measures reported include ABN/LG and off nest time. Blanket and passive nursing were infrequently observed in our sample. When observations from all sessions were summed, saline-treated dams displayed 2735.56 ± 480 s of ABN/LG and 3921.67 ± 385 s off nest time. PolyI:C-treated dams displayed 3077.27 ± 315 s of ABN/LG and 3365.46 ± 353 s off nest time. A repeated measures ANOVA revealed no effect of Treatment (F(1, 18) = 0.23, NS), Day (F(6, 108) = 1.26, NS) or a Treatment × Day interaction (F(6, 108) = 1.77, NS) on ABN/LG. Similarly, no effect of Treatment (F(1, 18) = 0.87, NS), Day (F(6, 108) = 1.91, NS) or a Treatment × Day interaction (F(6, 108) = 0.87, NS) was found for off nest time.
3.3. Operant training
The effects of Treatment and Sex on the number of days required for operant training were considered. Inspection of the data revealed that female rats took longer to complete training regardless of their Treatment condition (days of FR1: males 3.32 ± 0.6, females 4.09 ± 0.3; days of retractable lever: males 5.32 ± 0.1, females 6.09 ± 0.3; omits: males 1.55 ± 0.4, females 3.00 ± 0.4). These differences were significant for the number of days required for retractable lever training (F(1, 40) = 4.84, p = 0.034) and omitted trials during the last training day (F(1, 40) = 7.73, p = 0.008). No significant effect of Sex was observed for FR1 training (F(1, 40) = 2.83, NS) and no effect of PolyI:C Treatment or interactions between Sex and PolyI:C Treatment were found for days required for FR1 training, retractable lever training, or omitted trials (all F’s < 1.1, NS).
3.4. Visual-cue discrimination
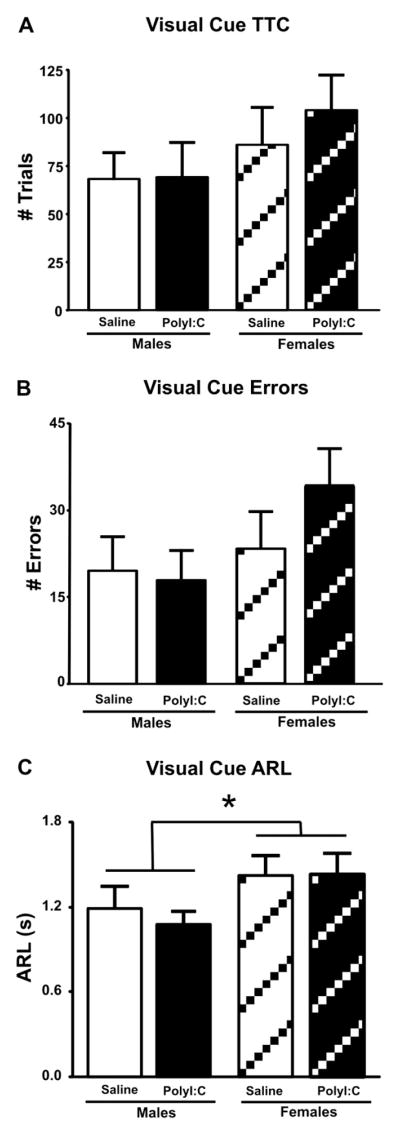
The number of TTC and errors were similar for all groups during the visual-cue discrimination day (Fig. 2A, B). These impressions were confirmed statistically, with insignificant effects of Treatment (TTC: F(1, 40) = 0.28, NS; Errors: F(1, 40) = 0.57, NS), Sex (TTC: F(1, 40) = 2.25, NS; Errors: F(1, 40) = 2.74, NS), and Sex × Treatment interactions (TTC: F(1, 40) = 0.22, NS; Errors: F(1, 40) = 1.05, NS). While average response latencies did not differ as a result of treatment (F(1, 40) = 0.17, NS), they differed for sex with female rats taking significantly longer than males to respond when the levers were inserted (Fig. 2C; F(1, 40) = 4.70; p = 0.03). Taken together, these results suggest that PolyI:C treatment did not affect the learning of visual-cue discrimination, although female rats, regardless of treatment condition, were slower than male rats to respond to insertion of the levers.
Fig. 2.
Performance of rats in the PolyI:C or saline-treated groups on the visual-cue discrimination day. A. Trials to criterion (TTC). B. Total errors. C. Average response latencies (ARL’s) to press a lever. Female rats had significantly increased ARL’s regardless of treatment. Saline: n = 11 males, 10 females; PolyI:C: n = 11 males, 12 females. * denotes p < 0.05.
3.5. Shift to response discrimination
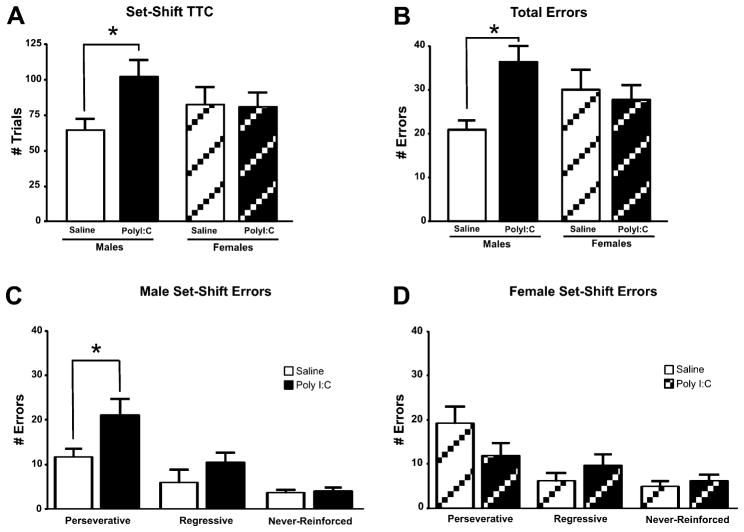
On this test day, rats were required to shift their strategy from responding to the light cue to using an egocentric spatial strategy. Inspection of the data (Fig. 3A) revealed that males treated prenatally with PolyI:C required more TTC (102.46 ±12 trials) than males treated prenatally with saline (64.73 ± 8 trials). In contrast, female rats performed similarly regardless of treatment (PolyI:C group: 80.92 ± 10 trials; saline group: 82.50 ± 13). A two-way ANOVA revealed in significant main effects of Treatment (F(1, 40) = 2.90; NS) and Sex (F(1, 40) = 0.03; NS) while a trend toward a significant Sex × Treatment interaction was observed (F(1, 40) = 3.43; p = 0.07). Further analysis of the data for each sex revealed that, when considered alone, the male PolyI:C-treated offspring took significantly more trials to reach criterion (t(20) = −2.71, p = 0.01). No difference for TTC was observed when the females were considered alone (t(20) = 0.01, NS). Average response latencies were significantly slower for the female rats (F(1, 40) = 7.55, p = 0.009) with no significant main effect for Treatment (F(1, 40) = 1.37, NS) or a Sex × Treatment interaction (F(1, 40) = 0.37, NS). Due to a significant sex difference for ARL, analysis of treatment effects on TTC with ARL as a covariate was performed. This analysis showed that PolyI:C male rats were significantly slower in reaching criterion than saline-treated male rats (F(1, 19) = 5.209; p = 0.03) whereas female rats required a similar number of TTC regardless of treatment (F(1, 19) = 0.03; NS).
Fig. 3.
Effects of prenatal PolyI:C or saline treatment on set-shifting from a visual to response based strategy. A. Effects of prenatal PolyI:C treatment on trials to criterion (TTC). B. Total errors committed during set-shifting. Male offspring prenatally treated with PolyI:C took significantly more trials to reach criterion and made significantly more total errors than saline-treated offspring. C. Error subtypes committed by the males. The impairment in set-shifting for the PolyI:C-treated males was due to a significant increase in perseverative errors. D. Error subtypes committed by the females. * denotes p < 0.05.
The animals did not differ on the total number of errors (Fig. 3B) made when either Treatment (F(1, 40) = 2.33, NS) or Sex (F(1, 40) = 0.03, NS) were considered; however, there was a significant Sex by Treatment interaction (F(1, 40) = 4.50, p = 0.04). Male PolyI:C-treated offspring made significantly more errors (post-hoc, p < 0.05) than saline-treated offspring whereas no significant difference existed for female offspring. When errors are broken down into perseverative, regressive and never-reinforced subtypes, a repeated measures ANOVA revealed no main effect of Sex (F(2, 80) = 0.30, NS) or Treatment (F(2, 80) = 0.58, NS) but a significant Sex by Treatment by Error interaction (F(2, 80) = 4.15, p = 0.02). Post-hoc comparisons indicated that PolyI:C-treated males committed significantly more perseverative errors than saline-treated males (Fig. 3C; p < 0.05). No significant differences were noted for regressive or never-reinforced errors in the male rats or any of the error subtypes for the female subjects (Fig. 3D). Hence, when compared to the saline-treated males, PolyI:C-treated males displayed impaired set-shifting as a result of increased perseverative errors.
3.6. Reversal learning
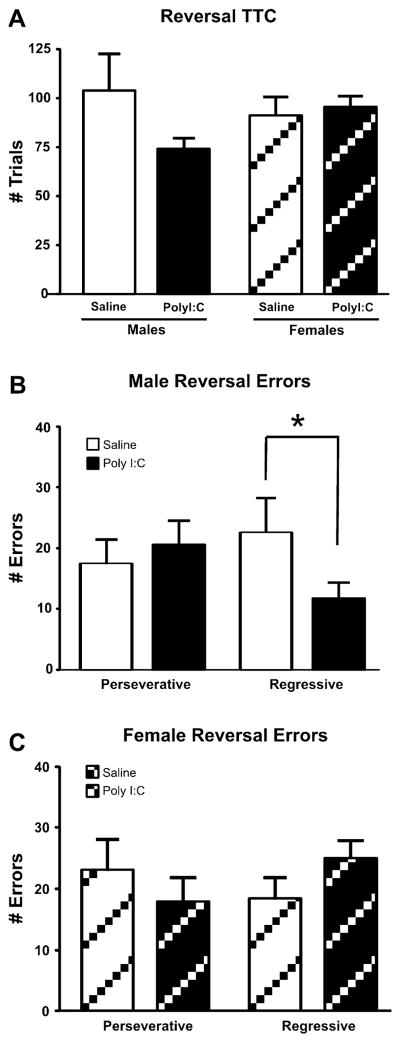
PolyI:C-treated male offspring required fewer TTC (Fig. 4A, 74.00 ± 6 trials) than saline-treated male adult offspring (104.00 ± 18) whereas the female rats showed similar performance regardless of treatment (PolyI:C group: 95.50 ± 6 trials; saline group: 91.40 ± 9 trials). ANOVA revealed that these differences were not significant for either TTC (Sex: F(1, 40) = 0.17; Treatment: F(1, 40) = 1.41; Sex × Treatment interaction: F(1, 40) = 2.45, all NS) or total errors (Sex: F(1, 40) = 0.45; Treatment: F(1, 40) = 0.36; Sex × Treatment interaction: F(1, 40) = 0.77, all NS). Similar to the other test days, females were significantly slower to respond to insertion of the levers than males regardless of treatment condition (F(1, 40) = 11.11, p = 0.002). An ANCOVA performed on TTC with ARL as the covariate revealed the same insignificant effects noted above (statistics not shown).
Fig. 4.
Effects of prenatal PolyI:C or saline treatment reversal learning. A. Effects of prenatal PolyI:C treatment on trials to criterion (TTC). B. Analysis of the subtypes of errors made by the male animals. Male PolyI:C-treated offspring made significantly more regressive errors during the reversal learning phase of the task. C. Subtypes of errors made by the females. * denotes p < 0.05.
Analysis of error subtype revealed a significant Sex × Treatment × Error interaction (F(1, 40) = 4.10, p = 0.049) without significant main effects for Treatment (F(1, 40) = 0.03, NS) or Sex (F(1, 40) = 0.21, NS). PolyI:C-treated male offspring made significantly fewer regressive errors than saline-treated male offspring (Fig. 4B; p < 0.05) while the female groups did not differ (Fig. 4C). Therefore, during reversal learning, PolyI:C-treated male rats made fewer regressive errors than saline-treated males, whereas female adult offspring performed similarly regardless of treatment.
4. Discussion
The present experiments yielded a number of novel results. Most importantly, acute prenatal injection of PolyI:C significantly impaired strategy set-shifting in male, but not female, offspring (Fig. 3A). Analyses of the error subtypes committed revealed that male pups treated with PolyI:C made significantly more perseverative errors during set-shifting than saline-treated rats (Fig. 3C). No differences were observed between the groups for learning the initial visual-cue strategy (Fig. 2A). During the reversal learning phase, PolyI:C-treated male rats took fewer trials to reach criterion than saline-treated rats although this difference did not reach significance (Fig. 4A). However, PolyI:C-treated males made significantly fewer regressive errors than the saline-treated male rats (Fig. 4B). PolyI:C treatment did not significantly affect the behaviour of female offspring in any of the tasks although the females were significantly slower to respond to the levers than the male offspring regardless of treatment. These results demonstrate a sex-specific effect of infection during pregnancy on executive function in young adulthood.
4.1. Effects of PolyI:C treatment on dams and pups
Systemic administration of PolyI:C caused significant weight loss and febrile responses in pregnant rats (Fig. 1). Similar results have been reported in a number of species including rats (Ellis et al., 2006; Fortier et al., 2004; Zuckerman et al., 2003; Zuckerman and Weiner, 2005), although a recent report failed to find an effect of PolyI:C injection on GD15 on weight of pregnant rats (Wolff and Bilkey, 2010). Consistent with the present results are also reports that treatment with PolyI:C (4.0 mg/kg; i.v.) does not alter litter number or weight at birth (Wolff and Bilkey, 2010; Zuckerman et al., 2003; Zuckerman and Weiner, 2005).
It should be noted that the rats tested in the present study were from timed pregnant dams shipped from a remote supplier on GD 7. Prenatal stress in rodents has been shown to induce behavioural and neurochemical changes in the offspring associated with psychiatric illness (Koenig et al., 2002; Meyer et al., 2009). While the potential effects of stress related to shipment on the dams and fetuses used in the present study cannot be determined, similar performance of the same operant tasks by our saline-treated offspring and rats in other studies (Floresco et al., 2009) suggest that transportation did not have major effects on the offspring.
Patterns of maternal care affect the cognitive and behavioral development of rodents (Barha et al., 2007; Meyer et al., 2006b, 2008a; Schwendener et al., 2009; Zhang et al., 2005). For example, pups reared by dams exhibiting naturally occurring low levels of licking and grooming during the first week of postnatal rearing have reduced prepulse inhibition in adulthood (Zhang et al., 2005). Cognizant of these potential effects of maternal behaviour, we quantified several patterns of maternal care and found no significant difference between the PolyI:C and saline-treated dams during the first postnatal week. To the best of our knowledge, this is the first time such measurements have been made in rats treated with PolyI:C. Studies in mice suggest cross-fostering untreated pups to surrogate dams that were treated with PolyI:C on GD 17 results in behavioural, pharmacological, and neuropathological changes in the adult offspring (Meyer et al., 2006b, 2008b) that may be mediated by changes in maternal behaviour induced by PolyI:C treatment during pregnancy (Schwendener et al., 2009). A more thorough analysis of maternal behaviour (including additional measures and observations during the dark phase; Schwendener et al., 2009) may reveal subtle alterations in maternal behaviour of rats following PolyI:C treatment.
4.2. Effects of prenatal PolyI:C treatment on strategy set-shifting and reversal learning in the male offspring
Performance of rats on the operant-based task in the present study was comparable to previous reports also using similar methods in male Long-Evans rats (Floresco et al., 2008, 2009; Haluk and Floresco, 2009). The present results are the first reported for female rats, which were consistently slower to respond during the trials and took longer to reach criterion during the initial operant training days. Similar performance of all groups during visual-cue discrimination test day (Fig. 2) demonstrates that prenatal treatment with PolyI:C does not affect relatively simple discrimination learning, motivation, or gross locomotor behaviour in the adult offspring.
The present experiments are the first to examine the effects of prenatal infection on strategy set-shifting in rodents. Male, but not female, PolyI:C-treated rats were impaired during set-shifting from a visual-cue strategy to an egocentric spatial strategy (Fig. 3A). Such an impairment suggests that PolyI:C treatment disturbs normal function of the distributed circuit mediating strategy set-shifting that includes prefrontal cortical, thalamic, and striatal areas (Block et al., 2007; Floresco et al., 2008, 2009; Haluk and Floresco, 2009; Ragozzino et al., 1999; Ragozzino, 2007). The increase in perseverative errors during set-shifting for male rats (Fig. 3C) is consistent with the effects of medial PFC or midline thalamic lesions (Block et al., 2007; Floresco et al., 2008; Ragozzino et al., 1999) and dopaminergic receptor manipulations in the mPFC (Floresco et al., 2006; Ragozzino, 2002) and nucleus accumbens (Haluk and Floresco, 2009). A lack of effect of PolyI:C treatment on regressive errors suggests that alterations in the dorsomedial striatum may not be involved as lesions of this area selectively increase regressive errors (Ragozzino, 2007). Considerable research demonstrates significant pharmacological, neurochemical, electrophysiological, and neuropathological alterations of PFC and nucleus accumbens following prenatal infection (Dickerson et al., 2010; Meyer et al., 2006a, 2008a, 2009; Ozawa et al., 2006; Zuckerman et al., 2003). Thus, abnormalities in these areas likely underlie the detrimental effects of prenatal PolyI:C treatment on set-shifting and may underlie the impairments in set-shifting typically observed in schizophrenia patients (Brown et al., 2009; Leeson et al., 2009; Pantelis et al., 1999).
Reversal learning depends on a circuit including orbital frontal cortex and striatum with discrete roles for dopamine and serotonin (Boulougouris et al., 2007; Boulougouris and Robbins, 2010; Clarke et al., 2004; Ghods-Sharifi et al., 2008; Kehagia et al., 2010; Kim and Ragozzino, 2005; McAlonan and Brown, 2003; Schoenbaum et al., 2002). Interestingly, PolyI:C treatment has been shown to both facilitate and impair reversal learning in different studies. In one study, Wistar rats treated with PolyI:C (GD15 or 17) displayed enhanced reversal learning using two tasks in a water maze, an effect reversed by acute treatment with the atypical antipsychotic clozapine (Zuckerman and Weiner, 2005). However, male mice treated on GD17, but not GD9, with PolyI:C have impaired reversal learning in a water maze-based task (Meyer et al., 2006a). In the present study, significant differences were not observed between groups for TTC during reversal learning; however, there was a tendency for PolyI:C-treated male rats to take fewer trials than saline-treated male rats to reach criterion (Fig. 4A). PolyI:C-treated male rats committed significantly fewer regressive errors than the saline-treated male rats (Fig. 4B) suggesting that PolyI:C-treated rats are better at maintaining a new strategy under these conditions. The lack of a significant effect of PolyI:C treatment on reversal learning TTC may be due to factors including species and strain differences (Long-Evans vs. Wistar rats), variability in performance of the male saline-treated offspring, or the specifics of the operant task we employed. Given that the PolyI:C-treated male rats were slower to acquire the egocentric spatial strategy during set-shifting the previous day, they may have maintained responding with the correct strategy during reversal learning more easily once initial perseveration ceased. Future studies that test the effects of prenatal PolyI:C treatment on reversal learning in the absence of a prior strategy shift (for example, see (Haluk and Floresco, 2009)) will be important for assessing this possibility directly. Given that patients with schizophrenia consistently show impaired reversal learning (Leeson et al., 2009; McKirdy et al., 2009; Murray et al., 2008; Pantelis et al., 1999; Waltz and Gold, 2007), further study is warranted to validate this aspect of the prenatal infection model.
Impaired behavioural flexibility has been reported in other developmental rodent models relevant to schizophrenia. Neonatal ventral hippocampal lesions increase TTC and perseverative errors during strategy set-shifting in a T-maze task (Brady, 2009) and prenatal treatment with methylazoxymethanol acetate impairs extra-dimensional set-shifting and reversal learning in an attention-based task (Featherstone et al., 2007). Notably, both of these studies restricted their analyses to male rats. Together with the present results, these findings suggest that a variety of early developmental insults alter behavioural flexibility and produce cognitive symptoms consistent with schizophrenia.
4.3. Lack of effects of prenatal PolyI:C treatment on female offspring
The lack of effect observed in female offspring of PolyI:C-treated dams was unexpected. Few studies have tested the cognitive effects of prenatal infection on female rat offspring or the abilities of female rats in set-shifting tasks, although the set-shifting abilities of female rats are impaired following sub-chronic PCP administration in adulthood (McLean et al., 2008) or early experiences such as artificial rearing (Lovic and Fleming, 2004). Prenatal infection of rats and mice causes sex-specific changes for some behavioural tasks (Bitanihirwe et al., 2010a; Lante et al., 2007; Schwendener et al., 2009) but not others (Meyer et al., 2006a, 2008a). In one study, cognitive inflexibility was inferred from an abnormally persistent latent inhibition observed in male but not female mice treated with PolyI:C late in gestation (GD17; Bitanihirwe et al., 2010a). These behavioural findings correlated with partly sex-specific differences in neurotransmitter levels in the medial prefrontal cortex and hippocampus (Bitanihirwe et al., 2010b). Most studies examining sex-specific effects of prenatal infection have been conducted following infection later in the gestational period (GD15-19). Given that the precise timing of prenatal infection influences the specific array of behavioural alterations produced (Meyer et al., 2006a, 2009), examining the potential sex-specific effects of prenatal infection earlier in gestation may produce unique cognitive phenotypes in the female offspring.
The lack of effect following prenatal infection on set-shifting for female rats is interesting in light of human literature showing male schizophrenic patients perform worse than female schizophrenic patients on tests of executive function such as the Wisconsin Card Sorting Task (Goldstein et al., 1998; Longenecker et al., 2010; Seidman et al., 1997) although sex differences in the cognitive symptoms of schizophrenia and the potential mechanisms underlying these effects remain controversial (Bozikas et al., 2010; Leung and Chue, 2000).
5. Conclusions
Prenatal infection of rats on GD 15 with PolyI:C alters executive functions including set-shifting and reversal learning in the male offspring. The present findings support the face and construct validity of the prenatal infection model for neurodevelopmental disorders such as schizophrenia. Thus, the prenatal infection model may be suitable for testing the therapeutic efficacy of novel agents for the treatment of psychiatric disorders.
Acknowledgments
We thank three anonymous reviewers for constructive comments on a previous version of this manuscript. The present work was supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression, Saskatchewan Health Research Foundation New Investigator Award, and University of Saskatchewan Molstad Trust Intramural Research Award to JGH.BNC and CAT are recipients of National Sciences and Engineering Research Council of Canada scholarships.
References
- Barha CK, Pawluski JL, Galea LA. Maternal care affects male and female offspring working memory and stress reactivity. Physiol Behav. 2007;92:939–950. doi: 10.1016/j.physbeh.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology. 2010a;35:2462–2478. doi: 10.1038/npp.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Weber L, Feldon J, Meyer U. Cognitive impairment following prenatal immune challenge in mice correlates with prefrontal cortical AKT1 deficiency. Int J Neuropsychopharmacol. 2010b;13:981–996. doi: 10.1017/S1461145710000192. [DOI] [PubMed] [Google Scholar]
- Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- Boksa P. Maternal infection during pregnancy and schizophrenia. J Psychiatry Neurosci. 2008;33:183–185. [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;30:930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozikas VP, Kosmidis MH, Peltekis A, Giannakou M, Nimatoudis I, Karavatos A, Fokas K, Garyfallos G. Sex differences in neuropsychological functioning among schizophrenia patients. Aust N Z J Psychiatry. 2010;44:333–341. doi: 10.3109/00048670903489833. [DOI] [PubMed] [Google Scholar]
- Brady AM. Neonatal ventral hippocampal lesions disrupt set-shifting ability in adult rats. Behav Brain Res. 2009;205:294–298. doi: 10.1016/j.bbr.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol. 2011;93:23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Susser ES. In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev. 2002;8:51–57. doi: 10.1002/mrdd.10004. [DOI] [PubMed] [Google Scholar]
- Brown AS, Vinogradov S, Kremen WS, Poole JH, Deicken RF, Penner JD, McKeague IW, Kochetkova A, Kern D, Schaefer CA. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. Am J Psychiatry. 2009;166:683–690. doi: 10.1176/appi.ajp.2008.08010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaranello AL, Ciaranello RD. The neurobiology of infantile autism. Annu Rev Neurosci. 1995;18:101–128. doi: 10.1146/annurev.ne.18.030195.000533. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Dickerson DD, Wolff AR, Bilkey DK. Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. J Neurosci. 2010;30:12424–12431. doi: 10.1523/JNEUROSCI.3046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S, Mouihate A, Pittman QJ. Neonatal programming of the rat neuroimmune response: stimulus specific changes elicited by bacterial and viral mimetics. J Physiol. 2006;571:695–701. doi: 10.1113/jphysiol.2005.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- Featherstone RE, Rizos Z, Nobrega JN, Kapur S, Fletcher PJ. Gestational methylazoxymethanol acetate treatment impairs select cognitive functions: parallels to schizophrenia. Neuropsychopharmacology. 2007;32:483–492. doi: 10.1038/sj.npp.1301223. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Geyer MA, Gold LH, Grace AA. Developing predictive animal models and establishing a preclinical trials network for assessing treatment effects on cognition in schizophrenia. Schizophr Bull. 2005;31:888–894. doi: 10.1093/schbul/sbi041. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2004;287:R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem. 2008;89:567–573. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Goodman JM, Koren D, Lee H, Weintraub S, Tsuang MT. Are there sex differences in neuropsychological functions among patients with schizophrenia? Am J Psychiatry. 1998;155:1358–1364. doi: 10.1176/ajp.155.10.1358. [DOI] [PubMed] [Google Scholar]
- Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Fenton WS. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr. Bull. 2007;33:912–920. doi: 10.1093/schbul/sbm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JI, Kirkpatrick B, Lee P. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology. 2002;27:309–318. doi: 10.1016/S0893-133X(01)00396-7. [DOI] [PubMed] [Google Scholar]
- Lante F, Meunier J, Guiramand J, Maurice T, Cavalier M, De Jesus Ferreira MC, Aimar R, Cohen-Solal C, Vignes M, Barbanel G. Neurodevelopmental damage after prenatal infection: role of oxidative stress in the fetal brain. Free Radic Biol Med. 2007;42:1231–1245. doi: 10.1016/j.freeradbiomed.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, Joyce EM. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Longenecker J, Dickinson D, Weinberger DR, Elvevag B. Cognitive differences between men and women: a comparison of patients with schizophrenia and healthy volunteers. Schizophr Res. 2010;120:234–235. doi: 10.1016/j.schres.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovic V, Fleming AS. Artificially-reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task–reversal of effects with maternal-like licking stimulation. Behav Brain Res. 2004;148:209–219. doi: 10.1016/s0166-4328(03)00206-7. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McKirdy J, Sussmann JE, Hall J, Lawrie SM, Johnstone EC, McIntosh AM. Set shifting and reversal learning in patients with bipolar disorder or schizophrenia. Psychol Med. 2009;39:1289–1293. doi: 10.1017/S0033291708004935. [DOI] [PubMed] [Google Scholar]
- McLean SL, Beck JP, Woolley ML, Neill JC. A preliminary investigation into the effects of antipsychotics on sub-chronic phencyclidine-induced deficits in attentional set-shifting in female rats. Behav Brain Res. 2008;189:152–158. doi: 10.1016/j.bbr.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009;33:1061–1079. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immunoprecipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006a;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology. 2008a;33:441–456. doi: 10.1038/sj.npp.1301413. [DOI] [PubMed] [Google Scholar]
- Meyer U, Schwendener S, Feldon J, Yee BK. Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Exp Brain Res. 2006b;173:243–257. doi: 10.1007/s00221-006-0419-5. [DOI] [PubMed] [Google Scholar]
- Meyer U, Spoerri E, Yee BK, Schwarz MJ, Feldon J. Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia. Schizophr Bull. 2008b;36:607–623. doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GK, Cheng F, Clark L, Barnett JH, Blackwell AD, Fletcher PC, Robbins TW, Bullmore ET, Jones PB. Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull. 2008;34:848–855. doi: 10.1093/schbul/sbn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyper-function and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res. 1999;37:251–270. doi: 10.1016/s0920-9964(98)00156-x. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Neuroscience. Maternal effects on schizophrenia risk. Science. 2007;318:576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Pearce BD. Schizophrenia and viral infection during neurodevelopment: a focus on mechanisms. Mol Psychiatry. 2001;6:634–646. doi: 10.1038/sj.mp.4000956. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The effects of dopamine D(1) receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learn Mem. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schwendener S, Meyer U, Feldon J. Deficient maternal care resulting from immunological stress during pregnancy is associated with a sex-dependent enhancement of conditioned fear in the offspring. J Neurodevelopmental Disord. 2009;1:15–32. doi: 10.1007/s11689-008-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Goldstein JM, Goodman JM, Koren D, Turner WM, Faraone SV, Tsuang MT. Sex differences in olfactory identification and Wisconsin Card Sorting performance in schizophrenia: relationship to attention and verbal ability. Biol Psychiatry. 1997;42:104–115. doi: 10.1016/S0006-3223(96)00300-9. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Schizophrenia as a neurodevelopmental disorder: a review of the concept. In: Hirsch SR, Weinberger DR, editors. Schizophrenia. Blackwood; London: 1995. pp. 293–323. [Google Scholar]
- Wolff AR, Bilkey DK. Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring. Behav Brain Res. 2008;190:156–159. doi: 10.1016/j.bbr.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. The maternal immune activation (MIA) model of schizophrenia produces pre-pulse inhibition (PPI) deficits in both juvenile and adult rats but these effects are not associated with maternal weight loss. Behav Brain Res. 2010;213:323–327. doi: 10.1016/j.bbr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Wong AH, Van Tol HH. Schizophrenia: from phenomenology to neurobiology. Neurosci Biobehav Rev. 2003;27:269–306. doi: 10.1016/s0149-7634(03)00035-6. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Chretien P, Meaney MJ, Gratton A. Influence of naturally occurring variations in maternal care on prepulse inhibition of acoustic startle and the medial prefrontal cortical dopamine response to stress in adult rats. J Neurosci. 2005;25:1493–1502. doi: 10.1523/JNEUROSCI.3293-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Post-pubertal emergence of disrupted latent inhibition following prenatal immune activation. Psychopharmacology (Berl) 2003;169:308–313. doi: 10.1007/s00213-003-1461-7. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]