Abstract
Exposure to acute stress alters cognition; however, few studies have examined the effects of acute stress on executive functions such as behavioral flexibility. The goal of the present experiments was to determine the effects of acute periods of stress on two distinct forms of behavioral flexibility: set-shifting and reversal learning. Male Sprague-Dawley rats were trained and tested in an operant-chamber-based task. Some of the rats were exposed to acute restraint stress (30 min) immediately before either the set-shifting test day or the reversal learning test day. Acute stress had no effect on set-shifting, but it significantly facilitated reversal learning, as assessed by both trials to criterion and total errors. In a second experiment, the roles of glucocorticoid (GR) and mineralocorticoid receptors (MR) in the acute-stress-induced facilitation of reversal learning were examined. Systemic administration of the GR-selective antagonist RU38486 (10 mg/kg) or the MR-selective antagonist spironolactone (50 mg/kg) 30 min prior to acute stress failed to block the facilitation on reversal learning. The present results demonstrate a dissociable effect of acute stress on set-shifting and reversal learning and suggest that the facilitation of reversal learning by acute stress may be mediated by factors other than corticosterone.
Keywords: Executive functions, Behavioral flexibility, Glucocorticoid receptor, Mineralocorticoid receptor, Corticosterone, Prefrontal cortex
Considerable research has focused on the effects of acute stress on declarative-type memories mediated by regions of the medial temporal lobe (Cazakoff, Johnson, & Howland, 2010; Howland & Wang, 2008; Kim & Diamond, 2002). One consistent finding from this literature is that an out-of-context acute stressor experienced immediately before learning or memory retrieval impairs performance of tasks such as the water maze (de Quervain, Roozendaal, & McGaugh, 1998; Diamond, Park, Heman, & Rose, 1999; Wong et al., 2007) and object recognition (Baker & Kim, 2002; Howland & Cazakoff, 2010). These findings (and others) have led to a focus on the negative effects of stress for cognition. However, acute stress has complex effects on cognition that depend on the nature of the stressor, its timing, the sex of the subjects, and the specific task being examined (Cazakoff et al., 2010; Diamond, Campbell, Park, Halonen, & Zoladz, 2007; Joëls, Krugers, & Karst, 2008; Joëls, Pu, Wiegert, Oitzl, & Krugers, 2006; Shors, 2006), raising the question of whether detrimental effects of acute stress are observed across different cognitive domains.
Executive functions are a category of cognitive processes commonly ascribed to the functioning of prefrontal cortex (PFC; Robbins, 2007). Behavioral flexibility, an aspect of executive function, refers to the ability of an organism to change behavioral strategies to optimize survival or adapt to the environment (Floresco, Zhang, & Enomoto, 2009; Robbins, 2007). Two distinct forms of behavioral flexibility are set-shifting and reversal learning. Set-shifting, mediated in part by medial PFC (mPFC) in rodents, is the ability to change response patterns between different dimensions of complex stimuli (Birrell & Brown, 2000; Floresco, Block, & Tse, 2008; Ragozzino, Detrick, & Kesner, 1999; Rich & Shapiro, 2007). Reversal learning requires changes in responses between stimuli on the basis of one of their dimensions and is mediated in part by orbitofrontal cortex (OFC; Ghods-Sharifi, Haluk, & Floresco, 2008; McAlonan & Brown, 2003). Given that stress is well known to affect PFC function (Arnsten, 2009; Holmes & Wellman, 2009), a thorough examination of the effects of stress on executive functions is warranted. The effects of chronic stress on set-shifting and reversal learning have been examined previously. Chronic stress with a variety of paradigms impairs attentional set-shifting in rats (Bondi, Jett, & Morilak, 2010; Bondi, Rodriguez, Gould, Frazer, & Morilak, 2008; Liston et al., 2006; Nikiforuk, 2012; Nikiforuk & Popik, 2011), an effect that is correlated with morphological alterations in the pyramidal cells of mPFC (Liston et al., 2006; Radley et al., 2006). Either no effect (Liston et al., 2006), an inconsistent impairment (Bondi et al., 2008), or an impairment (Danet, Lapiz-Bluhm, & Morilak, 2010; Lapiz-Bluhm, Soto-Pina, Hensler, & Morilak, 2009) of reversal learning has been reported following various chronic-stress protocols. One recent study using a touch-screen assay in mice demonstrated that three days of swim stress enhanced reversal learning during late acquisition after severe perseveration had ceased (Graybeal et al., 2011). Notably, a single day of swim stress was not sufficient to alter reversal learning in this paradigm (Graybeal et al., 2011), although improved spatial reversal learning following a single acute stressor has been reported (Dong et al., in press). However, a systematic assessment of the potential effects of acute stress on set-shifting and reversal learning has yet to be conducted.
Acute stress exerts potent effects on brain function via numerous mediators, including steroid hormones such as corticosterone in rodents (Joëls & Baram, 2009). Glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs) are the primary receptors though which corticosterone exerts its actions. MRs have an affinity for corticosterone approximately ten times higher than do GRs and are mostly occupied by basal circulating levels of corticosterone, whereas GRs require higher levels of corticosterone to be activated, such as the levels that follow exposure to acute stress or the peaks of ultradian pulses of corticosterone (de Kloet, Joëls, & Holsboer, 2005; Joëls & Baram, 2009; Lightman et al., 2008). The expression patterns of the two receptors differ in the cortical–thalamic–striatal–limbic circuitry known to mediate set-shifting and reversal learning (de Kloet et al., 2005; Groeneweg, Karst, de Kloet, & Joëls, 2011), thereby raising the possibility that blocking the receptors may have different effects on these tasks.
The present experiments examined the effects of acute stress on set-shifting and reversal learning using an automated operant-chamber-based task (Floresco et al., 2008, 2009; Zhang, Cazakoff, Thai, & Howland, 2012). Given the demonstrated role of corticosterone in mediating the effects of acute (Butts, Weinberg, Young, & Phillips, 2011; Yuen et al., 2009) and chronic (Nikiforuk & Popik, 2011) stress on the cognitive operations governed by PFC, the roles of GRs and MRs in mediating the effects of acute stress were also examined with the administration of RU38486, a GR antagonist, and spironolactone, an MR antagonist.
Materials and methods
Subjects
A group of 78 adult male Sprague-Dawley rats (225–275 g on arrival; Charles River, Quebec, Canada) were used in this experiment. Upon arrival, the rats were pair-housed in plastic cages and kept on a 12:12-h light:dark cycle in a temperature-controlled (21 °C) colony room. Initially, the rats were allowed ad libitum access to food (Purina Rat Chow) and water for five days to acclimatize them to the facility. The rats were then housed singly and food restricted to 85 % of their free-feeding weight until the commencement of lever training. Rats were tested in six squads of 12–16 over a period of approximately 12 months. The first two squads tested included animals in the control, acute-stress/set-shifting, and acute-stress/reversal learning groups (n = 22). Subsequent squads included subjects in all groups (n = 56). All procedures were approved by the University of Saskatchewan Animal Research Ethics Board and were conducted in accordance with the standards of the Canadian Council on Animal Care.
Apparatus
Rats were trained and tested in eight operant chambers (30.5 × 21 × 24 cm [l × w × h], ENV-008, Med Associates, St. Albans, VT). Each chamber was located in a wooden sound-attenuating box (63.6 × 75.6 × 35.6 cm) containing a fan that provided background noise and ventilation. Each chamber contained a food receptacle where food reward pellets (Dustless Precision Pellets, 45 mg, Rodent Purified Diet; BioServ, Frenchtown, NJ) were delivered via an automated pellet dispenser. A retractable lever was positioned on either side of the receptacle, along with a stimulus light positioned above each lever. A house light (100 mA) was positioned in the center of the wall opposite the food receptacle and near the top. The chamber floor comprised a removable metal grid with a detachable metal tray. The presentation of trials and the recording of data from the operant chambers were controlled via an interface box connected to a personal computer.
Lever training
The behavioral training and testing protocols closely followed those described previously (Floresco et al., 2009; Zhang et al., 2012). The day prior to the commencement of lever training, rats were given ~20 food reward pellets in their home cages. On the first day of lever training, ~2–3 sugar pellets were crushed into a fine powder and placed on an extended lever in the operant chamber. The rats were then trained on a fixed-ratio 1 (FR1) schedule on each lever with a criterion of 45 presses in 30 min. Rats were trained on one lever and, upon reaching criterion, were trained on the other lever during the following day (the left/right order of lever training was counterbalanced between subjects).
Following the successful completion of FR1 training on both levers, the rats were familiarized with lever insertion on subsequent days. The rats were required to respond by pressing a lever within 10 s of its being inserted into the chamber. Training consisted of 90 trials each day. A new trial began every 20 s with illumination of the house light and insertion of one of the levers into the chamber. If a rat pressed the lever within 10 s following insertion, the trial was scored as a response, a food reward pellet was delivered to the food receptacle, and the house light remained on for an additional 4 s. Failure to respond within 10 s resulted in the trial being scored as an omission, retraction of the lever, and the house light immediately being extinguished. The order of presentation of the left and right levers was randomized. However, each lever was presented once in every pair of trials, such that the left and right levers were inserted for equal numbers of trials (i.e., 45 trials each) during each training session. The criterion to pass retractable lever training was ≤5 omissions in a 90-trial session. The rats were trained in this phase of the experiment for a minimum of 5 days or until the criterion was reached. Upon reaching criterion, the lever side bias of each rat was determined. To determine side bias, both levers were inserted into the chamber, and pressing either lever resulted in delivery of a food reward pellet. Following the initial trial, rats were required to alternate pressing the left and right levers to obtain further food reward. The side preference session concluded when seven reward pellets had been delivered. Side preference was determined by comparing the total left and right leverpresses. If the total presses were comparable (<2:1 ratio for total presses), the lever pressed on the first trial was recorded as the rat’s biased lever. If the total presses were not comparable (≥2:1 ratio), the lever that was pressed more was recorded as the biased lever. The stimulus lights above each lever were never used during training or side preference sessions. Three days of testing commenced the day following the final training session.
Visual-cue discrimination
In the first phase of testing, rats were required to press the lever underneath the illuminated stimulus light to receive a food reward pellet. Visual-cue discrimination sessions began with neither lever being inserted and with all lights extinguished (the intertrial state). Every 20 s, a trial began with one of the stimulus lights being illuminated. After 3 s, the house light was illuminated and both levers were inserted. A correct response was scored if the rat pressed the lever underneath the illuminated stimulus light. This led to the retraction of both levers, extinguishing of the stimulus light, delivery of a food reward pellet, and the house light remaining on for an additional 4 s before the chamber returned to the intertrial state. An incorrect response (error) was scored when the rat pressed the lever underneath the stimulus light that was not illuminated. This response caused the retraction of both levers, extinguishing of the stimulus and house lights, and an immediate return to the intertrial state. Failure to press a lever within 10 s of insertion was scored as an omission and also resulted in the immediate return to the intertrial state. The left and right stimulus lights were each illuminated once in every pair of trials, with the order randomized within each pair of trials. The criterion to pass this phase required rats to make at least ten consecutive correct choices in either the first 30 trials or within 150 trials. Failure to reach criterion within 150 trials resulted in the rats being tested in visual-cue discrimination again during subsequent days until criterion was reached. During visual-cue discrimination testing, the lever that the rat chose, the illuminated stimulus light, and the latency to press a lever were recorded. The raw data were used to determine the trials to criterion (TTC) and the total errors committed by each rat. Strategy set-shift discrimination commenced the day following successful completion of visual-cue discrimination.
Strategy set shift (shift to response discrimination)
This next phase of testing required rats to ignore the stimulus light (visual cue) and only to respond to the lever opposite each rat’s lever bias (spatial cue) to continue receiving a food reward. With the exception of how correct and incorrect responses were scored, the trial format and criterion to pass were identical to those described for visual-cue discrimination. Regardless of which stimulus light was illuminated, correct responses were scored when the rat pressed its nonbiased lever. Incorrect responses were scored whenever the rat pressed its biased lever. In addition, errors during this phase of testing were divided into three categories, as described previously (Floresco et al., 2009; Zhang et al., 2012). Perseverative errors were scored when a rat continued to follow the previously rewarding but currently unrewarding strategy (i.e., following the stimulus light). In this phase of testing, eight out of every 16 trials allowed a rat to make a response error (i.e., pressing the biased lever when the illuminated stimulus light was above it). With the exception of the first block of eight trials, when all errors were scored as perseverative, perseverative errors were counted when a rat made six or more errors in every block of eight trials. If a rat made five or fewer errors in subsequent blocks of eight trials, these errors were then scored as regressive errors. Never-reinforced errors were scored when a rat made a response that had not been previously rewarded (i.e., pressing the biased lever when the illuminated stimulus light was over the nonbiased lever). These error subtypes, along with the total errors and TTC, were recorded. Response-reversal testing commenced the day following successful completion of set-shift testing.
Response reversal
During this final phase of testing, rats were required to shift responding from the nonbiased lever to the biased lever to receive food rewards. With the exception of how correct and incorrect responses were scored, the trial format and criterion to pass were identical to those on the visual-cue and set-shift discrimination days. A correct trial was now scored whenever a rat pressed its biased lever, while an incorrect trial was scored when a rat pressed its nonbiased lever. Scoring of perseverative and regressive errors was similar to that described for set-shift days (Floresco et al., 2009; Zhang et al., 2012). However, with the exception of the first block of 16 trials, in which all errors were scored as perseverative, perseverative errors were counted when a rat made ten or more errors within a block of 16 trials. If a rat made fewer than ten errors per block of 16 trials, subsequent errors were then counted as regressive. Error subtypes were totaled for each rat along with the TTC and total errors.
Stress and drug treatment
Rats in the stress groups were placed in standard Plexiglas rat restraint tubes (Model 544-RR, Fisher Scientific) for 30 min in a novel brightly lit room immediately prior to either set-shift or response-reversal testing. This stress procedure has been shown to significantly increase levels of circulating corticosterone in our laboratory (Macdougall & Howland, 2012) and others (Koolhaas et al., 2011). Unstressed rats remained in their home cages until testing commenced. Some rats were injected 60 min prior to response-reversal testing with vehicle (50:50 DMSO:95 % ethanol; 2 ml/kg; i.p.), RU38486 (10 mg/kg; i.p.; Sigma), or spironolactone (50 mg/kg; s.c.; Tocris Biochemicals). These doses were chosen on the basis of previous studies that had shown that they are sufficient to block GR-mediated (Cazakoff & Howland, 2010; Macdougall & Howland, 2012; Pugh, Fleshner, & Rudy, 1997; Zhou et al., 2010) and MR-mediated (Kumar et al., 2007; Mackowiak et al., 2008; Zhou et al., 2010) effects in rodents. Average response latencies of the rats were not affected by either stress or drug treatment in any of the experiments (data not shown).
Data analysis
The data are presented as group means ± standard errors of the means. Statistical analyses were conducted using an α level of .05. Statistical analysis of the effects of acute stress on set-shifting and reversal days was performed using one-way ANOVAs with Stress (no stress, stress on set-shifting day, or stress on reversal day) as a between-subjects factor. The dependent variables for visual-cue discrimination were TTC and the total number of errors committed. The dependent variables for set-shifting were TTC, total errors, perseverative errors, regressive errors, and never-reinforced errors. The dependent variables for response reversal discrimination were TTC, total errors, and perseverative and regressive errors. Statistical analysis of the effects of acute stress and GR and MR antagonists on reversal learning was performed using 2 × 3 between-subjects ANOVAs with Stress (no stress or stress) and Drug (vehicle, RU38486, or spironolactone) as factors. These analyses used the same dependent variables as the one-way ANOVAs mentioned above. Post-hoc comparisons were made with the Neuman–Keuls test, where appropriate.
Results
Effects of acute stress on set-shifting and reversal learning
Visual-cue discrimination (Fig. 1a and b)
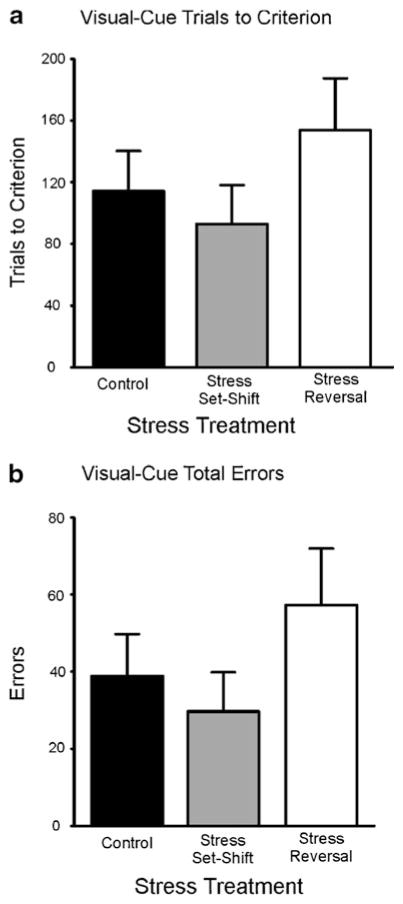
Fig. 1.
Performance of rats in the no-stress, acute-stress-before-set-shifting, and acute-stress-before-reversal-learning groups on visual-cue discrimination. a Trials to criterion (TTC). b Total errors. No significant differences were found between groups on either TTC or total errors. No stress, n = 16; stress before set-shifting, n = 10; stress before reversal learning, n = 13
Rats were assigned to one of the three treatment groups (no-stress control, n = 16; stress prior to set-shifting, n = 10; stress prior to reversal, n = 13) in a counterbalanced manner based on when they completed lever training. The statistical analysis revealed no significant differences among the groups on TTC or total errors for the visual-cue discrimination test day [Fs(2, 36) = 0.86 and 0.98, respectively, both n.s.].
Set-shifting (Fig. 2a–c)
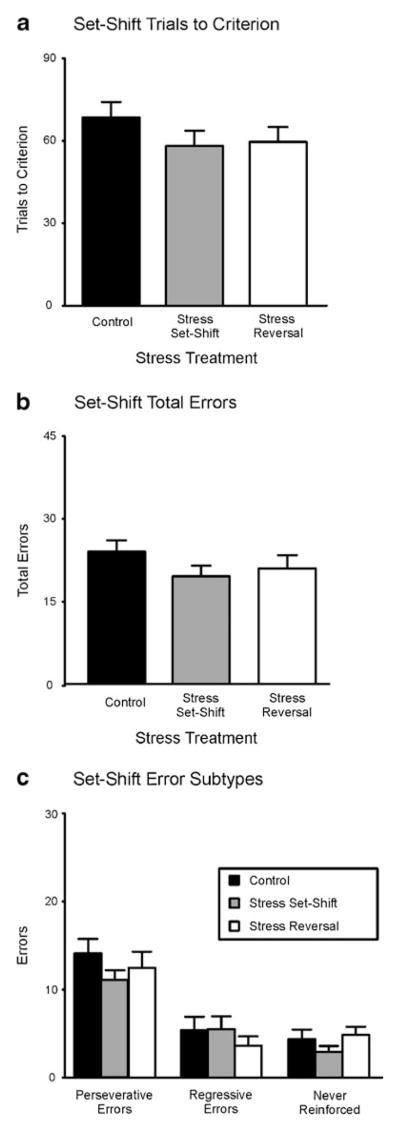
Fig. 2.
Effect of acute stress on set-shifting. a Trials to criterion. b Total errors. c Error subtypes. The performance of rats acutely stressed prior to set-shifting was not significantly different than the performance of rats in the other two groups (neither of which were subjected to acute stress on set-shifting day). No stress, n = 16; stress before set-shifting, n = 10; stress before reversal learning, n = 13
Rats in the group subjected to acute stress immediately before set-shifting, as compared to rats not subjected to acute stress, required a similar number of trials to reach criterion (58.10 ± 5.6) and committed a similar number of total errors (19.50 ± 2.0) (TTC means: control, 70.00 ± 6.3; stressed on reversal, 63.49 ± 3.5; total error means: control, 24.88 ± 2.2; stressed on reversal, 20.92 ± 2.4). The statistical analysis of the data confirmed these observations, with no significant main effect of acute stress on set-shifting for TTC or total errors [Fs(2, 36) = 1.23 and 1.53, respectively, both n.s.]. Analysis of the error subtypes also revealed that acute stress prior to set-shifting had no significant effect on perseverative, regressive, or never-reinforced errors [Fs(2, 36) = 0.54, 0.49, and 1.17, respectively, all n.s.].
Response reversal (Fig. 3a–c)
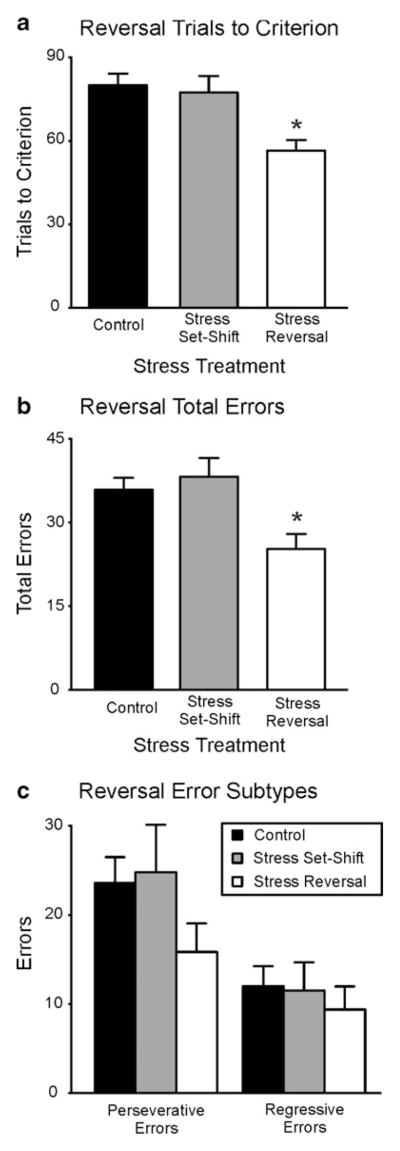
Fig. 3.
Effect of acute stress on reversal learning. a Trials to criterion. b Total errors. c Error subtypes. The rats acutely stressed prior to reversal learning reached criterion significantly faster and with fewer total errors than did rats from the other two groups. However, the numbers of perseverative and regressive errors committed were not significantly different between the groups. No stress, n = 16; stress before set-shifting, n = 10; stress before reversal learning, n = 13. *p < .05
In contrast, rats exposed to acute stress before reversal learning required fewer TTC (56.54 ± 3.8) and committed fewer total errors (25.23 ± 2.7) than did rats not subjected to acute stress (TTC means: control, 80.00 ± 4.4; stressed on set shift, 77.40 ± 5.9; total error means: control, 36.06 ± 2.4; stressed on set shift, 38.20 ± 3.3). It is noteworthy that rats subjected to acute stress prior to set-shifting performed similarly to those rats never subjected to acute stress, thereby demonstrating that a single bout of acute stress does not alter performance of the reversal component of the task 24 h later. Statistical analysis for response reversal discrimination confirmed these observations by revealing significant effects of acute stress on both TTC [F(2, 36) = 7.87, p = .001] and total errors [F(2, 36) = 6.28, p = .005]. Rats stressed prior to reversal learning reached criterion significantly faster and with fewer errors than did the rats of the other two treatment groups (post-hoc, p < .05). However, there was no specific effect of stress on either perseverative or regressive subtypes of errors [Fs(2, 36) = 1.74 and 0.24, respectively, both n.s.], indicating that neither error subtype preferentially contributed to the reduced total error rate of the rats stressed prior to reversal learning.
Effects of GR and MR antagonists on the acute stress facilitation of response reversal
Visual-cue discrimination and set-shifting (Table 1)
Table 1.
Behavior of rats in the RU38486 and spironolactone groups on the visual-cue and set-shifting test days
| Visual-Cue Trials to Criterion | Visual-Cue Total Errors | Set-Shift Trials to Criterion | Set-Shift Total Errors | |
|---|---|---|---|---|
| Vehicle + no stress (n = 16) | 120.56 (30.1) | 41.56 (12.6) | 70.00 (6.3) | 24.88 (2.2) |
| Vehicle + stress (n = 13) | 153.92 (33.5) | 57.23 (14.7) | 59.62 (5.4) | 20.92 (2.4) |
| RU38486 + no stress (n = 10) | 154.60 (33.4) | 56.00 (14.5) | 59.80 (4.9) | 18.90 (2.5) |
| RU38486 + stress (n = 11) | 127.45 (29.0) | 43.09 (11.4) | 63.18 (6.0) | 20.55 (2.5) |
| Spironolactone + no stress (n = 10) | 87.20 (13.9) | 26.40 (5.8) | 60.90 (4.7) | 20.30 (1.5) |
| Spironolactone + stress (n = 8) | 118.25 (33.4) | 42.13 (13.9) | 65.75 (9.6) | 19.63 (3.3) |
Stress refers only to stress on the reversal test day (see Fig. 4). Group means and SEMs are shown
The effects of acute stress and the pharmacological manipulations on the reversal learning day were not due to differences in the rats’ abilities to learn the visual-cue discrimination or set-shifting aspects of the task, as there were no significant differences among the groups on either the visual-cue or set-shifting test days (statistics not shown).
Response reversal (Fig. 4a and b)
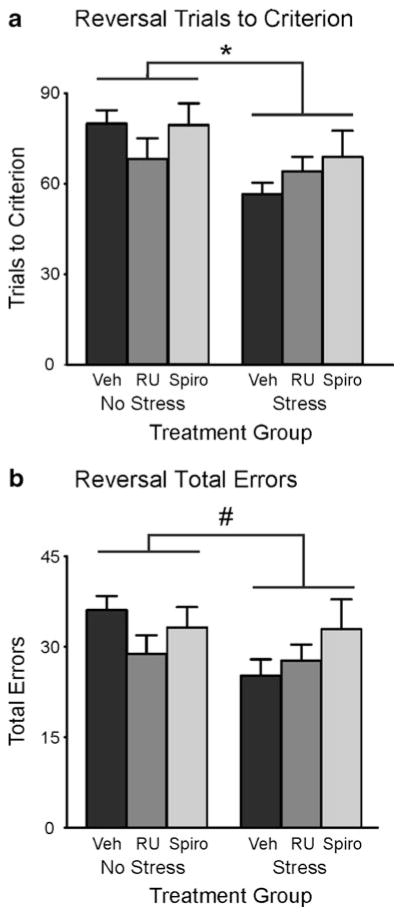
Fig. 4.
Effects of RU38486 (RU) or spironolactone (Spiro) treatment on the performance of stressed and unstressed rats during reversal learning. a Trials to criterion. b Total errors. Rats exposed to acute stress took significantly fewer trials to reach criterion than did unstressed rats, regardless of drug treatment. Rats in the acute-stress group tended to commit fewer total errors than did rats that were not stressed; however, this effect did not reach significance. One rat in the stress + spironolactone group performed particularly poorly. When this rat was removed, the effect of acute stress on total errors reached significance (p = .026; see the Results for further details). Vehicle + no stress, n = 16; vehicle + stress, n = 13; RU38486 + no stress, n = 10; RU38486 + stress, n = 11; spironolactone + no stress, n = 10; spironolactone + stress, n = 8. *p < .05. #p = .11
On the response reversal test day, the statistical analysis of TTC with a 2 × 3 between-subjects ANOVA revealed a significant main effect of stress [F(1, 62) = 7.19, p = .009], whereby stressed rats reached reversal criterion significantly faster than did unstressed rats (Fig. 4a). The main effect of drug [F(2, 62) = 0.89] and the Stress × Drug interaction term [F(2, 62) = 1.66] failed to reach significance. When total errors were analyzed with an ANOVA, no significant effects were observed for stress [F(1, 62) = 2.58], drug [F(2, 62) = 1.05], or the Stress × Drug interaction [F(2, 62) = 2.02]. Inspection of the data revealed that one rat in the spironolactone + stress group performed poorly on the reversal day relative to the other animals in the group (TTC = 115, total errors = 62). Reanalysis of the data with that animal removed revealed significant main effects of stress on TTC [F(1, 61) = 10.58, p = .002] and total errors [F(1, 61) = 5.19, p = .026]. Separate analyses of the perseverative and regressive error subtypes revealed no significant effects of stress or drug treatment (statistics not shown). Taken together, these analyses demonstrate that treatment with GR and MR antagonists was ineffective in blocking the facilitation of reversal learning by acute stress.
Discussion
In the present experiments, we examined the effects of acute stress on set-shifting and reversal learning. Treatment with 30 min of acute restraint stress immediately before testing had no significant effect on set-shifting. However, acute stress significantly facilitated reversal learning, as rats stressed prior to response reversal reached criterion in significantly fewer trials and with fewer total errors. Thus, in a second experiment, we sought to investigate whether GRs or MRs were involved in mediating the acute stress effect on reversal learning. To that end, we injected rats with the GR antagonist RU38486 or the MR antagonist spironolactone to examine whether either compound could block the stress-induced facilitation of reversal learning. Neither RU38486 nor spironolactone significantly affected reversal learning or its facilitation by acute stress. Thus, activation of GRs and MRs may not be involved in mediating the acute stress-induced facilitation of reversal learning.
Differential effects of acute stress on set-shifting and reversal learning
To our knowledge, the present study is the first to examine the effects of acute stress on set-shifting and reversal learning in rodents. We found that acute restraint stress had no effect on set-shifting using an operant-based task (Fig. 2a–c). In contrast, acute restraint stress significantly facilitated reversal learning as measured by TTC and total errors (Fig. 3a and b). The stress-induced facilitation of reversal learning was not due to reductions of a specific error subtype, although the number of perseverative errors was reduced more dramatically than that of regressive errors (Fig. 3c). The lack of an effect of stress on set-shifting was surprising, given that set-shifting depends on cortico–thalamic–striatal circuits including the mPFC in rodents (Floresco et al., 2009; Robbins & Roberts, 2007), and these circuits are exquisitely sensitive to acute stress (Arnsten, 2009; Butts et al., 2011; Holmes & Wellman, 2009; Yuen et al., 2009). Chronic stress also reliably impairs set-shifting in rodents (Bondi et al., 2008, 2010; Liston et al., 2006; Nikiforuk, 2012; Nikiforuk & Popik, 2011), an effect associated with decreased arborization of the apical dendrites of mPFC pyramidal neurons (Liston et al., 2006). Antidepressant drugs from a variety of classes also block the effects of chronic stress on set-shifting (Bondi et al., 2008, 2010; Nikiforuk, 2012; Nikiforuk & Popik, 2011).
While it is possible that the restraint stress protocol used in the present experiments was not sufficiently intense to impair set-shifting, the same stressor did alter behavior during the reversal learning component of the task. An alternative possibility is that task difficulty, and not the cognitive process involved, led to the observed results. The reversal learning component of the task required more trials for criterion (~80 trials) than did the set-shifting component (~70 trials) in the present data set and in another from our laboratory (Zhang et al., 2012; but see also Floresco et al., 2009). The reversal learning task also required the rats to press the lever that they were biased toward during the side preference assessment. Thus, it is possible that acute stress biased the rats toward a stimulus–response strategy instead of a spatial strategy, as has been shown in mice (Schwabe, Schächinger, de Kloet, & Oitzl, 2010) and humans (Schwabe et al., 2007; Schwabe, Oitzl, Richter, & Schächinger, 2009).
Consistent with these findings, acute elevated platform stress was reported as facilitating spatial reversal learning in the water maze, an effect correlated with stress-facilitated long-term depression in the dorsal CA1 region of the hippocampus (Dong et al., in press). While the stress-induced facilitation of reversal learning is also similar to the findings recently reported in mice using a touch-screen-based task (Graybeal et al., 2011), there are considerable differences between the studies. In Graybeal et al.’s study, mice were trained on a visual discrimination task and a subsequent reversal over a period of weeks. Mice subjected to swim stress for three days, but not for one day, before the initiation of reversal learning showed no difference in reversal learning during the early stages, when perseveration was high. However, previously stressed mice committed fewer errors than did unstressed mice later in reversal learning, when perseveration had ceased (i.e., accuracy ≥ 50 %), an effect prevented by brain-derived neurotrophic factor infusions into the ventral medial prefrontal cortex (Graybeal et al., 2011). The effects of other, longer chronic-stress protocols on reversal learning have been inconsistent (Bondi et al., 2008; Liston et al., 2006), although impaired reversal learning is consistently observed following some chronic stress protocols (Danet et al., 2010; Lapiz-Bluhm et al., 2009). The time frame of the acute stress-induced facilitation of reversal learning was much shorter in the present experimental design: Rats were subjected to acute stress for 30 min immediately prior to reversal learning, which took place during a single session of approximately 1 h. Thus, as sufficient time did not exist in our design for the longer-term morphological changes caused by stress to occur, we examined the effects of GR and MR antagonists on the acute stress-induced facilitation.
Lack of effect of GR or MR antagonism on the acute stress-induced facilitation of reversal learning
Pretreatment of rats with the GR antagonist RU38486 or the MR antagonist spironolactone failed to significantly affect the acute stress-induced enhancement of reversal learning (Fig. 4a and b). While the doses of RU38486 and spironolactone used in these studies have been effective in previous behavioral and electrophysiological experiments (Cazakoff & Howland, 2010; Kumar et al., 2007; Macdougall & Howland, 2012; Mackowiak et al., 2008; Pugh et al., 1997; Zhou et al., 2010), we cannot rule out the possibility that higher doses of the drugs would have blocked the facilitation of reversal learning by acute stress (Adamec, Muir, Grimes, & Pearcey, 2007; Koenig & Olive, 2004). Inspection of the data revealed that drug treatment did subtly alter behavior in the absence of stress. For example, treatment of the rats with RU38486 alone reduced TTC (68.10 trials), as compared to either vehicle treatment (80.00 trials) or spironolactone treatment alone (79.30 trials), albeit this effect failed to reach significance with the present statistical design and sample size.
A role for increased circulating corticosterone in the effects of stress on tasks that are dependent on mPFC has been shown for a number of specific tasks, including the disruptive effects of chronic stress on set-shifting (Nikiforuk & Popik, 2011) and the disruptive (Butts et al., 2011) and facilitative (Yuen et al., 2009) effects of acute stress on working memory. The actions of corticosterone in prefrontal areas involved in reversal learning, such as the OFC, are less thoroughly understood. Interestingly, a dissociation in the effects of chronic restraint stress has been shown in the PFC, whereby chronic stress increases dendritic arborization in OFC (Liston et al., 2006), whereas it decreases arborization in mPFC (Liston et al., 2006). Whether acute stressors have dissociable effects on OFC and mPFC function remains an open question, although these findings from the chronic-stress literature are intriguing in this context.
Other neurotransmitter systems responsive to periods of acute stress may be involved in the acute-stress-induced facilitation of reversal learning. For example, a recent report has shown that infusions of corticotropin-releasing factor (CRF) into the locus coeruleus produce a complex modulation of set-shifting and reversal learning in rats (Snyder, Wang, Han, McFadden, & Valentino, 2012): Rats infused with higher doses of CRF displayed enhanced reversal learning, whereas a low dose enhanced the set-shifting component of the same task (Snyder et al., 2012). Serotonergic, dopaminergic, and glutamatergic systems are also involved in reversal learning (Balschun et al., 2010; Bannerman, Deacon, Seeburg, & Rawlins, 2003; Boulougouris & Robbins, 2010; Floresco et al., 2009; Lapiz-Bluhm et al., 2009; Robbins & Roberts, 2007). Therefore, a direction for further research could be to manipulate these neurotransmitter systems in order to assess the roles that they have, if any, in mediating the acute stress-induced facilitation of reversal learning.
Conclusion
The effects of acute stress on cognition are complex and depend on the nature of the task examined, the duration and type of stressor, the subjects, and the context of the stressor (Cazakoff et al., 2010; Diamond et al., 2007; Joëls et al., 2006, 2008; Shors, 2006). The present experiments demonstrated that acute stress facilitates reversal learning without affecting set-shifting in male rats. A role for adrenal steroid hormones in this effect was not supported; however, other components of the hypothalamic–pituitary–adrenal axis or neurotransmitters may be involved.
Acknowledgments
This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) to J.G.H. J.G.H. is a National Alliance for Research on Schizophrenia and Depression Young Investigator and a Canadian Institutes of Health Research New Investigator. C.A.T. was the recipient of an NSERC USRA Award.
Contributor Information
Chester A. Thai, Department of Psychology, University of Saskatchewan, Rm. 154, 9 Campus Dr., Saskatoon, SK, S7N 5A5, Canada
Ying Zhang, Department of Psychology, University of Saskatchewan, Rm. 154, 9 Campus Dr., Saskatoon, SK, S7N 5A5, Canada.
John G. Howland, Department of Physiology, University of Saskatchewan, 107 Wiggins Rd, Rm. A302, Saskatoon, Saskatchewan, S7N 5E5, Canada
References
- Adamec R, Muir C, Grimes M, Pearcey K. Involvement of noradrenergic and corticoid receptors in the consolidation of the lasting anxiogenic effects of predator stress. Behavioural Brain Research. 2007;179:192–207. doi: 10.1016/j.bbr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learning and Memory. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Moechars D, Callaerts-Vegh Z, Vermaercke B, Van Acker N, Andries L, D’Hooge R. Vesicular glutamate transporter VGLUT1 has a role in hippocampal long-term potentiation and spatial reversal learning. Cerebral Cortex. 2010;20:684–693. doi: 10.1093/cercor/bhp133. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Seeburg PH, Rawlins JN. GluR-A-Deficient mice display normal acquisition of a hippocampus-dependent spatial reference memory task but are impaired during spatial reversal. Behavioral Neuroscience. 2003;117:866–870. doi: 10.1037/0735-7044.117.4.866. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set-shifting in the rat. Journal of Neuroscience. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Jett JD, Morilak DA. Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1 adrenergic receptors in medial prefrontal cortex. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:913–923. doi: 10.1016/j.pnpbp.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. Journal of Neuroscience. 2010;30:930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts KA, Weinberg J, Young AH, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proceedings of the National Academy of Sciences. 2011;108:18459–18464. doi: 10.1073/pnas.1111746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazakoff BN, Howland JG. Acute stress disrupts paired pulse facilitation and long-term potentiation in rat dorsal hippocampus through activation of glucocorticoid receptors. Hippocampus. 2010;20:1327–1331. doi: 10.1002/hipo.20738. [DOI] [PubMed] [Google Scholar]
- Cazakoff BN, Johnson KJ, Howland JG. Converging effects of acute stress on spatial and recognition memory in rodents: A review of recent behavioural and pharmacological findings. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:733–741. doi: 10.1016/j.pnpbp.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Danet M, Lapiz-Bluhm S, Morilak DA. A cognitive deficit induced in rats by chronic intermittent cold stress is reversed by chronic antidepressant treatment. International Journal of Neuropsychopharmacology. 2010;13:997–1009. doi: 10.1017/S1461145710000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes–Dodson law. Neural Plasticity. 2007;2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9:542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dong Z, Bai Y, Wu X, Li H, Gong B, Howland JG, Wang YT. Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology. 64:65–73. doi: 10.1016/j.neuropharm.2012.06.027. (in press) [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behavioural Brain Research. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behavioural Brain Research. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiology of Learning and Memory. 2008;89:567–573. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: Rescue with BDNF. Nature Neuroscience. 2011;14:1507–1509. doi: 10.1038/nn.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joëls M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. Journal of Endocrinology. 2011;209:153–167. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neuroscience & Biobehavioral Reviews. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland JG, Cazakoff BN. Effects of acute stress and GluN2B-containing NMDA receptor antagonism on object and object–place recognition memory. Neurobiology of Learning and Memory. 2010;93:261–267. doi: 10.1016/j.nlm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Howland JG, Wang YT. Synaptic plasticity in learning and memory: Stress effects in the hippocampus. Progress in Brain Research. 2008;169:145–158. doi: 10.1016/S0079-6123(07)00008-8. [DOI] [PubMed] [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. Nature Reviews Neuroscience. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Krugers H, Karst H. Stress-induced changes in hippocampal function. Progress in Brain Research. 2008;167:3–15. doi: 10.1016/S0079-6123(07)67001-0. [DOI] [PubMed] [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: How does it work? Trends in Cognitive Sciences. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews Neuroscience. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Koenig HN, Olive MF. The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psychoneuroendocrinology. 2004;29:999–1003. doi: 10.1016/j.psyneuen.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Fuchs E. Stress revisited: A critical evaluation of the stress concept. Neuroscience & Biobehavioral Reviews. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kumar G, Couper A, O’Brien TJ, Salzberg MR, Jones NC, Rees SM, Morris MJ. The acceleration of amygdala kindling epileptogenesis by chronic low-dose corticosterone involves both mineralocorticoid and glucocorticoid receptors. Psychoneuroendocrinology. 2007;32:834–842. doi: 10.1016/j.psyneuen.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Soto-Pina AE, Hensler JG, Morilak DA. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology. 2009;202:329–341. doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, Conway-Campbell BL. The significance of glucocorticoid pulsatility. European Journal of Pharmacology. 2008;583:255–262. doi: 10.1016/j.ejphar.2007.11.073. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. Journal of Neuroscience. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdougall MJ, Howland JG. Acute stress, but not corticosterone, disrupts short- and long-term synaptic plasticity in rat dorsal subiculum via glucocorticoid receptor activation. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak M, Grzegorzewska M, Budziszewska B, Chocyk A, Hess G, Wedzony K. Cocaine decreases the expression of PSA-NCAM protein and attenuates long-term potentiation via glucocorticoid receptors in the rat dentate gyrus. European Journal of Neuroscience. 2008;27:2928–2937. doi: 10.1111/j.1460-9568.2008.06255.x. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set-shifting in the rat. Behavioural Brain Research. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A. Selective blockade of 5-HT7 receptors facilitates attentional set-shifting in stressed and control rats. Behavioural Brain Research. 2012;226:118–123. doi: 10.1016/j.bbr.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Popik P. Long-lasting cognitive deficit induced by stress is alleviated by acute administration of antidepressants. Psychoneuroendocrinology. 2011;36:28–39. doi: 10.1016/j.psyneuen.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Fleshner M, Rudy JW. Type II glucocorticoid receptor antagonists impair contextual but not auditory-cue fear conditioning in juvenile rats. Neurobiology of Learning and Memory. 1997;67:75–79. doi: 10.1006/nlme.1996.3741. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic–infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. Journal of Neuroscience. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich EL, Shapiro ML. Prelimbic/infralimbic inactivation impairs memory for multiple task switches, but not flexible selection of familiar tasks. Journal of Neuroscience. 2007;27:4747–4755. doi: 10.1523/JNEUROSCI.0369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: Fronto–striatal substrates, neurochemical modulation and clinical implications. Philosophical Transactions of the Royal Society B. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Roberts AC. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cerebral Cortex. 2007;17(Supp 1):i151–i160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Oitzl MS, Philippsen C, Richter S, Bohringer A, Wippich W, Schachinger H. Stress modulates the use of spatial versus stimulus–response learning strategies in humans. Learning and Memory. 2007;14:109–116. doi: 10.1101/lm.435807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Oitzl MS, Richter S, Schächinger H. Modulation of spatial and stimulus–response learning strategies by exogenous cortisol in healthy young women. Psychoneuroendocrinology. 2009;34:358–366. doi: 10.1016/j.psyneuen.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Schächinger H, de Kloet ER, Oitzl MS. Corticosteroids operate as a switch between memory systems. Journal of Cognitive Neuroscience. 2010;22:1362–1372. doi: 10.1162/jocn.2009.21278. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Stressful experience and learning across the lifespan. Annual Review of Psychology. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder K, Wang WW, Han R, McFadden K, Valentino RJ. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology. 2012;37:520–530. doi: 10.1038/npp.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TP, Howland JG, Robillard JM, Ge Y, Yu W, Titterness AK, Wang YT. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proceedings of the National Academy of Sciences. 2007;104:11471–11476. doi: 10.1073/pnas.0702308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cazakoff BN, Thai CA, Howland JG. Prenatal exposure to a viral mimetic alters behavioural flexibility in male, but not female, rats. Neuropharmacology. 2012;62:1299–1307. doi: 10.1016/j.neuropharm.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Bakker EH, Velzing EH, Berger S, Oitzl M, Joëls M, Krugers HJ. Both mineralocorticoid and gluco-corticoid receptors regulate emotional memory in mice. Neurobiology of Learning and Memory. 2010;94:530–537. doi: 10.1016/j.nlm.2010.09.005. [DOI] [PubMed] [Google Scholar]