Abstract
The subiculum (SUB) serves as the major output structure of the hippocampus; therefore, exploring synaptic plasticity within this region is of great importance for understanding the dynamics of hippocampal circuitry and hippocampal–cortical interactions. Previous research has shown exposure to acute stress dramatically alters synaptic plasticity within the hippocampus proper. Using in vivo electrophysiological recordings in urethane-anesthetized adult male Sprague–Dawley rats, we tested the effects of either acute restraint stress (30 min) or corticosterone (CORT) injections (3 mg/kg; s.c.) on short- and long-term forms of synaptic plasticity in the Cornu Ammonis 1–SUB pathway. Paired-pulse facilitation and two forms of long-term plasticity (long-term potentiation and late-developing potentiation) were significantly reduced after exposure to acute stress but not CORT treatment. Measurements of plasma CORT confirmed similar levels of circulating hormone in animals exposed to either acute stress or CORT treatment. The disruptive effects of acute stress on both short- and long-term forms of synaptic plasticity are mediated by glucocorticoid receptor (GR) activation as these disruptions were blocked by pre-treatment with the selective GR antagonist RU38486 (10 mg/kg; s.c.). The present results highlight the susceptibility of subicular plasticity to acute stress and provide evidence that GR activation is necessary but not sufficient for mediating these alterations.
Keywords: hippocampus, in vivo electrophysiology, late-developing potentiation, long-term potentiation, paired-pulse facilitation
Introduction
Patterns of synaptic plasticity within the hippocampal formation consistent with long-term potentiation (LTP) and long-term depression (LTD) are necessary for cognitive operations such as learning and memory (Martin et al. 2000; Malenka and Bear 2004; Massey and Bashir 2007; Collingridge et al. 2010). The mammalian hippocampal formation consists of several anatomically distinct regions: entorhinal cortex (EC), dentate gyrus (DG), hippocampus proper (Cornu Ammonis (CA) subfields, CA3 and CA1), and subiculum (SUB; O’Mara et al. 2001; Andersen et al. 2006; Witter 2006; van Strien et al. 2009). Although research implicating hippocampal synaptic plasticity in learning and memory has focused primarily on the EC, DG, and hippocampus proper, the contributions of SUB have largely been neglected (O’Mara 2005; Behr et al. 2009). The relatively paucity of research on subicular synaptic plasticity is surprising given the important role of SUB, along with the CA1 subfield, in providing the majority of hippocampal output to cortical and subcortical regions (Witter and Groenewegen 1990; O’Mara 2006; van Strien et al. 2009) and contributions to learning and memory (Morris et al. 1990; Deadwyler and Hampson 2004; O’Mara 2006; Sharp 2006; Behr et al. 2009; O’Mara et al. 2009; Potvin et al. 2010). Distinct roles for the CA1 subfield and SUB in learning and memory are proposed (Deadwyler and Hampson 2004; Witter 2006; van Strien et al. 2009); therefore, a more detailed understanding of the potentially divergent mechanisms governing synaptic plasticity in these regions will contribute to theories of medial temporal lobe function.
Pyramidal cells in the dorsal CA1 subfield send dense, topographically organized projections to the dorsal SUB (Witter and Groenewegen 1990; O’Mara et al. 2001; Andersen et al. 2006; van Strien et al. 2009) and studies using electrophysiological recordings have revealed several distinct forms of synaptic plasticity at these synapses (Behr et al. 2009). Short-term synaptic plasticity, in the form of paired-pulse facilitation (PPF), has been reported in the CA1–SUB pathway (Commins et al. 1998a). Long-term alterations including classical LTP are induced in SUB following trains of high-frequency stimulation (HFS) delivered to CA1 either in vitro or in vivo (Boeijinga and Boddeke 1996; Commins et al. 1998b). Interestingly, a form of late-developing potentiation has also been reported within the CA1–SUB pathway following low-frequency stimulation (LFS) in vitro as well as in vivo (Anderson et al. 2000; Huang and Kandel 2005; Fidzinski et al. 2008).
Acute stress is well known to alter patterns of synaptic plasticity in the hippocampus proper, and these alterations are proposed as the basis of the effects of acute stress on cognitive processes such as spatial learning and memory (Diamond and Rose 1994; Kim and Diamond 2002; Shors 2006; Howland and Wang 2008; Collingridge et al. 2010). For example, in the CA1 region, acute stress disrupts classical LTP and facilitates long-term depression (LTD; Foy et al. 1987; Diamond et al. 1994; Kim et al. 1996; Xu et al. 1997; Wong et al. 2007; Cazakoff and Howland 2010), effects reportedly mediated by glucocorticoid receptor (GR) activation (Xu et al. 1998; Cazakoff and Howland 2010). Acute stress also disrupts PPF in CA1 via GR activation (Cazakoff and Howland 2010). The role of corticosterone (CORT), a steroid hormone released by the adrenal cortex, on hippocampal synaptic plasticity has also been examined. An inverted U-shaped function between synaptic plasticity in the CA1 region and peripheral CORT levels has been characterized (Diamond et al. 1992; Lupien and McEwen 1997) and related to spatial memory performance and emotionality (Park et al. 2006).
Given the established susceptibility of the hippocampus to acute stress, the aim of this study was to explore the effects of acute restraint stress and acute CORT administration on three well-characterized forms of SUB plasticity (Commins et al. 1998a,b; Anderson et al. 2000), all of which have received sparse experimental attention in the context of acute stress. In a second series of experiments, we examined the necessity of GR activation for the disruptive effects of acute stress on these forms of subicular plasticity using the selective GR antagonist RU38486 (Xu et al. 1998; Cazakoff and Howland 2010).
Materials and Methods
Subjects
Adult male Sprague–Dawley rats (>300 g; Charles River Laboratories, Quebec, Canada) were pair housed in plastic cages with ad libitum access to food and water. Rats were housed under a 12:12 h light:dark cycle (lights on at 07:00) in a temperature and humidity controlled vivarium. Experimentation was initiated during the first 4 h of the light phase. After arrival at the facility, rats were given at least 5 days to acclimatize before experiments were initiated. All experiments were conducted in accordance with the Canadian Council on Animal Care and were approved by the University of Saskatchewan Animal Research Ethics Board.
In Vivo Electrophysiology
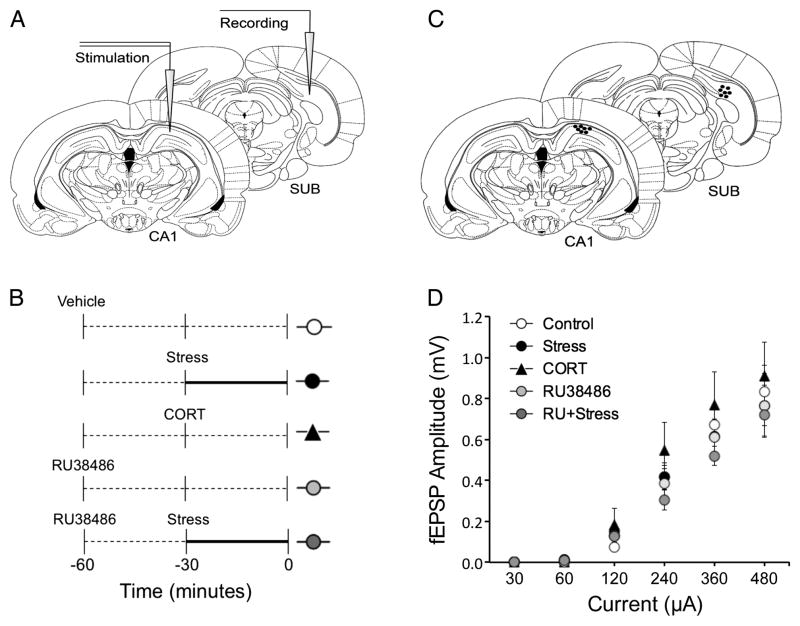
Rats were anesthetized using urethane (1.5–2.0 mg/kg, i.p., Sigma-Aldrich, Oakville, ON, Canada) and placed in a stereotaxic frame (David Kopf, CA, United States of America). A grounded homeothermic temperature control unit (Harvard Instruments, MA, United States of America) was used to maintain the rectal temperature of the rats at 37°C ± 1°C during the experimental sessions. A monopolar recording electrode (insulated platinum iridium wire, 125 μm, AM Systems, WA, United States of America) was lowered into the dorsal SUB through a bored hole in the skull (AP = −6.8 mm, ML = 4.00 mm, DV = −2.5 mm). A concentric-ring bipolar stimulating electrode (NE-100, Rhodes Medical Instruments, Inc., Tunjunga, CA, United States of America; tip separation: 0.5 mm) was lowered into the dorsal CA1 region through a more anterior hole in the skull (AP = −4.5 mm, ML = 2.5 mm, DV = −2.5 mm; Fig. 1A). A reference wire for the recording electrode was secured to the skull anterior to bregma with a jeweller’s screw. SUB field excitatory postsynaptic potentials (fEPSPs) were initially evoked by stimulation of CA1 stratum radiatum (pulse width = 0.12 ms, 200 μA, 0.2 Hz) and were recorded at varying depths. Final electrode placement was determined by maximal field response and recordings were initiated 15–20 min following optimization of electrode placements. First, an input–output curve (Fig. 1D) was generated by applying five consecutive stimuli at each of the following intensities: 30, 60, 120, 240, 360, and 480 μA. Following the input–output curve, electrical current was adjusted to elicit fEPSPs of 50–60% of the maximal response for each rat, which remained at that level for the duration of the experiment. No differences were observed between treatment groups in the amount of current used during the experiments (data not shown).
Figure 1.
(A) A schematic of the experimental design with stimulating electrode placed in dorsal CA1 and recording electrode placed in dorsal subiculum (SUB). (B) A schematic of the experimental conditions. Dotted line represents time spent in home cage and bold line represents time in restraint. All rats were anesthetized at time zero. (C) Representative electrode placements as indicated by black dots. (D) Input–output curve showing the amount of stimulating current (μA) and the evoked fEPSP responses (mV) for the experimental conditions obtained prior to the first paired-pulse facilitation recordings.
PPF was then measured by delivering 5 pairs of pulses to CA1 at inter-pulse intervals (IPIs) of 25, 50, 100, and 200 ms. Immediately following PPF, baseline fEPSPs were obtained by administering single pulses of stimulation at 0.07 Hz until a stable baseline was achieved for 20 min. Potentiation was induced by 2 tetanus protocols: the HFS protocol consisted of 10 bursts of 20 pulses at 200 Hz with an interburst interval of 2 s (Commins et al. 1998b), while the LFS protocol consisted of 900 pulses delivered at 1 Hz for 15 min (Anderson et al. 2000). In all experiments, the baseline stimulation frequency was resumed following the tetanus and responses were recorded for 60 min after which another input–output curve and series of PPF ratios was generated as described above.
Acute Stress Protocol
Acute stress was accomplished by immobilizing rats in a Plexiglas restraint tube (544-RR, Fisher Scientific, Ottawa, ON, Canada) in a brightly lit novel room for 30 min. Rats exposed to acute stress consistently displayed high levels of urination, defecation, and piloerection. All rats were anesthetized immediately following acute stress and mounted on a stereotaxic frame in preparation for electrophysiological recordings (Fig. 1B).
Pharmacology
CORT (Sigma-Aldrich) was suspended in vegetable oil (Crisco) and injected (3 mg/kg; s.c.) 30 min prior to anesthesia. Rats were returned to their home cages for 30 min before being anesthetized (Fig. 1B). To examine the role of GR activation, some rats received an injection of vehicle (50:50 DMSO:95% ethanol; 2 ml/kg; s.c.) or an injection of the selective GR antagonist RU38486 (10 mg/kg; s.c.; Xu et al. 1998; Cazakoff and Howland 2010) 60 min prior to anesthesia. Vehicle- and RU38486-treated rats were then randomly assigned either to the stress or to a non-stress condition. Rats in the stress group received the acute stress protocol 30 min post-injection, whereas the rats in the non-stress condition remained in their home cages for 60 min before being anesthetized (Fig. 1B).
Corticosterone Assay
Plasma CORT levels were determined using tail blood samples collected from control, acute stress, and CORT groups at two distinct time points: immediately after anesthesia and before tetanization. Blood samples were then left to sit for 15–20 min at room temperature. Blood fractionation was achieved by spinning the blood samples at 2000 rotations per minute for 20 min using a centrifuge (Fisher Scientific, accuSpin Micro 17). The supernatant was then collected and stored in plastic tubes at −80°C until subsequent analysis. All samples were analyzed using the commercially available enzyme-linked immunosorbent assay (ELISA) CORT kit (ADI-900-097; Enzo Life Sciences, Farmingdale, NY, United States of America) according to the manufacturer’s specifications.
Histology
Following the recordings, electrolytic lesions were created by administering direct current (0.2 mA, 20 s) through each of the electrodes. Rats were then transcardially perfused with 30 mL of physiological saline and their brains removed and stored in a 10% formalin–10% sucrose solution. Brains were sectioned using a sliding microtome and electrode placements were verified (Fig. 1C) with the aid of a rat brain atlas (Paxinos and Watson 1997) and a compound light microscope (Fisher Scientific).
Statistical Analysis
Statistical tests were conducted using SPSS Version 18 for Windows and Graphpad Prism 5.0. All descriptive values are reported as mean ± standard error of the mean. P values of less than or equal to 0.05 were considered statistically significant. PPF is expressed as percent change in the second evoked fEPSP slope relative to the first fEPSP slope. For comparisons of pre- and post-tetanus PPF values, difference scores were calculated as post-tetanus PPF minus pre-tetanus PPF. One sample t-test revealed that significant PPF was only elicited reliably for trials with 25 and 50 ms IPIs (P < 0.05). Thus, we only report the 25 and 50 ms IPIs. Omnibus repeated measures ANOVA revealed no significant effect of Tetanus (F1,52 = 0.523, P = 0.473); therefore, PPF data were combined for HFS and LFS groups in all subsequent analyses. The magnitude of long-term plasticity was normalized and expressed as the percent change in fEPSP slope from baseline (the 20 min of recording immediately prior to tetanization). For each group, comparisons between the average fEPSP slope for the last 5 min of baseline and the last 5 min of the 1-hour decay period were made using paired sample t-tests (P < 0.05 for all groups except the stress alone groups; statistics not shown). ANOVAs were used to determine differences between experimental conditions followed by post hoc comparisons using Tukey’s LSD where appropriate.
Results
Acute Stress and Acute Corticosterone Injections Elevate Circulating Corticosterone Levels
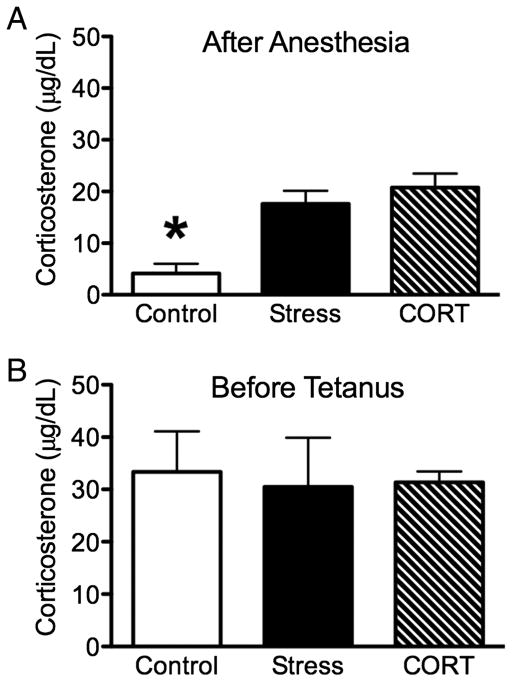
Plasma CORT levels were determined using tail blood samples collected at two time points (following anesthesia and prior to tetanization) from a subset of animals. Control rats displayed low levels of circulating hormone (4.17 ± 1.84 μg/dL; n = 4) relative to rats exposed to acute stress (17.56 ± 2.56 μg/dL; n = 5) and acute CORT injections (20.79 ± 2.70 μg/dL; n = 10) immediately following anesthesia (Fig. 2A). Rats exposed to acute stress and CORT injections displayed statistically equivalent levels of CORT (P > 0.05). Surprisingly, control rats displayed an increase in basal levels of circulating hormone (33.36 ± 7.75 μg/dL) prior to tetanization, as did rats exposed to acute stress (30.46 ± 9.45 μg/dL) and acute CORT injections (31.37 ± 2.10 μg/dL; Fig. 2B). These results were confirmed by a significant main effect of time [F1,16 = 25.41, P = 0.005], while the group by time interaction approached significance (F1,16 = 3.35, P = 0.059). When data from the two time points were analyzed separately, a significant effect of group was found for the initial plasma levels following anesthesia (F2,19 = 7.16, P = 0.006) but not subsequent measurements taken prior to tetanization (F2,19 = 0.117, P = 0.89). Post hoc comparisons revealed that the control group differed significantly from both stress and CORT groups (P < 0.05) immediately following anesthesia.
Figure 2. Corticosterone (CORT) Assay.
(A) Plasma CORT levels taken immediately after anesthesia for control, acute stress, and CORT groups. (B) Plasma CORT levels taken prior to tetanization for control, acute stress, and CORT groups. Asterisk denotes P < 0.05 relative to all other groups.
Exposure to Acute Stress, But Not Corticosterone, Disrupts Paired-pulse Facilitation Within the Cornu Ammonis1–Subiculum Pathway
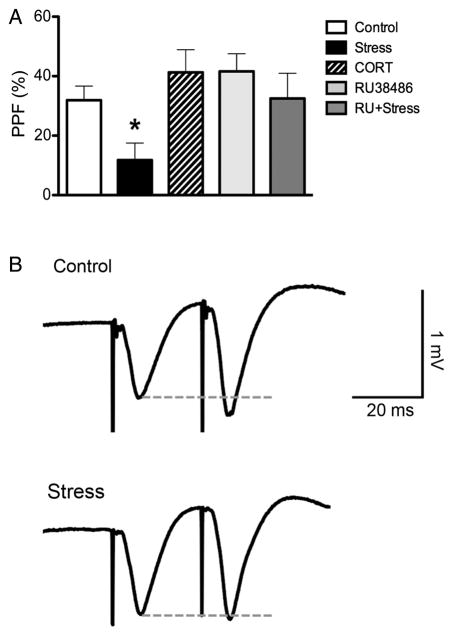
In the present experiments, the effects of acute stress and CORT were examined on short-term plasticity using initial pre-tetanus PPF values (averaged 25 and 50 ms IPIs). Significant PPF was observed for the control (30.44 ± 4.69%; n = 17) and CORT (41.61 ± 5.98%; n = 10) groups, whereas the stress group (11.76 ± 5.78%; n = 11) did not display significant PPF (Fig. 3A,B; 1 sample t-test not shown). An ANOVA revealed a significant effect of group for the pre-tetanus PPF (F2,37 = 6.24, P = 0.005). Post hoc comparisons revealed that control and CORT groups displayed significantly higher levels of PPF than the stress group (P < 0.05). These results demonstrate that exposure to acute stress, but not CORT injections, disrupts PPF in the CA1–SUB pathway.
Figure 3. Initial paired-pulse facilitation (PPF).
(A) Acute stress, but not corticosterone injections, disrupts pre-tetanus PPF values for the averaged 25 and 50 ms inter-pulse intervals, an effect reversed by pre-treatment with RU38486 (10 mg/kg). Asterisk denotes P < 0.05 relative to all other groups. (B) Representative fEPSP traces for control and stress conditions.
Next, we used the selective GR antagonist RU38486 to elucidate the role of GR activation in the effects of acute stress on PPF. Robust PPF was observed in rats treated with RU38486 (32.52 ± 8.84%; n = 11) as well as rats treated with RU38486 and exposed to acute stress (41.30 ± 7.98%; n = 11; Fig. 3A). A 2 × 2 ANOVA with stress and RU38486 revealed a significant main effect of RU38486 (F1,46 = 5.81, P = 0.020) as well a significant stress by RU38486 interaction (F1,46 = 4.39, P = 0.042). Post hoc comparisons revealed that the stress group displayed significantly lower PPF than the other groups (P < 0.05).
High-frequency Stimulation of Cornu Ammonis 1 Induces Long-Term Potentiation in Subiculum That is Disrupted Following Exposure to Acute Stress, But not Corticosterone Injections, in a Glucocorticoid Receptor-dependent Manner
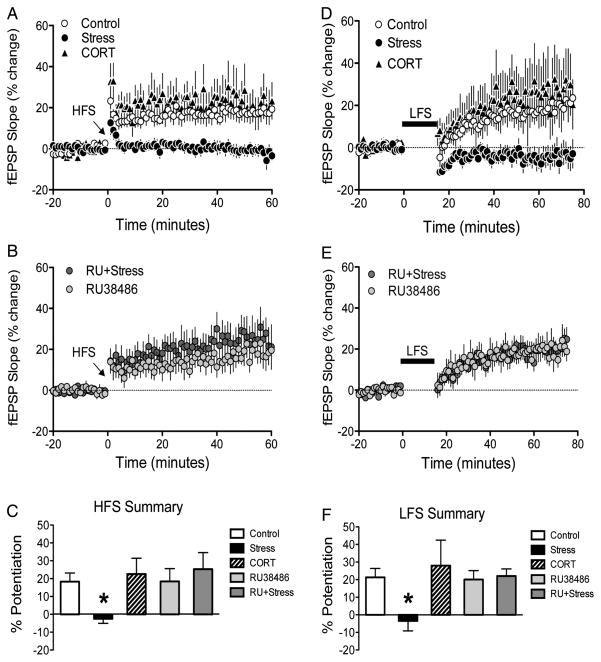
We observed that HFS of the CA1–SUB pathway induces reliable LTP with a magnitude of 18.31 ± 4.46% (n = 7; Fig. 4A). Exposure to 30 min of restraint stress completely abolished this synaptic potentiation (−4.33 ± 2.47%; n = 5), while rats treated with acute CORT injections had similar patterns of plasticity as controls (22.55 ± 8.51; n = 5; Fig. 4A). These impressions were confirmed by a significant effect of Group (F2,16 = 6.26, P = 0.011). Post hoc analyses revealed that the acute stress alone group displayed significantly lower potentiation than the other groups (P < 0.05). Next, we examined the role of GR activation in the disruptive effects of acute stress using RU38486. Rats injected with RU38486 displayed significant LTP (18.81 ± 8.06%; n = 5), as did rats that received both RU38486 treatment and exposure to acute stress (25.28% ± 8.91; n = 6; Fig. 4B). A 2 × 2 ANOVA with stress and RU38486 as factors revealed a significant main effect of RU38486 (F1,19 = 5.55, P = 0.029) and a significant stress by RU38486 interaction (F1,19 = 5.18, P = 0.035). Post hoc comparisons revealed that the stress group displayed significantly lower PPF than the other groups (P < 0.05). These results demonstrate that HFS-induced potentiation is significantly disrupted following exposure to acute stress, but not CORT injections, and that this disruptive effect is mediated via GR activation (Fig. 4C).
Figure 4. Long-term plasticity.
(A) High-frequency stimulation-induced LTP is present in control (open circle) and CORT (black triangles) rats but not acutely stressed rats (black circles). (B) High-frequency stimulation-induced LTP following RU38486 treatment (light gray circles) and RU38486-treated rats exposed to acute stress (dark gray circles). (C) Summary of the effects of acute stress, CORT, and RU38486 treatment on HFS-induced long-term plasticity. (D) Low-frequency stimulation-induced late-developing potentiation is present in control (open circles) and CORT (black triangles) but not acutely stressed rats (black circles). (E) Low-frequency stimulation-induced late-developing potentiation following RU38486 treatment (light gray circles) and RU38486 treated rats exposed to acute stress (dark gray circles). (F) Summary of the effects of acute stress, CORT, and RU38486 treatment on LFS-induced long-term plasticity. Asterisk denotes P < 0.05 relative to all other groups.
Low-frequency Stimulation of Cornu Ammonis 1 Induces Late-Developing Potentiation in Subiculum That is Disrupted Following Exposure to Acute Stress, But Not Corticosterone, in a Glucocorticoid Receptor-dependent Manner
LFS of the CA1–SUB pathway induced late-developing potentiation (21.30 ± 4.97%; n = 8) in SUB that was disrupted following exposure to 30 min of restraint stress (−4.68 ± 7.30%; n = 6). Interestingly, rats that received acute CORT injections demonstrated similar patterns of potentiation as control animals (28.25 ± 14.81%; n = 5; Fig. 4D). An ANOVA revealed a significant effect of Group (F2,19 = 3.82, P = 0.044). Post hoc analyses revealed that the acute stress alone group displayed significantly lower potentiation than the other groups (P < 0.05). Again, we examined the role of GR activation in the disruptive effects of acute stress using RU38486. Rats injected with RU38486 displayed significant potentiation (20.17 ± 4.70%; n = 6), as did rats that received both RU38486 treatment and exposure to acute stress (22.04 ± 4.29%; n = 5; Fig. 4E). A 2 × 2 ANOVA with stress and RU38486 as factors revealed significant main effects of stress (F1,21 = 4.85, P = 0.039), RU38486 (F1,21 = 5.46, P = 0.029), and a significant stress by RU38486 interaction (F1,21 = 6.47, P = 0.019). Post hoc comparisons revealed that the stress group displayed significantly lower PPF than the other groups (P < 0.05). In summary, similar to the HFS-induced LTP within the CA1–SUB pathway, the LFS-induced late-developing potentiation is significantly disrupted following exposure to acute stress, but not CORT injections, and this disruptive effect is also mediated via GR activation (Fig. 4F).
Reduced Paired-pulse Facilitation in the Cornu Ammonis1–Subiculum Pathway Following Long-Term Synaptic Potentiation is Abolished by Acute Stress
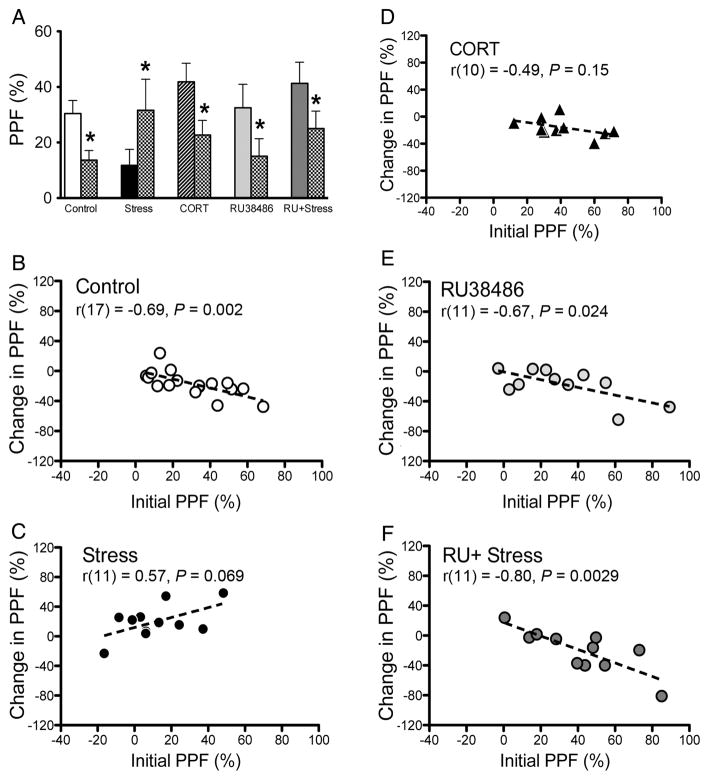
The magnitude of PPF (averaged 25 and 50 ms IPIs) changed following the induction of HFS- and LFS-induced potentiation, as measured after the 60 min decay following tetanization. A repeated measures ANOVA on the pre-tetanus and post-tetanus PPF values revealed a significant main effect of time (F1, 46 = 8.51, P = 0.005), a time by stress interaction (F1, 46 = 12.95, P = 0.001), a time by RU38486 interaction (F1, 46 = 12.19, P = 0.001), as well as a time by stress by RU38486 interaction (F1, 46 = 11.37, P = 0.002). Difference scores revealed that PPF was decreased in control (−16.82 ± 4.16%), CORT (−17.44 ± 0.76%), RU38486 (−17.45 ± 6.84%), and RU + Stress (−16.26 ± 3.32%) groups, while it was significantly increased in the stress alone group (19.84 ± 7.16%; Fig. 5A). Post hoc comparisons on the PPF difference scores revealed that the change in PPF following tetanization was significantly different in the stress-alone group compared with all other groups (P < 0.05). These results demonstrate that the reduced PPF following the induction of potentiation is abolished by exposure to acute stress, but not CORT injections, and that antagonism of GRs eliminates this effect.
Figure 5. Changes in paired-pulse facilitation (PPF) following tetanic stimulation.
(A) Initial pre-tetanus PPF values (left bars) compared with post-tetanus PPF values (chequered bars). Values are the average PPF for the 25 and 50 ms inter-pulse intervals. Asterisk denotes P < 0.05 relative to pre-tetanus value. (B–F) Correlation and regression plots showing changes in PPF following the induction of potentiation across different experimental conditions. The initial pre-tetanus PPF is plotted on the x-axis and the PPF difference scores are plotted on the y-axis.
Changes in PPF exhibited an inverse relationship with initial pre-tetanus PPF values for control (Fig. 5B; r2 = 0.48, P = 0.002, y = −0.59x + 1.30), RU38486 (Fig. 5E; r2 = 0.45, P = 0.024, y = −0.52x – 0.60), and RU + stress (Fig. 5F; r2 = 0.64, P = 0.003, y = −0.90x + 17.53) groups but not stress (Fig. 5C; r2 = 0.32, P = 0.069, y = 0.67x + 11.95), or CORT (Fig. 5D; r2 = 0.24, P = 0.15, y = −0.35x - 1.50) groups. These data indicate that larger initial PPF is associated with decreases in PPF, while smaller initial PPF is associated with little to no change in PPF following the induction of potentiation in the CA1–SUB pathway.
Discussion
The present experiments provide novel results regarding the effects of acute stress on patterns of both short- and long-term synaptic plasticity within the CA1–SUB pathway. Exposure to 30 min of restraint stress, but not acute treatment with CORT, disrupted PPF (Fig. 3) and two forms of long-term plasticity (Fig. 4). Critically, both the stress and CORT manipulations resulted in similar plasma levels of CORT before the surgical procedure was initiated (Fig. 2). Acute stress also reversed the typically observed reduction in PPF at this synapse following the induction of long-term synaptic potentiation (Fig. 5). We provide strong evidence that the disruptive effects of acute stress are mediated by GR activation, as the disruptions are fully blocked by pre-treatment with the selective GR antagonist RU38486. Taken together, our results demonstrate for the first time the necessity, but not sufficiency, of GR activation for the changes in subicular plasticity caused by acute stress.
Acute Stress and Corticosterone Injections Elevate Circulating Corticosterone Levels
Plasma CORT measurements were taken from animals in all three groups near the same time of day (first 4 h of the light cycle); therefore, we are confident that basal levels of CORT were similar between the groups and near the levels of the controls reported in Figure 2A (~4 μg/dL). Plasma levels of circulating CORT are well known to increase in response to acute stress (McEwen 1994; Habib et al. 2001; Ulrich-Lai and Herman 2009). In this study, exposure to acute stress significantly enhanced circulating CORT relative to control levels as measured immediately after anesthesia (Fig. 2A). In order to test the effects of elevated CORT alone on subicular synaptic plasticity, we injected CORT systemically and found that a dose of 3 mg/kg produced comparable levels of circulating hormone to acute stress shortly following anesthesia (Fig. 2A), as has been shown previously in rats (de Quervain et al. 1998). Unexpectedly, CORT measurements made after surgery and prior to tetanization revealed an increase in circulating levels within the control group (Fig. 2B), as well as sustained elevations in the CORT and acute stress groups. Previous studies have noted post-operative elevations in circulating CORT in rats (Goldkuhl et al. 2010; Nyuyki et al. 2012), and it is therefore likely that the surgery itself is responsible for the observed CORT elevations. Further experimentation is required to better monitor these hormonal changes over time.
Effects of Acute Stress on Paired-pulse Facilitation in Dorsal Subiculum
In this study, we found evidence for acute stress-induced disruptions of PPF in the CA1–SUB pathway before tetanic stimulation, which were prevented by pre-treatment with RU38486. The magnitude of PPF of the control group at 25 and 50 ms IPIs is similar to that reported in other in vivo studies (~30–50%; Commins et al. 1998a, 2001; Commins and O’Mara 2000), although it should be noted that we did not observe significant PPF at longer intervals (100 and 200 ms). Previous studies are contradictory regarding the effects of acute stress on initial subicular PPF before tetanus. One in vivo study reported no change in PPF following exploration of a novel box for 30 min (Commins and O’Mara 2000), an experience used previously as a stressor in studies of CA1 synaptic plasticity (Xu et al. 1997). Another in vivo study demonstrated significantly disrupted PPF (50 ms IPI) 4 h following lipopolysaccharide treatment (Commins et al. 2001). It is difficult to specify why the study of Commins and O’Mara (2000) failed to demonstrate a significant effect, although differences between the stressors may be involved. Our results also indicate that increased circulating CORT is not sufficient to alter PPF; therefore, other factors related specifically to stress such as heightened emotionality or arousal may be critical for synaptic plasticity to be disrupted (see below). GR-dependent disruptions of PPF have also been reported in vivo in the CA3–CA1 pathway following acute stress induced by exposure to an elevated platform (Cazakoff and Howland 2010).
Acute Stress and Long-Term Plasticity in Dorsal Subiculum
Two forms of long-term synaptic potentiation have been shown to exist within the CA1–SUB pathway in vivo following either HFS (Commins et al. 1998b) or LFS (Anderson et al. 2000) protocols. Interestingly, these forms of synaptic plasticity are thought to rely upon distinct induction mechanisms (Fidzinski et al. 2008; Wozny et al. 2008) as demonstrated using patch-clamp electrophysiology. Our data demonstrate that acute stress disrupts both forms of synaptic plasticity and that these disruptions are prevented by pre-treatment with RU38486. Specifically, HFS-induced LTP is disrupted following exposure to acute restraint stress, which we show is dependent on GR activation (Fig. 4C), similar to in vivo reports from CA1 (Xu et al. 1998; Cazakoff and Howland 2010). In SUB, LFS-induced late-developing potentiation also undergoes GR-dependent disruption following exposure to acute stress (Fig. 4F). This is in dramatic contrast to the typically observed enabling of LTD following LFS in CA1 (Xu et al. 1997; Wong et al. 2007). Interestingly, neither the HFS-induced LTP nor the LFS-induced late-developing potentiation suffered disruptions following acute CORT administration. Taken together, our data provide novel and compelling evidence that GR-activation is a necessary, but not a sufficient, component of the observed stress-induced disruptions on patterns of long-term plasticity within the CA1–SUB pathway.
Although a limited number of studies have examined long-lasting changes in synaptic strength within the CA1–SUB pathway in the context of acute stress, disruptions in HFS-induced LTP within this pathway have previously been reported in vivo in rats exposed to the bacterial endotoxin lipopolysaccharide, which is thought to activate similar neural and endocrine responses as those activated by acute stress exposure (Commins et al. 2001). Moreover, exposure to a novel open field environment has been shown to facilitate the induction of LTD within this pathway following a 10 Hz stimulation protocol in an anesthetized preparation (Commins and O’Mara 2000). However, to our knowledge, we are the first to examine and provide evidence for a mechanism by which acute stress disrupts HFS-induced LTP and LFS-induced late-developing potentiation within this pathway.
A Presynaptic Locus for Potentiation in the Dorsal Subiculum
Whether the mechanisms governing synaptic plasticity are expressed pre- or post-synaptically is a topic of rigorous debate among researchers (Krueger and Fitzsimonds 2006; Lisman 2009). It has been argued that changes in PPF following the induction of potentiation suggest the involvement of a presynaptic locus to the expression of the synaptic plasticity in question (Schulz et al. 1994). Commins et al. (1998a) have previously demonstrated reductions in PPF following LTP over a small range of IPIs (30–100 ms) in the CA1–SUB pathway in vivo. The present results demonstrate similar changes in PPF for 25 and 50 ms IPIs following LTP and extend these findings to include the late-developing potentiation (Fig. 5A). These results suggest a presynaptic locus exists for both forms of potentiation within the CA1–SUB pathway. Specifically, we show significant decreases in PPF values following the induction of potentiation (Fig. 5), which may indicate increase in transmitter release following the first stimulation (Zucker and Regehr 2002). Interestingly, rats exposed to acute restraint stress displayed significantly lower levels of pre-tetanus PPF, whereas an increase in PPF following long-term synaptic potentiation was observed (Fig. 5A). Importantly, blocking GR activation abolished these effects of acute stress on PPF before and after the tetanus. The increase in PPF following tetanization in the acute stress group may be attributed to either the tetanic stimulation itself or the transient nature of the stress effects on synaptic release probability. Thus, our data thus provide evidence that acute stress may trigger an initial positive shift in the release probability at hippocampal output synapses (Karst et al. 2005).
Functional Implications
It is well established that exposure to acute stress disrupts forms of synaptic plasticity within the hippocampus both in vivo and in vitro (Shors and Thompson 1992; Diamond et al. 1994; Kim et al. 1996; Xu et al. 1997; Kim and Diamond 2002; Yang et al. 2004, 2005; Cazakoff and Howland 2010; Chen et al. 2010) as well as memory performance on a variety of hippocampal-dependent tasks (Diamond and Rose 1994; Kim and Diamond 2002; Wong et al. 2007; Howland and Wang 2008; Park et al. 2008; Cazakoff et al. 2010). Although GR activation is necessary for the acute stress-induced disruptions in plasticity, several lines of evidence suggest that elevated circulating CORT is not a sufficient physiological parameter for disrupting hippocampal processing (Kim and Diamond 2002). For example, inactivation of the amygdala during acute stress exposure prevents stress-induced disruption of hippocampal LTP in vitro and spatial learning (Kim et al. 2005), despite the fact that circulating plasma levels of CORT are statistically similar as rats with intact amygdalae (Kim et al. 2001). In addition, Diamond and colleagues demonstrated that acute predatory stress, but not other behavioral approaches capable of elevating CORT, such as exposure to a sexually receptive female, disrupts memory performance on a hippocampal-dependent task (Woodson et al. 2003).
On the basis of the previous empirical work examining the dissociation between elevated CORT and fear-provoking stressors, it has been suggested that heightened emotionality or arousal, involving the amygdala, plays an integral role in the stress-inducible disruptions of hippocampal processing (Kim and Diamond 2002; McGaugh 2004; Park et al. 2006). Importantly, amygdalo-hippocampal bundles project directly to CA1 and SUB while indirect projections via the EC to numerous regions of the hippocampal formation have also been demonstrated (Pikkarainen et al. 1999). Thus, there are numerous routes through which the amygdala influences hippocampal information processing following stressful circumstances (Kim and Diamond 2002). Our data fit well into these observations and it may be the case that stress-induced emotionality and heightened arousal, thereby recruiting the amygdala, is a necessary and concurrent component to the observed stress-induced disruptions of subicular plasticity.
In conclusion, the present experiments highlight the susceptibility of subicular plasticity to acute stress and demonstrate a mechanism by which this hippocampal output structure undergoes synaptic modification under challenging circumstances. Moreover, we show for the first time that GR-activation is a necessary, but not a sufficient, physiological parameter for the effects of acute stress of subicular plasticity. The specific downstream signaling mechanisms, involvement of the amygdala, and behavioural consequences of synaptic remodeling in SUB following exposure to acute stress remain important and open questions.
Acknowledgments
This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada to J.G.H. J.G.H. is a National Alliance for Research on Schizophrenia and Depression Young Investigator and a Canadian Institutes of Health Research (CIHR) New Investigator. M.J.M. is the recipient of CIHR Masters Award and University of Saskatchewan Dean’s Scholarship. The authors would like to thank Brittany N. Cazakoff, John R. Gordon, Wojciech Dawicki, and Brittany Klischuk for technical assistance with these experiments.
Footnotes
Conflict of Interest: None declared.
References
- Andersen P, Morris R, Bliss T, Amaral D, O’Keefe J. The Hippocampus Book. New York: Oxford University Press US; 2006. [Google Scholar]
- Anderson M, Commins S, O’Mara SM. The effects of low frequency and two-pulse stimulation protocols on synaptic transmission in the CA1–subiculum pathway in the anaesthetized rat. Neurosci Lett. 2000;279:181–184. doi: 10.1016/s0304-3940(99)00996-9. [DOI] [PubMed] [Google Scholar]
- Behr J, Wozny C, Fidzinski P, Schmitz D. Synaptic plasticity in the subiculum. Prog Neurobiol. 2009;89:334–342. doi: 10.1016/j.pneurobio.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Boeijinga PH, Boddeke HWGM. Activation of 5-HT1B receptors suppresses low but not high frequency synaptic transmission in the rat subicular cortex in vitro. Brain Res. 1996;721:59–65. doi: 10.1016/0006-8993(96)00149-7. [DOI] [PubMed] [Google Scholar]
- Cazakoff BN, Howland JG. Acute stress disrupts paired pulse facilitation and long-term potentiation in rat dorsal hippocampus through activation of glucocorticoid receptors. Hippocampus. 2010;20:1327–1331. doi: 10.1002/hipo.20738. [DOI] [PubMed] [Google Scholar]
- Cazakoff BN, Johnson KJ, Howland JG. Converging effects of acute stress on spatial and recognition memory in rodents: a review of recent behavioural and pharmacological findings. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:733–741. doi: 10.1016/j.pnpbp.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Chen C-C, Yang C-H, Huang C-C, Hsu K-S. Acute stress impairs hippocampal mossy fiber-CA3 long-term potentiation by enhancing cAMP-specific phosphodiesterase 4 activity. Neuropsychopharmacology. 2010;35:1605–1617. doi: 10.1038/npp.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- Commins S, Gigg J, Anderson M, O’Mara SM. Interaction between paired-pulse facilitation and long-term potentiation in the projection from hippocampal area CA1 to the subiculum. NeuroReport. 1998a;9:4109–4113. doi: 10.1097/00001756-199812210-00019. [DOI] [PubMed] [Google Scholar]
- Commins S, Gigg J, Anderson M, O’Mara SM. The projection from hippocampal area CA1 to the subiculum sustains long-term potentiation. NeuroReport. 1998b;9:847–850. doi: 10.1097/00001756-199803300-00015. [DOI] [PubMed] [Google Scholar]
- Commins S, O’Mara SM. Interaction between paired-pulse facilitation, low-frequency stimulation, and behavioral stress in the pathway from hippocampal area CA1 to the subiculum: dissociation of baseline synaptic transmission from paired-pulse facilitation and depression of the same pathway. Psychobiology. 2000;28:1–11. [Google Scholar]
- Commins S, O’Neill LA, O’Mara SM. The effects of the bacterial endotoxin lipopolysaccharide on synaptic transmission and plasticity in the CA1–subiculum pathway in vivo. Neuroscience. 2001;102:273–280. doi: 10.1016/s0306-4522(00)00498-x. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and gluco-corticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE. Differential but complementary mnemonic functions of the hippocampus and subiculum. Neuron. 2004;42:465–476. doi: 10.1016/s0896-6273(04)00195-3. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennet MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Fleshner M, Rose GM. Psychological stress repeatedly blocks hippocampal primed burst potentiation in behaving rats. Behav Brain Res. 1994;62:1–9. doi: 10.1016/0166-4328(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Rose GM. Stress impairs LTP and hippocampal-dependent memory. Ann NY Acad Sci. 1994;746:411–414. doi: 10.1111/j.1749-6632.1994.tb39271.x. [DOI] [PubMed] [Google Scholar]
- Fidzinski P, Shor O, Behr J. Target-cell-specific bidirectional synaptic plasticity at hippocampal output synapses. Eur J Neurosci. 2008;27:1111–1118. doi: 10.1111/j.1460-9568.2008.06089.x. [DOI] [PubMed] [Google Scholar]
- Foy MR, Stanton ME, Levine S, Thompson RF. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behav Neural Biol. 1987;48:138–149. doi: 10.1016/s0163-1047(87)90664-9. [DOI] [PubMed] [Google Scholar]
- Goldkuhl R, Klockars A, Carlsson HE, Hau J, Abelson KSP. Impact of surgical severity and analgesic treatment on plasma corticosterone in rats during surgery. Eur Surg Res. 2010;44:117–123. doi: 10.1159/000264962. [DOI] [PubMed] [Google Scholar]
- Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinology and Metabolism Clinics of North America. 2001;30:695–728. doi: 10.1016/s0889-8529(05)70208-5. [DOI] [PubMed] [Google Scholar]
- Howland JG, Wang YT. Chapter 8 Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog Brain Res. 2008;169:145–158. doi: 10.1016/S0079-6123(07)00008-8. [DOI] [PubMed] [Google Scholar]
- Huang Y, Kandel E. Theta frequency stimulation up-regulates the synaptic strength of the pathway from CA1 to subiculum region of hippocampus. Proc Natl Acad Sci USA. 2005;102:232–237. doi: 10.1073/pnas.0408368102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioural stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Koo JW, Lee HJ, Han J. Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. J Neurosci. 2005;25:1532–1539. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Han J, Packard MG. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger S, Fitzsimonds RM. Remodeling the plasticity debate: the presynaptic locus revisited. Physiology. 2006;21:346–351. doi: 10.1152/physiol.00013.2006. [DOI] [PubMed] [Google Scholar]
- Lisman JE. The pre/post LTP debate. Neuron. 2009;63:281–284. doi: 10.1016/j.neuron.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute efffects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RGM. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Massey PV, Bashir ZI. Long-term depression: multiple forms and implications for brain function. Trends Neurosci. 2007;30:176–184. doi: 10.1016/j.tins.2007.02.005. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Corticosteroids and hippocampal plasticity. Ann NY Acad Sci. 1994;746:134–142. doi: 10.1111/j.1749-6632.1994.tb39223.x. [DOI] [PubMed] [Google Scholar]
- Morris RG, Schenk F, Tweedie F, Jarrard LE. Ibotenate lesions of hippocampus and/or subiculum: Dissociating components of allocentric spatial learning. Eur J Neurosci. 1990;2:1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Nyuyki KD, Maloumby R, Reber SO, Neumann ID. Comparison of corticosterone responses to acute stressors: chronic jugular vein versus trunk blood samples. Stress. 2012:1–9. doi: 10.3109/10253890.2012.655348. Early Online. [DOI] [PubMed] [Google Scholar]
- O’Mara SM. Controlling hippocampal output: the central role of subiculum in hippocampal information processing. Behav Brain Res. 2006;174:304–312. doi: 10.1016/j.bbr.2006.08.018. [DOI] [PubMed] [Google Scholar]
- O’Mara SM. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. J Anat. 2005;207:271–282. doi: 10.1111/j.1469-7580.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mara SM, Commins S, Anderson M, Gigg J. The subiculum: a review of form, physiology and function. Prog Neurobiol. 2001;64:129–155. doi: 10.1016/s0301-0082(00)00054-x. [DOI] [PubMed] [Google Scholar]
- O’Mara SM, Sanchez-Vives MV, Brotons-Mas JR, O’Hare E. Roles for the subiculum in spatial information processing, memory, motivation and the temporal control of behaviour. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:782–790. doi: 10.1016/j.pnpbp.2009.03.040. [DOI] [PubMed] [Google Scholar]
- Park CR, Campbell AM, Woodson JC, Smith TP, Fleshner M, Diamond DM. Permissive influence of stress in the expression of a U-shaped relationship between serum corticosterone levels and spatial memory errors in rats. Dose–Response. 2006;4:55–74. doi: 10.2203/dose-response.004.01.005.Park. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CR, Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn Mem. 2008;15:271–280. doi: 10.1101/lm.721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1997. [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory bed nuclei of the amygdala to the hippocampal formation. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Potvin O, Lemay F, Dion M, Corado G, Doré FY, Goulet S. Contribution of the dorsal subiculum to memory for temporal order and novelty detection using objects, odors, or spatial locations in the rat. Neurobiol Learn Mem. 2010;93:330–336. doi: 10.1016/j.nlm.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Schulz PE, Cook EP, Johnston D. Changes in paired-pulse facilitation suggests presynaptic involvement in long-term potentiation. J Neurosci. 1994;14:5325–5337. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. Subicular place cells generate the same “map” for different environments: comparison with hippocampal cells. Behav Brain Res. 2006;174:206–214. doi: 10.1016/j.bbr.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Stressful experience and learning across the lifespan. Annu Rev Psychol. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Thompson RF. Acute stress impairs (or induces) synaptic long-term potentiation (LTP) but does not affect paired-pulse facilitation in the stratum radiatum of rat hippocampus. Synapse. 1992;11:262–265. doi: 10.1002/syn.890110311. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Strien NM, Cappaert NLM, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Witter MP. Connections of the subiculum of the rat: topography in relation to columnar and laminar organization. Behav Brain Res. 2006;174:251–264. doi: 10.1016/j.bbr.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen JH. The subiculum: cytoarchitectonically a simple structure, but hodologically complex. Prog Brain Res. 1990;83:47–58. doi: 10.1016/s0079-6123(08)61240-6. [DOI] [PubMed] [Google Scholar]
- Wong TP, Howland JG, Robillard JM, Ge Y, Yu W, Titterness AK, Brebner K, Liu L, Weinberg J, Christie BR, et al. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proc Natl Acad Sci USA. 2007;104:11471–11476. doi: 10.1073/pnas.0702308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson JC, Macintosh D, Fleshner M, Diamond DM. Emotion-induced amnesia in rats: working memory-specific impairment, corticosterone-memory correlation, and fear versus arousal effects on memory. Learn Mem. 2003;10:326–336. doi: 10.1101/lm.62903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozny C, Maier N, Schmitz D, Behr J. Two different forms of long-term potentiation at CA1-subiculum synapses. J Physiol. 2008;586:2725–2734. doi: 10.1113/jphysiol.2007.149203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Behavioral stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- Xu L, Holscher C, Anwyl R, Rowan MJ. Glucocorticoid receptor and protein/RNA synthesis-dependent mechanisms underlie the control of synaptic plasticity by stress. Proc Natl Acad Sci USA. 1998;95:3204–3208. doi: 10.1073/pnas.95.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. Behavioral stress enhances hippocampal CA1 long-term depression through the blockade of the glutamate uptake. J Neurosci. 2005;25:4288–4293. doi: 10.1523/JNEUROSCI.0406-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. Behavioral stress modifies hippocampal synaptic plasticity through corticosterone-induced sustained extracellular signal-regulated kinase/mitogen-activated protein kinase activation. J Neurosci. 2004;24:11029–11034. doi: 10.1523/JNEUROSCI.3968-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]