Abstract
The thalamus, with its cortical, subcortical, and cerebellar connections, is a critical node in networks supporting cognitive functions known to decline in normal aging, including component processes of memory and executive functions of attention and information processing. The macrostructure, microstructure, and neural connectivity of the thalamus changes across the adult lifespan. Structural and functional magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) have demonstrated, regional thalamic volume shrinkage and microstructural degradation, with anterior regions generally more compromised than posterior regions. The integrity of selective thalamic nuclei and projections decline with advancing age, particularly those in thalamofrontal, thalamoparietal, and thalamolimbic networks. This review presents studies that assess the relations between age and aging and the structure, function, and connectivity of the thalamus and associated neural networks and focuses on their relations with processes of attention, speed of information processing, and working and episodic memory.
Keywords: thalamus, aging, MRI, DTI, connectivity, thalamocortical, memory, executive functions, attention
Overview
This review focuses on the relations among thalamic macrostructure, microstructure, and select cognitive processes and age and aging as significant moderators. The organization of the studies reviewed is based on findings from structural and functional magnetic resonance imaging (MRI) used to assess aspects of regional brain and neuronal health, including volume, tissue quality, and structural and functional network integrity. Taken together, these different imaging modalities enable investigation of the association between age-related neuronal differences (cross-sectional study) and changes (longitudinal study) and the extent, nature, and pattern of cognitive, motor, and sensory deficits observed in normal aging. We also note limitations of these studies and how newly developing high-resolution neuroimaging modalities can further age-related thalamic research.
Introduction
The thalamus comprises upwards of 60 cytoarchitectonically and functionally distinct nuclei, each of which has a different pattern of anatomical connections to subcortical, cortical, and cerebellar structures and regions (Behrens et al., 2003; Schmahmann and Pandya, 2008; Strick, 1985). Traditionally, the thalamus was characterized as the central sensory and motor relay station of the brain, reciprocally communicating and relaying signals between subcortical and cerebellar regions and the cortex, playing a role in sleep, arousal, and primary sensory processing (Adams and Victor, 1993).
Similarly, the role of the thalamus in cognitive processes was historically considered to be passive, serving primarily as a “triage center” for information, determining what information would be passed on to higher cortical regions for processing (Van Der Werf et al., 2003). Appreciation of the neural complexity and distributed brain networks underlying cognitive, sensory, motor, and behavior processes and the relevance of integrated neural networks to higher cognitive processes has resulted in increased efforts in explicating the subcortical contributions, including the thalamus as a critical node of interconnected cortical, subcortical, and cerebellar circuits (Alexander et al., 1990; Haber and McFarland, 2001). Over the past two decades, the characterization of the passive thalamus has been debunked by myriad studies (Aggleton, 2014; Pergola and Suchan, 2013; Schmahmann and Pandya, 2008), and the contribution of the thalamus to cognitive processes, including attention, speed of information processing, and memory has become evident (Van Der Werf et al., 2001).
Some of the earliest studies linking the thalamus, in particular the pulvinar nuclei, and cognitive processes involved attentional processes. The pulvinar nuclei of the dorsal thalamus account for approximately 25% of the total mass of the thalamus (Grieve et al., 2000) and comprise 8–10 anatomically divided subdivisions. The medial and lateral regions are associated with cortical targets, and the inferior and lateral regions are associated with striate and extrastriate cortices (Figure 1). The pulvinar plays a role in selective attention (LaBerge and Buchsbaum, 1990), including visual attentional filtering, the ability to focus on a target object in an environment containing distractors (Grieve et al., 2000). LaBerge posited that expression, preparation, and maintenance of attentional processes relied on a triangular brain circuit composed of the prefrontal cortex, pulvinar, and posterior cortical neurons (LaBerge, 2000).
Figure 1.
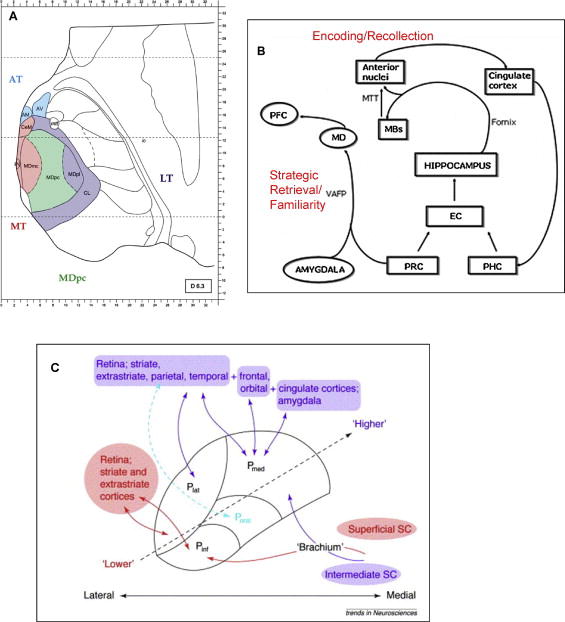
(A) Modified from Pergola et al. (2012) Neuropsychologia Vol. 50: 2477–2491, Figure 1. Thalamo-cortical connectivity. Spatial arrangements of the anterior thalamus (AT), medial thalamus (MT), parvocellular mediodorsal nucleus (MDpc), and lateral thalamus (LT). The section is 6.3 mm superior to the intercommissural plane. Modified from Morel (2007). AM-anteromedial nucleus; AV-anteroventral nucleus; CeM-centromedial nucleus; CL-centrolateral nucleus; MDmc-magnocellular mediodorsal nucleus; MDpl-paralamellar mediodorsal nucleus; mtt-mammillothalamic tract; Pv-paraventricular nucleus (B) Modified from Carlesimo et al. (2011) Neuropsychologia Vol 49: 777–789, Figure 1. A diagram showing principal thalamic nuclei connections to other relevant structures in declarative, including episodic, memory functioning. EC-entorhinal cortex; MBs-mammillary bodies; MD-mediodorsal nucleus; MTT-mammillothalamic tract; PFC-prefrontal cortex; PHC-perihippocampal cortex; PRC-perirhinal cortex; VAFP-ventral amygdalofugal pathway.
(C) Taken from Grieve et al. (2000) Trends in Neurosciences Vol 23: 35–39, Figure 1. The major cortical and subcortical inputs to the inferior, lateral, and medial pulvinar nuclei. ‘Lower” vision and near-striate visual cortices are in red, while ‘higher’ cortices, visual association such as parietal and prefrontal and non-sensory association cortices including frontal and cingulated are in purple. P inf-inferior pulvinar; P lat-lateral pulvinar; P med-medial pulvinar; P oral-oral pulvinar; SC-superior colliculus.
Disruption of interrelated and interconnected brain networks can occur with age, and the resulting compromised connectivity between regions and structures has been hypothesized to be at the heart of age-related cognitive decline (Tisserand and Jolles, 2003); however, exactly how normal or typical aging affects thalamic networks is incompletely understood. Disrupted connections between brain systems or regions necessary to perform specific cognitive processes are often associated with microstructural changes, including demyelination of white matter fibers. This “disconnection” concept is not a new one, being initially championed almost 50 years ago by Geschwind as a possible underlying neuropathological mechanism of neurological conditions (Geschwind, 1965). Although the initial focus of disconnection studies was on cortico-cortical pathways, white matter and subcortical regions and related neural pathways are now recognized to play a critical role in age-related cognitive deficits (Filley, 2001). Damage to white matter pathways or subcortical structures relevant or critical in the communication of a network such as the thalamus, regardless of cortical integrity, can be responsible for behavioral impairments (Chanraud et al., 2010). A recent spate of studies has demonstrated associations between thalamic structure and integrity and higher-order cognitive processes that typically decline with aging, including component processes of episodic memory (encoding and retrieval) and executive functions (attention, working memory, and speed of information processing) (Philp et al., 2014).
Compromise in memory functions is one of the most consistently reported complaints in older adults. Memory is not a unitary process, with different types of memory (e.g., explicit/declarative, implicit/procedural) associated with dissociable neural systems, traditionally involving medial temporal, lateral prefrontal, and even cerebellar hemisphere structures (Graf and Schacter, 1985; Squire et al., 1993; Tulving and Markowitsch, 1997; Tulving and Schacter, 1990). Animal and human lesion studies also provide strong evidence for the role of specific thalamic nuclei in selective component processes of memory (Aggleton, 2014; Aggleton and Brown, 1999; Cipolotti et al., 2008) (Figure 1). Thalamic nuclei and broader regions have been shown to be selectively associated with encoding, retrieval, recollection and familiarity (Van Der Werf et al., 2003). Specifically, the anterior thalamic nucleus, which is directly connected to the hippocampus via the fornix and indirectly connected to the mammillary bodies via the mammillothalamic tract, is associated with encoding content and contextual information and recollective processes; the medial dorsal thalamic nucleus with its connections to the prefrontal cortex and the limbic system (amygdala via the ventroamygdalofugal pathway) is related to executive aspects of memory, including strategic memory retrieval of information to be remembered and familiarity processes; and the intralaminar/midline thalamic nuclei, which is connected to the parietal lobe, has a role in attention, arousal, and awareness, and activation of cortical regions necessary for the processing of information to be remembered (Llinas and Pare, 1997; Van Der Werf et al., 2003).
After a review of 23 studies, Pergola and Suchan concluded that the thalamo-prefrontal and thalamo-retrosplenial networks contribute to dissociable component processes of recognition and recall (Pergola and Suchan, 2013). Specifically, the thalamo-prefrontal network is involved in goal-directed memory acquisition and encoding under conscious/intentional control, whereas the thalamo-retrosplenial network is primarily associated with the retrieval phase of memory processing. These reviewed studies, as well as numerous reported cases of severe memory impairment following focal thalamic stroke (Carlesimo et al., 2011) and Korsakoff’s syndrome (the amnesia associated with prolonged and severe alcoholism with concomitant thiamine deficiency) (Kopelman, 1995), provide substantial evidence for the relevance of thalamic regions and related neural networks to memory processes.
Thalamic Macrostructure and Regional Subnuclei, Aging, and Cognition
Thalamic macrostructure or brain tissue morphology can be examined with a number of MRI procedures. Structural MRI methods measure the distribution of hydrogen atoms in water to create images depicting gray matter (approximately 80% water), white matter (approximately 70% water), and CSF (approximately 99% water). Images processed from MRI data reveal the size, shape, tissue, and fluid composition of brain structures and regions of interest.
Cross-sectional studies of normal aging have reported smaller thalamic volumes in older than younger adults (Cherubini et al., 2009; Hughes et al., 2012; Sullivan et al., 2004; Van Der Werf et al., 2001; Walhovd et al., 2005); however, not all studies, including ones reliant on unsupervised, automated quantification methods, have observed this relation (see Jernigan et al., 1991; Jernigan et al., 2001). Of the studies that reported age-thalamus relations, several also reported thalamus volume – cognitive process relationships, generally involving executive functions or memory processes as they relate to normal aging.
Thalamus-age-cognitive relations were observed in a study of 57 healthy adults categorized into three age groups: young adults (21–45 years), middle-aged adults (46–60 years), and older adults (61–82 years) (Van Der Werf et al., 2001). Thalamic volumetric analyses based on inversion recovery and 3D T1-weighted, fast-field echo images (1.5-mm thick slices in the coronal plane) showed that thalamic volumes were smaller in the older age groups. Volume differences between groups could not be attributed to variation in intracranial volume, which can reflect sex and cohort effects, and thalamic volumes were corrected for the effects of intracranial volume, brain size, and age to avoid possible confounds of these variables in relation to cognitive scores. Smaller thalamic volumes in the middle-aged adults were associated with slower information processing speed, assessed with the Stroop (Stroop, 1935). Smaller thalamic volume in the young adults (21–45 years) was associated with poorer Stroop and Paper and Pencil Memory Scanning Test (Van Der Elst et al., 2007). Although the associations reported by Van Der Werf (2001) suggest a relationship between thalamic volume and speed of processing in young and middle-aged adults (21–60 years old), selectivity of this brain-behavior relation could not be determined because the thalamus was the only brain structure examined (cf. Fama and Sullivan, 2014; Salthouse, 2011).
Differential effects of aging on cortical, subcortical (including the thalamus), and CSF regions were examined in a study of 73 men and women spanning the adult age range (20–88 years old) using an automated segmentation and labeling technique (Walhovd et al., 2005). Of the neuroanatomical regions assessed, which included cortical gray matter, cerebral white matter, hippocampus, amygdala, thalamus, accumbens, caudate, putamen, pallidus, brainstem, cerebellar cortex and cerebellar white matter, all showed age-related volume decline, with the exception of the pallidum. Older age was most strongly related to thalamus (Spearman rho=−.79) and cortical gray matter (Spearman rho=−.78) volumes, with thalamus volume showing a linear decline with age and cortical gray matter volume showing a curvilinear decline. Analyses assessing tissue shrinkage over the lifespan showed an approximately 23.5% difference in thalamus volume between 20 and 90 years olds.
Subregions of the thalamus are differentially affected by age. Structural MRI showed bilateral shape differences in the anterior thalamus between younger and older adults (Hughes et al., 2012). Smaller anterior and dorsomedial thalamic volumes, regions whose primary connections are with the frontal cortex, but not lateral posteroventral thalamic volume, a region whose primary connections are with the parietal cortex, were associated with older age. This study of 86 right-handed men and women (20–74 years of age) also found selective vulnerability of the anterior thalamus to age-related cognitive decline in executive functions including attention, processing speed, and working memory processes as assessed by the Stroop Test (Hughes et al., 2012). Despite group differences in thalamic structural characteristics, the scatter of brain volume measures was particularly heteroscedastic in older ages, indicative of heterogeneity among subjects in the older age range, a common feature of cross-sectional measures of age-related effects (cf. Walhovd et al., 2005; Walhovd et al., 2011). Hughes and colleagues also examined thalamic microstructure and connectivity, and these results will be reviewed in a later section.
A longitudinal MRI study of 55 men and 67 women (20 to 85 years) demonstrated that older age was associated with a decline in thalamus volume, and that this decline accelerated with age (60+ years) (Pfefferbaum et al., 2013). Longitudinal studies enable assessment of change within individuals, as opposed to cross-sectional studies that can only address differences between age groups (Fjell et al., 2013; Raz and Lindenberger, 2011; Raz et al., 2005). Subjects underwent 2 to 6 MRIs over a time period of approximately 1 to 8 years, and individual trajectories across 18 ROIs, including the thalamus, were computed. A quadratic model, which is indicative of accelerated changes in older age, best modeled thalamus volume trajectories, where men exhibited a more rapid decline than women in the later years. Other subcortical regions including the caudate, pallidum, and amygdala also demonstrated this age-related pattern, whereas cortical regions including the precentral and postcentral cortices demonstrated a linear decline. When adolescent data were added (Sullivan et al., 2011), extending the age range down to 10 years old, the thalamic trajectory was best fit by a higher order function (Figure 2) (Pfefferbaum et al., 2013).
Figure 2.
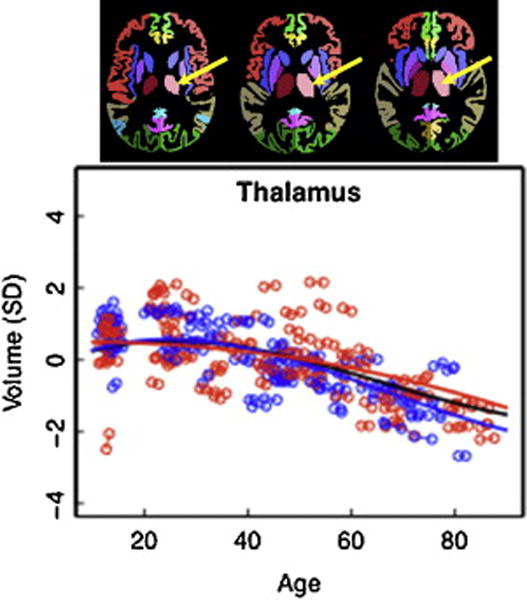
Modified from Pfefferbaum et al. (2013) NeuroImage Vol. 65: 176–193, Figures 1 and 4.
Top figure: Parcellated segmentation of the thalamus (depicted in light and dark pink) from the SRI24 atlas (http://nitc.org/projects/sri24).
Scatterplot: Volume of the thalamus, expressed as standardized residuals (Z-score) or standard deviations (SD), after correction for supratentorial volume of regional brain structures of the adult plus adolescent samples, with boys and men (blue) and girls and women (red) and best-fit functions over age for each sex. The black fit is the combined group irrespective of sex.
Examination of the thalamus with structural MRI has been fraught with problems of partial voluming, resulting in poor gray/white matter differentiation and difficulty in delineating specific nuclei (Magnotta et al., 2000). Recently, enhanced visualization of intra-thalamic nuclei has been demonstrated with ultra high field MRI imaging at 7T (Saranathan et al., 2014; Tourdias et al., 2014). Optimization of the 3D MPRAGE (magnetization-prepared rapidly-acquired gradient echo) enabled 3D delineation of 15 substructures, including the mediodorsal nucleus, pulvinar, ventral lateral posterior nucleus, lateral dorsal nucleus, and the mammillothalamic tract. This image acquisition strategy can identify thin bands between nuclei, reflective of myelin layers that separate small thalamic subregions (Tourdias et al., 2014) (Figure 3). Thus, despite low intrinsic contrast between adjacent nuclei, direct anatomical visualization of dissociable thalamic nuclei showed high correspondence with histological plates of identified areas based on the Morel atlas (Morel et al., 1997). Spatial resolution at this level will enable in vivo examination and enhance the potential of (1) identifying regional thalamic integrity that change with aging or disease and (2) delineating individual thalamic nuclei and related circuitry, thereby setting the stage to test their relations with component processes of cognitive, motor, and sensory functions affected in aging and age-related disease.
Figure 3.
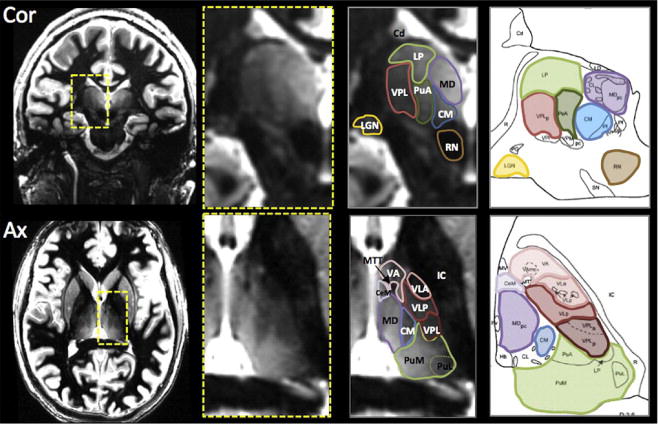
Modified from Tourdias et al. (2014) NeuroImage Vol. 84: 534–545 Figure 7 and Saranathan et al. (2014) Magnetic Resonance in Medicine (2014) in press, Figure 8. White-matter-nulled MRI images in Coronal (top) and Axial (bottom) orientations from two different subjects. Zoomed insets (yellow rectangle) of the thalamic region show the different nuclei, visualized due to improved contrast between adjacent substructures. The nuclei can then be segmented and labeled. Corresponding histological plates (Morel et al., 1997) are also shown for comparison highlighting the excellent correspondence between MR segmentation and the atlas. Cd-caudate nucleus ; CeM-central medial nucleus; CM-center median nucleus ; IC-internal capsule; LGN-lateral geniculate nucleus ; LP-lateral posterior nucleus ; MD-mediodorsal nucleus; MTT-mammillothalamic tracts; PuA-anterior pulvinar ; PuM-medial pulvinar; PuL-lateral pulvinar; RN-red nucleus; VA-ventral anterior nucleus; VLA-ventral lateral anterior nucleus; VLP-ventral lateral posterior nucleus; VPL-ventral posterior lateral nucleus ;
Thalamic Microstructure, Aging, and Cognition
Brain tissue quality and fiber integrity can be assessed with diffusion tensor imaging (DTI), an MRI modality that measures the magnitude and orientation of water diffusion within image volume units (i.e., voxels) (Basser and Pierpaoli, 2011; Basser and Pierpaoli, 1996; Jones et al., 1999; Jones et al., 2013). With DTI, differences in tissue quality and integrity can be observed in cases where regional brain volume and tissue quality appear normal with standard structural MRI imaging (O’Sullivan et al., 2001; Pfefferbaum and Sullivan, 2002). DTI metrics include fractional anisotropy (FA) which measures the orientation of water diffusivity and assesses the linear organization of fibers and mean diffusivity (MD) which measures the magnitude of diffusivity independent of orientation and assesses freely diffusing water in tissue as occurs in white matter hyperintensities. Mean diffusivity can further be parsed as axial diffusivity (also referred to as longitudinal diffusivity, λL; coursing parallel to axonal fibers) and radial diffusivity (also referred to as transverse diffusivity, λT; coursing perpendicular to axonal fibers) (Le Bihan, 2003; Song et al., 2002; Sullivan and Pfefferbaum, 2006; Sullivan and Pfefferbaum, 2010).
DTI indices, particularly FA, are often used as measures of connectivity between brain regions, assessing the integrity of white matter fibers, although the microstructure of gray matter can also be assessed with this modality (Truong et al., 2014; Whitwell et al., 2010). Given that white matter and gray matter differ in microstructural components, DTI metrics likely reflect different processes in these two tissue groups. In white matter, FA and MD values reflect integrity of fiber components, such as axons and myelin that are commonly negatively correlated with one another (e.g., white matter pathology generally results in a decrease in FA and axial diffusivity, reflecting loss of axonal integrity, and an increase in MD and radial diffusivity, reflecting myelin degradation). By contrast, in gray matter, FA and MD, likely reflect the integrity of neuronal cell wall and intercellular membranes (Song et al., 2002), which may be less strongly related to one another than FA and MD measured in white matter. MD in subcortical gray matter structures is also sensitive to iron, which has a lower content in older than younger healthy adults in the thalamus but a higher content in older than younger adults in caudate, putamen, red nucleus and dendate nucleus of the cerebellum (Pfefferbaum et al., 2010).
Using DTI, age-related differences in thalamic microstructure have been reported in men (Ota et al., 2007) and women (Abe et al., 2008). Lower FA and higher MD were reported in a study of healthy older than younger women (n=73 ranging in age from 22 to 70 years) (Abe et al., 2008). A study examining thalamic subregions (anterior lateral, anterior medial, central lateral, central medial, and pulvinar) in 28 healthy right-handed men, aged 21 to 71 years, revealed that older age was associated with lower FA in bilateral anterior medial regions of the thalamus and higher MD in right anterior lateral and bilateral central medial and central lateral thalamic subregions, areas with connections to frontal and pre- and postcentral gyri (Ota et al., 2007). Older age was associated with greater radial (perpendicular), but not axial (parallel), diffusivity in anterior lateral and bilateral central lateral thalamic subregions, which might be related to age-related decreases in number of myelinated neural fibers (cf., Peters et al., 2010). No significant age-related differences in FA or MD, however, were observed in posterior thalamic subregions/pulvinar. Age-related MD differences were more consistently observed than age-related FA differences (Ota et al., 2007), possibly attributable to partial voluming associated with greater CSF presence, which also occurs in edematous tissue of older adults (cf., (Pfefferbaum and Sullivan, 2003). In the Ota et al. study, thalamic subregions were determined on percentage-based divisions of the total area of the thalamus rather than with neuroanatomical markers and results need to be replicated using anatomically segmented thalamic volumes. Nonetheless, the pattern of age-related relations indicates vulnerability of anterior and lateral thalamic regions, which have connections to frontal, motor, and sensory cortices. These disrupted or “freyed” connections affecting thalamic regions have the potential of impairing functions subserved by associated thalamic-cortical circuitry.
Smaller thalamic and striatal (caudate and putamen) volumes and higher regional MD were observed in older age in a multimodal imaging (MRI/DTI) study of men and women (n=100) ranging from 20 to 70 years (Cherubini et al., 2009). Thalamic microstructural damage and atrophy, along with iron deposition in the putamen, were the best predictors of aging. Regression analyses indicated that although thalamic volume and MD values were not significantly correlated, together they accounted for 57% of the age variance in the thalamus, and each made independent contributions to the variance. No age relations emerged on any imaging metric for the hippocampus, amygdala, globus pallidus, and accumbens.
The relevance of the thalamus to frontal-parietal networks was demonstrated in a study of 87 healthy adults, age 20 to 73 years, that used a voxel-based approach to isolate regional white matter on structural MRI data for DTI analysis (Grieve et al., 2007). Quantification revealed that lower FA in frontal, temporal, and parietal lobes, but not occipital lobes, in older than younger participants that was greatest in frontal regions. Significant bilateral clusters of voxels extending from the prefrontal to parietal lobes with extensions to the anterior thalamus were associated with performance on an executive maze task, assessed as time to complete the maze two times consecutively without any mistakes. This measure reflected planning and execution processes and error monitoring and correction.
Overall, these studies demonstrate age-related differences in the microstructure of thalamic gray matter, particularly in the anterior thalamus, and related white matter pathways, particularly those associated with frontal and parietal regions. How these age-related microstructural changes translate into age-related changes in cognition may be better answered by examining how gray and white matter structural integrity affects the function of brain networks.
Structural and Functional Thalamic Networks, Aging, and Cognition
Aging affects structural and functional brain network organization (Meunier et al., 2014; Stam, 2014; Wu et al., 2012), and alterations of functional connectivity of these networks has been associated with cognitive decline in aging (Ystad et al., 2011). Distributed brain networks include nodes and hubs, regions that are highly connected to other brain regions possibly relevant to structurally-based network connections. The thalamus is connected to cortical, subcortical, and cerebellar structures and regions, and the effects of aging on these interacting and interconnecting networks are still under investigation. Thus far, a number of specific connections between the thalamus and cerebral cortex have been identified in vivo via fully automated probabilistic tractography (Behrens et al., 2003). Behrens and colleagues were the first to provide quantitative inferences of connectivity between human gray matter structures using DTI data. Their probabilistic tractography algorithms differed from traditional maximum-likelihood approaches, which were hampered by low FA as fibers entered gray matter structures. The alternative method determined the probability of each thalamic voxel to be connected to identified ipsilateral cortical areas, thereby overcoming the problem of low FA. Identified thalamocortical projections include (1) prefrontal cortex with mediodorsal nuclei, (2) motor and premotor cortex with ventral anterior and ventral lateral nuclei, (3) somatosensory cortex with ventral posterior nucleus, and (4) occipital and partial cortices with lateral pulvinar nucleus (Behrens et al., 2003). These anatomically distinct regions inferred from patterns of thalamocortical connectivity resulting from seeding thalamic voxels were reproducible across subjects and are consistent with histological studies of thalamic connections to the cortex (Behrens et al., 2003; Johansen-Berg et al., 2005).
Functional connectivity of the thalamus has been inferred with the use of task-activated and resting-state paradigms that measure highly correlated activity based on complex correlational analyses of blood oxygen level dependent (BOLD) fMRI data (Zhang et al., 2008). These functionally connected thalamic networks demonstrate high overall correspondence to structural thalamic connectivity measures as assessed with DTI tractography, although results based on structural and functional connectivity are not always identical (Zhang et al., 2008; Zhang et al., 2010) (Figure 4). In summary, there is growing evidence that probabilistic DTI tractography and fcMRI (functional connectivity magnetic resonance imaging) reflect thalamocortical connectivity pathways and demonstrate that it is possible to trace connections between the thalamus and cortical gray matter regions using in vivo multimodal neuroimaging.
Figure 4.
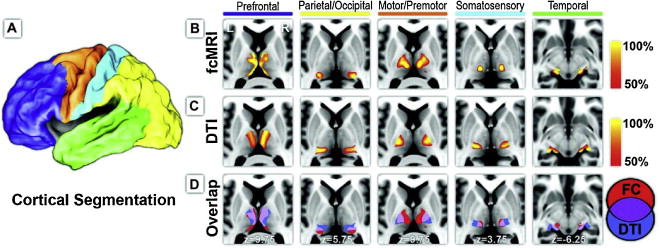
From Zhang et al. (2010) Cerebral Cortex. Vol. 20 Issue 5: 1187–1194, Figure 1. Structural and functional connectivity between cerebral cortex and thalamus. (A) The cortex is partitioned on the basis of major anatomical landmarks into 5 nonoverlapping regions using surface-based ROI definition from CARET (Van Essen 2005; Van Essen et al. 2001). (B) Each cortical area demonstrated specific correlations in its intrinsic neuronal activity with distinct areas of the thalamus. (C) Probabilistic tractography likewise demonstrated specificity of tracking white matter fiber tracks between the thalamus and each cortical area, similar to Behrens et al. (2003). (D) Structural and functional mapping results demonstrated considerable overlap in their connectivity profiles (purple).
Age-related differences in thalamic connections with selective cortical targets was reported by Hughes and colleagues (Hughes et al., 2012) (Figure 5). Volumes of thalamofrontal projections, normalized for brain volume, were smaller in older than younger adults. Specifically, lower FA and higher MD in bilateral thalamofrontal tracts and higher MD in thalamoparietal tracts were related to older age. Further, smaller bilateral thalamofrontal projection volumes and older age were associated with longer times to complete an interference task, assessed with the Stroop.
Figure 5.
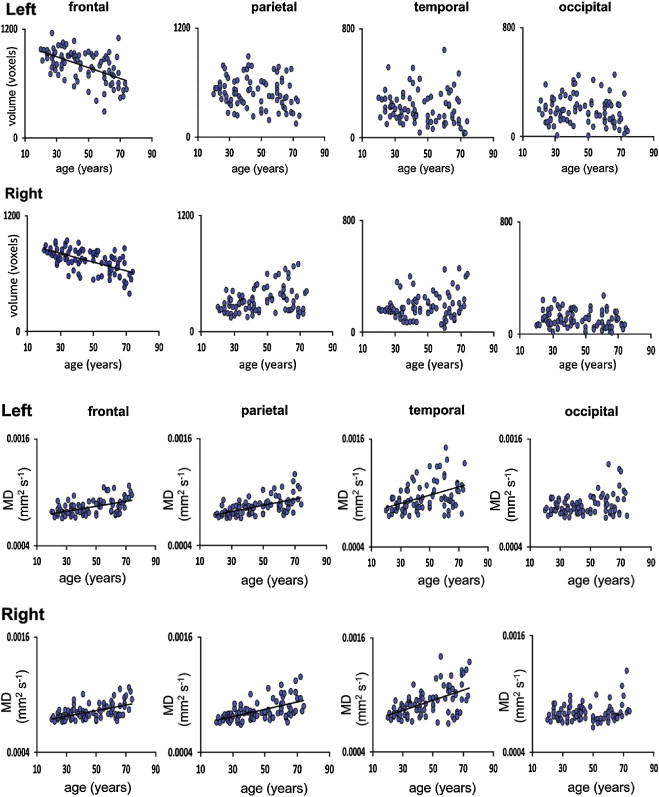
Modified from Hughes et al (2012) NeuroImage Vol. 63: 1134–1142, Figures 2–3. (A) Linear regression plots for normalized volume (voxels) in the left and right thalamo-frontal, thalamo-parietal, thalamo-temporal, and thalamo-occipital projections with age. A regression line is shown for those regions where a significant robust linear regression with age was found (Bonferroni correction; p<0.05/22). (B) Linear regression plots for mean MD (mm2/s) of the left and right thalamo-frontal, thalamo-parietal, thalamo-temporal, and thalamo-occipital projections with age. A regression line is shown for those regions where a significant robust linear regression with age was found (Bonferroni correction; p<0.05/22).
Using resting state fMRI and high model order spatial independent component analysis, relations between verbal episodic memory and thalamic connectivity were reported in a study of 100 healthy middle-aged and older adults, age 49–80 years (Ystad et al., 2010). Total verbal learning, long and short delay free recall, and total recognition discrimination assessed with the California Verbal Learning Test, were negatively related to level of functional connectivity in the thalamus and the superior putamen. Further, functional connectivity strength between the dorsomedial nucleus of the thalamus and parts of the striatum, namely the putamen and head of the caudate nucleus, was negatively related to verbal episodic memory functioning. Taken together, the cross-sectional studies of Hughes et al (2012) and Ystad et al. (2010) suggest that age-related differences in thalamocortical connectivity can contribute to age-related changes in attention, working memory, and episodic memory processes.
Limitation of Studies
The majority of the studies conducted thus far examining the interrelations of the thalamus, aging, and cognition have been cross-sectional in design. Cross-sectional studies take a “snap shot” of structure and function at a single time and can address differences between age groups; however, incipient disease, which is a particular concern in aging studies, and change are only detectable over time, issues that can be addressed in longitudinal studies. Thus, understanding the effects of age and aging on brain structure and function and their relations is best suited for longitudinal studies, although there are practicality concerns, both financial and logistical, which have limited these studies.
Generalizability across studies is often in question, as study participants may not be reflective of the general population, and many studies either do not include an equal number of men and women or differ on demographic variables such as age or education that can be related to the dependent variables assessed. Further, although associations between the thalamus and cognition have been reported, additional studies demonstrating selectivity of these relations are needed to ensure specificity between thalamus and associated pathways and specific working memory, information processing speed, and episodic memory components [for discussion on association, dissociation, and double dissociation models see (Fama and Sullivan, 2014)]. Even in cases where selectivity is demonstrated, however, causation cannot be inferred. As Salthouse cautioned, determination of mediation of brain volume to the relation between thalamus and cognition must be demonstrated in order to have confidence that age-related thalamic changes underlie age-related cognitive changes (for a detailed review see Salthouse, 2011).
Brain imaging studies, regardless of whether they are cross-sectional or longitudinal in design, have inherent limitations. Over the years, in vivo examination of the thalamus and its related networks has been limited by the level of spatial and temporal resolution of existing imaging methodologies. Due to the small size and deep subcortical location of the thalamus, thalamic nuclei have been difficult to assess, even in animal models (Cassel et al., 2013). Methodological issues concerning imaging acquisition and processing as well as heterogeneity among individuals in shape and volume of thalami may contribute to inconsistencies among studies (Hughes et al., 2012). Additionally, despite their usefulness for inference, imaging modalities to date do not have adequate resolution to represent the true distribution of fiber structures or portray real fiber pathways (Le Bihan and Johansen-Berg, 2012). Tractography studies are particularly hindered by partial voluming issues that may result in less than accurate interpretation of network related data (Jones et al., 2013). Advances in imaging acquisition, hardware, and analysis address a number of these limitations (Saranathan et al., 2014; Tourdias et al., 2014), providing greater spatial resolution that will enhance our understanding of the thalamus, its networks, and their relevance to higher cognitive processes (Metzger et al., 2013; Pergola and Suchan, 2013) and resting state network connectivity (Ystad et al., 2011).
Conclusion
Multimodal brain imaging using structural MRI, DTI, and fcMRI has revealed age-related thalamic macrostructure, microstructure, and connectivity differences and changes over the adult lifespan. Smaller thalamic volume, particularly in anterior and medial nuclei, is associated with older age, and size of these nuclei correlate with component processes of episodic memory and executive functions including speed of information processing, directed attention, and working memory. Higher MD and lower FA in regions of the thalamus and its projections, particularly in thalamofrontal and thalamoparietal networks, suggestive of compromised tissue integrity, also occur in older age and are associated with age-related information processing differences between younger and older adults. Taken together, the studies reviewed demonstrate the relevance of the thalamus as a critical node in cognitive networks and suggest that age-related disruption of thalamic connectivity with frontal, parietal, and medial temporal regions, likely play a role in age-related cognitive decline. More refined depiction of the interconnections and interrelationships of large and small brain networks intersecting with the thalamus will be forthcoming with further advances in in vivo structural and functional brain imaging techniques. These refinements will allow for continued investigation of the relationships between age and aging, specific thalamic nuclei, and selective cognitive processes.
Highlights.
Multimodal imaging shows age-related differences in thalamic nuclei and projections
Normal aging differentially affects dissociable thalamic regions and networks
Thalamic connectivity changes may contribute to age-related cognitive decline
Anterior thalamic networks related to encoding and recollective memory processes
Medial thalamic networks related to retrieval and familiarity memory processes
Acknowledgments
This research was supported by AA010723, AA017628, and AA012388
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Masutani Y, Kato N, Kato N, Ohtomo K. Aging in the CNS: comparison of gray/white matter volume and diffusion tensor data. Neurobiology of Aging. 2008;29:102–116. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Adams RD, Victor M. Principles of Neurology. 7. McGraw-Hill, Inc; New York: 1993. [Google Scholar]
- Aggleton JP. Looking beyond the hippocampus: old and new neurological targets for understanding memory disorders. Proceeding of the Royal Society: Biological Sciences. 2014:281. doi: 10.1098/rspb.2014.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behavioral Brain Science. 1999;22(3):425–44. discussion 444–89. [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progress in Brain Research. 1990;85:119–146. [PubMed] [Google Scholar]
- Basser P, Pierpaoli C. Recollections about our 1996 JMR paper on diffusion anisotropy. Journal of Magnetic Resonance. 2011;213:571–572. doi: 10.1016/j.jmr.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative diffusion tensor MRI. Journal of Magnetic Resonance Series B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 2003;6(7):750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Lombardi MG, Caltagirone C. Vascular thalamic amesia: a reappraisal. Neuropsychologia. 2011;49:777–789. doi: 10.1016/j.neuropsychologia.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Cassel J-C, de Vasconcelos AP, Loureiro M, Cholvin T, Dalrymple-Alford JC, Vertes RP. The reuniens and rhomboid nuclei: neuroanatomy, electrophysiological characteristics and behavioral implications. Progress in Neurobiology. 2013;111(34–52) doi: 10.1016/j.pneurobio.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Zahr NM, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: A window into white matter integrity of the working brain. Neuropsychology Review. 2010;20(2):209–225. doi: 10.1007/s11065-010-9129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini A, Peran P, Caltagirone C, Sabatini U, Spalletta G. Aging of subcortical nuclei: microstructural, mineralization and atrophy modifications measured in vivo using MRI. NeuroImage. 2009;48:29–36. doi: 10.1016/j.neuroimage.2009.06.035. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Husain M, Crinion J, Bird CM, Khan SS, Losseff N, Howard RS, Leff AP. The role of the thalamus in amnesia: a tractography, high-resolution MRI and neuropsychological study. Neuropsychologia. 2008;46:2745–2758. doi: 10.1016/j.neuropsychologia.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Fama R, Sullivan EV. Methods of association and dissociation for establishing selective brain-behavior relations. In: Sullivan EV, Pfefferbaum A, editors. Handbook of Clinical Neurology. Elsevier; Oxford: 2014. pp. 175–181. Alcohol and the Nervous System. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley CM. The Behavioral Neurology of White Matter. Oxford University Press; Oxford: 2001. [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Holland D, Dale AM, Walhovd KB, Initiative ADN. Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiology of Aging. 2013;34:2239–2247. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man: Part 1. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Graf P, Schacter DL. Implicit and explicit memory for new associations in normal and amnesic subjects. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1985;11:501–518. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- Grieve KL, Acuna C, Cudeiro J. The primate puulvinar nuclei: vision and action. Trends in Neuroscience. 2000;23:35–39. doi: 10.1016/s0166-2236(99)01482-4. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. American Journal of Neuroradiology. 2007;28(2):226–235. [PMC free article] [PubMed] [Google Scholar]
- Haber S, McFarland NR. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist. 2001;7(4):315–324. doi: 10.1177/107385840100700408. [DOI] [PubMed] [Google Scholar]
- Hughes EJ, Bond J, Svrckova P, Makropoulos A, Ball G, Sharp DJ, Edwards AD, Hajnal JV, Counsell SJ. Regional changes in thalamic shape and volume with increasing age. NeuroImage. 2012;63:1134–1142. doi: 10.1016/j.neuroimage.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI .1. Localization of age-related changes. Biological Psychiatry. 1991;29(1):55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22(4):581–94. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TEJ, Sillery EL, Ciccarelli O, Thompson AJ, Smith SM, Matthews PM. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cerebral Cortex. 2005;15:31–39. doi: 10.1093/cercor/bhh105. [DOI] [PubMed] [Google Scholar]
- Jones D, Simmons A, Williams S, Horsfield M. Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magnetic Resonance in Medicine. 1999;42:37–41. doi: 10.1002/(sici)1522-2594(199907)42:1<37::aid-mrm7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. The Korsakoff Syndrome. British Journal of Psychiatry. 1995;166:154–173. doi: 10.1192/bjp.166.2.154. [DOI] [PubMed] [Google Scholar]
- LaBerge D. Networks of Attention. In: Gazzaniga MS, editor. The New Cognitive Neurosciences. MIT Press; Cambridge: 2000. pp. 711–724. [Google Scholar]
- LaBerge D, Buchsbaum MS. Positron emission tomographic measurements of pulvinar activity during an attention task. The Journal of Neuroscience. 1990;10(2):613–619. doi: 10.1523/JNEUROSCI.10-02-00613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4(6):469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Johansen-Berg H. Diffusion MRI at 25: Exploring brain tissue structure and function. NeuroImage. 2012;61:324–341. doi: 10.1016/j.neuroimage.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Pare D. Coherent oscillations in specific and non-specific thalamocortical networks and their role in cognition. In: Steriade M, Jones EG, McCormick DA, editors. Thalamus. II. Elsevier; Amsterdam: 1997. pp. 501–516. [Google Scholar]
- Magnotta VA, Gold S, Andreasen NC, Ehrhardt JC, Yuh WTC. Visualization of subthalamic nuclei with cortex attentuated inversion recovery MR imaging. NeuroImage. 2000;11:341–346. doi: 10.1006/nimg.2000.0552. [DOI] [PubMed] [Google Scholar]
- Metzger CD, Van Der Werf YD, Walter M. Functional mapping of thalamic nuclei and their integration into cortico-striatal-thalamo-cortical loops via ultra-high resolution imaging - from animal anatomy to in vivo imaging in humans. Frontiers in Neuoscience. 2013:7. doi: 10.3389/fnins.2013.00024. [DOI] [PMC free article] [PubMed]
- Meunier D, Stamatakis EA, Tyler LK. Age-related functional reorganization, structural changes, and presered cognition. Neurobiology of Aging. 2014;35:42–54. doi: 10.1016/j.neurobiolaging.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and Stereotactic Atlas of the Human Thalamus. The Journal of Comparative Neurology. 1997;387:588–630. doi: 10.1002/(sici)1096-9861(19971103)387:4<588::aid-cne8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones D, Summers P, Morris R, Williams S, Markus H. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Ota M, Obata T, Akine Y, Ito H, Matsumoto R, Ikehira H, Asada T, Suhara T. Laterality and aging of thalamic subregions measured by diffusion tensor imaging. NeuroReport. 2007;18(10):1071–1075. doi: 10.1097/WNR.0b013e3281c10e27. [DOI] [PubMed] [Google Scholar]
- Pergola G, Suchan B. Associative learning beyond the medial temporal lobe: many actors on the memory stage. Frontiers in Behavioral Neuroscience. 2013 doi: 10.3389/fnbeh.2013.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB. How the primate fornix is affected by age. The Journal of Comparative Neurology. 2010;518:3962–3980. doi: 10.1002/cne.22434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. Diffusion tensor imaging of deep gray matter brain structures: Effects of age and iron concentration. Neurobiology of Aging. 2010;31(3):482–493. doi: 10.1016/j.neurobiolaging.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. NeuroImage. 2013;65:176–193. doi: 10.1016/j.neuroimage.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage. 2002;15:708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: Relationship to anisotropy and partial voluming. Magnetic Resonance in Medicine. 2003;49:953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- Philp DJ, Korgaonkar MS, Grieve SM. Thalamic volume and thalamo-cortical white matter tracts correlate with motor and verbal memory performance. NeuroImage. 2014;91:77–83. doi: 10.1016/j.neuroimage.2013.12.057. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U. Only time will tell: cross-sectional studies offer no solution to the age-brain-cognition triangle: comment on Salthouse (2011) Psychological Bulletin. 2011;137(5):790–795. doi: 10.1037/a0024503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences, and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychological Bulletin. 2011;137(5):753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saranathan M, Tourdias T, Bayram E, Ghanouni P, Rutt BK. Optimization of white-matter-nulled magnetization prepared rapid gradient echo (MP-RAGE) Imaging. Magnetic Resonance in Medicine. 2014 doi: 10.1002/mrm.25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex. 2008;44(8):1037–66. doi: 10.1016/j.cortex.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Squire LR, Knowlton B, Musen G. The structure and organization of memory. Annual Review of Psychology. 1993;44:453–495. doi: 10.1146/annurev.ps.44.020193.002321. [DOI] [PubMed] [Google Scholar]
- Stam CJ. Modern network science of neurological disorders. Nature Reviews Neuroscience. 2014;15:683–695. doi: 10.1038/nrn3801. [DOI] [PubMed] [Google Scholar]
- Strick PL. How do the basal ganglia and cerebellum gain access to the cortical motor areas? Behavioural Brain Research. 1985;18:107–123. doi: 10.1016/0166-4328(85)90067-1. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience and Biobehavioral Review. 2006;30(6):749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging in aging and age-related disorders. In: Jones DK, editor. Diffusion MRI: Theory, Methods and Applications. Oxford University Press; Oxford: 2010. in press. [Google Scholar]
- Sullivan EV, Pfefferbaum A, Rohlfing T, Baker FC, Padilla ML, Colrain IM. Developmental change in regional brain structure over 7 months in early adolescence: Comparison of approaches for longitudinal atlas-based parcellation. NeuroImage. 2011;57:214–224. doi: 10.1016/j.neuroimage.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Serventi KL, Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiology of Aging. 2004;25:185–192. doi: 10.1016/s0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex. 2003;39(4–5):1107–28. doi: 10.1016/s0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- Tourdias T, Saranathan M, Levesque IR, Su J, Rutt BK. Visualization of intra-thalamic nuclei with optimized white-matter-nulled MPRAGE at 7T. NeuroImage. 2014;84:534–545. doi: 10.1016/j.neuroimage.2013.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong T-K, Guidon A, Song AW. Cortical depth dependence of the diffusion anistropy in the human cortical gray matter in vivo. PLOS ONE. 2014;9:e91424. doi: 10.1371/journal.pone.0091424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ. Memory beyond the hippocampus. Current Opinion in Neurobiology. 1997;7(2):209–216. doi: 10.1016/s0959-4388(97)80009-8. [DOI] [PubMed] [Google Scholar]
- Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;247:301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- Van Der Elst W, Van Boxtel MP, Van Breukele GJ, Jolles J. Assessment of information processing in working memory in applied settings; the paper and pencil memory scannning test. Psychological Medicine. 2007;37:1335–1344. doi: 10.1017/S0033291707000360. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Jolles J, Witter MP, Uylings HBM. Contributions of thalamic nuclei to declarative memory functioning. Cortex. 2003;39:1047–1062. doi: 10.1016/s0010-9452(08)70877-3. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Tisserand DJ, Visser PJ, Hofman PA, Vuurman E, Uylings HB, Jolles J. Thalamic volume predicts performance on tests of cognitive speed and decreases in healthy aging. A magnetic resonance imaging-based volumetric analysis. Cognitive Brain Research. 2001;11(3):377–385. doi: 10.1016/s0926-6410(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of Aging. 2005;26(9):1261–70. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275–8. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang INR, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM. Consistent neuroanatomical age-relared volume differences across multiple samples. Neurobiology of Aging. 2011;32:916–932. doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J, Avula R, Senjem M, Kantarci K, Weigand S, Samikoglu A, Edmonson H, Vemuri P, Knopman D, Boeve B, Petersen R, Josephs K, Jack C. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology. 2010;74:1279–1287. doi: 10.1212/WNL.0b013e3181d9edde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Taki Y, Sato K, Kinomura S, Goto R, Okada K, Kawashima R, He Y, Evans AC, Fukuda H. Age-related changes in topological organization of strutural brain networks in healthy individuals. Human Brain Mapping. 2012;33:552–568. doi: 10.1002/hbm.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad M, Eichele T, Lundervold AJ, Lundervold A. Subcortical functional connectivity and verbal episodic memory in healthy adults - a resting state fMRI study. NeuroImage. 2010;52:379–388. doi: 10.1016/j.neuroimage.2010.03.062. [DOI] [PubMed] [Google Scholar]
- Ystad M, Hodneland E, Adolfsdottir S, Haasz J, Lundervold AJ, Eichele T, Lundervold A. Cortico-striatal connectivity and cognition in normal aging: A combined DTI and resting state fMRI study. NeuroImage. 2011;55:24–31. doi: 10.1016/j.neuroimage.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sandbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. Journal of Neurophysiology. 2008;100:1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalmocortical system. Cerebral Cortex. 2010;20:1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]


