Abstract
Backgound
Antibodies (Abs) to the HPV16 proteome increase risk for HPV-associated OPC (HPVOPC). The goal of this study was to investigate the association of a panel of HPV16 Abs with risk for OPC as well as the association of these Abs with tumor HPV and smoking status among patients with OPC.
Methods
IgG Abs to the HPV16 antigens E1, E2, E4, E5, E6, E7, L1, L2 were quantified using a programmable ELISA assay. Sera were obtained from 258 OPC patients at diagnosis and 250 healthy controls. HPV16 tumor status was measured by PCR for 137 cases. Multivariable logistic regression was used to calculate odds ratios for the association of HPV16 Abs with risk for OPC.
Results
HPV16 E1, E2, E4, E5, E6, E7 and L1-specific IgG levels were elevated in OPC patients compared to healthy controls (p<0.05). After multivariable adjustment, Ab positivity for NE2, CE2, E6, and/or E7 was associated with OPC risk (OR [95% CI], 249.1 [99.3–624.9]). Among patients with OPC, Ab positivity for these antigens was associated with tumor HPV status, especially among never or light smokers (OR [95% CI], 6.5 [2.1–20.1] and OR [95% CI], 17.5 [4.0–77.2], respectively).
Conclusions
Antibodies to HPV16 proteins are associated with increased risk for HPVOPC. Among patients with OPC, HPV16 Abs are associated with tumor HPV status, in particular among HPV positive patients with no or little smoking history.
Keywords: Serology, antibodies, biomarker, HPV, oropharyngeal cancer, head and neck cancer
1. Introduction
Oropharyngeal cancer (OPC) is a subset of head and neck cancer, which is ranked as the sixth most common cancer worldwide with 405,000 new cases and 200,000 deaths annually1. Tobacco-associated OPC, which is associated with somatic mutations in p53, is decreasing in incidence, but human papillomavirus-associated OPC (HPVOPC) has increased in the U.S. by 225% between 1984 and 20042. HPV type 16 accounts for 85–90% of HPV-associated cases of OPC3,4. Epidemiological evidence supports a causal role for HPV in OPC, including the association with lifetime numbers of vaginal and oral sex partners3,5 and presence of HPV DNA in oral exfoliated cells3,6–10. HPV is detectable and persistent in tumors11–14, contains viral oncogenes15–17, can transform target cells18,19, and induce tumors in transgenic mice20,21.
Unlike cervical cancer, there exists no sensitive and selective screening method for the early detection of HPVOPC. The HPV16 genome consists of six early genes (E1, E2, E4, E5, E6, and E7) and two late genes (L1 and L2) that constitute the viral capsid. Serum antibodies (Abs) to HPV16 E6 and E7 have been detected in a subset of patients with OPC22,23, with Abs to E6 and/or E7 present in 67% of HPV-positive OPC cases24. Seropositivity for HPV16 E6 and E7 are strongly associated with increasing odds of HPV-positive OPC (OR 58–67)25,26 and with improved prognosis27–29. HPV16 Abs to E6 have been detected in 34.8% of OPC patients up to 10 years prior to clinical diagnosis11, suggesting that HPV serology may yield biomarkers for early detection of OPC. In a pilot study, we detected Abs to both E6 and E7 proteins in OPC patient sera, but also detected Abs to the HPV16 E1 and E2 proteins22. This suggests that serology of additional HPV antigens may improve detection of HPVOPC.
In this study, we investigated the association of a panel of HPV16 Abs with risk of OPC. We used an extensive collection of sera from newly-diagnosed OPC patients and cancer-free controls to evaluate the association between HPV16 proteome-wide serology and disease status as well as tumor HPV and smoking status among cases.
2. Material and Methods
2.1 Patient Sera
Patients with newly diagnosed, histopathologically confirmed, and previously untreated OPC who were participating in a large ongoing molecular epidemiology study of head and neck cancer at the University of Texas MD Anderson Cancer Center in Houston, TX were eligible for the study. All participants were recruited between January 2006 and September 2008. Participants provided demographic and exposure history, including smoking and alcohol use, using a standardized questionnaire and provided a blood sample for biological testing. Sera used in this analysis were collected from OPC patients prior to initiation of treatment (n=258).
Healthy control sera were collected from genetically unrelated visitors or companions of patients to the head and neck clinic during the same time period. Controls were frequency matched to cases on age (±5 years), gender, and race (n = 250). Approximately 93% of cases and 85% of controls who were eligible agreed to participate in the study. All samples were collected using a standardized sample collection protocol and stored at −80°C until use. Written informed consent was obtained from all subjects under institutional review board approval.
2.2 HPV DNA cloning and expression
Plasmids containing HPV16 genes30 were expressed as a C-terminal GST-fusion protein using human HeLa cell lysate31 (Thermo Scientific, Waltham, MA) per manufacturer’s instructions. The HPV16 E2 gene was expressed as N- and C-terminal fragments for optimal protein expression22. GST was expressed as a negative control protein. All recombinant DNA research was performed in accord with NIH guidelines under institutional biologic safety review and approval.
2.3 Programmable Protein (RAPID) ELISA
ELISAs were performed essentially as described32, with modifications33. Protein was expressed from template cDNA and captured onto 96-well plates coated with anti-GST Ab (GE Healthcare, Piscataway, NJ). Sera were diluted 1:100 and blocked with E. coli lysate. Cases and controls were analyzed simultaneously in duplicate. Horseradish peroxidase (HRP) anti-human IgG Abs (Jackson ImmunoResearch Laboratories, West Grove, PA) were added at 1:10,000, and detected using Supersignal ELISA Femto Chemiluminescent substrate (Thermo Scientific). Luminescence was detected as relative light units (RLU) on a Glomax 96 Microplate Luminometer (Promega, Madison, WI) at 425 nm. To control for non-specific and GST-specific antibodies, the ratio of RLU for individual HPV-specific Abs to the RLU for the control GST-antigen was measured. To establish cut-off values, an RLU ratio > (the mean +3 standard deviations) of 125 randomly chosen control samples was designated positive. These levels were E1: 2.66; NE2: 2.29; CE2: 4.35; E4: 2.58; E5: 1.69; E6: 2.01; E7: 1.99; L1: 1.83; L2: 3.57. The controls chosen to establish cut-off values were not statistically significantly different with respect to age, sex, race, smoking, or alcohol use from the controls not used.
2.4. Reproducibility of RAPID ELISA
To determine the reproducibility of the RAPID ELISA assay, replicates for E6 serology were performed with 8 replicates on 4 consecutive days. RLU ratios (E6-GST: GST control) among RAPID ELISAs processed on the same day showed intra-assay CVs of 0.2–7%, and RLU ratios between plate-based arrays processed on 4 consecutive days demonstrated inter-assay CVs of 12–17%.
2.5. Tumor HPV DNA detection by PCR
Diagnostic in-house paraffin-embedded tissue was obtained following histopathologic confirmation of the diagnosis for determination of tumor HPV status34. DNA was extracted using a tissue DNA extraction kit (Qiagen Inc., Valencia, CA). Tumor tissue from the study subjects was tested for the presence of HPV16 E6 or E7 regions using PCR-based type-specific assays and each subject was classified as HPV-positive or HPV-negative based on these results. Samples were run in triplicate with positive (Siha cell line) and negative (TPC-1 cell line) controls and β-actin as DNA quality control.
2.6 Statistical Analysis
Categorical variables were created to describe study subjects’ demographic, clinical, and exposure (smoking and alcohol) history. A subject was considered an ever-smoker if they had smoked at least 100 cigarettes during their lifetime and an ever-drinker if they had drunk alcoholic beverages at least once a week for a year or more during their lifetime. Subjects who previously smoked or drank alcohol but had not done so in the year prior to their diagnosis were considered former-smokers and former-drinkers, respectively.
Demographic and clinical variables of interest were analyzed using standard descriptive statistical methods. Differences between groups were compared using chi-square or Fisher’s exact (when cell frequencies <5) tests for categorical variables and Student’s t-test, with adjustment for unequal variances where appropriate, for continuous variables. Mean Ab values were plotted for cases and controls and compared using Mann-Whitney nonparametric analysis (GraphPad Prism version 5.0c, San Diego, CA). Odds ratios (OR) with 95% confidence intervals (CI) were calculated using logistic regression models with adjustment for possible confounding factors to determine the association between pre-treatment Ab status and OPC. The association between pre-treatment Ab status and tumor HPV16 and smoking status among cases was also evaluated (Stata 12.0, StataCorp, College Station, TX). A p-value of <.05 was considered significant and all tests were 2-sided.
3. Results
3.1 Subject Characteristics
Ab levels specific for HPV16 proteins and GST control protein were compared in sera from 258 cases of OPC and 250 age-, gender- and race-matched controls. Two cases were omitted from further study because they presented with distant metastases at diagnosis, leaving a sample size of 256 cases. The demographics of cases and controls are presented in Table 1. There were no significant differences between cases and controls with respect to age, gender, and race (Table 1). However, cases were more likely to be current smokers while controls were more likely to be current alcohol drinkers (p=0.007 and p<0.001, respectively; Table 1). The majority of cases was derived from the tonsils or base of tongue (95%) and was stage III/IV (92.6%) at presentation. Among the 256 cases, HPV16 status (as determined by tumor HPV PCR for E6 and E7) were available for 137 cases, with 111 cases positive for HPV16 (81%).
Table 1.
Demographic, exposure, and clinical characteristics of cases and controls
| Cases N=256 |
Controls N=250 |
p | |
|---|---|---|---|
| Age, mean (SD) | 56.5 (9.6) | 56.5 (9.6) | .963 |
| Age, median | 55 | 55.5 | .930 |
| N (%) | N (%) | ||
| Sex | .913 | ||
| Male | 220 (85.9) | 214 (85.6) | |
| Female | 36 (14.1) | 36 (14.4) | |
| Race | .691 | ||
| White | 233 (91.0) | 230 (92.0) | |
| Other | 23 (9.0) | 20 (8.0) | |
| Alcohol | <.001 | ||
| Never | 71 (27.7) | 111 (44.4) | |
| Former | 66 (25.8) | 47 (18.8) | |
| Current | 119 (46.5) | 92 (36.8) | |
| Smoking | .007 | ||
| Never | 116 (45.3) | 131 (52.4) | |
| Former | 93 (36.3) | 97 (38.8) | |
| Current | 47 (18.4) | 22 (8.8) | |
| Smokinga | |||
| ≤10 pack years | 149 (59.6) | ||
| >10 pack years | 101 (40.4) | ||
| HPV statusb | |||
| Negative | 26 (19.0) | ||
| Positive | 111 (81.0) | ||
| Subsite | |||
| Tonsil | 122 (47.7) | ||
| Base of tongue | 121 (47.3) | ||
| Other oropharynx | 9 (3.5) | ||
| Other site (not oropharynx) | 4 (1.6) | ||
| Stage | |||
| I–II | 19 (7.4) | ||
| III–IV | 237 (92.6) | ||
| T category | |||
| 0–1 | 76 (29.7) | ||
| 2 | 110 (43.0) | ||
| 3 | 41 (16.0) | ||
| 4 | 29 (11.3) | ||
| N category | |||
| 0 | 29 (11.3) | ||
| 1–2a | 42 (16.4) | ||
| 2b | 126 (49.2) | ||
| 2c-3 | 59 (23.1) | ||
| Gradec | |||
| Well to moderate | 76 (34.5) | ||
| Moderately Poor to poor | 144 (65.5) | ||
Total N=250 cases due to missing data (N=6)
Total N=137 cases due to missing data (N=119)
Total N=220 cases due to missing data (N=36)
3.2 Detection of HPV16 Abs by programmable ELISA
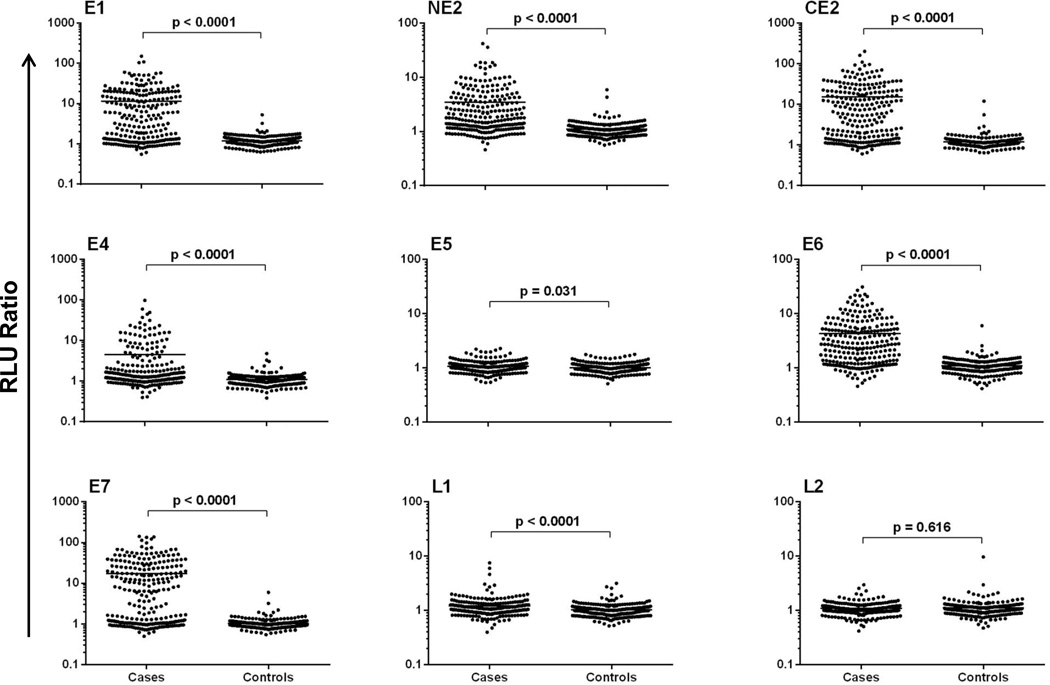
Results of the RAPID ELISA for serum IgG Abs to HPV16 antigens for OPC cases and healthy controls are shown in Figure 1. The medians and ranges of these values for each individual antigen are presented in Supplemental Table 1. In the total OPC samples (unselected by HPV status), all HPV16 Abs except L2 were significantly higher among patients with OPC than healthy controls (p<0.05, Supplementary Table 1 and Figure 1). A higher proportion of patients with OPC than healthy controls were seropositive for each of these Abs (Table 2). Using cut-off values derived from the healthy controls (n=125, >3 SDs over the mean), at least one HPV16 early gene Ab was detected in the sera of 210/256 (82.0%) of OPC cases, compared with 10/250 (4.0%) of healthy controls (Table 2). A high proportion of patients were positive for IgG Abs specific for HPV16 E1 (144/256, 56.3%), NE2 (104/256, 40.6%), CE2 (137/256, 53.5%), E4 (61/256, 23.8%), E6 (148/256, 57.8%), and/or E7 (148/256, 57.8%). In comparison, only 9 cases (3.5%) were positive for E5 Abs, 15 cases (5.9%) were positive for L1 Abs, and none were positive for L2 Abs.
Figure 1.
Specific detection of multiple HPV16 antibodies in patients with HPV-associated OPC HPV16 proteins were expressed as GST-fusion proteins and captured onto anti-GST coated plates. The RLU ratio (RLU of HPV antigen/RLU of GST-control) of IgG detected in HPV-associated OPC sera (n=256) and controls (n=250) is shown.
Table 2.
Association of pre-treatment antibody status with case-control status
| Cases N=256 |
Controls N=250 |
Pb | Crude OR (95% CI) |
Adjusted ORc (95% CI) |
|
|---|---|---|---|---|---|
| No. + (%) | No. + (%) | ||||
| E1 | 144 (56.3) | 2 (0.8) | <0.001 | 159.4 (38.8–655.1) | 170.6 (41.3–705.0) |
| NE2 | 104 (40.6) | 2 (0.8) | <0.001 | 84.8 (20.6–348.8) | 85.7 (20.7–354.2) |
| CE2 | 137 (53.5) | 2 (0.8) | <0.001 | 142.8 (34.7–586.5) | 157.5 (38.0–653.2) |
| NE2 and/or CE2a | 144 (56.3) | 2 (0.8) | <0.001 | 159.4 (38.8–655.1) | 177.5 (42.8–736.9) |
| E4 | 61 (23.8) | 3 (1.2) | <0.001 | 25.8 (8.0–83.3) | 26.9 (8.3–87.4) |
| E5 | 9 (3.5) | 2 (0.8) | 0.063 | 4.5 (1.0–21.1) | 4.7 (1.0–22.8) |
| E6 | 148 (57.8) | 3 (1.2) | <0.001 | 112.8 (35.2–361.8) | 130.6 (40.1–425.5) |
| E7 | 148 (57.8) | 3 (1.2) | <0.001 | 112.8 (35.2–361.8) | 147.4 (45.0–483.1) |
| E6 and/or E7a | 194 (75.8) | 6 (2.4) | <0.001 | 127.2 (53.9–300.4) | 241.4 (92.6–629.4) |
| NE2, CE2, E6, and/or E7a | 203 (79.3) | 7 (2.8) | <0.001 | 133.0 (59.2–298.9) | 249.1 (99.3–624.9) |
| Any Ea | 210 (82.0) | 10 (4.0) | <0.001 | 109.6 (53.9–222.5) | 243.7 (101.4–586.0) |
| L1 | 15 (5.9) | 4 (1.6) | 0.017 | 3.8 (1.3–11.7) | 4.0 (1.3–12.3) |
| L2 | 0 | 1 (0.4) | 0.494 | NC | NC |
| Any La | 15 (5.9) | 5 (6.3) | 0.038 | 3.0 (1.1–8.5) | 3.1 (1.1–8.8) |
| Any E and/or La | 210 (82.0) | 12 (4.8) | <0.001 | 90.5 (46.7–175.5) | 193.8 (85.0–441.5) |
Any positive vs. all negative
Fisher’s exact test
Adjusted for age, smoking, and alcohol status
NC, not calculable due to zero cells
Risk for OPC was estimated by calculating OR (95%CI), adjusted for age, smoking, and alcohol drinking status (Table 2). The OR for E1, E2, E6, and E7 Abs ranged from 86–171. While the 95%CI are wide, an extremely high risk of OPC was associated with seropositivity for the E1 protein (OR = 171), the C-terminal portion of E2 (OR = 86), the E6 protein (OR = 131), and for the E7 protein (OR = 147). Being seropositive to any antigen was associated with a 194-fold increased risk of OPC, while being seropositive to an early protein was associated with a 244-fold increased risk of OPC, and being seropositive to a late protein was associated with a 3-fold increased risk of OPC.
3.3. Association of serology with tumor HPV status and smoking
Among the 137 cases with tumor tissue tested for HPV16 E6 or E7 DNA by PCR, 111 (81.0%) were determined to be tumor HPV positive, and 26 (19.0%) were tumor HPV negative. The association between serology and tumor HPV status among the cases are presented in Table 3. Of the tumor HPV positive cases, 94/111 (84.7%) were serologically positive for at least one HPV early antigen. However, 16/26 (61.5%) of tumor HPV negative cases were also serologically positive for at least one HPV early antigen (p=0.008). The presence of Abs to NE2, CE2, E6, and/or E7 was associated with tumor HPV status (OR [95%CI], 6.5 [2.1–20.1]; Table 3), and this was particularly true among those who smoked less than or equal to 10 pack years (OR [95%CI], 17.5 [4.0–77.2]; Table 3). Being seropositive to the early proteins CE2 or E7 was associated with HPV tumor status among the cases with ≤ 10 pack-years of smoking but this association was not observed for late proteins (Table 3). Among smokers (> 10 pack-years), no consistent association between seropositivity and HPV tumor status was identified (Table 3).
Table 3.
Association of pre-treatment antibody status with tumor HPV and smoking status among cases
| HPV- (Reference) N=26 |
HPV+ N=111 |
p | Adjusted ORc (95% CI) |
HPV+ >10 pack yearsd N=47 |
Pe | Adjusted ORef (95% CI) |
HPV+ ≤10 pack yearsd N=63 |
Pe | Adjusted ORef (95% CI) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| No. + (%) | No. + (%) | No. + (%) | No. + (%) | |||||||
| E1 | 13 (50.0) | 66 (59.5) | 0.380 | 1.1 (0.4–2.9) | 21 (44.7) | 0.663 | 0.7 (0.2–1.9) | 44 (69.8) | 0.076 | 1.5 (0.5–4.2) |
| NE2 | 8 (30.8) | 50 (45.1) | 0.185 | 1.5 (0.6–4.0) | 19 (40.4) | 0.413 | 1.6 (0.5–4.8) | 31 (49.2) | 0.111 | 1.5 (0.5–4.3) |
| CE2 | 11 (42.3) | 68 (61.3) | 0.078 | 1.9 (0.7–5.0) | 20 (42.6) | 0.984 | 1.0 (0.3–2.8) | 47 (74.6) | 0.004 | 2.9 (1.0–8.1) |
| NE2 and/or CE2a | 11 (42.3) | 72 (64.9) | 0.034 | 2.3 (0.9–6.0) | 22 (46.8) | 0.711 | 1.2 (0.4–3.5) | 49 (77.8) | 0.001 | 3.4 (1.2–9.6) |
| E4 | 6 (23.1) | 27 (24.3) | 0.893 | 0.9 (0.3–2.6) | 10 (21.3) | 0.859 | 0.8 (0.2–2.6) | 16 (25.4) | 0.818 | 0.7 (0.2–2.4) |
| E5 | 2 (7.7) | 4 (3.6) | 0.319b | 0.3 (0–1.9) | 3 (6.4) | 1.0b | 0.6 (0.1–4.3) | 1 (1.6) | 0.203b | 0.2 (0–2.4) |
| E6 | 10 (38.5) | 66 (59.5) | 0.052 | 2.1 (0.8–5.3) | 24 (51.1) | 0.301 | 1.6 (0.6–4.6) | 42 (66.7) | 0.014 | 2.5 (0.9–7.0) |
| E7 | 11 (42.3) | 68 (61.3) | 0.078 | 1.8 (0.7–4.7) | 20 (42.6) | 0.984 | 0.9 (0.3–2.6) | 47 (74.6) | 0.004 | 2.9 (1.0–8.1) |
| E6 and/or E7a | 12 (46.2) | 88 (79.3) | 0.001 | 4.4 (1.6–12.4) | 30 (63.8) | 0.143 | 2.0 (0.7–5.7) | 57 (90.5) | <0.001 | 8.4 (2.5–27.8) |
| NE2, CE2, E6, and/or E7a | 13 (50.0) | 94 (84.7) | <0.001 | 6.5 (2.1–20.1) | 33 (70.2) | 0.087 | 2.5 (0.8–7.5) | 60 (95.2) | <0.001 | 17.5 (4.0–77.2) |
| Any Ea | 16 (61.5) | 94 (84.7) | 0.008 | 3.4 (1.0–11.4)) | 33 (70.2) | 0.450 | 1.4 (0.4–4.4) | 60 (95.2) | <0.001 | 8.0 (1.8–35.7) |
| L1 | 3 (11.5) | 6 (5.4) | 0.371b | 0.4 (0.1–1.8) | 3 (6.4) | 0.659b | 0.5 (0.1–2.7) | 3 (4.8) | 0.352b | 0.3 (0.1–2.0) |
| L2 | 0 | 0 | NC | NC | 0 | NC | NC | 0 | NC | NC |
| Any La | 3 (11.5) | 6 (5.4) | 0.371b | 0.4 (0.1–1.8) | 3 (6.4) | 0.659b | 0.5 (0.1–2.7) | 3 (4.8) | 0.352b | 0.3 (0.1–2.0) |
| Any E and/or La | 16 (61.5) | 94 (84.7) | 0.008 | 3.4 (1.0–11.5) | 33 (70.2) | 0.450 | 1.4 (0.4–4.4) | 60 (95.2) | <0.001 | 8.0 (1.8–35.7) |
Any positive vs. all negative
Fisher’s exact test
Adjusted for age, smoking (≤10 pack years vs. >10 pack years), N category (N0-N2a vs. N2b-N3), and subsite (tonsil/base of tongue vs. other)
One HPV+ case is missing pack years
Referent group is HPV- cases
Adjusted for age, N category (N0-N2a vs. N2b-N3), and subsite (tonsil/base of tongue vs. other)
NC, not calculable due to zero cells
4. Discussion
Due to the rising incidence of HPV-associated oropharyngeal cancers, there is an urgent clinical need for biomarkers for early detection, diagnosis, prognosis, and monitoring of these patients. Prior studies have demonstrated that a subset of patients (~64–74%) with HPVOPC have detectable Abs to HPV16 E6 and/or E7 Abs in their sera26,35. In the present study we evaluated Abs specific for a panel of eight HPV16 antigens as potential biomarkers for the diagnosis of OPC. We demonstrate that Abs to multiple HPV16 early antigens (E1, E2, E4, E5, E6, and E7) are specifically detected in the sera of patients compared with age- gender- and race-matched controls. Using these stringent cutoffs, Abs to the capsid protein L1 are rarely observed (and L2 Abs are not observed) in HPVOPC. Our data support the hypothesis that HPV16 antibody signatures may be specific and clinically useful biomarkers of HPVOPC, and potentially for other HPV- associated malignancies.
Our data confirms and extends published studies from the Pawlita laboratory demonstrating detection of E6 and E7 Abs in patients with newly diagnosed HPVOPC26,35. E6 Abs have been detected over 10 years prior to clinical diagnosis36,37, but in only 35% of patients with undefined tissue HPV status, limiting clinical applicability. Here, we detect E6 and/or E7 Abs in 79% of HPVOPC patients (n=111). Our results are similar to our findings of E6 and/or E7 Abs in 76% of patients (n=119) with HPVOPC from the independent, multicenter HOTSPOT study33, suggesting that there is limited regional or technical variation in serologic detection of HPVOPC. This also suggests that differences in the definition of HPV status (PCR used in this study vs p16/ISH in HOTSPOT) do not significantly impact these results. Using the full panel of early antigens, the proportion of patients seropositive was increased to 85%, strongly supporting the use of a multiparametric signature for HPVOPC detection.
The OPC cases presented here are representative of the head and neck clinic at MD Anderson Cancer Center, as over 90% of eligible patients consented to the study. In subset analysis, there were no correlations between age, gender, and race with seropositivity. Patients who were heavy smokers (>10 pack-years) had both lower frequencies of seropositivity and no association of serology with tumor HPV16 status. Because smoking has a higher association of tumors with p53 mutation, this may represent a subpopulation where serologic biomarkers are less reliable.
One limitation of this study is the rapid evolution of methods and standards for the detection of HPV in tumor tissue in the 10 years during which these patients were enrolled38. There is no current standard of care for tissue biomarkers of HPV, although p16 expression detected by immunohistochemistry (IHC) is commonly used as a surrogate marker, with or without ISH for HR HPV nucleic acid39. Assignment of HPV status in this study was determined by PCR for HPV16 E6 and E734. Only a small subset of these cases had tissue available for p16 IHC (n=22). Therefore, we did not have the ability to confirm the tissue HPV status with p16 testing.
Within the subset of cases with tissue HPV16 status identified by PCR (n=137), Abs to HPV16 early antigens (E1, E2, E4, E5, E6, E7) were detected in 85% of cases who also had tumors that tested HPV16 positive by PCR, but 62% of cases that were negative by tissue HPV PCR were still positive by serology. These serologic responses were similar in strength and in antigenic specificity as the responses in tissue HPV PCR positive cases (data not shown). Since 10–15% of HPVOPC are associated with HPV subtypes other than HPV16, we cannot exclude the possibility that the serologic assay is cross-reacting with other subtypes of HPV in the seropositive, PCR negative population. A second possibility is that the HPV16 PCR assay used in this study has limited sensitivity (false negatives), which cannot be confirmed by p16 testing in this study. We cannot exclude that we are detecting false-positive serologic responses in the HPV-negative OPC cases, but we observe no significant differences in sero-reactivity between these healthy controls from MD Anderson (n=250) and Oregon Health Sciences University (n=78, data not shown).
A notable finding from our data is the heterogeneity of the serologic response to HPV16 in these patients, which is of unknown biologic or clinical significance. The majority (79%) of patient sera have Abs to E6 and/or E7, but Abs to multiple other early genes, including E1, E2, and E4 are also specifically detected. This is unique to HPVOPC, as these Abs are rarely detected in sera from patients with invasive cervical cancer (not shown). E1 Abs are strongly correlated with E2 Abs, and E4 Abs were only detected in a subset (25.7%) of patients with E7 Abs. Five percent of patients had isolated Abs to E1/E2 antigens, without E6/E7 Abs.
A key observation from our study is the high frequency of E1 and E2 Abs in HPVOPC sera, which has not been observed in cervical disease40. Because humoral immunity is induced by antigen expression, we predict that the variation in the serologic response to individual HPV antigens is a result of differences in antigen expression in the tumor. Since expression of E2 decreases due to viral integration and de-repression of E6/E741, we predict that Abs to E2 may inversely correlate with viral integration. As a result, E1/E2 Abs may be detected earlier in HPVOPC development than E6/E7 Abs. In summary, panels of antibodies to HPV16 early proteins may be highly specific biomarkers for the diagnosis of HPVOPC.
Supplementary Material
Highlights.
Antibodies to the HPV16 proteome were measured in 256 cases of OPC and 250 controls.
Early gene Abs were detected in the sera of 85% of HPVOPC cases and 4% of controls.
Seropositivity is strongly associated with OPC risk (OR 249).
Seropositivity is associated with tumor HPV16 status.
ACKNOWLEDGEMENTS
This study was supported by a research grant from the Early Detection Research Network (EDRN) U01CA117374 and Arizona State University institutional funds (KSA), NIH/NCI R03 Grant CA128110-01A1 (EMS) and NCI R25T Grant CA57730 (Shine Chang, PhD, Principal Investigator)(KRD).
Abbreviations
- Abs
antibodies
- CV
coefficient of variation
- HR HPV
High Risk Human Papilloma Virus
- ISH
in situ hybridization
- IVTT
in vitro transcription/translation
- OPC
oropharyngeal cancer
- OR
odds ratio
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Dr. Anderson is a consultant, hold stock options, and serves on the scientific advisory board of Provista Diagnostics. All other authors report no conflicts of interest.
References
- 1.Duvvuri U, Myers JN. Cancer of the head and neck is the sixth most common cancer worldwide. Current problems in surgery. 2009;46(2):114–117. doi: 10.1067/j.cpsurg.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. The New England journal of medicine. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 4.Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. The lancet oncology. 2010;11(8):781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heck JE, Berthiller J, Vaccarella S, Winn DM, Smith EM, Shan'gina O, et al. Sexual behaviours and the risk of head and neck cancers: a pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. International journal of epidemiology. 2010;39(1):166–181. doi: 10.1093/ije/dyp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith EM, Ritchie JM, Summersgill KF, Hoffman HT, Wang DH, Haugen TH, et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. Journal of the National Cancer Institute. 2004;96(6):449–455. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]
- 7.Hansson BG, Rosenquist K, Antonsson A, Wennerberg J, Schildt EB, Bladstrom A, et al. Strong association between infection with human papillomavirus and oral and oropharyngeal squamous cell carcinoma: a population-based case-control study in southern Sweden. Acta oto-laryngologica. 2005;125(12):1337–1344. doi: 10.1080/00016480510043945. [DOI] [PubMed] [Google Scholar]
- 8.Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. Journal of the National Cancer Institute. 2008;100(6):407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 9.Pintos J, Black MJ, Sadeghi N, Ghadirian P, Zeitouni AG, Viscidi RP, et al. Human papillomavirus infection and oral cancer: a case-control study in Montreal, Canada. Oral oncology. 2008;44(3):242–250. doi: 10.1016/j.oraloncology.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Tachezy R, Klozar J, Rubenstein L, Smith E, Salakova M, Smahelova J, et al. Demographic and risk factors in patients with head and neck tumors. Journal of medical virology. 2009;81(5):878–887. doi: 10.1002/jmv.21470. [DOI] [PubMed] [Google Scholar]
- 11.Kreimer AR, Johansson M, Waterboer T, Kaaks R, Chang-Claude J, Drogen D, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(21):2708–2715. doi: 10.1200/JCO.2012.47.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeudall WA, Paterson IC, Patel V, Prime SS. Presence of human papillomavirus sequences in tumour-derived human oral keratinocytes expressing mutant p53. European journal of cancer. Part B, Oral oncology. 1995;31B(2):136–143. doi: 10.1016/0964-1955(94)00030-8. [DOI] [PubMed] [Google Scholar]
- 13.Ferris RL, Martinez I, Sirianni N, Wang J, Lopez-Albaitero A, Gollin SM, et al. Human papillomavirus-16 associated squamous cell carcinoma of the head and neck (SCCHN): a natural disease model provides insights into viral carcinogenesis. Eur J Cancer. 2005;41(5):807–815. doi: 10.1016/j.ejca.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Steenbergen RD, Hermsen MA, Walboomers JM, Joenje H, Arwert F, Meijer CJ, et al. Integrated human papillomavirus type 16 and loss of heterozygosity at 11q22 and 18q21 in an oral carcinoma and its derivative cell line. Cancer research. 1995;55(22):5465–5471. [PubMed] [Google Scholar]
- 15.van Houten VM, Snijders PJ, van den Brekel MW, Kummer JA, Meijer CJ, van Leeuwen B, et al. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. International journal of cancer. Journal international du cancer. 2001;93(2):232–235. doi: 10.1002/ijc.1313. [DOI] [PubMed] [Google Scholar]
- 16.Jung AC, Briolat J, Millon R, de Reynies A, Rickman D, Thomas E, et al. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. International journal of cancer. Journal international du cancer. 2010;126(8):1882–1894. doi: 10.1002/ijc.24911. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann M, Scheunemann D, Fazel A, Gorogh T, Kahn T, Gottschlich S. Human papillomavirus and p53 polymorphism in codon 72 in head and neck squamous cell carcinoma. Oncology reports. 2009;21(3):809–814. [PubMed] [Google Scholar]
- 18.Smeets SJ, van der Plas M, Schaaij-Visser TB, van Veen EA, van Meerloo J, Braakhuis BJ, et al. Immortalization of oral keratinocytes by functional inactivation of the p53 and pRb pathways. International journal of cancer. Journal international du cancer. 2011;128(7):1596–1605. doi: 10.1002/ijc.25474. [DOI] [PubMed] [Google Scholar]
- 19.Lace MJ, Anson JR, Klussmann JP, Wang DH, Smith EM, Haugen TH, et al. Human papillomavirus type 16 (HPV-16) genomes integrated in head and neck cancers and in HPV-16-immortalized human keratinocyte clones express chimeric virus-cell mRNAs similar to those found in cervical cancers. Journal of virology. 2011;85(4):1645–1654. doi: 10.1128/JVI.02093-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strati K, Pitot HC, Lambert PF. Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(38):14152–14157. doi: 10.1073/pnas.0606698103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strati K, Lambert PF. Role of Rb-dependent and Rb-independent functions of papillomavirus E7 oncogene in head and neck cancer. Cancer research. 2007;67(24):11585–11593. doi: 10.1158/0008-5472.CAN-07-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson KS, Wong J, D'Souza G, Riemer AB, Lorch J, Haddad R, et al. Serum antibodies to the HPV16 proteome as biomarkers for head and neck cancer. British journal of cancer. 2011;104(12):1896–1905. doi: 10.1038/bjc.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubenstein LM, Smith EM, Pawlita M, Haugen TH, Hamsikova E, Turek LP. Human papillomavirus serologic follow-up response and relationship to survival in head and neck cancer: a case-comparison study. Infectious agents and cancer. 2011;6:9. doi: 10.1186/1750-9378-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith EM, Pawlita M, Rubenstein LM, Haugen TH, Hamsikova E, Turek LP. Risk factors and survival by HPV-16 E6 and E7 antibody status in human papillomavirus positive head and neck cancer. International journal of cancer. Journal international du cancer. 2010;127(1):111–117. doi: 10.1002/ijc.25015. [DOI] [PubMed] [Google Scholar]
- 25.Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, Balaram P, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95(23):1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 26.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 27.Smith EM, Rubenstein LM, Ritchie JM, Lee JH, Haugen TH, Hamsikova E, et al. Does pretreatment seropositivity to human papillomavirus have prognostic significance for head and neck cancers? Cancer Epidemiol Biomarkers Prev. 2008;17(8):2087–2096. doi: 10.1158/1055-9965.EPI-08-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith EM, Pawlita M, Rubenstein LM, Haugen TH, Hamsikova E, Turek LP. Risk factors and survival by HPV-16 E6 and E7 antibody status in human papillomavirus positive head and neck cancer. International journal of cancer. Journal international du cancer. 2010;127(1):111–117. doi: 10.1002/ijc.25015. [DOI] [PubMed] [Google Scholar]
- 29.Rubenstein LM, Smith EM, Pawlita M, Haugen TH, Hamsikova E, Turek LP. Human papillomavirus serologic follow-up response and relationship to survival in head and neck cancer: a case-comparison study. Infect Agent Cancer. 2011;6:9. doi: 10.1186/1750-9378-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson KS, Sibani S, Wallstrom G, Qiu J, Mendoza EA, Raphael J, et al. Protein microarray signature of autoantibody biomarkers for the early detection of breast cancer. J Proteome Res. 2011;10(1):85–96. doi: 10.1021/pr100686b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Festa F, Rollins SM, Vattem K, Hathaway M, Lorenz P, Mendoza EA, et al. Robust microarray production of freshly expressed proteins in a human milieu. Proteomics Clin Appl. 2013;7(5–6):372–377. doi: 10.1002/prca.201200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson KS, Wong J, Vitonis A, Crum CP, Sluss PM, Labaer J, et al. p53 autoantibodies as potential detection and prognostic biomarkers in serous ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 2010;19(3):859–868. doi: 10.1158/1055-9965.EPI-09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Souza G, Gross ND, Pai SI, Haddad R, Anderson KS, Rajan S, et al. Oral Human Papillomavirus (HPV) Infection in HPV-Positive Patients With Oropharyngeal Cancer and Their Partners. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 doi: 10.1200/JCO.2014.55.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. International journal of cancer. Journal international du cancer. 2007;121(11):2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 35.Smith EM, Ritchie JM, Pawlita M, Rubenstein LM, Haugen TH, Turek LP, et al. Human papillomavirus seropositivity and risks of head and neck cancer. International journal of cancer. Journal international du cancer. 2007;120(4):825–832. doi: 10.1002/ijc.22330. [DOI] [PubMed] [Google Scholar]
- 36.Mork J, Lie AK, Glattre E, Hallmans G, Jellum E, Koskela P, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344(15):1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 37.Kreimer AR, Johansson M, Waterboer T, Kaaks R, Chang-Claude J, Drogen D, et al. Evaluation of Human Papillomavirus Antibodies and Risk of Subsequent Head and Neck Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 doi: 10.1200/JCO.2012.47.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang C, Marsit CJ, McClean MD, Nelson HH, Christensen BC, Haddad RI, et al. Biomarkers of HPV in head and neck squamous cell carcinoma. Cancer research. 2012;72(19):5004–5013. doi: 10.1158/0008-5472.CAN-11-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24(10):1295–1305. doi: 10.1038/modpathol.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luevano M, Bernard HU, Barrera-Saldana HA, Trevino V, Garcia-Carranca A, Villa LL, et al. High-throughput profiling of the humoral immune responses against thirteen human papillomavirus types by proteome microarrays. Virology. 2010;405(1):31–40. doi: 10.1016/j.virol.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue Y, Bellanger S, Zhang W, Lim D, Low J, Lunny D, et al. HPV16 E2 is an immediate early marker of viral infection, preceding E7 expression in precursor structures of cervical carcinoma. Cancer research. 2010;70(13):5316–5325. doi: 10.1158/0008-5472.CAN-09-3789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.