Abstract
Brain pathologies of Alzheimer’s, cerebrovascular and Lewy body diseases are common in old age, but the relationship of these pathologies with progression from normal cognitive function to the various stages of cognitive impairment is unknown. In this study, we fit latent Markov models from longitudinal cognitive data to empirically derive three latent stages corresponding to no impairment, mild impairment, and moderate impairment; then, we examined the associations of common neuropathologies with the rates of transition among these stages. Cognitive and neuropathological data were available from 653 autopsied participants in two ongoing cohort studies of aging who were cognitively healthy at baseline (mean baseline age 79.1 years) and had longitudinal cognitive data. On average, participants in these analyses developed mild impairment 5 years after enrollment, progressed to moderate impairment after an additional 3.4 years, and stayed impaired for 2.8 years until death. AD and chronic macroscopic infarcts were associated with a higher risk of progression to mild impairment and subsequently to moderate impairment. By contrast, Lewy bodies were associated only with progression from mild to moderate impairment. The 5-year probability of progression to mild or moderate impairment was 20% for persons without any of these three pathologies, 38% for AD only, 51% for AD and macroscopic infarcts, and 56% for AD, infarcts and Lewy bodies. Thus, the presence of AD pathology alone nearly doubles the risk of developing cognitive impairment in late life, and the presence of multiple pathologies further increases this risk over multiple years prior to death.
Keywords: Neuropathologies, Progression of Cognitive Impairment, Latent Markov Model
INTRODUCTION
Neuropathologic diagnoses of Alzheimer’s (AD), cerebrovascular (CVD) and Lewy bodies’ diseases (LBD) are common in older persons (Markesbery, 2010, Rahimi and Kovacs, 2014). Cross-sectional studies show that these common pathologies are associated with cognitive impairment and dementia (Cholerton, et al., 2013, Guillozet, et al., 2003, Nelson, et al., 2012, Sonnen, et al., 2007, Troncoso, et al., 2008). Separately, data from longitudinal studies suggest that the pathologies are also associated with faster cognitive decline (Nelson, et al., 2009, Pietrzak, et al., 2015, Smits, et al., 2015). However, most prior studies assume a linear decline in cognition such that the associations of pathologies with rate of decline are constant throughout the trajectory. Accumulating evidence suggests that the profile of cognitive aging may follow a nonlinear trajectory (Johnson, et al., 2009, Reiman, et al., 2011, Small and Backman, 2007, Sperling, et al., 2011). As neuropathology accumulates, dementia begins with a preclinical stage with little or no manifestation of cognitive impairment, progresses through an intermediate stage characterized by a slow but steady decline in cognition, and ends with a terminal stage proximate to death during which a precipitous drop in cognition is evident (Wilson, et al., 2012). This evidence of nonlinearity poses a new question regarding the extent to which common neuropathologies are associated with rates of progression from normality to the various stages of cognitive impairment.
Limited data are available about the relationship of various neuropathologies to cognition across the spectrum from no impairment to clear impairment. One prior study showed that trajectories of cognitive decline differed between AD and vascular dementia, where the onset of decline was earlier in AD dementia, but the rate of decline was faster after diagnosis in vascular dementia (Laukka, et al., 2012). We previously showed that infarct pathology was associated with early (or pre-terminal) cognitive decline; by contrast, Lewy bodies were more strongly associated with later (or terminal) decline (Wilson, et al., 2010). These findings suggest the hypothesis that there may be differential associations of neuropathologies with rates of progression from no impairment to mild impairment, and subsequently to moderate impairment.
To investigate this hypothesis, we analyzed annual longitudinal cognitive data for up to 20 years from a group of community based older persons who were cognitively healthy at enrollment and underwent autopsy after death. We empirically determined three cognitive impairment stages: no impairment, mild impairment, and moderate impairment using latent Markov models. We estimated the mean sojourn time at each stage, as well as the rates of progression from one stage to the next, and examined how pathologic AD, macroscopic cerebral infarcts, and Lewy bodies were associated with the rates of progression.
METHODS
Participants
Participants came from two ongoing clinical pathological cohort studies of aging and dementia, the Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP) (Bennett, et al., 2012a, Bennett, et al., 2012b). Both studies were approved by the Institutional Review Board of Rush University Medical Center. Each participant signed an informed consent and an anatomical gift act form, and agreed to annual neuropsychological and clinical evaluations, as well as to brain donation after death.
At time of these analyses, 1,385 ROS and MAP participants had died and 1,197 (86.4%) had undergone brain autopsies. Of the 1,197, 670 had no cognitive impairment (NCI) at baseline with at least 2 cognitive measures, and 17 participants were excluded due to incomplete neuropathological measures. This left a total of 653 participants whose data were analyzed in this study. The mean age at baseline was 79.1 years (standard deviation [SD] = 6.7 years); the mean age at death was 88.0 years (SD = 6.7 years); the mean level of education was 16.5 years (SD = 3.8 years); and 435 (66.6%) were females.
Neuropsychological Assessment of Cognition
Participants underwent uniform structured neuropsychological assessments at baseline and subsequent follow-up waves. The assessments include 17 cognitive tests which evaluate a broad range of cognitive abilities. Specifically, 7 tests (immediate and delayed recall of story A from logical memory, immediate and delayed recall of the east Boston story, word list memory, word list recall, and word list recognition) assess episodic memory, 3 tests (verbal fluency, Boston naming, and reading test) assess semantic memory; 3 tests (digit span forward and backward, and digit ordering) assess working memory; 2 tests (symbol digit modality and number comparison) assess perceptual speed, and the last 2 tests (standard progressive matrices and judgment of line orientation) assess visuospatial ability. Raw scores on each test were standardized using the mean and standard deviation at the baseline evaluation. These standardized scores were then averaged to obtain a composite measure of global cognition. A higher score indicates better cognitive performance. Every 1 unit represents 1 standard unit above (if positive) or below (if negative) the mean baseline cognition of the entire pooled cohort. The use of this composite measure is supported by results from previous factor analyses where the leading principal component accounts for a majority of the total variance. Psychometric properties of the measure have been described previously (Krueger, et al., 2009, Wilson, et al., 2003, Wilson, et al., 2005, Wilson, et al., 2002).
Cognitive assessments were administered annually up to 20 years. The composite measure of cognition is assigned missing if participants skipped an assessment due to logistic difficulty or could not complete at least 6 tests during the assessment. Of the 653 participants included, 100 participants had 1 missing cognitive measure, and 28 had 2 or more missing cognitive measures. Together, these missing measures account for about 3% of the total cognitive data in these analyses. The mean duration between consecutive visits was 1.04 years (SD 0.28, Interquartile Range [IQR] 0.99–1.03), and the mean duration between last evaluation and death was 0.81 years (SD 1.02, IQR 0.33–0.91).
Clinical Diagnosis of Dementia and Mild Cognitive Impairment
Annual clinical diagnosis was performed by clinicians blinded to previous diagnoses (Bennett, et al., 2005). Clinical diagnosis of dementia follows the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association. A dementia diagnosis requires a history of cognitive decline and evidence of impairment in multiple cognitive domains (McKhann, et al., 1984). Participants who did not meet criteria for dementia but showed impairment in at least one cognitive domain were classified as having mild cognitive impairment (MCI).
Neuropathologic Assessment of AD, Infarcts and Lewy Bodies
Brain autopsies were performed following a standard procedure (Schneider, et al., 2009). Multiple neuropathology measures were examined, including pathological AD diagnosis, chronic macroscopic infarcts and Lewy bodies. Pathological AD diagnosis was based on the National Institute on Aging-Reagan criteria which requires an intermediate likelihood or a high likelihood of AD (Hyman and Trojanowski, 1997). Fixed slabs and/or pictures of the brains were visually examined for the presence of chronic macroscopic infarcts, which were verified after dissection and histologic review (Schneider, et al., 2003). The presence of nigral, limbic and neocortical Lewy bodies were identified using alpha-synuclein immunohistochemistry (Zymed, 1:100; or Wako #015-25191; 1:20,000).
Statistical Analysis
In this study, we used latent Markov models to investigate the associations of common neuropathologies with the progression from cognitive normality, through mild impairment, to more severe impairment or dementia prior to death. Markov models provide a popular statistical approach for capturing the progression of chronic diseases (Aalen, et al., 1997, Corpechot, et al., 2000, Deuffic-Burban, et al., 2002, Gentleman, et al., 1994, Hsieh, et al., 2002, Kay, 1986, Longini, et al., 1989, Rangel-Frausto, et al., 1998). The models are also widely applied to investigate neurodegenerative disease processes such as AD dementia (Abner, et al., 2012, Commenges, et al., 2004, Harezlak, et al., 2003, Kryscio, et al., 2006, Salazar, et al., 2007, Yu, et al., 2010). The central structure of the model is represented by a transition probability or intensity (i.e. instantaneous probability) matrix which determines the rates of transition between various disease states. Transition probabilities are estimated and can be further modeled as functions of risk factors.
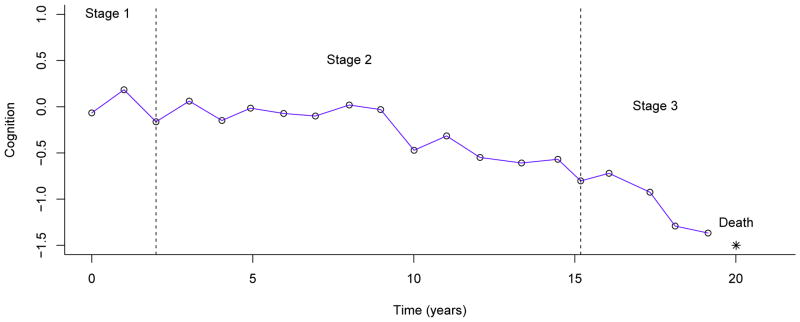
Standard Markov models rely on observed disease states (e.g. clinical diagnosis) which are subject to misclassification. A latent or hidden Markov model estimates disease progression by utilizing data for a disease marker (e.g. cognitive performance) whose probability distribution changes as the underlying unobserved disease state progresses (Jackson and Sharples, 2002). In this study, we chose latent Markov models by using data on longitudinal measure of cognition to characterize a three-stage progression of cognitive impairment (Figure 1). The number of cognitive stages (N=3) was decided a priori to coincide with clinical diagnoses of NCI, MCI and dementia. As cognition declined, participants progressed from less impaired stages to more impaired stages. The model assumes that the observed cognitive scores within each stage share the same mean and standard deviation (SD), which were estimated from the model. In addition, we included a fourth stage for death as a participant could die in any of the 3 cognitive stages. Simultaneously, the transitions between the latent stages as well as death were modeled using the following transition intensity matrix:
Figure 1.
illustrates latent Markov model by using longitudinal cognitive data (open circles) of a representative participant. The asterisk shows time of death. Two vertical dotted lines show the most likely visits for the onset of transitions to mild and moderate impairment, based on the fitted model.
Each element inside the matrix, denoted by qrs, is an unknown parameter represents the instantaneous transition probability from stage r to stage s. We named these stages as (1) no impairment, (2) mild impairment, (3) moderate impairment and (4) death. As a result, q12 is the instantaneous probability of transition from no impairment to mild impairment. Other qs can be interpreted similarly. We hypothesize that cognitive decline is irreversible and the models assume no back transitions. The progressive nature of cognitive decline and the fact that death is an absorbing state determine the 0’s in the matrix. This transition intensity matrix also determines the transition probabilities over a given time period (Jackson and Sharples, 2002).
The associations of neuropathologies with the transitions were examined via proportional hazard models of the form . Here e(βrs) estimates the hazard ratio (HR) of instantaneous transition from stage r to stage s in the presence of a pathological index z. A positive and significant estimate of βrs would indicate that the presence of the pathology is associated with a higher risk of transition from r to s.
The model likelihood was maximized using numerical methods. We examined the stability of the numerical methods by assigning different sets of initial values, and the results were consistent. Statistical significance was set at α level of 0.05. The analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, NC) and the msm Package for R (Jackson, 2012).
RESULTS
All participants in this study were cognitive normal at baseline. On average, they were followed annually for 7.8 years (SD=4.3 years, range = 1–19 years) before death. At autopsy, pathologic AD was present in 379 (58.0%) individuals, 206 (31.6%) had chronic macroscopic infarcts, and 144 (22.1%) had Lewy bodies. Notably, more than 75% of the participants (N = 492) had at least one of these three pathologies, of whom 42% (N = 206) had multiple pathologies (Table 1).
Table 1.
Presence of neuropathologies in the study cases
| Neuropathology Findings | ||||
|---|---|---|---|---|
| AD1 | Macroscopic infarcts | Lewy bodies | Frequency | % |
| Absent | Absent | Absent | 161 | 24.66 |
| Absent | Absent | Present | 36 | 5.51 |
| Absent | Present | Absent | 61 | 9.34 |
| Absent | Present | Present | 16 | 2.45 |
| Present | Absent | Absent | 189 | 28.94 |
| Present | Absent | Present | 61 | 9.34 |
| Present | Present | Absent | 98 | 15.01 |
| Present | Present | Present | 31 | 4.75 |
| Total | 653 | 100% | ||
Pathologic diagnosis of AD according to NIA-Reagan criteria
Identifying Latent Stages of Cognitive Impairment
We first fit an unadjusted latent Markov model with longitudinal cognition as the outcome and time in years since baseline as the time-scale. This model provided estimates of the mean level of cognition for each latent stage. Specifically, the mean score for the stage of no impairment (Stage 1) was 0.39 (SD = 0.36), which was about 0.4 standard units higher than the baseline average of the whole cohort. The mean sojourn time, estimated from the transition matrix, for stage 1 was 5.0 years (95% confidence interval (CI) = 4.6–5.4 years), suggesting that participants remained cognitively intact for an average of 5 years before developing impairment or dying. The mean score for the stage of mild impairment (Stage 2) was −0.25 (SD = 0.27), which was a quarter unit below the cohort baseline average. The mean sojourn time for stage 2 was 3.4 years (95% CI = 3.0–3.7 years), suggesting that participants spent an average of 3.4 years before progressing to moderate impairment or dying. Finally, the mean score for the stage of moderate impairment (Stage 3) was −1.39 (SD=0.78), which was about 1.4 units below the cohort baseline average. The mean sojourn time for stage 3 was 2.8 years (95% CI = 2.4–3.2 years), suggesting that moderate impairment lasted for approximately 3 years before death occurred.
Influence of Neuropathologies on Progression of Cognitive Impairment
We fit a second latent Markov model to examine the association of pathologic AD, infarcts and Lewy bodies with progression of cognitive impairment, adjusted for age, sex and education. First, we assessed the instantaneous probabilities of progression from less impaired stages to more impaired stages, including death, by using the hazard ratios (Table 2). Relative to older persons without pathological AD, the likelihood of a transition from no impairment to mild impairment was 36% higher (HR=1.36, 95% CI=1.07–1.72) for persons with pathologic AD, and the likelihood of a transition from mild impairment to moderate impairment was almost doubled (HR=1.95, 95% CI=1.30–2.92). Similarly, chronic macroscopic infarcts also implies higher risk of progression from no impairment to mild impairment (HR=1.38, 95% CI=1.12–1.72), and subsequently from mild impairment to moderate impairment (HR=1.50, 95% CI=1.12–2.02). Interestingly, Lewy bodies were only associated with risk of progression from mild to moderate impairment (HR=1.48, 95% CI=1.08–2.03) and the association with the transition from no impairment to mild impairment was not significant. The instantaneous risk of death directly from no impairment (i.e. death without going through any impairment stages) was significantly lower for participants with neuropathologies, particularly AD, due to the association of pathology with cognitive impairment.
Table 2.
Hazard ratios (95% CI) for demographic and neuropathologic variables for transitions of cognitive impairment
| Stage 1→ Stage 2 | Stage 1→ Stage 4 | Stage 2→ Stage 3 | Stage 2→ Stage 4 | Stage 3→ Stage 4 | |
|---|---|---|---|---|---|
| Age at baseline | 1.08 (1.06–1.10)* | 0.99 (0.95–1.03) | 1.00 (0.97–1.02) | 1.02 (0.99–1.06) | 1.00 (0.98–1.02) |
| Male sex | 1.08 (0.86–1.36) | 1.15 (0.73–1.81) | 1.01 (0.72–1.41) | 0.87 (0.52–1.43) | 1.40 (1.04–1.89)* |
| Education | 0.93 (0.91–0.96)* | 1.01 (0.95–1.07) | 0.99 (0.95–1.03) | 1.00 (0.95–1.06) | 1.03 (1.00–1.07) |
| AD | 1.36 (1.07–1.72)* | 0.40 (0.24–0.66)* | 1.95 (1.30–2.92)* | 0.38 (0.23–0.61)* | 0.81 (0.57–1.16) |
| Macro infarcts | 1.38 (1.12–1.72)* | 0.56 (0.31–1.04) | 1.50 (1.12–2.02)* | 0.33 (0.18–0.62)* | 0.98 (0.75–1.28) |
| Lewy bodies | 1.17 (0.93–1.48) | 0.58 (0.29–1.14) | 1.48 (1.08–2.03)* | 0.60 (0.31–1.16) | 0.77 (0.58–1.03) |
1. Stage 1, no impairment; Stage 2, mild impairment; Stage 3, moderate impairment; Stage 4, death.
2. The reference group refers to female participants with age 80 at baseline, with 15 years of education, and without any of the three neuropathologic findings.
3. *Hazard ratios that were statistically significant at α=0.05.
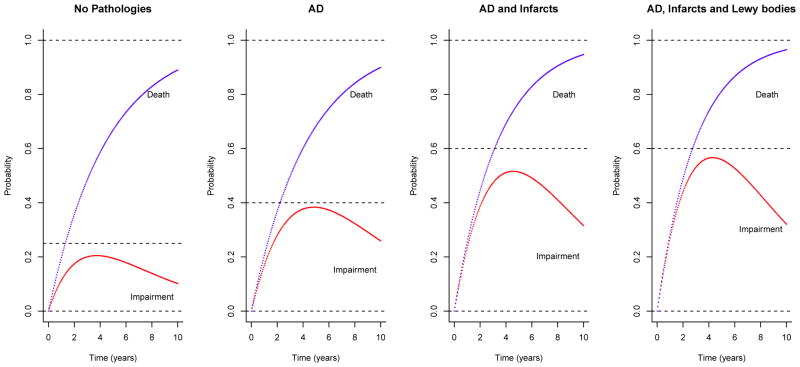
Next, we estimated transition probabilities from no impairment to mild/moderate impairment over multiple years prior to death. The results are illustrated in Figures 2 and 3. For participants with no AD, infarcts or Lewy body pathology, the 5-year probability of progressing from no to mild impairment was 16.6% (95% CI = 12.3%–20.4%), and the probability of progressing to moderate impairment was only 3.0% (95% CI = 1.8%–4.5%). That is, the likelihood of being alive and having developed cognitive impairment in 5 years was about 20% for participants free of the pathologic indices of most common causes of dementia. By contrast, the likelihood of developing cognitive impairment was almost double for participants with pathological AD diagnosis. The 5-year probability of progressing from no cognitive impairment to mild impairment among participants with AD was 28.0% (95% CI = 23.4%–32.0%), and the probability of progressing to moderate impairment was 10.4% (95% CI = 8.0%–12.9%). Adding these together, the 5-year probability of transitioning from no impairment to an impairment stage for participants with AD was 38.4%. The presence of multiple pathologies substantially increased the likelihood of developing more severe stage of impairment. For participants with both AD and macroscopic infarcts, the 5-year probabilities of progressing to mild and moderate impairment were 32.1% (95% CI = 26.5%–37.4%) and 19.2% (95% CI = 15.1%–23.3%) respectively; for participants with AD, infarcts and Lewy bodies, the 5-year probability of progressing to mild impairment was 26.2% (95% CI = 19.0%–33.0%), but the probability of progressing to moderate impairment was almost tripled (5-year probability = 29.7%, 95% CI = 23.3%–35.6%) compared to participants with AD only.
Figure 2.
illustrates estimated transition probabilities for persons with no common pathologies (Panel 1), AD only (Panel 2), AD and infarcts (Panel 3), and AD, infarcts and Lewy bodies diseases (Panel 4). The lower curves in each panel show the probabilities of transition from no impairment to cognitive impairment (mild and moderate combined) for a given time period (up to 10 years); and the upper curves show the cumulative transition probabilities from no impariment to impairment or death. The probabilities are shown for representative participants in the sample, that is, for females with 80 years old at baseline and with 15 years of education.
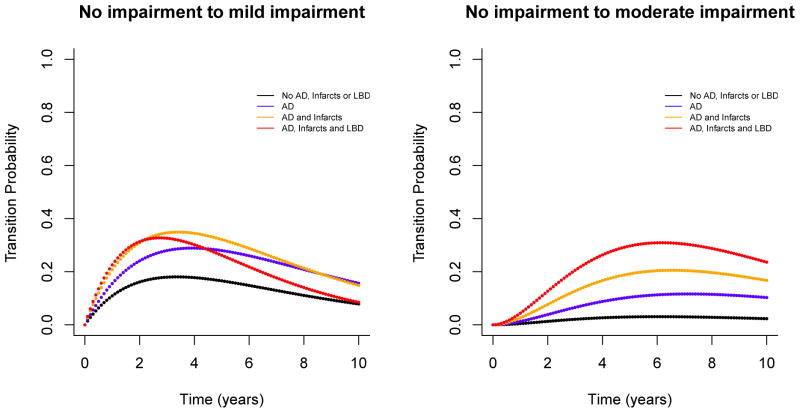
Figure 3.
illustrates estimated probabilities of transition from no impairment to mild impairment (left panel) and moderate impairment (right panel) for a given time period (up to 10 years). Within each panel, different curves represent transition probabilities for different burdens of common neuropathologies (black: no pathology; blue: AD only; orange: AD and macroscopic infarcts; red: AD, macroscopic infarcts and Lewy bodies). The probabilities are shown for representative participants in the sample, that is, for females with 80 years old at baseline and with 15 years of education.
Comparison of Latent Stages with Clinical Diagnoses
Based on the adjusted model, we estimated the most likely series of the latent stages from the longitudinal cognitive data for each participant. Over the course of follow-up, approximately a third of the participants (N=219, 33.5%) had no transition, a third (N=215, 32.9%) transitioned to mild impairment and remained mildly impaired, and a third (N=219, 33.6%) transitioned to moderate impairment proximate to death.
To facilitate comparison with annual clinical diagnoses, we looked at series of latent stages with annual clinical diagnoses (Table 3). Notably, as our annual clinical diagnoses are blinded to previous diagnoses, there were some back transitions. For example, 27.8% of the MCI diagnoses were followed by NCI diagnosis the next year, and 12.8% of dementia were diagnosed MCI the following year. For the purpose of comparison, we focused on forward transitions. As shown in Table 3, the year-to-year transitions were consistent between annual clinical diagnoses and latent stages derived from the transition model. For instance, using annual clinical diagnosis, 14.0% of the NCI diagnoses were followed by MCI the next year, 1.8% by dementia, and 7.4% by death. In comparison, using latent stages derived from the Markov model (Table 2), for visits of no impairment, 12.1% progressed to mild impairment in the following year; 0.6% progressed to moderate impairment; and 6.4% died. The findings for other forward transitions were also similar.
Table 3.
Clinical diagnoses and latent stages for pairs of consecutive evaluations
| Consecutive annual clinical diagnoses | |||||
|---|---|---|---|---|---|
| Prior visits | Subsequent visits | ||||
| Frequency Row Pct | NCI | MCI | Dementia | Death | Total |
| NCI | 3133 | 573 | 75 | 301 | 4082 |
| 76.75 | 14.04 | 1.84 | 7.37 | ||
| MCI | 286 | 397 | 173 | 172 | 1028 |
| 27.82 | 38.62 | 16.83 | 16.73 | ||
| Dementia | 10 | 58 | 204 | 180 | 452 |
| 2.21 | 12.83 | 45.13 | 39.82 | ||
| Total | 3429 | 1028 | 452 | 653 | 5562 |
| Consecutive latent stages | |||||
|---|---|---|---|---|---|
| Prior visits | Subsequent visits | ||||
| Frequency Row Pct | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Total |
| Stage 1 | 2755 | 413 | 21 | 219 | 3408 |
| 80.84 | 12.12 | 0.62 | 6.43 | ||
| Stage 2 | - | 1165 | 198 | 215 | 1578 |
| 73.83 | 12.55 | 13.62 | |||
| Stage 3 | - | - | 357 | 219 | 576 |
| 61.98 | 38.02 | ||||
| Total | 2755 | 1578 | 576 | 653 | 5562 |
1. NCI, no cognitive impairment; MCI, mild cognitive impairment
2. Stage 1, no impairment; Stage 2, mild impairment; Stage 3, moderate impairment; Stage 4, death.
3. Numbers in bold font correspond to forward transitions.
DISCUSSION
Identifying the profile of cognitive aging has long been considered essential to prevent cognitive decline in old age. The profile can be characterized by different stages where an asymptomatic, cognitively normal stage precedes a mild impairment stage which is followed by a dementia stage. However, little is known about the length of each stage, the likelihood of the risks of transition from one stage to the next, or how common neuropathologies influence risks of these transitions. The findings from this study shed some light on these questions.
First, we empirically dissected the cognitive trajectory into 3 stages of no impairment, mild impairment and moderate impairment, and estimated the average onset of mild and moderate cognitive impairment. The Markov models imply that on average, older persons in these analyses were cognitively normal for 5 years before developing impairment, they spent a mean of 3.4 years in a mildly impaired stage before progressing to a moderately impaired stage, and remained there for an additional 2.8 years until death. In general, these time intervals are consistent with findings obtained using other approaches. For example, using random change-point models, we previously reported that the acceleration of cognitive decline for subjects who were NCI at enrollment occurred about 5.6 years after baseline (Yu, et al., 2012). Separately, we also reported that a steeper terminal decline occurred 3 years prior to death (Yu, et al., 2013).
Second, prior literature has established the association of pathologies with an increased risk of dementia and faster rate of cognitive decline. In this study, we extend these results by investigating the effects of pathologies with respect to progression of cognitive impairment. For older persons with AD or chronic macroscopic infarcts, their history of longitudinal cognitive performance shows an increased risk of transition from no impairment to mild impairment as well as an increased risk of transition from mild to moderate impairment. Consistent with prior reports (Wilson, et al., 2010), this result suggests that the pathologies of AD and infarcts influence cognition many years prior to death. In comparison, older persons with Lewy bodies had an increased risk of transitioning only from mild to moderate impairment, suggesting that the impact of Lewy body pathology on cognition may be more potent during the later stage of cognitive aging. Another possibility is that Lewy bodies develop at later age.
In addition, the transition probabilities from no impairment to mild and moderate impairment over multiple years revealed an interesting pattern (Figure 3). All transition probabilities increase over the first few years, peak at a certain time point and then decrease, likely due to competing risk of death. Interestingly, for participants with AD, infarcts, and Lewy bodies, the multiple-year transition probability from no impairment to mild impairment decreased earlier than participants with no Lewy bodies. This result reflects that mild impairment is a more transient stage and participants with all three pathologies are likely to transition to more impaired stages over fewer years. Indeed, the probabilities of transitioning to moderate impairment for these participants are greatly increased over the same period.
We and others have previously explored random change-points models to allow the rate of cognitive decline to accelerate over the course of the follow-up and to estimate the onset of the acceleration as well as the rates of decline before and after such acceleration. While useful, change-point models are highly complex and computationally intensive, especially when multiple acceleration points are estimated. The application of the latent Markov models to longitudinal cognitive measures provides a complementary tool for investigating the progression of cognitive aging from normality to the various stages of impairment. We choose latent Markov model over regular Markov model for two major reasons. First, we observe a fair number of back transitions using annual clinical diagnoses (Table 3). The transitions (i.e. from MCI to NCI, and from dementia to MCI or NCI), while informative, likely are not reflective of the progressive nature of the dementia process. Second, the psychometric properties of our cognitive measures have been rigorously tested and are well-established. The same measures are administered across individuals at relatively evenly spaced time intervals up to 20 years. The follow-up rate among survivors is extraordinarily high, and there is very little missing data. These desirable features of the cognitive measures allow us to securely and reliably capture trajectories of cognitive decline.
Limitations of the study are noted. First, we used postmortem pathological indices in the analysis. While these indices are useful in differentiating associations with progression of cognitive impairment and are important determinants of cognitive decline many years prior to death, eventually it may prove possible to measure evolution of the neuropathology in vivo and to correlate dynamic changes in pathology with longitudinal cognitive assessments. Second, regional distributions of particular pathologies may contribute differentially to the progression of cognitive impairment. For example, prior studies suggest that the influence of Lewy body pathology on cognitive decline is mostly driven by neocortical Lewy bodies (Schneider, et al., 2012, Wilson, et al., 2010). While our current sample size was insufficient to determine the influence of neocortical versus other Lewy body pathology, we will certainly revisit this important question in future analyses as more postmortem samples are being collected.
CONCLUSIONS
In this study, we characterized the cognitive trajectories of a group of community based older persons with three progressive stages: a cognitively normal stage, a mild impairment stage, and then a more severe impairment stage. We estimated the onsets of impairment, the rates of progression from cognitive normality to different stages of impairment, and examined how neuropathologies of AD, infarcts and Lewy bodies influenced the progression. We found that each neuropathology was associated with a higher risk for cognitive impairment in late life, the presence of multiple pathologies further increased such risk, and importantly, different sets of neuropathologies were implicated at different stages of progression.
We fit latent Markov models from longitudinal cognitive data to empirically derive three latent stages corresponding to no impairment, mild impairment, and moderate impairment.
We examined the associations of common neuropathologies with the rates of transition among these latent stages.
Participants developed mild impairment 5 years after enrollment, progressed to moderate impairment after an additional 3.4 years, and stayed impaired for 2.8 years before death.
AD pathology alone nearly doubles the risk of developing cognitive impairment in late life, and the presence of multiple pathologies further increases this risk over multiple years prior to death.
Acknowledgments
This study was supported by National Institute on Aging grants R01AG17917, R01AG34374, R01AG15819, R01AG038651 and P30AG10161. We thank all the participants of the Religious Order Study and the Rush Memory and Aging Project, as well as the staff at the Rush Alzheimer’s Disease Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aalen OO, Farewell VT, De Angelis D, Day NE, Gill ON. A Markov model for HIV disease progression including the effect of HIV diagnosis and treatment: application to AIDS prediction in England and Wales. Statistics in medicine. 1997;16(19):2191–210. doi: 10.1002/(sici)1097-0258(19971015)16:19<2191::aid-sim645>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Abner EL, Kryscio RJ, Cooper GE, Fardo DW, Jicha GA, Mendiondo MS, Nelson PT, Smith CD, Van Eldik LJ, Wan L, Schmitt FA. Mild cognitive impairment: statistical models of transition using longitudinal clinical data. International journal of Alzheimer’s disease. 2012;2012:291920. doi: 10.1155/2012/291920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Current Alzheimer research. 2012a;9(6):628. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64(5):834–41. doi: 10.1212/01.wnl.0000152982.47274.9e. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Current Alzheimer research. 2012b;9(6):646. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholerton B, Larson EB, Baker LD, Craft S, Crane PK, Millard SP, Sonnen JA, Montine TJ. Neuropathologic correlates of cognition in a population-based sample. Journal of Alzheimer’s disease: JAD. 2013;36(4):699–709. doi: 10.3233/JAD-130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commenges D, Joly P, Letenneur L, Dartigues JF. Incidence and mortality of Alzheimer’s disease or dementia using an illness-death model. Statistics in medicine. 2004;23(2):199–210. doi: 10.1002/sim.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot C, Carrat F, Bonnand AM, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis. Hepatology (Baltimore, Md) 2000;32(6):1196–9. doi: 10.1053/jhep.2000.20240. [DOI] [PubMed] [Google Scholar]
- Deuffic-Burban S, Poynard T, Valleron AJ. Quantification of fibrosis progression in patients with chronic hepatitis C using a Markov model. Journal of viral hepatitis. 2002;9(2):114–22. doi: 10.1046/j.1365-2893.2002.00340.x. [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Lawless JF, Lindsey JC, Yan P. Multi-state Markov models for analysing incomplete disease history data with illustrations for HIV disease. Statistics in medicine. 1994;13(8):805–21. doi: 10.1002/sim.4780130803. [DOI] [PubMed] [Google Scholar]
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Archives of neurology. 2003;60(5):729–36. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- Harezlak J, Gao S, Hui SL. An illness-death stochastic model in the analysis of longitudinal dementia data. Statistics in medicine. 2003;22(9):1465–75. doi: 10.1002/sim.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HJ, Chen TH, Chang SH. Assessing chronic disease progression using non-homogeneous exponential regression Markov models: an illustration using a selective breast cancer screening in Taiwan. Statistics in medicine. 2002;21(22):3369–82. doi: 10.1002/sim.1277. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. Journal of neuropathology and experimental neurology. 1997;56(10):1095–7. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Jackson CH. Multi-State Models for Panel Data: The msm Package for R. Journal of Statistical Software. 2012;38(8):1–29. [Google Scholar]
- Jackson CH, Sharples LD. Hidden Markov models for the onset and progression of bronchiolitis obliterans syndrome in lung transplant recipients. Statistics in medicine. 2002;21(1):113–28. doi: 10.1002/sim.886. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Archives of neurology. 2009;66(10):1254–9. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay R. A Markov model for analysing cancer markers and disease states in survival studies. Biometrics. 1986;42(4):855–65. [PubMed] [Google Scholar]
- Krueger KR, Wilson RS, Bennett DA, Aggarwal NT. A battery of tests for assessing cognitive function in older Latino persons. Alzheimer disease and associated disorders. 2009;23(4):384–8. doi: 10.1097/WAD.0b013e31819e0bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryscio RJ, Schmitt FA, Salazar JC, Mendiondo MS, Markesbery WR. Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology. 2006;66(6):828–32. doi: 10.1212/01.wnl.0000203264.71880.45. [DOI] [PubMed] [Google Scholar]
- Laukka EJ, Macdonald SW, Fratiglioni L, Backman L. Preclinical cognitive trajectories differ for Alzheimer’s disease and vascular dementia. Journal of the International Neuropsychological Society: JINS. 2012;18(2):191–9. doi: 10.1017/S1355617711001718. [DOI] [PubMed] [Google Scholar]
- Longini IM, Jr, Clark WS, Byers RH, Ward JW, Darrow WW, Lemp GF, Hethcote HW. Statistical analysis of the stages of HIV infection using a Markov model. Statistics in medicine. 1989;8(7):831–43. doi: 10.1002/sim.4780080708. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. Neuropathologic alterations in mild cognitive impairment: a review. Journal of Alzheimer’s disease: JAD. 2010;19(1):221–8. doi: 10.3233/JAD-2010-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kovari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. Journal of neuropathology and experimental neurology. 2012;71(5):362–81. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Kryscio RJ, Jicha GA, Abner EL, Schmitt FA, Xu LO, Cooper G, Smith CD, Markesbery WR. Relative preservation of MMSE scores in autopsy-proven dementia with Lewy bodies. Neurology. 2009;73(14):1127–33. doi: 10.1212/WNL.0b013e3181bacf9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Lim YY, Ames D, Harrington K, Restrepo C, Martins RN, Rembach A, Laws SM, Masters CL, Villemagne VL, Rowe CC, Maruff P Australian Imaging, B., Lifestyle Research, G. Trajectories of memory decline in preclinical Alzheimer’s disease: results from the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing. Neurobiology of aging. 2015;36(3):1231–8. doi: 10.1016/j.neurobiolaging.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimer’s research & therapy. 2014;6(9):82. doi: 10.1186/s13195-014-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Frausto MS, Pittet D, Hwang T, Woolson RF, Wenzel RP. The dynamics of disease progression in sepsis: Markov modeling describing the natural history and the likely impact of effective antisepsis agents. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 1998;27(1):185–90. doi: 10.1086/514630. [DOI] [PubMed] [Google Scholar]
- Reiman EM, McKhann GM, Albert MS, Sperling RA, Petersen RC, Blacker D. Alzheimer’s disease: implications of the updated diagnostic and research criteria. The Journal of clinical psychiatry. 2011;72(9):1190–6. doi: 10.4088/JCP.10087co1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar JC, Schmitt FA, Yu L, Mendiondo MM, Kryscio RJ. Shared random effects analysis of multi-state Markov models: application to a longitudinal study of transitions to dementia. Statistics in medicine. 2007;26(3):568–80. doi: 10.1002/sim.2437. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Annals of neurology. 2009;66(2):200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain: a journal of neurology. 2012;135(Pt 10):3005–14. doi: 10.1093/brain/aws234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, Bennett DA. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60(7):1082–8. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- Small BJ, Backman L. Longitudinal trajectories of cognitive change in preclinical Alzheimer’s disease: a growth mixture modeling analysis. Cortex; a journal devoted to the study of the nervous system and behavior. 2007;43(7):826–34. doi: 10.1016/s0010-9452(08)70682-8. [DOI] [PubMed] [Google Scholar]
- Smits LL, van Harten AC, Pijnenburg YA, Koedam EL, Bouwman FH, Sistermans N, Reuling IE, Prins ND, Lemstra AW, Scheltens P, van der Flier WM. Trajectories of cognitive decline in different types of dementia. Psychological medicine. 2015;45(5):1051–9. doi: 10.1017/S0033291714002153. [DOI] [PubMed] [Google Scholar]
- Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Annals of neurology. 2007;62(4):406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Annals of neurology. 2008;64(2):168–76. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Barnes L, Bennett D. Assessment of lifetime participation in cognitively stimulating activities. Journal of clinical and experimental neuropsychology. 2003;25(5):634–42. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. Journal of the International Neuropsychological Society: JINS. 2005;11(4):400–7. [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychology and aging. 2002;17(2):179–93. [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75(12):1070–8. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, Bennett DA. The natural history of cognitive decline in Alzheimer’s disease. Psychology and aging. 2012;27(4):1008–17. doi: 10.1037/a0029857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Boyle P, Schneider JA, Segawa E, Wilson RS, Leurgans S, Bennett DA. APOE epsilon4, Alzheimer’s disease pathology, cerebrovascular disease, and cognitive change over the years prior to death. Psychology and aging. 2013;28(4):1015–23. doi: 10.1037/a0031642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Boyle P, Wilson RS, Segawa E, Leurgans S, De Jager PL, Bennett DA. A random change point model for cognitive decline in Alzheimer’s disease and mild cognitive impairment. Neuroepidemiology. 2012;39(2):73–83. doi: 10.1159/000339365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Griffith WS, Tyas SL, Snowdon DA, Kryscio RJ. A nonstationary Markov transition model for computing the relative risk of dementia before death. Statistics in medicine. 2010;29(6):639–48. doi: 10.1002/sim.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]