Abstract
The T helper (Th) cell subsets are characterized by the type of cytokines produced and the master transcription factor expressed. Th1 cells participate in cell-mediated immunity, whereas Th2 cells promote humoral immunity. Furthermore, the two subsets can control each other. Thereby, Th1-Th2 balance offered a key paradigm in understanding the induction and regulation of immune pathology in autoimmune and other diseases. However, over the past decade, Th17 cells producing interleukin-17 (IL-17) have emerged as the major pathogenic T cell subset in many pathological conditions that were previously attributed to Th1 cells. In addition, the role of CD4+CD25+ T regulatory cells (Treg) in controlling the activity of Th17 and other T cell subsets has increasingly been realized. Thereby, examination of the Th17/Treg balance in the course of autoimmune diseases has significantly advanced our understanding of the pathogenesis of these disorders. The differentiation of Th17 and Treg cells from naïve T cells is inter-related and controlled in part by the cytokine milieu. For example, transforming growth factor β (TGFβ) is required for Treg induction, whereas the same cytokine in the presence of IL-6 (or IL-1) promotes the differentiation of Th17. Furthermore, IL-23 plays a role in the maintenance of Th17. Accordingly, novel therapeutic approaches are being developed to target IL-23/IL-17 as well as to modulate the Th17/Treg balance in favor of immune regulation to control autoimmunity.
Paradigm shift from Th1-Th2 to Th17-Treg
For over two decades, the T helper 1-T helper 2 (Th1-Th2) paradigm was used as a framework to characterize human diseases [1, 2]. After CD4+ T cells are activated by antigen, the cells expand and polarize into either Th1 cells that produce interferon-γ (IFNγ), interleukin-2 (IL-2), and lymphotoxin (LT) or Th2 that produce IL-4, IL-5, IL-9 and IL-13 [3]. The cytokine milieu present during T cell activation plays a role in the differentiation of naïve T cells into specific T cell subsets. IFNγ and IL-12 induce the differentiation of naïve T cells into Th1 cells, whereas IL-4 promotes the differentiation into Th2 cells. The Th1 cells participate in a cellular response that targets intracellular pathogens, whereas the Th2 cells provide help to the B cells leading to the production of antibodies, which target extracellular pathogens [2, 4]. The master transcription factor of Th1 cells is T-box transcription factor expressed in T-cells (T-bet) and it is inhibited by IL-4. In contrast, GATA-binding protein 3 (GATA-3) is necessary for Th2 differentiation and it is inhibited by IFNγ. The cross regulation of the two Th subsets is the hallmark of the Th1-Th2 paradigm.
The Th1-Th2 paradigm had to be expanded and revised with the discovery of IL-23, which shares the p40 subunit with IL-12, and thereby, was responsible for many of the immune effects that had previously been attributed to IL-12 [5]. IL-12 is a heterodimer composed of p35 and p40 subunits, whereas IL-23 is composed of a unique p19 and the shared p40 subunit, and IL-23 is very similar to IL-12 but is functionally different. Studies conducted in the mouse collagen-induced arthritis (CIA) model of human rheumatoid arthritis (RA) [6] revealed that mice lacking the p19 subunit of IL-23, but not those lacking the p35 subunit of IL-12, were protected from arthritis. It was also shown that IL-12-deficient mice had an increase in Th17 cells. Similar results were obtained in studies in the mouse experimental autoimmune encephalitis (EAE) model of human multiple sclerosis (MS) [5]. It was later shown that IL-23 did not directly induce the differentiation of Th17 cells, but it helped Th17 cells to expand and maintain their lineage [7]. Instead, it was shown that IL-6 and transforming growth factor-β (TGFβ) were necessary for murine Th17 cell differentiation [8]. Since inducible T regulatory cells (iTreg) are differentiated by TGFβ alone, a new paradigm has arisen in the form of Th17-Treg cell balance in controlling immune responses in health and disease.
The cellular source of IL-17 and its role in synovial inflammation and bone damage in arthritis
IL-17 is a pro-inflammatory cytokine (Table 1), which is known to be an important contributor to the development and progression of RA and other autoimmune diseases (Table 2) [9–11]. IL-17 is a signature cytokine of CD4+ Th17 cells. However, IL-17 also can be produced by CD8+ T cells, natural Th17 cells, innate lymphoid cells (ILCs), γδ T cells, natural killer (NKT) cells, and neutrophils [9, 12]. Of these, two subsets of cells deserve special mention. Natural Th17 cells are present in the skin and mucosa and they can produce IL-17 in response to IL-1 and IL-23 [9, 13]. As with adaptive Th17, natural Th17 also develop in the thymus. ILCs, which are found in the gut and skin, can produce IL-17 in response to inflammatory cytokines and stress [9, 14, 15]. Unlike natural Th17 cells, ILCs develop in the bone marrow.
Table 1.
IL-17 Family of Cytokines
| Member Name | Receptors | Homology with IL-17A (%) | Source |
|---|---|---|---|
| IL-17A | IL-17RA/RC | 100 | CD4+ T cells, CD8+ T cells, eosinophils, and neutrophils |
| IL-17B | IL-17RB | 24 | Pancreas, small intestine, stomach, and spinal cord |
| IL-17C | IL-17RA/RE | 26 | Human testis, thymus, spleen, and prostate |
| IL-17D | Unknown | 30 | Skeletal muscle, neuronal cells, prostate, and some resting CD4+ T lymphocytes |
| IL-17E (CD25) | IL-17RA/RB | 16 | Brain, lung, testis, prostate, and CD4+ T cells |
| IL-17F | IL-17RA/RC | 50 | CD4+ T cells |
| IL-17A/F (Heterodimer) | IL-17RA/RC | - | CD4+ T cells |
Table 2.
Examples of diseases associated with cytokines secreted by Th17 cells
| Disease category | Human disease | Animal model | Th17 cytokines involved | References |
|---|---|---|---|---|
| Autoimmunity | Rheumatoid arthritis (RA) | Collagen-induced arthritis, Adjuvant-induced arthritis, SKG model | IL-17A | [10, 25, 26] |
| Multiple sclerosis (MS) | Experimental autoimmune encephalomyelitis | IL-17A, IL-17F | [128, 129] [130] | |
| Systemic lupus erythematosus (SLE) | MRL/lpr, NZB/W F1, BXD2 | IL-17A | [131, 132] | |
| Psoriasis | Chronic proliferative dermatitis, transgenic cutaneous overexpression of cytokines, IL-23 injection i.d. | IL-17A, IL-22 | [133–135] | |
| Inflammatory bowel disease (IBD) | Trinitrobenzenesulfo nic acid-induced colitis, Dextran sulfate sodium-induced colitis | IL-17A, IL-17F | [136–138] | |
| Type 1 diabetes mellitus (T1D) | NOD mouse, STZ-induced Type 1 diabetes mellitus | IL-17A, IL-17F | [139, 140] | |
| Allergy | Bronchial asthma | Allergen sensitization in BALB/c; RSV-induced asthma exacerbation | IL-17E (IL-25), IL-17F | [141–143] |
| Fungal/Bacterial infections and host defense | Chronic mucocutaneous candidiasis (CMC) | Oral/dermal infection of mice with Candida albicans | IL-17A, IL-17F | [43, 45, 46, 144] |
| Pulmonary tuberculosis, pertussis, and pneumonia | Aerosol route to infect mouse, retropharyngeal instillation of the causative bacteria | IL-17A, IL-17F | [145, 146] [147] |
The IL-17 family of cytokines consists of 6 members, IL-17A-F (Table 1) [16] . The most widely studied of the family members is the IL-17A. IL-17A and IL-17F signal through a heterodimeric receptor complex that includes the IL-17RA and IL-17RC. IL-17RA is found ubiquitously but can only signal in the presence of IL-17RC [17]. The IL-17RA/RC complex signals through tumor necrosis factor receptor-associated factor 6 (TRAF6) leading to activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), mitogen-activated protein kinase (MAPK), and other pathways and production of inflammatory mediators [9, 18–20]. Various cytokines, chemokines and other mediators of inflammation increase the expression of IL-17. This cytokine has been shown to have a cooperative or an antagonistic relationship with other pro-inflammatory cytokines such as IFNγ [21–24].
IL-17 directly aggravates arthritic inflammation by binding to its receptor (IL-17RA/IL17RC) on immune cells as well as fibroblasts, osteoblasts, synoviocytes and endothelial cells, and stimulating them to produce more pro-inflammatory cytokines, chemokines and other inflammatory mediators including nitric oxide (NO), prostaglandins, and matrix metalloproteinases (MMPs) [10, 23, 25–28]. IL-17 works synergistically with other pro-inflammatory cytokines, mainly IL-1, IL-6 and tumor necrosis factor-α (TNF-α) to increase the production of diverse mediators of inflammation. IL-17 itself acts as a chemotactic factor and promotes immune cell infiltration into the arthritic synovium. In addition, IL-17 facilitates neovascularization by stimulating fibroblasts to secrete vascular endothelial growth factor (VEGF), by promoting blood vessel growth, and by activating endothelial cells.
Osteoclastic bone resorption is another characteristic feature of arthritis besides inflammation. Molecular pathways involving the receptor activator of NF-Kappa B (RANK) and its ligand, RANK ligand (RANKL), play a critical role in regulating osteoclastogenesis in the joints in RA. IL-17 plays a major role in driving osteoclastogenesis [10, 23, 25–28]. IL-17 upregulates RANK on osteoclast precursors and thereby increases their sensitivity to RANKL. Also, IL-17 induces the production of RANKL. Furthermore, IL-17 induces the expression of macrophage-colony stimulating factor (M-CSF), which is required for the differentiation and survival of osteoclasts.
IL-23 and the IL-23/IL-17 axis
IL-23 is a member of the IL-12 cytokine family and it is mainly secreted by activated macrophages and dendritic cells (DCs). The levels of IL-17 and IL-23 are elevated in the serum and synovial fluid of patients with RA, and these levels positively correlate with the disease severity [29–31]. However, these cytokines are barely detectable in healthy joints and in the joints of patients with osteoarthritis. There are other pieces of support for the strategic role of IL-23 in several autoimmune diseases including RA. For example, naïve T cells do not express the receptor for IL-23 (IL-23R), but this receptor is expressed on activated Th17 cells. IL-23 not only acts on conventional αβ T cells, but also on innate cells such as γδ T cells and cells of myeloid origin. Furthermore, IL-23 is required for the amplification and stabilization of Th17 cells. IL-23 signals through Janus kinases (e.g., JAK2 and non-receptor tyrosine-protein kinase (Tyk2)) and signal transducer and activator of transcription (STATs) (STAT3 and STAT4). Sustained IL-23 signaling in T cells is of importance for maintaining ongoing inflammation [7]. Differentiated Th17 cells are maintained and expanded primarily by IL-23 [7, 8, 32]. In γδ T cells, IL-23 promotes IL-17 production and to a lesser extent can do this without the engagement of the T cell receptor (TCR) [33]. Thus, both IL-23 and IL-17 form a new axis through Th17 cells, which play an important role in autoimmunity and chronic inflammation [9]. Examples of some diseases in which Th17 cells and their cytokine products play a role in disease pathogenesis are shown in Table 2 [9, 20, 34].
The cytokine milieu for Th17 differentiation and the activity of Th17 cells
A primary source of IL-17 is the Th17 cell, which is characterized by the expression of the master transcription factor retinoic acid receptor-related orphan receptor gamma t (RORγt). Th17 cells differentiate from naïve T cells in the presence of different cytokine combinations in mice and humans [35]. In mice, TGFβ and IL-6 have been shown to be sufficient for Th17 differentiation. IL-21 can also pair with TGFβ to differentiate murine Th17 cells [36]. In humans, Th17 differentiation occurs in the presence of IL-1β paired with either IL-6 or IL-23 [37, 38]. It was later shown that this type of differentiation occurs in T central memory cells, but that IL-21 and TGFβ combine to differentiate naïve T cells into Th17 cells [39]. IL-6 and IL-21 both signal through STAT3, highlighting the importance ofSTAT3 in Th17 differentiation. In addition, since Th17 cells can produce IL-21 besides IL-17, IL-21 may have an autocrine effect in promoting Th17 responses.
The Th17 cells direct immune responses against extracellular bacteria and fungi and some intracellular pathogens (Table 2). IL-17A and IL-17F produced by Th17 cells aid the migration of neutrophils and other myeloid cells to sites of infection by inducing the production of chemokines (e.g., IL-8) [40]. These cytokines play a vital role in immunity against fungal infections, such as those caused by Candida albicans [41–46]. Genetic deficiency of IL-17RA (autosomal recessive) or IL-17F (autosomal dominant) has been reported in a subset of patients with chronic mucocutaneous candidiasis (CMC) characterized by infection primarily by C. albicans and sometimes by Staphylococcus aureus [43, 44]. Recently, the role of natural Th17 and γδ T cells, but not by ILCs, in providing innate immunity against C. albicans infection in mice has been elaborated [45]. The clearance of S. aureus in the nasal mucosa has also been shown to be Th17-dependent and mediated via IL-17A production and neutrophil influx [47]. These findings are of relevance in understanding the pathogenesis of mucosal immunity against oral pathogens In other studies, it has been shown that mycobacteria and Pneumocystis carinii induce IL-23 production by infected antigen-presenting cells (APCs). Furthermore, knocking out p19 of IL-23 or neutralizing IL-17 prevents the clearance of P. carinii, further supporting the role of Th17 cells in anti-fungal immune response [48]. In addition, it has been shown that IFNα promotes Th17 effector response, while inhibiting Treg activity, thus favoring an inflammation-promoting milieu [49].
The phenotype, cellular differentiation, and activity of Treg cells
The Treg cells are of CD4+ CD25+ Foxp3+ phenotype. CD25 represents the IL-2 receptor alpha (IL-2Rα), whereas Forkhead box 3 (Foxp3) is the intracellular transcription factor expressed by these cells. Treg cells belong to two subsets: natural Tregs (nTreg) and inducible Tregs (iTreg). A large proportion of Treg cells develop in the thymus (nTreg) when a developing T cell binds to self-antigen with high-affinity. The second population of Treg cells differentiates in the periphery (iTreg) from naïve T cells following activation by an antigen [50]. Based on the location of their development, the terms thymically-derived Treg (tTreg) and peripheral Treg (pTreg) are now being used is favor of the terms nTreg and iTreg, respectively [50, 51].
The differentiation of Treg requires TGFβ [52]). TGFβ signals through the TGFβ receptor and SMAD family member proteins (human homologue of Drosophila Mad (Mad =Mothers against decapentaplegic) and the related Caenorhabditis elegans gene Sma) leading to Foxp3 expression. IL-2 has been shown to be indispensable for both Treg proliferation and differentiation [53]. IL-2 signals through the IL-2R and STAT5 to induce and maintain Foxp3 expression. This was evident following IL-2 treatment of cancer patients that resulted in an increase in Treg cells, followed by a decrease in Treg cells when IL-2 treatment was terminated [54]. Once the Treg cell differentiates, it upregulates the expression of CD25 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), but downregulates CD127 (representing IL-7Rα) (in humans) [55].
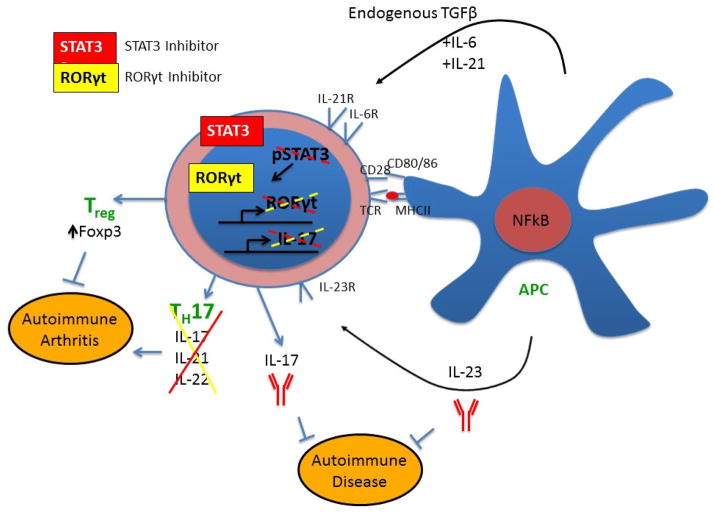
Treg cells play an important role in the induction of tolerance and protection against autoimmunity [53, 55–57]. Treg cells suppress effector cells through a number of secreted and surface-expressed proteins. Evidence from different studies supports at least four main mechanisms of suppression [58–64]: cell-cell contact involving inhibitory surface molecules (CTLA-4 and lymphocyte-activation gene 3 (LAG-3)); inhibitory cytokines (TGF-β, IL-10); cytolytic activity (granzyme/perforin); and metabolic disruption (IL-2 deprivation). First, Treg cells express CTLA-4, which, in mice, has the ability to trans-endocytose CD80/CD86 from dendritic cells (DCs). This prevents DCs from activating and expanding the effector T cell population. Similarly, LAG-3, which is a CD4-related molecule that binds the major histocompatibility complex (MHC) class II molecule, limits the expansion of activated T cells in part via inhibiting the TCR signaling [64]. Second, Tregs produce anti-inflammatory cytokines (e.g., IL-10, TGF-β, and IL-35). Third, Tregs can mediate cytolysis of target cells via granzyme/perforin. Fourth, high-level expression of IL-2R on Treg cells is believed to deprive the effector T cells of IL-2 and inhibit their proliferation [58, 63]. The balance between the effector T cells and the regulatory T cells determines whether or not autoreactive cells are able to induce and propagate an autoimmune response (Figure 1).
Figure 1. Targeting IL-17, IL-23, and the Th17/Treg imbalance for the control of autoimmune arthritis.
IL-17 drives inflammation and bone destruction in arthritis by promoting the cellular migration and activation of osteoclasts. One therapeutic approach in arthritis would be to limit the effects of IL-17. This can be achieved either by direct inhibition of IL-17 through the use of monoclonal antibodies against IL-17 or by indirect inhibition via blocking the function of the primary producer of IL-17, the Th17 cell. The use of inhibitors against STAT3 or RORγt could block the production of IL-17, and potentially might have the added effect of promoting the generation of inducible Treg (or pTreg) cells because of the antagonistic relationship between Th17 and Treg differentiation. Since pro-inflammatory Th17 cells express the IL-23R, antibodies against IL-23 may also be beneficial in targeting Th17-mediated autoimmune diseases.
The control of Th17 cells by Tregs and/or cytokines
The cytokines produced by Treg cells have been shown to inhibit Th17 development. Many cell types including Treg cells produce TGFβ. At higher doses and in the absence of inflammatory cytokines (IL-6 and IL-1), TGFβ promotes Foxp3 expression, which inhibits RORγt [65]. Recombinant IL-10 has been shown to limit Th17 differentiation in vitro, and IL-10−/− mice have increased expression of IL-17 and IL-22 [66]. This was confirmed by the finding that Th17 cells from an RA patient treated with IL-10 in vitro had reduced expression of IL-17 and RORC, but increased expression of Foxp3 [67]. An additional mechanism of Th17 inhibition by Tregs was discovered when two different TNFα-inhibitors, adalimumab and etanercept, were tested on immune cells from an active RA patient. IL-10 and TGFβ were sufficient to suppress IFNγ, but had much less effect on IL-17 production. Instead, the altered activation and expression of IL-6 by monocytes from RA patients correlated with the reduction in IL-17. Furthermore, co-culture of adalimumab-treated Treg cells and monocytes identified an IL-10-independent mechanism of Th17 inhibition [68].
Other cytokines not typically produced by Treg cells that control Th17 differentiation are IL-4 and IL-27. IL-4 can inhibit IL-17 production by Th17 cells in a STAT6-dependent inhibition of STAT3. IL-4 cannot cause the already differentiated Th17 cells to become Th2 cells, but it can inhibit IL-17 production. However, this is short-lived as Th17 cells exposed to IL-4 for long-term become sensitized and downregulate IL-4R expression [69]. Next, IL-27 has been shown to antagonize Th17 development in vivo in a STAT-1-dependent, IFNγ-independent mechanism [70]. We have also shown that the injection of recombinant IL-27 limits autoimmune arthritis and IL-17 production in the rat adjuvant arthritis (AA) model of RA [23].
The dynamics of the Th17/Treg balance in the course of arthritis
It has been reported that Th17 cells are significantly increased in the peripheral blood of RA patients compared to healthy controls [71–75]. However, the pattern of change in Treg cell number and/or function in RA is not fully clear [76]. Some investigators have shown either a decrease [71–73, 77] or no change [78] in the frequency of Tregs, while others have reported an increase [79] in Treg in the peripheral blood of RA patients [80]. Comparative evaluation of Treg frequency in the synovial tissue/joints versus peripheral blood has revealed two types of patters. Most of the reports indicate increased Treg in synovial tissue [78, 79, 81], while some others show reduced Treg [77, 82, 83] compared to peripheral blood.
It has been reported by several investigators that Treg are functionally defective in RA [84–87], while few investigators observed that Treg are not defective in their suppressive activity [78, 79]. The suppressive function of Treg cells is controlled by the phosphorylation of Foxp3, and TNFα has been shown to reduce this phosphorylation leading to an increase in IL-17 response [88]. Furthermore, the effect of TNFα could be reversed by anti-TNFα antibody treatment. Recent studies have shown that defective Treg function in RA might be attributable to dysregulated methylation of an enhancer region upstream of the Foxp3 locus [85]. Another set of studies have revealed that deficiency in Treg function is RA involves defects in the expression and function of CTLA-4 [86] and epigenetic modifications resulting in failure to activate the immunosuppressive indoleamine 2, 3-dioxygenase (IDO)-mediated pathway [87].
In general, the lower Th17/Treg ratio in healthy controls versus active RA patients supports the role of Treg in autoimmune pathogenesis in arthritis. A decrease in Treg function may be associated with an increase in the severity of arthritis. Interestingly, various treatment regimens such as non-depleting anti-CD4 antibody [89], IL-21 [90], or anti-IL-6 receptor antibody [91] in animal models of RA, and anti-IL-6 receptor antibody (tocilizumab) [92], anti-TNFα antibody [68, 88], or combination therapy using methotrexate and etanercept [73] in RA patients have been shown to correct the disturbed Th17/Treg imbalance [80]. Furthermore, a differential effect of anti-TNFα modalities was observed in a study comparing adalimumab and etanercept: Treg of patients treated with adalimumab, but not those treated with etanercept showed suppressive effect on Th17 cells [68]. The effect of anti-TNF therapy on Th17/Treg balance has also been observed in a model of uveitis [93]. In that study, anti-TNF therapy inhibited the differentiation of Th17 cells and thereby, altered the Th17/Treg balance in favor of immune suppression. Taken together, the results of the above-mentioned studies and the success of Treg adoptive therapy in animal models of RA and other autoimmune diseases have offered a convincing rationale for considering Treg supplementation as a therapeutic approach for RA [94, 95].
Paradoxical, regulatory role of IL-17 and IL-17-expressing T cells
We have described above the pathogenic role of IL-17 and Th17 cells. Three aspects of IL-17 and Th17 deserve special mention. First, that IL-17 is not equivalent to Th17, and vice versa [96]; second, both IL-17 and Th17 have been shown to expression anti-inflammatory activity under specific conditions [97, 98]; and third, a subset of Foxp3-expressing regulatory T cells have been shown to produce IL-17 [99, 100]. For example, IL-17-producing regulatory Th17 cells have been reported to modulate disease in an experimental model of type 1 diabetes (T1D) [97]. In another study, it has been shown that IL-17 displays an anti-inflammatory role in experimental autoimmune uveitis [98]. Finally, IL-17-producting regulatory T cells have been shown to possess suppressive activity [99, 100]. These new realizations show the complexities pertaining to the prototypic pro-inflammatory cytokine IL-17 and IL-17-producing T cells. Further studies are required to fully understand the dual role of these mediators of inflammation.
Therapeutic approaches based on IL-17/IL-23 and Th17/Treg balance
Neutralization of IL-17/IL-23 for the treatment of arthritis
Therapeutic efficacy of IL-17 neutralization has been investigated in experimental models of RA and in RA patients [101–104]. In rat AA and mouse CIA models, treatment with anti-IL-17A alleviated symptoms of arthritis and afforded protection against bone damage [101]. Two IL-17A-neutralizing antibodies, secukinumab- a fully human monoclonal antibody of the IgG1/γ isotype and ixekizumab- a humanized antibody of the IgG4 isotype, have been tested in the clinic. Both these antibodies improved clinical signs and symptoms, with no major adverse effects in RA, psoriatic arthritis (PsA), and ankylosing spondylitis [103, 105, 106]. Similarly, the efficacy and safety of brodalumab, a human monoclonal antibody against IL-17RA, was tested in a phase 2, randomized, double-blind, placebo-controlled study involving patients with PsA [104]. Brodalumab induced a marked improvement in patients with PsA. However, in one trial, this antibody was not much effective against RA [107]. IL-17RA is a common receptor subunit for IL-17 family member ligands (except for IL-17B), and blocking/inhibiting it may affect the functions of other members as well. Therefore, targeting the other receptor of IL-17, IL-17RC, is being considered for the control of autoimmune diseases.
Blocking IL-23, which is required for Th17 expansion and maintenance, has also been attempted as a potential therapeutic strategy. Ustekinumab, a human monoclonal antibody against IL-12/23 (p40 subunit), has been tested in patients with PsA and found to cause a significant reduction in the signs and symptoms of that disease [108–110]. Briakinumab and guselkumab are other antibodies against IL-23, which are being tested in different diseases including PsA and Crohn’s disease.
Inhibition of STAT3 and RORγt to control arthritis
STAT3 is a signaling protein that is activated by IL-6 and IL-21. It has been shown to bind to the IL-17A and IL-17F loci [111]. STAT3−/− mice display a severe reduction in RORγt expression and Th17 development, leading to increased Treg cells [112]. Taken together, STAT3 directly regulates the differentiation of Th17 cells and targeting STAT3 is one way to reset the Th17/Treg cell imbalance seen in RA (Figure 1). There are a few examples in animal models that confirm that STAT3 inhibition limits autoimmune arthritis. STA-21 is a small molecule that inhibits the DNA binding of STAT3 [113]. Furthermore, natural products can also limit STAT3 signaling. For example, epigallocatechin-3-gallate (EGCG) [114] and Celastrol [115] are bioactive components of green tea and Celastrus, respectively, and they alter the Th17/Treg ratio in favor of Treg and limit arthritis by blocking STAT3 phosphorylation. Similar effects have been reported for grape seed proanthocyanidin extract [116] and haloguginone [117]; the latter is a synthetic analog of a natural herbal alkaloid.
When activated by IL-6 or IL-21, STAT3 increases the expression of RORγt. As the master regulator of Th17 cells, RORγt is a prime target to alter autoimmune arthritis. Naturally-derived digoxin and its synthetic derivatives [118], and synthetic SR1001 [119] have been shown to inhibit RORγt, which resulted in a reduction in Th17 cells in vitro and in vivo. This reduction in Th17 cells correlated with reduced autoimmune disease in mice [118, 119]. In contrast, the effect on Treg cells is less correlative and is dependent on which inhibitor is used.
Concluding remarks
Defining the IL-23/IL-17 axis and elaborating the differentiation and function of Th17 and Treg cells represent major advancements in immunology in the past decade. Knowledge of the biochemical and molecular pathways and cellular events driven by these mediators of inflammation has filled many gaps that the Th1/Th2 paradigm could not fully explain. However, it would be an oversimplification, albeit incorrect, to conclude by any criteria that functionally Th17/Treg has supplanted Th1/Th2 activities. In fact, the two systems are known to cooperate in certain situations, while being adversaries in others. Furthermore, IL-17 and Th17 have been shown to display anti-inflammatory activity in some situations, and IL-17-producing Treg have also been reported. These issues need to be addressed in future research.
HIGHLIGHTS.
IL-17 produced by Th17 cells plays a vital role in the pathogenesis of arthritis.
Th17/Treg imbalance contributes to the initiation and progression of arthritis.
Neutralization of IL-17 and IL-23 is being explored for the treatment of arthritis.
Various synthetic/ natural products are being tested for resetting Th17/Treg balance
Acknowledgments
This work was supported by R01 AT004321 (KDM) and F31 AT007278 (BA) from NIH/NCCAM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 2.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual review of immunology. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 3.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunology today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 4.D'Elios M, Del Prete G. Th1/Th2 balance in human disease. Transplantation proceedings. 1998;30:2373–7. doi: 10.1016/s0041-1345(98)00659-9. [DOI] [PubMed] [Google Scholar]
- 5.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 6.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. The Journal of experimental medicine. 2003;198:1951–7. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181:5948–55. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 9.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nature reviews. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubberts E. Th17 cytokines and arthritis. Seminars in immunopathology. 2010;32:43–53. doi: 10.1007/s00281-009-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amarilyo G, Lourenco EV, Shi FD, La Cava A. IL-17 promotes murine lupus. J Immunol. 2014;193:540–3. doi: 10.4049/jimmunol.1400931. [DOI] [PubMed] [Google Scholar]
- 12.Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, et al. RORgammat+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nature immunology. 2011;12:320–6. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 13.Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, et al. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nature immunology. 2009;10:1125–32. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nature reviews. 2013;13:145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 15.Villanova F, Flutter B, Tosi I, Grys K, Sreeneebus H, Perera GK, et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. The Journal of investigative dermatology. 2014;134:984–91. doi: 10.1038/jid.2013.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine. 2013;64:477–85. doi: 10.1016/j.cyto.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–9. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 18.Awane M, Andres PG, Li DJ, Reinecker HC. NF-kappa B-inducing kinase is a common mediator of IL-17-, TNF-alpha-, and IL-1 beta-induced chemokine promoter activation in intestinal epithelial cells. J Immunol. 1999;162:5337–44. [PubMed] [Google Scholar]
- 19.Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. The Journal of experimental medicine. 2000;191:1233–40. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunological reviews. 2008;226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 21.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Annals of the New York Academy of Sciences. 2010;1183:211–21. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu CQ, Swart D, Alcorn D, Tocker J, Elkon KB. Interferon-gamma regulates susceptibility to collagen-induced arthritis through suppression of interleukin-17. Arthritis Rheum. 2007;56:1145–51. doi: 10.1002/art.22453. [DOI] [PubMed] [Google Scholar]
- 23.Rajaiah R, Puttabyatappa M, Polumuri SK, Moudgil KD. Interleukin-27 and interferon-gamma are involved in regulation of autoimmune arthritis. The Journal of biological chemistry. 2011;286:2817–25. doi: 10.1074/jbc.M110.187013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nindl V, Maier R, Ratering D, De Giuli R, Zust R, Thiel V, et al. Cooperation of Th1 and Th17 cells determines transition from autoimmune myocarditis to dilated cardiomyopathy. European journal of immunology. 2012;42:2311–21. doi: 10.1002/eji.201142209. [DOI] [PubMed] [Google Scholar]
- 25.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. The Journal of experimental medicine. 2006;203:2673–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koenders MI, Lubberts E, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Kolls JK, et al. Induction of cartilage damage by overexpression of T cell interleukin-17A in experimental arthritis in mice deficient in interleukin-1. Arthritis Rheum. 2005;52:975–83. doi: 10.1002/art.20885. [DOI] [PubMed] [Google Scholar]
- 27.Nanjundaiah SM, Astry B, Moudgil KD. Mediators of inflammation-induced bone damage in arthritis and their control by herbal products. Evid Based Complement Alternat Med. 2013;2013:518094. doi: 10.1155/2013/518094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venkatesha SH, Yu H, Rajaiah R, Tong L, Moudgil KD. Celastrus-derived Celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. The Journal of biological chemistry. 2011;286:15138–46. doi: 10.1074/jbc.M111.226365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu Al Fadl EM, Fattouh M, Allam AA. High IL-23 level is a marker of disease activity in rheumatoid arthritis. The Egyptian journal of immunology / Egyptian Association of Immunologists. 2013;20:85–92. [PubMed] [Google Scholar]
- 30.Guo YY, Wang NZ, Zhao S, Hou LX, Xu YB, Zhang N. Increased interleukin-23 is associated with increased disease activity in patients with rheumatoid arthritis. Chinese medical journal. 2013;126:850–4. [PubMed] [Google Scholar]
- 31.Metawi SA, Abbas D, Kamal MM, Ibrahim MK. Serum and synovial fluid levels of interleukin-17 in correlation with disease activity in patients with RA. Clinical rheumatology. 2011;30:1201–7. doi: 10.1007/s10067-011-1737-y. [DOI] [PubMed] [Google Scholar]
- 32.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Current opinion in immunology. 2007;19:652–7. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–41. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Zhu S, Qian Y. IL-17/IL-17 receptor system in autoimmune disease: mechanisms and therapeutic potential. Clin Sci (Lond) 2012;122:487–511. doi: 10.1042/CS20110496. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 36.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nature immunology. 2007;8:942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 38.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature immunology. 2007;8:950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–2. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luzza F, Parrello T, Monteleone G, Sebkova L, Romano M, Zarrilli R, et al. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J Immunol. 2000;165:5332–7. doi: 10.4049/jimmunol.165.9.5332. [DOI] [PubMed] [Google Scholar]
- 41.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. The Journal of experimental medicine. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez-Santos N, Gaffen SL. Th17 cells in immunity to Candida albicans. Cell host & microbe. 2012;11:425–35. doi: 10.1016/j.chom.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science (New York, NY. 2011;332:65–8. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puel A, Cypowyj S, Marodi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Current opinion in allergy and clinical immunology. 2012;12:616–22. doi: 10.1097/ACI.0b013e328358cc0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conti HR, Peterson AC, Brane L, Huppler AR, Hernandez-Santos N, Whibley N, et al. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. The Journal of experimental medicine. 2014;211:2075–84. doi: 10.1084/jem.20130877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milner JD, Holland SM. The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases. Nature reviews. 2013;13:635–48. doi: 10.1038/nri3493. [DOI] [PubMed] [Google Scholar]
- 47.Archer NK, Harro JM, Shirtliff ME. Clearance of Staphylococcus aureus nasal carriage is T cell dependent and mediated through interleukin-17A expression and neutrophil influx. Infect Immun. 2013;81:2070–5. doi: 10.1128/IAI.00084-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun. 2007;75:3055–61. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golding A, Rosen A, Petri M, Akhter E, Andrade F. Interferon-alpha regulates the dynamic balance between human activated regulatory and effector T cells: implications for antiviral and autoimmune responses. Immunology. 2010;131:107–17. doi: 10.1111/j.1365-2567.2010.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gratz IK, Rosenblum MD, Abbas AK. The life of regulatory T cells. Annals of the New York Academy of Sciences. 2013;1283:8–12. doi: 10.1111/nyas.12011. [DOI] [PubMed] [Google Scholar]
- 51.Sawant DV, Vignali DA. Once a Treg, always a Treg? Immunological reviews. 2014;259:173–91. doi: 10.1111/imr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. The Journal of experimental medicine. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–23. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 54.Wei S, Kryczek I, Edwards RP, Zou L, Szeliga W, Banerjee M, et al. Interleukin-2 administration alters the CD4+FOXP3+ T-cell pool and tumor trafficking in patients with ovarian carcinoma. Cancer Res. 2007;67:7487–94. doi: 10.1158/0008-5472.CAN-07-0565. [DOI] [PubMed] [Google Scholar]
- 55.Rudensky AY. Regulatory T cells and Foxp3. Immunological reviews. 2011;241:260–8. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Pillars article: immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995. J Immunol. 1995;186:3808–21. [PubMed] [Google Scholar]
- 57.Sawla P, Hossain A, Hahn BH, Singh RP. Regulatory T cells in systemic lupus erythematosus (SLE); role of peptide tolerance. Autoimmunity reviews. 2012;11:611–4. doi: 10.1016/j.autrev.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sega EI, Leveson-Gower DB, Florek M, Schneidawind D, Luong RH, Negrin RS. Role of lymphocyte activation gene-3 (Lag-3) in conventional and regulatory T cell function in allogeneic transplantation. PloS one. 2014;9:e86551. doi: 10.1371/journal.pone.0086551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nature immunology. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.You S, Alyanakian MA, Segovia B, Damotte D, Bluestone J, Bach JF, et al. Immunoregulatory pathways controlling progression of autoimmunity in NOD mice. Annals of the New York Academy of Sciences. 2008;1150:300–10. doi: 10.1196/annals.1447.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nature reviews. 2009;9:480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vignali DA. Mechanisms of T(reg) Suppression: Still a Long Way to Go. Frontiers in immunology. 2012;3:191. doi: 10.3389/fimmu.2012.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–13. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 65.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu Y, Yang J, Ouyang X, Liu W, Li H, Yang J, et al. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. European journal of immunology. 2008;38:1807–13. doi: 10.1002/eji.200838331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heo YJ, Joo YB, Oh HJ, Park MK, Heo YM, Cho ML, et al. IL-10 suppresses Th17 cells and promotes regulatory T cells in the CD4+ T cell population of rheumatoid arthritis patients. Immunology letters. 2010;127:150–6. doi: 10.1016/j.imlet.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 68.McGovern JL, Nguyen DX, Notley CA, Mauri C, Isenberg DA, Ehrenstein MR. Th17 cells are restrained by Treg cells via the inhibition of interleukin-6 in patients with rheumatoid arthritis responding to anti-tumor necrosis factor antibody therapy. Arthritis Rheum. 2012;64:3129–38. doi: 10.1002/art.34565. [DOI] [PubMed] [Google Scholar]
- 69.Cooney LA, Towery K, Endres J, Fox DA. Sensitivity and resistance to regulation by IL-4 during Th17 maturation. J Immunol. 2011;187:4440–50. doi: 10.4049/jimmunol.1002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nature immunology. 2006;7:929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 71.Chen J, Li J, Gao H, Wang C, Luo J, Lv Z, et al. Comprehensive evaluation of different T-helper cell subsets differentiation and function in rheumatoid arthritis. J Biomed Biotechnol. 2012;2012:535361. doi: 10.1155/2012/535361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang W, Shao S, Jiao Z, Guo M, Xu H, Wang S. The Th17/Treg imbalance and cytokine environment in peripheral blood of patients with rheumatoid arthritis. Rheumatol Int. 2012;32:887–93. doi: 10.1007/s00296-010-1710-0. [DOI] [PubMed] [Google Scholar]
- 73.Lina C, Conghua W, Nan L, Ping Z. Combined treatment of etanercept and MTX reverses Th1/Th2, Th17/Treg imbalance in patients with rheumatoid arthritis. Journal of clinical immunology. 2011;31:596–605. doi: 10.1007/s10875-011-9542-6. [DOI] [PubMed] [Google Scholar]
- 74.van Hamburg JP, Asmawidjaja PS, Davelaar N, Mus AM, Colin EM, Hazes JM, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73–83. doi: 10.1002/art.30093. [DOI] [PubMed] [Google Scholar]
- 75.Niu Q, Cai B, Huang ZC, Shi YY, Wang LL. Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol Int. 2012;32:2731–6. doi: 10.1007/s00296-011-1984-x. [DOI] [PubMed] [Google Scholar]
- 76.Mijnheer G, Prakken BJ, van Wijk F. The effect of autoimmune arthritis treatment strategies on regulatory T-cell dynamics. Current opinion in rheumatology. 2013;25:260–7. doi: 10.1097/BOR.0b013e32835d0ee4. [DOI] [PubMed] [Google Scholar]
- 77.Jiao Z, Wang W, Jia R, Li J, You H, Chen L, et al. Accumulation of FoxP3-expressing CD4+CD25+ T cells with distinct chemokine receptors in synovial fluid of patients with active rheumatoid arthritis. Scandinavian journal of rheumatology. 2007;36:428–33. doi: 10.1080/03009740701482800. [DOI] [PubMed] [Google Scholar]
- 78.Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clinical and experimental immunology. 2005;140:360–7. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 80.Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmunity reviews. 2014;13:668–77. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 81.Moradi B, Schnatzer P, Hagmann S, Rosshirt N, Gotterbarm T, Kretzer JP, et al. CD4(+)CD25(+)/highCD127low/(−) regulatory T cells are enriched in rheumatoid arthritis and osteoarthritis joints--analysis of frequency and phenotype in synovial membrane, synovial fluid and peripheral blood. Arthritis research & therapy. 2014;16:R97. doi: 10.1186/ar4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Behrens F, Himsel A, Rehart S, Stanczyk J, Beutel B, Zimmermann SY, et al. Imbalance in distribution of functional autologous regulatory T cells in rheumatoid arthritis. Annals of the rheumatic diseases. 2007;66:1151–6. doi: 10.1136/ard.2006.068320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raghavan S, Cao D, Widhe M, Roth K, Herrath J, Engstrom M, et al. FOXP3 expression in blood, synovial fluid and synovial tissue during inflammatory arthritis and intra-articular corticosteroid treatment. Annals of the rheumatic diseases. 2009;68:1908–15. doi: 10.1136/ard.2008.100768. [DOI] [PubMed] [Google Scholar]
- 84.Bernard NJ. Rheumatoid arthritis: Who knows why regulatory T cells are defective in RA ... IDO. Nat Rev Rheumatol. 2014;10:381. doi: 10.1038/nrrheum.2014.96. [DOI] [PubMed] [Google Scholar]
- 85.Kennedy A, Schmidt EM, Cribbs AP, Penn H, Amjadi P, Syed K, et al. A novel upstream enhancer of FOXP3, sensitive to methylation-induced silencing, exhibits dysregulated methylation in rheumatoid arthritis Treg cells. European journal of immunology. 2014;44:2968–78. doi: 10.1002/eji.201444453. [DOI] [PubMed] [Google Scholar]
- 86.Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19396–401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cribbs AP, Kennedy A, Penn H, Read JE, Amjadi P, Green P, et al. Treg cell function in rheumatoid arthritis is compromised by ctla-4 promoter methylation resulting in a failure to activate the indoleamine 2,3-dioxygenase pathway. Arthritis & rheumatology (Hoboken, NJ. 2014;66:2344–54. doi: 10.1002/art.38715. [DOI] [PubMed] [Google Scholar]
- 88.Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med. 2013;19:322–8. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- 89.Duarte J, Agua-Doce A, Oliveira VG, Fonseca JE, Graca L. Modulation of IL-17 and Foxp3 expression in the prevention of autoimmune arthritis in mice. PloS one. 2010;5:e10558. doi: 10.1371/journal.pone.0010558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niu X, He D, Zhang X, Yue T, Li N, Zhang JZ, et al. IL-21 regulates Th17 cells in rheumatoid arthritis. Human immunology. 2010;71:334–41. doi: 10.1016/j.humimm.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 91.Fujimoto M, Serada S, Mihara M, Uchiyama Y, Yoshida H, Koike N, et al. Interleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responses. Arthritis Rheum. 2008;58:3710–9. doi: 10.1002/art.24126. [DOI] [PubMed] [Google Scholar]
- 92.Samson M, Audia S, Janikashvili N, Ciudad M, Trad M, Fraszczak J, et al. Brief report: inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:2499–503. doi: 10.1002/art.34477. [DOI] [PubMed] [Google Scholar]
- 93.Sugita S, Kawazoe Y, Imai A, Yamada Y, Horie S, Mochizuki M. Inhibition of Th17 differentiation by anti-TNF-alpha therapy in uveitis patients with Behcet's disease. Arthritis research & therapy. 2012;14:R99. doi: 10.1186/ar3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Esensten JH, Wofsy D, Bluestone JA. Regulatory T cells as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:560–5. doi: 10.1038/nrrheum.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haque M, Fino K, Lei F, Xiong X, Song J. Utilizing regulatory T cells against rheumatoid arthritis. Frontiers in oncology. 2014;4:209. doi: 10.3389/fonc.2014.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O'Connor W, Jr, Zenewicz LA, Flavell RA. The dual nature of T(H)17 cells: shifting the focus to function. Nature immunology. 2010;11:471–6. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- 97.Singh B, Schwartz JA, Sandrock C, Bellemore SM, Nikoopour E. Modulation of autoimmune diseases by interleukin (IL)-17 producing regulatory T helper (Th17) cells. The Indian journal of medical research. 2013;138:591–4. [PMC free article] [PubMed] [Google Scholar]
- 98.Ke Y, Liu K, Huang GQ, Cui Y, Kaplan HJ, Shao H, et al. Anti-inflammatory role of IL-17 in experimental autoimmune uveitis. J Immunol. 2009;182:3183–90. doi: 10.4049/jimmunol.0802487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4793–8. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mercer F, Khaitan A, Kozhaya L, Aberg JA, Unutmaz D. Differentiation of IL-17-producing effector and regulatory human T cells from lineage-committed naive precursors. J Immunol. 2014;193:1047–54. doi: 10.4049/jimmunol.1302936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chao CC, Chen SJ, Adamopoulos IE, Davis N, Hong K, Vu A, et al. Anti-IL-17A therapy protects against bone erosion in experimental models of rheumatoid arthritis. Autoimmunity. 2011;44:243–52. doi: 10.3109/08916934.2010.517815. [DOI] [PubMed] [Google Scholar]
- 102.Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–9. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 103.Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62:929–39. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 104.Mease PJ, Genovese MC, Greenwald MW, Ritchlin CT, Beaulieu AD, Deodhar A, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. The New England journal of medicine. 2014;370:2295–306. doi: 10.1056/NEJMoa1315231. [DOI] [PubMed] [Google Scholar]
- 105.Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Annals of the rheumatic diseases. 2013;72(Suppl 2):ii116–23. doi: 10.1136/annrheumdis-2012-202371. [DOI] [PubMed] [Google Scholar]
- 106.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Science translational medicine. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 107.Martin DA, Churchill M, Flores-Suarez L, Cardiel MH, Wallace D, Martin R, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis research & therapy. 2013;15:R164. doi: 10.1186/ar4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009;373:633–40. doi: 10.1016/S0140-6736(09)60140-9. [DOI] [PubMed] [Google Scholar]
- 109.McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–9. doi: 10.1016/S0140-6736(13)60594-2. [DOI] [PubMed] [Google Scholar]
- 110.Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Annals of the rheumatic diseases. 2014;73:990–9. doi: 10.1136/annrheumdis-2013-204655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nature immunology. 2011;12:247–54. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. The Journal of biological chemistry. 2007;282:9358–63. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 113.Park JS, Kwok SK, Lim MA, Kim EK, Ryu JG, Kim SM, et al. STA-21, a promising STAT-3 inhibitor that reciprocally regulates Th17 and Treg cells, inhibits osteoclastogenesis in mice and humans and alleviates autoimmune inflammation in an experimental model of rheumatoid arthritis. Arthritis & rheumatology (Hoboken, NJ. 2014;66:918–29. doi: 10.1002/art.38305. [DOI] [PubMed] [Google Scholar]
- 114.Yang EJ, Lee J, Lee SY, Kim EK, Moon YM, Jung YO, et al. EGCG attenuates autoimmune arthritis by inhibition of STAT3 and HIF-1alpha with Th17/Treg control. PloS one. 2014;9:e86062. doi: 10.1371/journal.pone.0086062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Astry B, Venkatesha S, Moudgil KD. Celastrus-derived Celastrol modulates autoimmune arthritis through cellular immunoregulation Immunity & Tolerance: 78th Cold Spring Harbor Symposium on Quantitative Biology; May 2013; Cold Spring Harbor, New York: Cold Spring Harbor Laboratory; 2013. [Google Scholar]
- 116.Jhun JY, Moon SJ, Yoon BY, Byun JK, Kim EK, Yang EJ, et al. Grape seed proanthocyanidin extract-mediated regulation of STAT3 proteins contributes to Treg differentiation and attenuates inflammation in a murine model of obesity-associated arthritis. PloS one. 2013;8:e78843. doi: 10.1371/journal.pone.0078843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park MK, Park JS, Park EM, Lim MA, Kim SM, Lee DG, et al. Halofuginone ameliorates autoimmune arthritis in mice by regulating the balance between Th17 and Treg cells and inhibiting osteoclastogenesis. Arthritis & rheumatology (Hoboken, NJ. 2014;66:1195–207. doi: 10.1002/art.38313. [DOI] [PubMed] [Google Scholar]
- 118.Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature. 2011;472:486–90. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491–4. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ely LK, Fischer S, Garcia KC. Structural basis of receptor sharing by interleukin 17 cytokines. Nature immunology. 2009;10:1245–51. doi: 10.1038/ni.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–12. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 122.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nature reviews. 2009;9:556–67. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. The Journal of allergy and clinical immunology. 2001;108:430–8. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 124.Moore EE, Presnell S, Garrigues U, Guilbot A, LeGuern E, Smith D, et al. Expression of IL-17B in neurons and evaluation of its possible role in the chromosome 5q-linked form of Charcot-Marie-Tooth disease. Neuromuscular disorders : NMD. 2002;12:141–50. doi: 10.1016/s0960-8966(01)00250-4. [DOI] [PubMed] [Google Scholar]
- 125.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine & growth factor reviews. 2003;14:155–74. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 126.Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 127.Starnes T, Broxmeyer HE, Robertson MJ, Hromas R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J Immunol. 2002;169:642–6. doi: 10.4049/jimmunol.169.2.642. [DOI] [PubMed] [Google Scholar]
- 128.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–73. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 129.Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–30. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 130.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. The American journal of pathology. 2008;172:146–55. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen XQ, Yu YC, Deng HH, Sun JZ, Dai Z, Wu YW, et al. Plasma IL-17A is increased in new-onset SLE patients and associated with disease activity. Journal of clinical immunology. 2010;30:221–5. doi: 10.1007/s10875-009-9365-x. [DOI] [PubMed] [Google Scholar]
- 132.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nature immunology. 2008;9:166–75. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 133.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. The Journal of investigative dermatology. 2009;129:1339–50. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 134.Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. The Journal of investigative dermatology. 2008;128:1207–11. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 135.Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez-Farinas M, Fuentes-Duculan J, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. The Journal of experimental medicine. 2007;204:3183–94. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunological reviews. 2008;226:147–59. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 137.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, et al. Regulation of inflammatory responses by IL-17F. The Journal of experimental medicine. 2008;205:1063–75. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Grieco FA, Moore F, Vigneron F, Santin I, Villate O, Marselli L, et al. IL-17A increases the expression of proinflammatory chemokines in human pancreatic islets. Diabetologia. 2014;57:502–11. doi: 10.1007/s00125-013-3135-2. [DOI] [PubMed] [Google Scholar]
- 140.Shao S, He F, Yang Y, Yuan G, Zhang M, Yu X. Th17 cells in type 1 diabetes. Cell Immunol. 2012;280:16–21. doi: 10.1016/j.cellimm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 141.Kawaguchi M, Kokubu F, Fujita J, Huang SK, Hizawa N. Role of interleukin-17F in asthma. Inflammation & allergy drug targets. 2009;8:383–9. doi: 10.2174/1871528110908050383. [DOI] [PubMed] [Google Scholar]
- 142.Ota K, Kawaguchi M, Matsukura S, Kurokawa M, Kokubu F, Fujita J, et al. Potential involvement of IL-17F in asthma. Journal of immunology research. 2014;2014:602846. doi: 10.1155/2014/602846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Petersen BC, Dolgachev V, Rasky A, Lukacs NW. IL-17E (IL-25) and IL-17RB promote respiratory syncytial virus-induced pulmonary disease. Journal of leukocyte biology. 2014 doi: 10.1189/jlb.0913482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol. 2010;185:5453–62. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nature immunology. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 146.Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–9. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 147.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–81. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]