Abstract
Background
Growing evidence shows that metabolic syndrome (MetS) is already starting in childhood however there is no consensus regarding how to diagnose this condition in pediatric population. Studies in adults show that altered levels of specific micro-RNAs are related with components of the MetS.
Objective
We determined the plasma levels of four MetS-associated micro-RNAs (miR-126, miR-132, mir-145 and Let-7e) in 10 to 12 years old children with or without MetS traits.
Design
Pediatric subjects were selected from a cohort of 3325 school-age children, and clustered by the absence (control, n = 30), or the presence of 1 (n = 50), 2 (n = 41) or 3 (n = 35) MetS traits according to Cook´s criteria. Micro-RNAs were isolated from plasma, and levels of miR-126, miR-132, miR-145 and Let-7e were determined by Taqman qPCR.
Results
Regression analysis of the different MetS traits regarding the different miRNAs analyzed showed that Let-7e presented a negative association with HDL-C levels, but a positive correlation with the number of MetS traits. Levels of miR-126 presented a positive correlation with waist circumference, waist to hip ratio, BMI, and plasma triglycerides and VLDL-C. Levels of miR-132 showed a positive correlation with waist to hip ratio. Plasma levels of Let-7e were increased (~3.4 fold) in subjects with 3 MetS traits, and showed significant AUC (0.681; 95%CI = [0.58, 0.78]; p < 0.001) in the ROC analysis which were improved when miR-126 was included in the analysis (AUC 0.729; p < 0.001). In silico analysis of the interaction of proteins derived from mRNAs targeted by Let7 and miR-126 showed an important effect of both Let-7e and miR-126 regulating the insulin signaling pathway.
Conclusions
These results suggest that changes in the plasma levels of Let-7e and miR-126 could represent early markers of metabolic dysfunction in children with MetS traits.
Introduction
The metabolic syndrome (MetS) in adults is commonly defined as the concomitance of cardiometabolic alterations including central obesity, elevated fasting glucose, hypertriglyceridemia, low plasma HDL and arterial hypertension [1]. Due to its growing prevalence worldwide during the last decades efforts have been focused to unveil the underlying mechanisms that precede MetS. Initial studies aimed to discover the genetic basis of this disease; however evidence suggests that the MetS results from the interaction of genetic, life style, environmental and early life factors. In this context, compelling data shows that MetS risk is increased in subjects with altered fetal and early infancy growth, suggesting the premature establishment of MetS susceptibility [2].
Notably, an important increase in childhood obesity during the last years has emerged, leading to higher risk of MetS in these subjects [1]. At the present time there is no consensus whether children and adolescents could present MetS, and a clear criteria to define it in the pediatric population is lacking [1, 3]. Possible definitions have been suggested by Cook and colleagues [4], and the International Diabetes Federation (IDF) [1]which have proposed that the MetS can be considered in (1) children aged 6–10 years who present central obesity [defined as waist circumference (WC) ≥ 90th percentile] and have other relevant risk factors (i.e. family history of cardiometabolic disease), and in (2) children aged 10–16 years who are obese (defined as WC ≥ 90th percentile) and meet the adult metabolic syndrome criteria for triglycerides (TGs), HDL-cholesterol (HDL-C), blood pressure (BP), and glycaemia. However the consistency of these parameters used for the MetS in adults are in conflict with normal metabolic changes that take place during puberty and evade the fact that MetS risk factors are a continuum that begins with subtle alterations [3]. Altogether these data suggest the necessity for additional markers to improve the MetS diagnosis in the pediatric population.
During the last years plasma micro-RNAs (miRNAs) have emerged as potential biomarkers in diverse pathologic conditions due to their high stability in serum compared with other types of RNA [5]. Wide-screening studies in adult blood samples show that despite the presence of hundreds of circulating miRNAs, a few of them present altered levels in cardiovascular diseases and diabetes mellitus [6, 7]. Notably circulating levels of miR-126 levels are importantly reduced in subjects with impaired glucose tolerance or type 2 diabetes [8]. In contrast, Let-7e [9] and miR-145 [10, 11] are importantly increased in different vascular pathologies. Additionally an important role for miR-33b [12] and miR-132 [13] have been suggested in the development of obesity due to their participation in lipid and cholesterol metabolisms as well as significant expression in adipose tissue [14]. In order to determine whether MetS components are associated with altered levels of MetS-associated miRNAs, we determined the plasma levels of Let-7e, miR-33b, miR-126, miR-132 and miR-145 in 126 children with one or more MetS trait and 30 control subjects.
Methods
Ethics statement
This study was conducted according to the principles expressed in the “Declaration of Helsinki”. Parents or children representatives signed an informed consent form, and boys/girls an informed acceptance form. The study was approved by the Ethic committee of the School of Medicine of the Pontificia Universidad Católica de Chile, and Fondo Nacional de Desarrollo Científico y Tegnológico de Chile (FONDECYT, Chile).
Sample
The sample selected was part of a larger study of cardiovascular risk factors in children 10 to 14 years. Briefly, in a first stage the original study evaluated the number of MS components for 3329 children among other measurements. In a second stage, the students who presented certain pre-established combinations of MS components were eligible to conform groups to apply more specific measurements; about 20% of the cases were normal in their nutritional diagnoses and were included as controls.
These two stages were successively performed during a 3 year period. Plasma samples from the participants of the last year were used to determine the levels of 4 microRNAs: miRNA Let-7e, miRNA 126, miRNA 132, and miRNA145. The samples were processed blinded to the knowledge of the nutritional status or the cardiovascular risk factors of the studied subjects.
Subjects
This study considered a subgroup of 156 subjects (age 10 to 12 y.o., 90 females and 66 males) conveniently selected due to the presence of a combination of MetS traits (presence of altered lipids and glucose plasma levels, central obesity and elevated blood pressure according to Cook´s criteria) and a control group without any MetS trait, from a retrospective non concurrent cohort study of school-age children from 20 public schools (total number 3325) [15] managed by the Municipality of Puente Alto, Santiago, Chile. Groups analyzed included 30 control subjects with no MetS traits as described below, and subjects with one (n = 50), two (n = 41), and three or more (n = 35) MetS traits. Socio-economic status was estimated using as a proxy the number of maternal years of formal education.
Metabolic syndrome criteria
The criteria of Cook et al. were used to define MS in the studied children [4], when at least three out of five of its components were present, as defined by the following cut-off points: waist circumference (WC) ≥ 90th percentile; blood pressure (BP), either systolic (SBP) or diastolic (DBP), ≥ 90th percentile, low high density lipoprotein cholesterol (HDL-C) ≤ 40 mg/dL; triglycerides (TG) > 110 mg/dL, and glycaemia (GLU) ≥100 mg/ dL. The evaluation at each school was made by a trained Nurse and a Nutritionist. Height and weight were measured using a stadiometer and a beam-scale Seca, with an accuracy of 50 g, while being barefoot and lightly clothed. The final height and weight were the respective averages of three measurements; in the latter, the average weight of their clothes was deducted. Waist circumference was measured with inextensible tape on the upper lateral border of the right ilium in the mid-axillary line at the end of an exhalation; three measurements were averaged and we used ≥ 90th percentile as cut-off value [16]. A blood pressure monitor (Critikon Dinamap Pro 100) was used according to international norms and the averages of three measurements of SBP and DBP were obtained and classified as abnormal using the ≥ 90 percentile of the same reference [17]. A voluntary private self-report of pubertal status was requested by observation of standardized photos of breast development in girls and genitalia in boys, including the presence of pubic hair. All cut-off values applied to the subjects in this study were referred to curves generated from the whole cohort previously studied.
Blood sample analysis
Venous blood samples under fasting conditions were collected for determination of glucose (Gluco-quant method, Glucose / Hexokinase, Roche Diagnostics GmbH, Mannheim) and insulin (immunoassay direct luminometer chemotherapy, ADVIA Centaur XP. Bayer HealthCare LLC, Kyowa Medex Co, Japan), this method measures concentrations of insulin from 0.5 to 300 mUI / mL (sensitivity 0.5 mUI / mL) with a coefficient of variation of 3.48% and 6.17% for concentrations of 23.51 mUI / mL and 62.49 mUI / mL, respectively. HOMA was calculated using the formula [(Glucose (mmol / L) x insulinaemia (mUI / mL)) / 22.5]. Triglycerides and HDL-C were analyzed on the Modular Analytics P-800 platform (Roche Diagnostics GmbH, Mannheim, Germany).
RNA extraction
Plasma samples for micro-RNA determination and analysis were conducted as doubled blinded to prevent biased results. Total RNA was extracted from 250 μL of plasma of supernatant fraction according to the supplier instructions (miRNAesy extraction kit, Qiagen). For normalization, 250 pmol of syn-cel-miR-39 miScript miRNA Mimic (Qiagen) were added to the supernatant 1 fraction just before the denaturation step from the organic extraction [10].
Reverse transcription
Sequence-specific reverse transcription of hsa-miR-33b, hsa-miR-126, hsa-miR-132, hsa-miR-145, hsa-Let-7e and Cel-miR-39 were achieved with TaqMan microRNA Reverse Transcription kit (Applied Biosystem).
Preamplification and amplification
A pre-amplification step was carried out before the qPCR analysis, using TaqMan PreAmp Master Mix and TaqMan Universal PCR Master Mix II, respectively (Applied Biosystem). Relative quantity was calculated by mean 2-ΔΔCt method regarding the synthetic control syn-cel-miR-39.
Let7e and miR-126 targets prediction and interaction
Prediction of targets for Let-7e and miR-126 were independently analyzed using the previously validated web software TargetScan 6.2 (http://www.targetscan.org) [18, 19] and miRanda (http://www.microrna.org) [20]. Selection of targeted messenger RNAs was based on the presence of a low “context score” and a high “probability of conserved targeting” [19] and the coincidence between the two algorithms. Following, the interaction of the proteins which mRNA are targeted by the analyzed miRNAs were evaluated with the web software String 9.1 (http://string-db.org/) [21, 22] using the highest confidence score (0.900) for protein-protein interaction, a maximum of 20 interactors and excluding all the proteins not regulated by the miRNAs analyzed.
Statistics
Differences in anthropometry (WC, waist to hip ratio, BMI), blood sample (TG, VLDL, HDL, glycaemia, insulin, HOMA-IR) and miRNA (Let7e, 126, 132 and 145) averages by number of MS components were evaluated using one way ANOVA for independent samples. Post hoc comparisons were assessed using Tamane or Bonferroni correction. Additionally, linear association between continuous variables was evaluated using Pearson’s correlation test. To describe a possible additive effect of each MS component, ANOVA test for independent samples and its post hoc test was used between miRNA averages for 3 groups: without a specific MS component, with only the specific MS component and with the MS component and at least one more. To evaluate the diagnostic accuracy, we generated receiver operating characteristic (ROC) curve for the different miRNA studied. The sensitivity and specificity which maximized the area under the ROC curve (AUC) were used to assess the diagnostic accuracy of miRNA in cases and controls. For all analysis SPSS 17.0 was used. Significant P-value was defined as <0.05.
Results
Characteristics of selected children
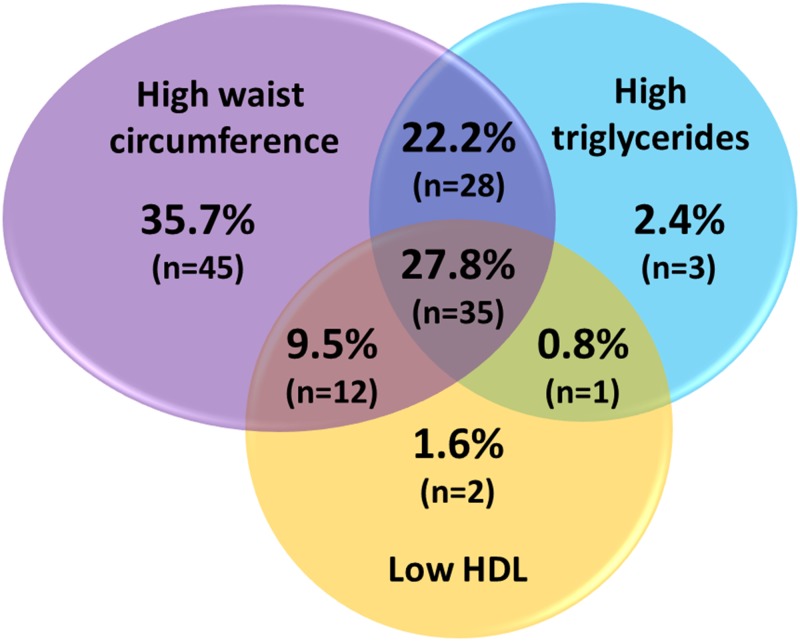
A comparable number of subjects (30–50) without or with one, two or three components of MetS were selected from a larger cohort. General characteristics of the children studied grouped by gender showed differences between girls and boys pubertal development, presence of overweight and altered triglycerides levels which were higher in girls than boys (Table 1). Additionally the presence of obesity and altered HOMA-IR were higher in the groups of boys compared to girls. Among the selected children with one or more MetS-related alterations, increased waist circumference as a single alteration was present in 45 of 50 subjects who showed 1 MetS trait (Fig 1). Conversely, altered plasma lipid levels (i.e. high triglycerides or low HDL) as a single MetS trait showed a low presence (5 of 50 subjects) and were mainly associated with increased waist circumference (75 of 126 showed high waist circumference together with altered lipid levels).
Table 1. General characteristics of the study group according to sex.
| Boys | Girls | Total | P-value | ||||
|---|---|---|---|---|---|---|---|
| N = 66 | N = 92 | N = 158 | |||||
| Age (years, mean [± SD]) | 11.63 | [0.92] | 11.65 | [0.90] | 11.64 | [0.90] | 0.920 |
| Pubertal (Tanner II-V, n [%]) | 54 | [81.8] | 91 | [98.9] | 145 | [91.8] | <0.001* |
| Weight (kg, mean [± SD]) | 57.3 | [12.1] | 55.3 | [11.4] | 56.2 | [11.7] | 0.285 |
| Height (cm, mean [± SD]) | 151 | [7.4] | 150 | [6.3] | 150.4 | [6.8] | 0.337 |
| BMI (mean [± SD]) | 24.9 | [4.0] | 24.4 | [4.0] | 24.6 | [4.0] | 0.432 |
| z-BMI (mean [± SD]) | 1.6 | [0.7] | 1.4 | [0.8] | 1.5 | [0.8] | 0.079 |
| Obesity (n [%]) | 46 | [71.9] | 41 | [44.6] | 87 | [55.8] | 0.001* |
| Overweight (n [%]) | 10 | [15.6] | 34 | [37.0] | 44 | [28.2] | 0.004* |
| WC ≥ 90th percentile (n [%]) | 52 | [81.3] | 68 | [73.9] | 120 | [76.9] | 0.285 |
| Triglycerides ≥ 110 mg/dL (n [%]) | 21 | [32.8] | 46 | [50.0] | 67 | [42.9] | 0.033* |
| HDL ≤ 40 mg/dL (n [%]) | 16 | [25.0] | 34 | [37.0] | 50 | [32.1] | 0.115 |
| HOMA-IR ≥ 90th percentile (n [%]) | 20 | [30.3] | 15 | [16.7] | 35 | [22.4] | 0.044* |
| MetS ≥3 components (n [%]) | 10 | [15.6] | 25 | [27.2] | 35 | [22.4] | 0.089 |
| RQ Let-7e (mean [± SD]) | 2.37 | [4.15] | 2.57 | [4.41] | 2.49 | [4.29] | 0.776 |
| RQ miR145 (mean [± SD]) | 1.57 | [2.72] | 1.89 | [4.53] | 1.74 | [3.87] | 0.646 |
| RQ miR132 (mean [± SD]) | 2.27 | [5.40] | 1.56 | [2.03] | 1.86 | [3.82] | 0.252 |
| RQ miR126 (mean [± SD]) | 1.78 | [2.28] | 2.07 | [3.23] | 1.95 | [2.87] | 0.535 |
WC, waist circumference; RQ, relative quantification.
Fig 1. Presence of different MetS traits in the children with cardiometabolic risk.
Venn diagram showing the percentage and total number (in parenthesis) of children with increased waits circumference, increased triglycerides and decreased HDL plasma levels alone and their combinations in the groups studied.
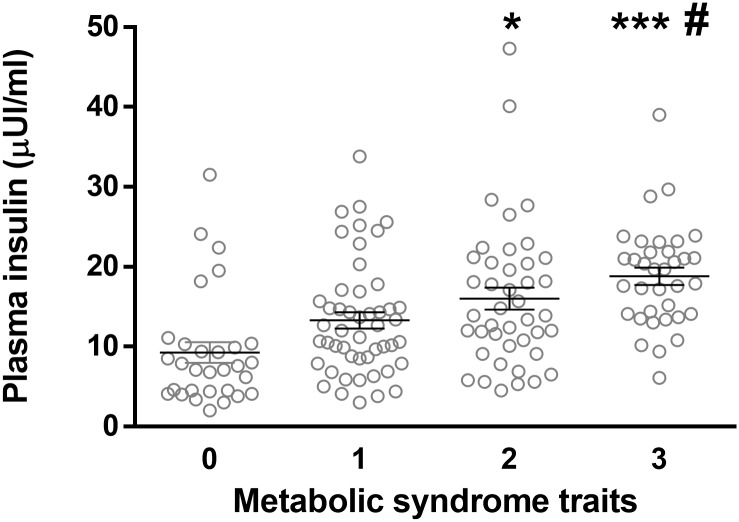
HDL-C in subjects with one or two MS traits were over the cut-off used (≤ 40 mg/dL) but significantly decreased (12% and 20%, respectively) compared with control subjects, furthermore subjects with two MetS present lower HDL-C levels than subjects with one trait. On the other hand, the presence of one or more MetS traits was associated with increased waist to hip ratio (~1.1 fold), body weight (~1.3 fold) as well as body mass index (~1.3 fold) (S1 Table). Among the subjects studied there were no differences in blood pressure (i.e. mean, systolic or diastolic) and fasting glycaemia. In contrast, fasting insulin plasma levels were not different between subjects with one MetS trait and controls, but increased in subjects with two (~1.7 fold) or three (~2.0 fold) MetS traits (Fig 2), and this was also observed for HOMA-IR index (data not shown).
Fig 2. Insulinaemia in children with and without MetS traits.
Insulinaemia in children without (0) and with one (1), two (2) or three (3) MetS traits. Values are mean ± SEM, *p < 0.05 and ***p < 0.001 vs. controls, and # p < 0.05 vs. one MetS trait, ANOVA.
Metabolic syndrome traits and micro-RNAs plasma levels
Four out of five miRNAs considered in this study were detected in plasma samples from children with or without MS traits. Namely Let-7e, miR-126, miR-132 and miR-145 were present, whilst miR-33b was not detectable in plasma, however it was found in a control sample of miRNAs from HEK-293 cells as reported (S1 Fig) [23, 24]. Mean plasma levels of Let-7e, miR-126, miR-132 and miR-145 were not different between female and male subjects (Table 1). Regression analysis of the different MetS traits analyzed as continuous variables regarding the different miRNAs analyzed (Table 2) showed that Let-7e presented a negative association with HDL-C levels, but a positive correlation with the number of MetS traits. Levels of miR-126 presented a positive correlation with waist circumference, waist to hip ratio, BMI, and plasma triglycerides and VLDL-C. Additionally plasma levels of miR-132 showed a positive correlation with waist to hip ratio. On the other hand, miR-145 did not show significant correlation with any trait determined (S2 Table). Similarly there were no correlation between body weight, insulinaemia, HOMA-IR and blood pressure with miRNAs analyzed (S2 Table).
Table 2. Significant correlations of different MetS traits with plasma levels of selected miRNAs.
| Let-7e | miR-126 | miR-132 | ||
|---|---|---|---|---|
| Waist circumference (cm) | Correlation coefficient | 0.210 | ||
| p | 0.008 | |||
| Waist to hip ratio (cm/cm) | Correlation coefficient | 0.220 | 0.183 | |
| p | 0.006 | 0.021 | ||
| BMI (kg/m 2 ) | Correlation coefficient | 0.185 | ||
| p | 0.020 | |||
| Triglycerides (mg/dL) | Correlation coefficient | 0.175 | ||
| p | 0.028 | |||
| HDL (mg/dL) | Correlation coefficient | -0.168 | ||
| p | 0.035 | |||
| VLDL (mg/dL) | Correlation coefficient | 0.173 | ||
| p | 0.030 | |||
| Number of MetS traits | Correlation coefficient | 0.255 | ||
| p | 0.001 |
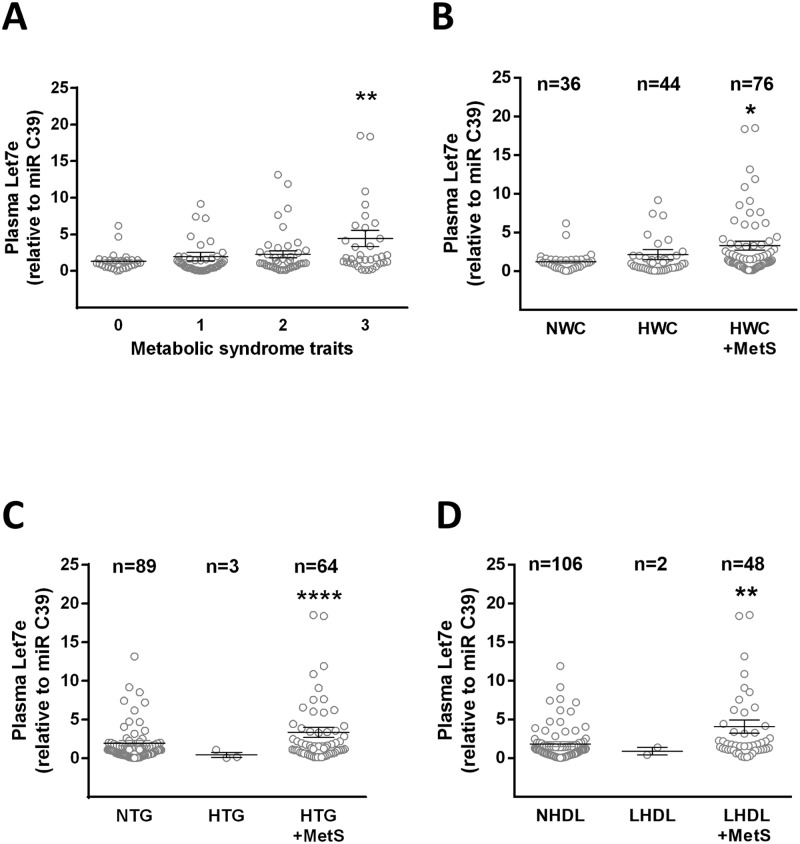
Comparison of Let-7e mean plasma levels according to the number of MetS traits showed an increased in subjects with three MetS traits (~4.5 fold) compared with control subjects (Fig 3A). Additionally Let-7e levels were compared in children with normal or altered waist circumference, high triglycerides and/or low HDL, independent of the presence of other MetS traits. Levels of Let-7e were not altered in subjects that only presented increased waist circumference compared with subjects with normal waist circumference, however were augmented (~3.3 fold) by the coexistence with any other MetS trait (Fig 3B). This was also observed in subjects with altered triglycerides or HDL levels (Fig 3C and 3D, respectively) where the addition of another MetS trait associated with increased levels of Let-7e. In contrast plasma levels of miR-126, miR-132 and miR-145 where not different among groups analyzed using this approach (data not shown).
Fig 3. Plasma levels of Let-7e according to the presence of MetS traits.
(A) Plasma levels of Let-7e according to the number of MetS traits, or (B) the presence of altered waist circumference (HWC), (C) elevated plasma triglycerides (HTG) and (D) low HDL plasma levels (LHDL) in combination with one other MetS trait (+MetS). Values are mean ± SEM, * p < 0.05, **p < 0.01, ****p < 0.0001 vs. controls, ANOVA.
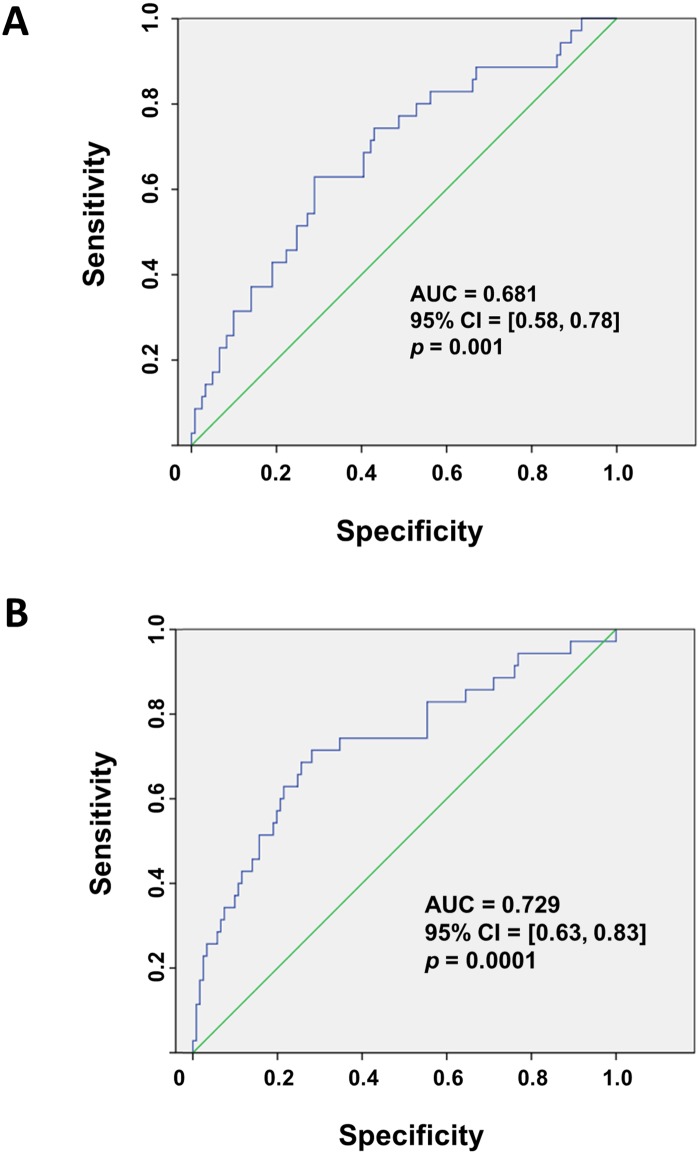
Further analysis of ROC curves to evaluate the potential diagnostic value of Let-7e and miR-126 levels for metabolic syndrome in children showed that Let-7e levels higher than 1.52 RQ presented a significant AUC (0.681) with a sensitivity of 62.9% and a specificity of 71.1% (Fig 4A), whilst miR-126 did not show a significant value (data not shown). However when RQs for Let-7e and miR-126 were considered together there was an improvement in the sensitivity (71.4%) and AUC (0.729) without changes in the specificity (71.9%) (Fig 4B).
Fig 4. Operating characteristic (ROC) curve for Let-7e and the combination with miR-126.
(A) ROC curve for plasma levels of Let-7e alone or (B) in combination with levels of miR-126 in pediatric subjects with and without metabolic syndrome according to Cook´s criteria.
In silico analysis of potential pathways compromised
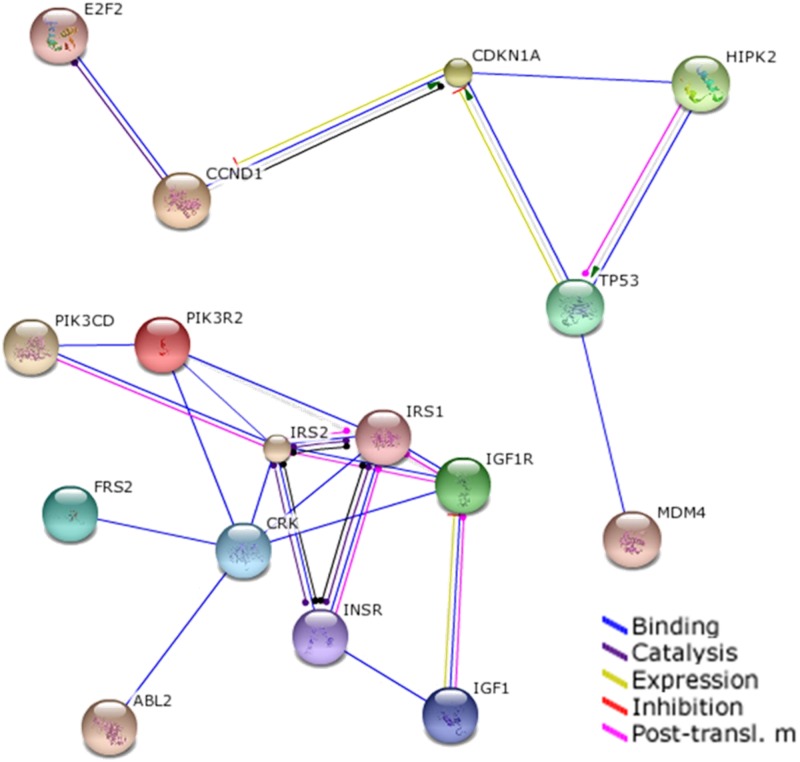
Since plasma levels of miR-126 showed a significant positive correlation with several MetS traits, and Let-7e was increased in subjects with increased waist circumference along with other trait risk, an in silico analysis searching potential targets for both miRNAs and their interactions was performed using two different algorithms. Analysis for potential targets of Let-7 and miR-126 in human showed 340 mRNA targets for Let-7, and 102 targets for miR-126 of which 46 were targeted by both miRNAs. In contrast interaction analysis using the highest confidence score values showed that 15 of 442 proteins (~3%) derived from these mRNA were potential interacting targets (Fig 5) which were mainly related with cell cycle, apoptosis, growth factors and metabolism (Table 3). Notably, most of these proteins are involved in the signaling pathway of insulin and IGF1 (Fig 5).
Fig 5. Convergence of Let-7e and miR-126-regulated signalling pathways at protein level.
In silico analysis of the interactions of proteins derived from mRNA targeted by Let-7e and miR-126. Interacting proteins are linked by colored lines which denote binding (blue), catalytic effect (purple), expression regulation (yellow), inhibition (red end) and post-translational modification (fuchsia).
Table 3. RNAs targeted by Let-7 and miR-126 whose proteins present functional interactions.
| Protein | Name | Function | Targeted by |
|---|---|---|---|
| ABL2 | Abelson tyrosine-protein kinase 2 | Cytoskeleton, cell motility | Let-7 |
| CCND1 | Cyclin D1 | Cell cycle/apoptosis | Let-7 |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A | Cell cycle/apoptosis | Let-7 |
| CRK | Adapter molecule crk | Cell cycle/apoptosis | miR-126 |
| E2F2 | E2F transcription factor 2 | Cell cycle/apoptosis | Let-7 |
| FRS2 | Fibroblast growth factor receptor substrate 2 | Growth factor/metabolism | Let-7 |
| HIPK2 | Homeodomain interacting protein kinase 2 | Cell cycle/apoptosis | Let-7 |
| IGF1 | Insulin-like growth factor 1 | Growth factor/metabolism | Let-7 |
| IGF1R | Insulin-like growth factor 1 receptor | Growth factor/metabolism | Let-7 |
| INSR | Insulin receptor | Growth factor/metabolism | Let-7 |
| IRS1 | Insulin receptor substrate 1 | Growth factor/metabolism | miR-126 |
| IRS2 | Insulin receptor substrate 2 | Growth factor/metabolism | Let-7/miR-126 |
| MDM4 | Mdm4 p53 binding protein homolog | Cell cycle/apoptosis | Let-7/miR-126 |
| PIK3CD | Phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit delta | Cell signaling kinase/phosphatase | miR-126 |
| PIK3R2 | Phosphoinositide-3-kinase, regulatory subunit 2 (beta) | Cell signaling kinase/phosphatase | Let-7/miR-126 |
| TP53 | Tumor protein p53 | Cell cycle/apoptosis | Let-7 |
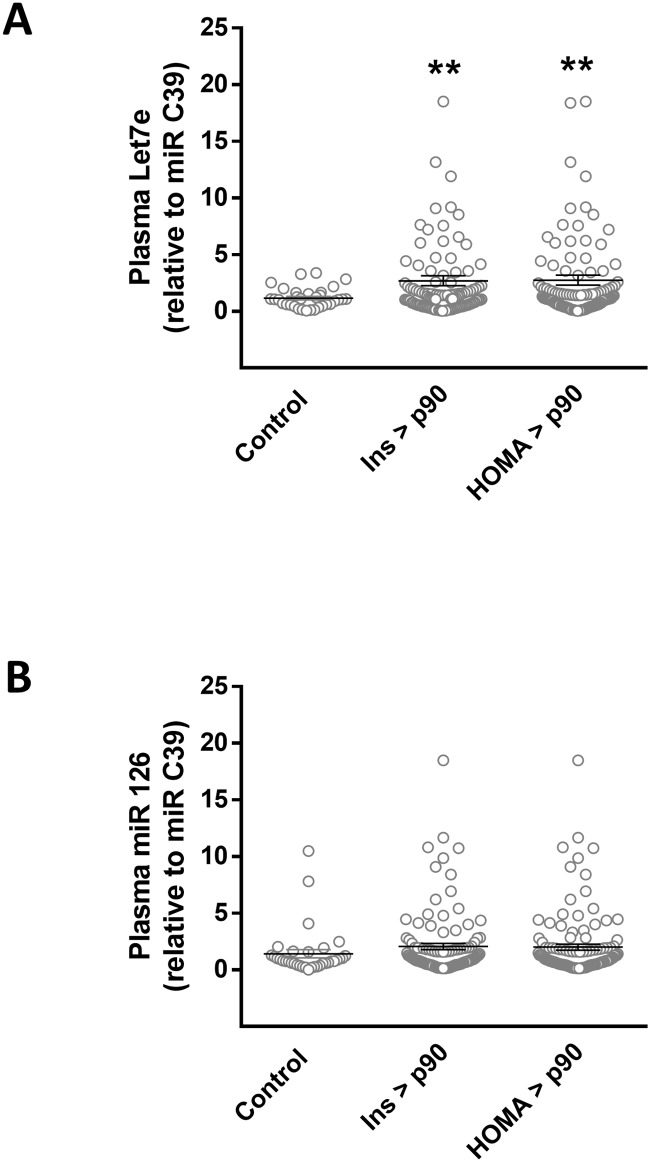
To address whether circulating Let-7e and miR-126 associated with markers of insulin resistance, plasma levels of both miRNAs were compared between controls and children with altered insulinaemia and HOMA-IR (over 90th percentile regarding the whole cohort previously studied [15]). Subjects with increased insulinaemia and HOMA-IR showed a ~2 fold increase in Let-7e levels compared with control (Fig 6A), without significant changes in miR-126 (p = 0.118 for insulin, p = 0.158 for HOMA-IR, versus control) (Fig 6B).
Fig 6. Circulating levels of Let-7e and miR-126 and insulin resistance.
(A) Plasma levels of Let-7e and (B) miR-126 in controls (open bars) and children with insulinaemia (light grey bars) and HOMA-IR (solid bars) over 90th percentile (light grey bars). Values are mean ± SEM. **p < 0.01. T-test.
Discussion
This study demonstrates for the first time in pediatric subjects that MetS components are associated with increased plasma levels of miRNAs that have been associated to MetS in adults. These results were obtained in a selected pediatric population, from a larger cohort, in which central obesity was the main parameter altered along with elevated insulinaemia and HOMA-IR. The analysis of continuous variables demonstrated that plasma levels of miR-126 significantly correlates with waist circumference, waist to hip ratio, BMI, plasma triglycerides and VLDL. This is also observed for miR-132 regarding waist to hip ratio. On the other hand Let-7e plasma levels showed a significant increase in subjects with three MetS traits, and positively correlated with the number of MetS traits present. Increased levels of Let-7e occurred mainly in subjects that present increased waist circumference along with one other altered parameter. Evaluation of Let-7e and miR-126 ROC curves as markers of metabolic syndrome in children showed that only Let-7e plasma levels presented a potential predictive value which was improved when miR-126 levels were considered. Finally, in silico analysis of the potential targets of the most significantly altered miRNAs (i.e. Let-7e and miR-126) showed that both converge in regulating the insulin signaling pathway, which connects with the increased insulinaemia and HOMA index present in these subjects.
Metabolic syndrome along with the individual cardiometabolic conditions associated to it, represent the most prevalent diseases in adults [25], however growing evidence shows that the presence of MS traits in children and adolescents associates to increased risk of developing cardiometabolic diseases in adult life [26]. There is no consensus regarding the diagnose of MS in pediatric population, mainly due to the absence of clear cut-off values for the classical parameters used for its diagnosis in adults [1, 3]. In this study, using Cook´s et al. criteria, we evaluated in children the association of different MS traits with plasma levels of miRNAs that have been reported altered in adults with cardiometabolic dysfunction. In order to provide insight on new markers associated to the risk of developing MS, we aimed to determine the plasma levels of specific miRNA in children with altered cardiometabolic traits. The strength of plasma miRNAs as molecular markers relies on several characteristics of these molecules. First, the stability of miRNAs in plasma is comparable to proteins, and higher than mRNAs, which in combination with the use of quantitative PCR on their detection rises the possibility to measure small changes in their levels with higher precision than proteins and other biomarkers [5, 6]. At cellular level it is known that a single miRNA can control the expression of several mRNA and the transcriptional activity of genes [19, 27], which in most of the cases affects the gene expression of specific molecular pathways [28]. Additionally part of plasma miRNAs circulate associated to vesicles with potential endocrine effects [29, 30] which is nowadays under study [5]. In spite of the fact that the precise role of circulating miRNAs is unknown, evidence shows that their levels are altered in several pathological conditions, and they change in response to pharmacological treatments earlier than routine clinical markers [31, 32]. These characteristics have led to propose the use of circulating miRNA as molecular markers in cancer, metabolic disorders and cardiovascular diseases [5, 6, 33].
Studies characterizing the profile of circulating miRNAs show that among hundreds of miRNAs normally present in plasma, there is a small proportion of them altered in adults with cardiovascular and metabolic diseases [8–11, 34, 35]. In fact a recent review shows that Let-7e and miR-126 along with other 6 miRNAs are among the most prominent miRNAs implicated in cardiometabolic disorders [36]. Moreover little is known about the levels of miRNAs in normal children, and especially those that have been highlighted as markers of cardiometabolic dysfunction in adults. In order to determine whether some miRNAs reported as altered in adults with MS-related diseases we determined the levels of Let-7e, miR-33b, miR-126, miR132 and miR-145.
Results show that plasma levels of Let-7e were increased in children with three MS traits, and positively correlated with the number of traits. Conversely mean plasma levels of miR-126, miR-132 and miR-145 were unchanged in children grouped according to the number of MS traits. Despite that miR-126 has been reported decreased in vascular diseases [6] and the fact that the subjects recruited in this study did not present vascular alterations (i.e. increased blood pressure), we found that miR-126 positively correlated with several metabolic traits (i.e. waist circumference, BMI and triglycerides). On the other hand an important role for miR-33b has been suggested in the development of obesity due to its participation in lipid and cholesterol metabolism and is highly expressed in adipose tissue [14], however miR-33b was not detectable in plasma samples from children as reported for adults [24].
Considering that Let-7e and miR-126 showed important associations with MS traits in the children studied, an in silico analysis was performed to identify common potential targets regulated by both miRNAs. Notably Let-7e and miR-126 showed genes related with insulin signaling pathway as common targets, which could reflect the higher insulinaemia and HOMA-IR present in children with two or more MS traits. In this context, circulating levels of Let-7e were importantly increased in subjects with altered insulinaemia and HOMA-IR as well as showed a significant diagnostic value in ROC analysis, whilst there was a trend in miR-126 levels supporting the role of this miRNAs as a marker for insulin resistance in pediatric population. Conversely when plasma levels of both miRNAs were considered in the analysis, the predictive value of Let-7e was improved from less predictive to moderately predictive [37], suggesting that a potential use of these markers as diagnostic tool should include the levels of the two miRNAs in order to have a significant clinical value. Insulin plays a key role in different conditions associated with MS including glucose and triglyceride metabolism, and immune and vascular function [38]. In this context several studies have shown that Let-7 family regulates insulin-related genes in pancreas [39], adipocytes [40] and skeletal muscle [41]. Additionally Let-7 regulates the gene expression of inflammatory mediators in T-cells [42] and endothelium [43]. Interestingly Let-7 overexpression in mice induces insulin resistance repressing the expression of several mediators of insulin signaling pathway [41]. On the other hand compelling data show that miR-126 plays a key role regulating endothelial function [44, 45]. Notably it is reported in a cohort of 822 adults that plasma levels of miR-126 is decreased in subjects that present impaired glucose tolerance or type-2 diabetes [8]. These results contrast with those found in this study where miR-126 levels positively correlated with MS traits in children, suggesting that reduced miR-126 in adults with type 2 diabetes could represent a final stage of metabolic dysfunction, whilst in the early stages, during childhood, a compensatory increase in miR-126 would be observed.
The present data suggest that circulating levels of Let-7e and miR-126 associate with different traits of metabolic syndrome in children with a potential role as biomarkers. There are few reports showing levels of circulating miRNAs in children with or without altered metabolic parameters [46, 47]. In one of these studies the identification of differentially expressed miRNAs was based on array wide screening and further validation by qPCR utilizing endogenous miRNAs for quantification. None of the miRNAs reported as altered in these studies includes those founded in this work suggesting a possible bias in analyzing selected miRNAs. However a validation method for miRNAs levels is not well established, and there is no consensus regarding a set of endogenous miRNAs for quantitative normalization. In this context the use of an external miRNA for quantification of miRNAs (spike-in) in biological fluids represents at the moment the best choice [48]. Thus despite that the miRNAs studied here were selected from studies conducted in adults, the normalization procedure increases the reliability of the results, which along with ROC analysis argue for a potential application of these miRNAs (i.e. Let-7e and 126) as biomarkers of metabolic syndrome in children. Nowadays metabolic alterations in these patients are well characterized by accessible and direct measurements (i.e. waist circumference, waist to hip ratio and BMI) however circulating miRNAs point to veiled subclinical conditions. In this context pre-clinical data show that overexpression of Let-7 in mice leads to impaired glucose tolerance and insulin secretion [49], suggesting that increased levels of Let-7e could represent a basis rather a consequence of altered insulin response in these children. Considering that these results represent levels of specific miRNAs in a selected group of pediatric children from a carefully characterized cohort, its clinical significance will require further validation in a larger population.
In summary, this study demonstrates for the first time that children at age 10 to 12 y.o. with increased risk of MS do not present changes in plasma levels of miRNAs related with altered vascular (i.e. miR-145) and adipocyte (i.e. miR-132) functions. However they show increased plasma levels of Let-7e and miR-126, which could be actively participating in the development of insulin resistance, agreeing with the increased insulinaemia and HOMA-IR observed in these subjects.
Supporting Information
Representative amplification plot for Cel-miR-39 in HEK-293 (a) and plasma (b), and Hsa-miR-33b in HEK-293 (c) and plasma showing that levels of the later miRNA were almost undetectable on the plasma samples studied.
(TIF)
(DOCX)
(DOCX)
Acknowledgments
Special thanks to Dr. Stefanie Dimmeler for providing the protocol for miRNA isolation and quantification.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Dirección de Investigación de la Escuela de Medicina, Pontificia Universidad Católica de Chile, PI 07-12 (http://medicina.uc.cl/concursos/inv-cfp). Vicerrectoría de Investigación Pontificia Universidad Católica de Chile, VRI 1898 (http://investigacion.uc.cl/Fondos-concursables/fondos-concursables.html). Fondo Nacional de Desarrollo Científico y Tegnológico, FONDECYT Chile 1090594, 1120928, 1130801 (http://www.conicyt.cl/fondecyt/).
References
- 1. Weiss R, Bremer AA, Lustig RH. What is metabolic syndrome, and why are children getting it? Ann N Y Acad Sci. 2013;1281:123–40. Epub 2013/01/30. 10.1111/nyas.12030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bruce KD, Hanson MA. The developmental origins, mechanisms, and implications of metabolic syndrome. J Nutr. 2010;140(3):648–52. Epub 2010/01/29. 10.3945/jn.109.111179 . [DOI] [PubMed] [Google Scholar]
- 3. Marcovecchio ML, Chiarelli F. Metabolic syndrome in youth: chimera or useful concept? Current diabetes reports. 2013;13(1):56–62. Epub 2012/10/12. 10.1007/s11892-012-0331-2 . [DOI] [PubMed] [Google Scholar]
- 4. Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157(8):821–7. Epub 2003/08/13. 10.1001/archpedi.157.8.821 . [DOI] [PubMed] [Google Scholar]
- 5. Muller G. Microvesicles/exosomes as potential novel biomarkers of metabolic diseases. Diabetes, metabolic syndrome and obesity: targets and therapy. 2012;5:247–82. Epub 2012/08/28. 10.2147/DMSO.S32923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Empel VP, De Windt LJ, da Costa Martins PA. Circulating miRNAs: reflecting or affecting cardiovascular disease? Curr Hypertens Rep. 2012;14(6):498–509. Epub 2012/09/22. 10.1007/s11906-012-0310-7 . [DOI] [PubMed] [Google Scholar]
- 7. Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol. 2013;9(9):513–21. Epub 2013/05/01. 10.1038/nrendo.2013.86 . [DOI] [PubMed] [Google Scholar]
- 8. Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107(6):810–7. Epub 2010/07/24. 10.1161/CIRCRESAHA.110.226357 . [DOI] [PubMed] [Google Scholar]
- 9. Li S, Zhu J, Zhang W, Chen Y, Zhang K, Popescu LM, et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation. 2011;124(2):175–84. Epub 2011/06/22. 10.1161/CIRCULATIONAHA.110.012237 . [DOI] [PubMed] [Google Scholar]
- 10. Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107(5):677–84. Epub 2010/07/03. 10.1161/CIRCRESAHA.109.215566 . [DOI] [PubMed] [Google Scholar]
- 11. Meder B, Keller A, Vogel B, Haas J, Sedaghat-Hamedani F, Kayvanpour E, et al. MicroRNA signatures in total peripheral blood as novel biomarkers for acute myocardial infarction. Basic Res Cardiol. 2011;106(1):13–23. Epub 2010/10/05. 10.1007/s00395-010-0123-2 . [DOI] [PubMed] [Google Scholar]
- 12. Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478(7369):404–7. Epub 2011/10/21. 10.1038/nature10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heneghan HM, Miller N, McAnena OJ, O'Brien T, Kerin MJ. Differential miRNA expression in omental adipose tissue and in the circulation of obese patients identifies novel metabolic biomarkers. J Clin Endocrinol Metab. 2011;96(5):E846–50. Epub 2011/03/04. 10.1210/jc.2010-2701 . [DOI] [PubMed] [Google Scholar]
- 14. Rottiers V, Naar AM. MicroRNAs in metabolism and metabolic disorders. Nat Rev Mol Cell Biol. 2012;13(4):239–50. Epub 2012/03/23. 10.1038/nrm3313 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mardones F, Arnaiz P, Barja S, Giadach C, Villarroel L, Dominguez A, et al. Nutritional status, metabolic síndrome and insulin resistance in children from Santiago, Chile. Nutricion Hospitalaria. 2013;In press. [PubMed] [Google Scholar]
- 16. Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. The Journal of pediatrics. 2004;145(4):439–44. Epub 2004/10/14. 10.1016/j.jpeds.2004.06.044 . [DOI] [PubMed] [Google Scholar]
- 17. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–76. Epub 2004/08/03. . [PubMed] [Google Scholar]
- 18. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome research. 2009;19(1):92–105. Epub 2008/10/29. 10.1101/gr.082701.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nature structural & molecular biology. 2011;18(10):1139–46. Epub 2011/09/13. 10.1038/nsmb.2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramasamy S, Velmurugan G, Shanmugha Rajan K, Ramprasath T, Kalpana K. MiRNAs with Apoptosis Regulating Potential Are Differentially Expressed in Chronic Exercise-Induced Physiologically Hypertrophied Hearts. PLoS One. 2015;10(3):e0121401 Epub 2015/03/21. 10.1371/journal.pone.0121401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Szklarczyk D, Franceschini A, Kuhn M, Simonovic M, Roth A, Minguez P, et al. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic acids research. 2011;39(Database issue):D561–8. Epub 2010/11/04. 10.1093/nar/gkq973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic acids research. 2013;41(Database issue):D808–15. Epub 2012/12/04. 10.1093/nar/gks1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takwi AA, Li Y, Becker Buscaglia LE, Zhang J, Choudhury S, Park AK, et al. A statin-regulated microRNA represses human c-Myc expression and function. EMBO molecular medicine. 2012;4(9):896–909. Epub 2012/08/14. 10.1002/emmm.201101045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao W, He HW, Wang ZM, Zhao H, Lian XQ, Wang YS, et al. Plasma levels of lipometabolism-related miR-122 and miR-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids in health and disease. 2012;11:55 Epub 2012/05/17. 10.1186/1476-511X-11-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999–2006. Diabetes Care. 2011;34(1):216–9. Epub 2010/10/05. 10.2337/dc10-0879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: a need for screening tools to target interventions. Nutrition. 2013;29(2):379–86. Epub 2012/10/02. 10.1016/j.nut.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433(7027):769–73. Epub 2005/02/03. 10.1038/nature03315 . [DOI] [PubMed] [Google Scholar]
- 28. Chu CY, Rana TM. Small RNAs: regulators and guardians of the genome. J Cell Physiol. 2007;213(2):412–9. Epub 2007/08/04. 10.1002/jcp.21230 . [DOI] [PubMed] [Google Scholar]
- 29. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9(6):654–9. Epub 2007/05/09. 10.1038/ncb1596 . [DOI] [PubMed] [Google Scholar]
- 30. Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3(11):e3694 Epub 2008/11/13. 10.1371/journal.pone.0003694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Boer HC, van Solingen C, Prins J, Duijs JM, Huisman MV, Rabelink TJ, et al. Aspirin treatment hampers the use of plasma microRNA-126 as a biomarker for the progression of vascular disease. Eur Heart J. 2013. Epub 2013/02/07. 10.1093/eurheartj/eht007 [DOI] [PubMed] [Google Scholar]
- 32. Dickinson BA, Semus HM, Montgomery RL, Stack C, Latimer PA, Lewton SM, et al. Plasma microRNAs serve as biomarkers of therapeutic efficacy and disease progression in hypertension-induced heart failure. European journal of heart failure. 2013;15(6):650–9. Epub 2013/02/08. 10.1093/eurjhf/hft018 . [DOI] [PubMed] [Google Scholar]
- 33. Olivieri F, Rippo MR, Procopio AD, Fazioli F. Circulating inflamma-miRs in aging and age-related diseases. Frontiers in genetics. 2013;4:121 Epub 2013/06/28. 10.3389/fgene.2013.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karolina DS, Tavintharan S, Armugam A, Sepramaniam S, Pek SL, Wong MT, et al. Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab. 2012;97(12):E2271–6. Epub 2012/10/04. 10.1210/jc.2012-1996 . [DOI] [PubMed] [Google Scholar]
- 35. Willeit P, Zampetaki A, Dudek K, Kaudewitz D, King A, Kirkby NS, et al. Circulating microRNAs as novel biomarkers for platelet activation. Circ Res. 2013;112(4):595–600. Epub 2013/01/04. 10.1161/CIRCRESAHA.111.300539 . [DOI] [PubMed] [Google Scholar]
- 36. Hulsmans M, Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc Res. 2013;100(1):7–18. Epub 2013/06/19. 10.1093/cvr/cvt161 . [DOI] [PubMed] [Google Scholar]
- 37. Vanagas G. Receiver operating characteristic curves and comparison of cardiac surgery risk stratification systems. Interactive cardiovascular and thoracic surgery. 2004;3(2):319–22. Epub 2007/08/03. 10.1016/j.icvts.2004.01.008 . [DOI] [PubMed] [Google Scholar]
- 38. Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339(6116):172–7. Epub 2013/01/12. 10.1126/science.1230721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liao X, Xue H, Wang YC, Nazor KL, Guo S, Trivedi N, et al. Matched miRNA and mRNA signatures from an hESC-based in vitro model of pancreatic differentiation reveal novel regulatory interactions. J Cell Sci. 2013;126(Pt 17):3848–61. Epub 2013/07/03. 10.1242/jcs.123570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perez LM, Bernal A, San Martin N, Lorenzo M, Fernandez-Veledo S, Galvez BG. Metabolic rescue of obese adipose-derived stem cells by lin28/let7 pathway. Diabetes. 2013;62(7):2368–79. Epub 2013/02/21. 10.2337/db12-1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhu H, Shyh-Chang N, Segre AV, Shinoda G, Shah SP, Einhorn WS, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147(1):81–94. Epub 2011/10/04. 10.1016/j.cell.2011.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Polikepahad S, Knight JM, Naghavi AO, Oplt T, Creighton CJ, Shaw C, et al. Proinflammatory role for let-7 microRNAS in experimental asthma. J Biol Chem. 2010;285(39):30139–49. Epub 2010/07/16. 10.1074/jbc.M110.145698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, et al. FGF regulates TGF-beta signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell reports. 2012;2(6):1684–96. Epub 2012/12/04. 10.1016/j.celrep.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nazari-Jahantigh M, Wei Y, Schober A. The role of microRNAs in arterial remodelling. Thrombosis and haemostasis. 2012;107(4):611–8. Epub 2012/03/01. 10.1160/TH11-12-0826 . [DOI] [PubMed] [Google Scholar]
- 45. Wei Y, Schober A, Weber C. Pathogenic arterial remodeling: the good and bad of microRNAs. Am J Physiol Heart Circ Physiol. 2013;304(8):H1050–9. Epub 2013/02/12. 10.1152/ajpheart.00267.2012 . [DOI] [PubMed] [Google Scholar]
- 46. Prats-Puig A, Ortega FJ, Mercader JM, Moreno-Navarrete JM, Moreno M, Bonet N, et al. Changes in circulating microRNAs are associated with childhood obesity. J Clin Endocrinol Metab. 2013;98(10):E1655–60. Epub 2013/08/10. 10.1210/jc.2013-1496 . [DOI] [PubMed] [Google Scholar]
- 47. Carolan E, Hogan AE, Corrigan M, Gaotswe G, O'Connell J, Foley N, et al. The impact of childhood obesity on inflammation, innate immune cell frequency, and metabolic microRNA expression. J Clin Endocrinol Metab. 2014;99(3):E474–8. Epub 2014/01/16. 10.1210/jc.2013-3529 . [DOI] [PubMed] [Google Scholar]
- 48. McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras-Schimnich A. Analysis of circulating microRNA: preanalytical and analytical challenges. Clin Chem. 2011;57(6):833–40. Epub 2011/04/14. 10.1373/clinchem.2010.157198 . [DOI] [PubMed] [Google Scholar]
- 49. Frost RJ, Olson EN. Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs. Proc Natl Acad Sci U S A. 2011;108(52):21075–80. Epub 2011/12/14. 10.1073/pnas.1118922109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative amplification plot for Cel-miR-39 in HEK-293 (a) and plasma (b), and Hsa-miR-33b in HEK-293 (c) and plasma showing that levels of the later miRNA were almost undetectable on the plasma samples studied.
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.