Abstract
Background
Human papillomavirus (HPV) tumor status and surgical salvage are associated with improved prognosis for patients with recurrent oropharyngeal squamous cell carcinoma (OPSCC). Current data regarding types of surgery and the impact of surgery for distant metastatic disease are limited.
Methods
A retrospective analysis of patients with recurrent OPSCC from two institutions between 2000-2012 was performed. P16 immunohistochemistry and/or in situ hybridization, as clinically available, were used to determine HPV tumor status. Clinical characteristics, distribution of recurrence site and treatment modalities were compared by HPV tumor status. Overall survival was examined by Kaplan-Meier and Cox proportional hazards methods.
Results
The study included 108 patients with 65 locoregional and 43 distant metastatic first recurrences. The majority were HPV-positive (n=80). HPV-positive tumor status was associated with longer time to recurrence (p<0.01). Anatomic site distribution of recurrences did not differ by HPV tumor status. HPV-positive tumor status (adjusted HR [aHR] 0.23 (95%CI 0.09-0.58), p=0.002), longer time to recurrence (≥1 year; aHR 0.36 (0.18-0.74), p=0.006), and surgical salvage (aHR 0.26 (0.12-0.61), p=0.002) were independently associated with overall survival after recurrence. Surgical salvage was independently associated with improved overall survival compared to non-surgical treatment in both locoregional (aHR 0.15 (0.04-0.56), p=0.005) and distant metastatic recurrence (aHR 0.19 (0.05-0.75), p=0.018).
Conclusions
Surgical salvage is associated with improved overall survival for recurrent locoregional and distant metastatic OPSCC, independent of HPV tumor status. Further prospective data is needed to confirm the role of surgical salvage for distant metastases.
Keywords: Oropharyngeal Neoplasms, Squamous Cell Carcinoma of the head and neck, Human Papillomavirus, Salvage Therapy, Recurrence, Neoplasm Metastasis
Introduction
Human papillomavirus (HPV) is responsible for a growing subset of oropharyngeal squamous cell carcinoma (OPSCC) in the United States and abroad.1-3 At primary diagnosis, HPV-positive tumor status is associated with improved response to chemoradiation, progression-free survival, and overall survival.4,5 Despite improved prognosis, locoregional and distant metastatic recurrences still pose a significant disease burden. Within three years of diagnosis, approximately 24-27% of HPV-positive patients experience recurrence.5,6
While the unique clinical features of HPV-positive OPSCC are now well characterized, few studies have addressed the clinical implications of recurrent disease.7 Earlier reports suggested unusual clinical presentations for HPV-associated recurrences.8-11 Recent prospective data from Radiation Therapy Oncology Group (RTOG) 0129 and 0522 trials demonstrated that the site distribution and time to recurrence does not differ by HPV tumor status.12
Recently, HPV-positive tumor status12-14 and receipt of surgical salvage12 have been shown to be independent markers of improved prognosis in recurrent OPSCC. Although type of recurrence (locoregional or distant) was accounted for in RTOG analysis, few patients (n=5) received surgical salvage for distant metastatic disease and details of salvage therapies were limited.12 The study populations in EXTREME and SPECTRUM trials were restricted to patients who were ineligible for surgical or radiation salvage.13-15 Therefore, we evaluated both surgical and non-surgical salvage therapies for recurrent locoregional and distant metastatic OPSCC and their prognostic role in the context of HPV tumor status.
Patients and Methods
Study population
This was an Institutional Review Board approved retrospective study of patients treated at Johns Hopkins Hospital and Greater Baltimore Medical Center between 2000 and 2012. Patients diagnosed with recurrent OPSCC or unknown primary, and known HPV tumor status were eligible. Unknown primaries were included as these tumors are frequently HPV-related.16 Recurrence was defined as diagnosis of local, regional or distant disease after completion of primary treatment with curative intent and a post-treatment disease-free interval of three months. Patients with second primaries or persistent disease (without disease-free interval) were excluded.
HPV tumor status
HPV tumor status was based upon clinically available HPV in situ hybridization (ISH; n=105) or p16 immunohistochemistry (IHC; n=94), an established surrogate marker for HPV in OPSCC,17 at primary diagnosis. HPV tumor status for recurrent lesions was reported based on p16 IHC (n=44) or HPV ISH (n=43) as clinically available. Recurrent tumors with discordant HPV status were excluded as second primaries (n=2).18,19
Clinical data
Clinical data was obtained by medical record abstraction. Variables of interest at the time of primary diagnosis were age, gender, race, history of tobacco and/or alcohol use, TNM stage, and treatment. At the time of first recurrence, location of recurrence (locoregional and/or distant), single or multiple sites of recurrence, anatomic site of distant metastasis, diagnostic method, treatment, and survival data were obtained. Surgical salvage data included surgical procedure, margin status and length of hospitalization. Margin status was based upon final pathology report. Close margins were considered negative.
The date of recurrence was defined by date of pathologic diagnosis or PET imaging if pathology was unavailable. Diagnostic methods of recurrence were categorized as clinical exam (at scheduled appointment), patient symptoms (prompting clinical evaluation), or imaging studies (surveillance studies in the absence of abnormal exam or symptoms). Anatomic site distribution of distant metastases at first and later recurrences was determined. Time to recurrence was defined from date of primary diagnosis to date of recurrence.
Overall survival after diagnosis of recurrence was the primary outcome. Additional survival data was also obtained from public social security records for patients with last date of follow up prior to July 2013. Survival analysis was restricted to patients who received treatment for recurrence.
Statistical analysis was performed using StataIC12 (College Station, Tx). Chi-squared tests were used for categorical data and Wilcoxon rank-sum tests for comparison of medians. Time to recurrence and survival were estimated by Kaplan Meier method and compared by log-rank test. Univariate and multivariate survival analysis was performed with Cox proportional hazards regression models. Two-sided p-values less than 0.05 were considered significant. Parsimonious variable selection for multivariate cox model was based on clinical and univariate significance. Non-significant variables that changed hazard ratios by more than 10% when removed from the model were also retained.20
Results
Patient characteristics
A total of 108 patients met study criteria (Table 1). The majority of patients were HPV-positive (n=80, 74.1%). HPV-positive patients were more likely to be white (p <0.01), male (p <0.01) and never smokers (p=0.06) compared with HPV-negative patients. HPV-positive patients were also more likely to receive non-surgical primary treatment (83.8 % vs. 53.6%, p<0.01).
Table 1. Characteristics of patients with recurrent oropharyngeal cancer by human papillomavirus (HPV) tumor status.
| HPV-positive (N=80) n (%) | HPV-negative (N=28) n (%) | p-value1 | |
|---|---|---|---|
| Age at diagnosis | 0.952 | ||
| Median (range) | 58 (34-79) | 58.5 (30-80) | |
|
| |||
| Race | |||
| White | 74 (92.5) | 16 (57.1) | <0.01 |
| Black | 4 (5.0) | 11(39.3) | |
| Other | 2 (2.5) | 1 (3.6) | |
|
| |||
| Gender | |||
| Male | 70 (87.5) | 18 (64.3) | <0.01 |
| Female | 10 (12.5) | 10 (35.7) | |
|
| |||
| Subsite of primary | |||
| Base of tongue | 36 (45.0) | 15 (53.6) | 0.65 |
| Tonsil | 35 (43.8) | 9 (35.7) | |
| Unknown primary | 4 (5.0) | 1 (3.6) | |
| Other | 5 (6.3) | 3 (10.7) | |
|
| |||
| Smoking history at diagnosis | |||
| Ever smoker | 47 (58.8) | 22 (78.6) | 0.06 |
| Never smoker | 33 (41.2) | 6 (21.4) | |
|
| |||
| History of alcohol use at diagnosis (>2drinks/day) | 0.25 | ||
| Yes | 30 (37.5) | 14 (50) | |
| No | 50 (62.5) | 14 (50) | |
|
| |||
| Tumor stage at diagnosis | |||
| T0 | 4 (5.3) | 0 (0) | 0.22 |
| T1 | 7 (9.3) | 7 (25.9) | |
| T2 | 24 (32.0) | 8 (29.6) | |
| T3 | 24 (32.0) | 7 (25.9) | |
| T4 | 16 (21.3) | 5 (18.5) | |
| Nodal stage at diagnosis | 0.47 | ||
| N0-N2a | 25 (31.2) | 11 (39.3) | |
| N2b-N3 | 52 (65.0) | 17 (60.7) | |
| Unknown | 3 (3.8) | 0 | |
| Overall stage at diagnosis | 0.48 | ||
| I or II | 5 (6.3) | 4 (14.8) | |
| III or IV | 71 (93.7) | 23 (85.2) | |
|
| |||
| Primary treatment | |||
| Surgery | 13 (16.2) | 12 (42.9) | 0.043 |
| alone | 5(6.2) | 3 (10.7) | 0.004 |
| w/adjuvant | 8 (10.0) | 9 (32.1) | |
| Non-surgical therapy | |||
| Primary CRT | 61 (76.3) | 14 (50.0) | 0.91 |
| Radiation only | 3 (3.8) | 1 (3.6) | |
| Chemotherapy only | 3 (3.8) | 1 (3.6) | |
|
| |||
| Site of first recurrence | |||
| Local only | 23 (29.5) | 8 (28.6) | 0.83 |
| Regional only | 13 (16.7) | 4 (14.3) | |
| Locoregional | 15 (19.2) | 4 (14.3) | |
| Locoregional and Distant | 5 (6.4) | 1 (3.6) | |
| Distant only | 22 (28.2) | 11 (39.3) | |
Chi-squared test unless otherwise indicated
Wilcoxon rank-sum test
Comparing distribution of all primary treatment modalities overall.
Patterns of disease recurrence
Patterns of recurrence were compared by HPV tumor status. HPV tumor status for recurrent disease was available for 45 of 78 (57.7%) HPV-positive patients and confirmed HPV-positive tumor status in all recurrences. There was no difference in the distribution of disease recurrence (local, locoregional or distant) by HPV tumor status (Table 1). Similar proportions of HPV-positive and HPV-negative patients had local (29.5 vs. 28.6%, p=0.93), locoregional (35.9% vs. 28.6%, p=0.48) and distant metastatic (34.6 vs. 42.8%, p=0.44) disease at the time of first recurrence (Table 1). Twelve patients had multiple sites of disease at the time of recurrence, and distribution was similar for HPV-positive and HPV-negative patients (11.5% vs. 12.0%, p=0.91)
Among patients with any history of distant metastases (n=57), lung was the most common site for both HPV-positive and HPV-negative patients (p=0.18, Table 2). Mediastinal lymph nodes were the second most common site of distant recurrence, followed by bone and skin. The distribution of distant metastatic sites did not differ by HPV tumor status (p=0.41).
Table 2. Patterns of distant metastatic disease*.
| Anatomic site | HPV-positive (n=43) | HPV-negative (n=14) | p-value |
|---|---|---|---|
| Lung | 30 (69.7) | 7 (50.0) | 0.41 |
| Mediastinal/hilar lymph nodes | 5 (11.6) | 3 (21.4) | |
| Bone | 5 (11.6) | 3 (21.4) | |
| Skin/dermal | 2 (4.6) | 2 (14.3) | |
| Liver | 6 (13.9) | 1 (7.1) | |
| Brain | 4 (9.3) | 1 (7.1) | |
| Thyroid | 2 (4.6) | 1 (7.1) | |
| Adrenal | 0 (0) | 1 (7.1) |
in the follow-up period.
Treatment of recurrence
The majority of patients received treatment at the time of initial recurrence (Table 3). Surgical salvage was the most common treatment (n=61, 61.6%) and frequently administered with adjuvant radiation (n=42, 68.9%). Non-surgical treatment (n=33) consisted of chemotherapy (45.5%), radiation therapy (24.2%) and chemoradiation (30.3%), and did not differ by HPV tumor status (Table 3).
Table 3. Treatment of recurrence1.
| HPV-positive N=78 | HPV-negative N=28 | p-value | |
|---|---|---|---|
| Any Salvage Therapy | 0.12 | ||
| Yes | 72 (92.3%) | 22 (78.6%) | |
| No | 3 (3.8) | 2 (7.1) | |
| Unknown | 3 (3.8) | 4 (14.2) | |
| Surgical salvage2 | 0.25 | ||
| Yes | 49 (68.1) | 12 (54.5) | |
| No | 23 (31.9) | 10 (45.5) | |
| Surgery only | 16 (32.7) | 2 (16.7) | 0.28 |
| Surgery + adjuvant | 33 (67.3) | 10 (83.3) | |
| Non-surgical salvage | |||
| Chemotherapy only | 9 (39.1) | 6 (60.0) | 0.25 |
| Radiation only | 5 (21.7) | 3 (30.0) | |
| Chemoradiation | 9 (39.1) | 1 (10.0) |
Patients with metastatic disease at primary diagnosis were excluded, n=106
n=94, treated patients only
Patients receiving surgical salvage did not differ by age (median: 56 vs. 58, p=0.28) or primary treatment modality (p=0.64). However, a greater proportion of patients who underwent surgical salvage were disease-free for ≥1 year as compared to patients who received non-surgical treatment (75.4% vs. 54.5%, p=0.038). For patients with distant metastatic recurrence, surgical salvage was also associated with longer disease-free interval (p=0.016, n=38). Patients with multiple sites of disease were more likely to receive non-surgical treatment compared to patients with a single disease site (80.0% vs. 29.8%, p=0.002).
Details of operative procedures were available for all 61 patients who underwent surgical salvage for locoregional or distant metastatic recurrence. Surgical salvage for locoregional recurrence (46 of 61, 75.4%) included wide local excision with or without neck dissection (n=29, including TORS n=4), total laryngectomy with or without total glossectomy (n=7), and neck dissection only (n=10). Twenty-one of 46 (45.7%) of these locoregional salvage surgeries were extensive enough to require tracheostomy (n=13), gastrostomy tube (n=9), and/or free flap reconstruction (n=14). Average postoperative length of stay was 6.5 days (n=42; range 0 to 28). There was no difference in prevalence of positive margins (n=42) by HPV tumor status (34.3% vs. 30.0%, p=0.80).
Surgical salvage for distant metastasis was performed for 16 patients, 14 of whom were HPV-positive. These procedures were predominantly pulmonary resections (9 of 16, 56.3%), seven (77.8%) were video-assisted thorascopic surgeries (VATS) and three (33.3%) were for multiple lung nodules. Four patients underwent mediastinal lymphadenectomy, including three with concomitant pulmonary resections. Additional procedures included craniotomy, dermal excision, hepatectomy and laminectomy. Average length of stay was 3.8 days (range 1-9 days, n=11).
Time to recurrence
Diagnosis of recurrence was significantly later for HPV-positive patients than HPV-negative patients (median time to recurrence 19.6 vs. 9.9 months, p<0.001, Figure 1). HPV-positive patients had increased time to recurrence for both locoregional (p=0.07) and distant metastatic recurrence (p<0.001) in Kaplan-Meier analysis. In multivariate analysis, HPV-positive tumor status was independently associated with longer time to recurrence (adjusted HR 0.39, 95%CI 0.23-0.68, p=0.001). Factors at primary diagnosis not associated with time to recurrence included age, gender, race, smoking and alcohol history, tumor stage, nodal stage, and primary treatment modality (p>0.10).
Figure 1. Time to recurrence from primary diagnosis of oropharyngeal cancer compared by human papillomavirus (HPV) tumor status.
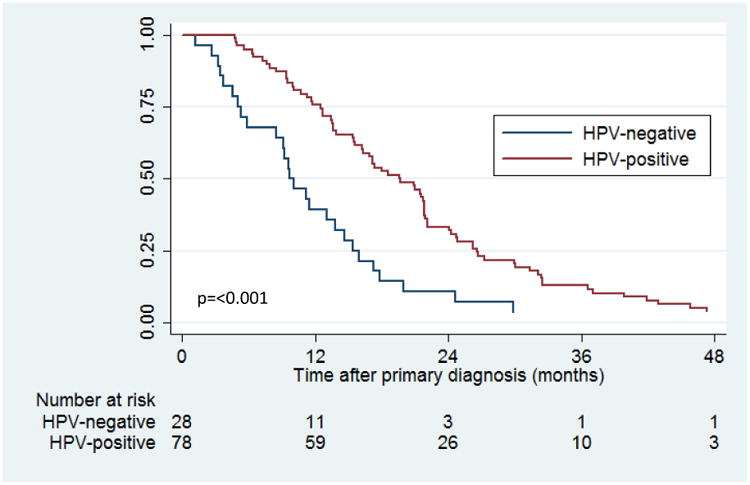
A majority of recurrences occurred within two years after primary diagnosis for both HPV-positive (66.0%, 95%CI 55.4-76.3) and HPV-negative patients (89.3%, 95%CI 74.9-97.3). The method of diagnosis of recurrence differed by HPV tumor status (p=0.02). HPV-positive recurrences were primarily diagnosed by imaging (47.4%) whereas clinical examination was the main method of diagnosis for HPV-negative recurrences (46.4%). Distant metastatic disease was more likely than locoregional disease to be diagnosed by imaging (73.7% vs. 24.2%, p <0.001). Method of diagnosis was not associated with time to recurrence (p=0.46).
Survival analysis
Median follow-up time after recurrence was 15.8 months (range 0.2-105.8 months). HPV-positive patients had significantly improved overall survival (OS) after recurrence as compared with HPV-negative patients (3-year OS: 55.7% vs. 25.2%, p=0.01; Figure 2A). Median survival was longer for HPV-positive than HPV-negative patients (82.6 vs. 23.1 months).
Figure 2. Kaplan Meier curves showing overall survival after recurrence.
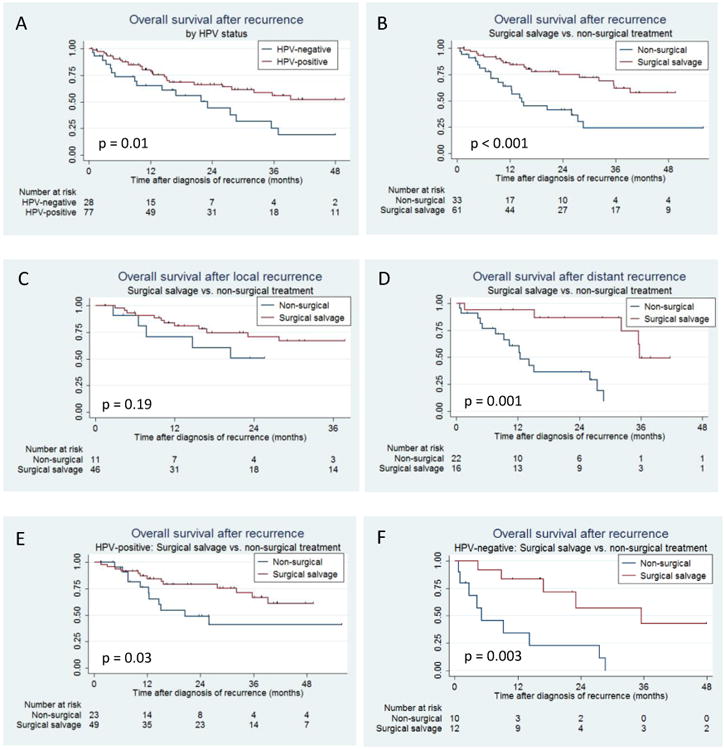
Censored patients are denoted by tick mark.
(A) Overall survival (OS) after recurrence by human papillomavirus (HPV) tumor status. 2-year OS: 66.3%(95%CI 53.2-76.5%) vs. 44.0%(95%CI 22.6-63.6%); 3-year OS: 55.7%(41.0-68.2%) vs. 25.2%(8.4-46.3%).
(B) Overall survival after recurrence by surgical salvage. 2-year OS: 74.9%(60.2-84.8) vs. 41.4%(23.3-58.6%); 3-year OS 61.8%(44.6-75.1%) vs. 24.1%(8.8-43.6%).
(C) Overall survival after locoregional recurrence by surgical salvage. 2-year OS: 70.7% (52.6-83.0) vs. 50.5%(18.7-75.7); 3-year OS: 66.8%(48.0-80.1) vs. 50.5%(18.7-75.7).
(D) Overall survival after distant recurrence by surgical salvage. 2-year OS: 86.5% (55.8-96.5) vs. 36.3%(15.5-57.7); 3-year OS: 49.5%(16.0-76.3) vs. 9.7%(0.7-33.5)
(E) Overall survival after recurrence by surgical salvage for HPV-positive disease. 2-year OS: 78.9%(63.2-88.5) vs. 49.0%(25.8-68.8); 3-year OS: 66.6%(47.2-80.3) vs. 40.9%(18.0-62.6)
(F) Overall survival after recurrence by surgical salvage for HPV-negative disease. 2-year OS: 57.1%(20.1-82.3) vs. 22.9%(3.5-52.2); 3-year OS: 42.9%(10.8-72.4), unable to assess for non-surgical group.
Factors associated with overall survival after recurrence in univariate analysis included time to recurrence (≥1 year: HR 0.41, 95%CI 0.23-0.73, p=0.002), HPV-positive tumor status (HR 0.47, 95%CI 0.26-0.88, p=0.02), multiple sites of recurrent disease (HR 3.22, 95%CI 1.53-6.79, p=0.002) and treatment with surgical salvage (HR 0.32, 95%CI 0.17-0.60, p<0.001; Figure 2B). Locoregional recurrence was associated with a non-significant reduction in risk of death compared with distant recurrence (HR 0.57, 95%CI 0.31-1.02, p=0.055). All other variables were not associated with overall survival (age, gender, race, smoking history, initial nodal status, primary treatment modality; p>0.10).
Patients who underwent surgical salvage had significantly improved overall survival compared to patients who received non-surgical treatment for recurrence (3-year OS 61.8% vs. 24.1%, p<0.001; Figure 2B). Median survival time was 101.5 months for patients who received salvage surgery and 14.7 months for those who received non-surgical treatment.
Patients with locoregional recurrence treated with surgical salvage had a non-significant improvement in survival compared to non-surgical treatment (p=0.19; Figure 2C). However, surgical salvage was associated with improved survival in patients with distant metastatic recurrence (p=0.001; Figure 2D). Median survival for patients with distant metastatic disease who received surgical salvage was 35.7 months as compared to 12.5 months for non-surgical treatment.
Surgical salvage improved overall survival for both HPV-positive (p=0.03; Figure 2E) and HPV-negative patients (p=0.003; Figure 2F) compared with non-surgical treatment. For patients with distant metastatic recurrence, surgical salvage was associated with improved overall survival for both HPV-positive (p=0.03) and HPV-negative patients (p=0.05), although the number of HPV-negative patients in this group was limited (n=11).
In multivariate analysis, HPV-positive tumor status (adjusted HR [aHR] 0.23, p=0.002), longer time to recurrence (≥1 year; aHR 0.36, p=0.006), primary surgical treatment (aHR 0.21, p=0.007), and treatment with surgical salvage (aHR 0.26, p=0.002) were each independently associated with improved overall survival (Table 4). When patients were stratified by type of recurrence, surgical salvage was independently associated with improved survival for both locoregional (aHR 0.15, 95%CI 0.04-0.56, p=0.005) and distant metastatic recurrence (aHR 0.19, 95%CI 0.05-0.75, p=0.018).
Table 4. Multivariate analysis of factors associated with overall survival after recurrence*.
| Variables | HR(95%CI) | P-value |
|---|---|---|
| HPV tumor status (Positive vs. negative) | 0.23(0.09-0.58) | 0.002 |
| Age at diagnosis (per year) | 1.04(0.99-1.09) | 0.062 |
| Race (White vs. non-white^) | 0.57(0.23-1.44) | 0.24 |
| Smoking history (Ever vs. never) | 0.72(0.33-1.61) | 0.43 |
| Initial nodal stage (2b-3 vs. 0-2a) | 0.91(0.35-1.61) | 0.84 |
| Time to recurrence (≥1 year vs. <1 year) | 0.36(0.18-0.74) | 0.006 |
| Site of recurrence (Locoregional vs. distant) | 2.31(0.83 -6.39) | 0.11 |
| Number of recurrences (Multiple vs. single) | 3.27(1.07-10.01) | 0.038 |
| Primary treatment (Surgical vs. non-surgical) | 0.21(0.066-0.65) | 0.007 |
| Salvage treatment (Surgical vs. non-surgical) | 0.26(0.12-0.61) | 0.002 |
Restricted to patients who received salvage therapy (n=92). HR=Hazard ratio, CI=confidence interval.
Black and other
Discussion
This study suggests a robust survival advantage associated with surgical salvage for patients with recurrent OPSCC even for distant metastatic disease, building on recent findings.12 Until recently, recurrent OPSCC was associated with poor overall survival, and the role of salvage surgery, especially for distant metastatic disease, was questioned in the context of this relatively poor overall survival and high potential morbidity.21-23 Previous studies on surgical salvage of recurrent OPSCC focused primarily on locoregional recurrence and did not account for HPV tumor status.22,24-26 In this analysis overall survival was evaluated after locoregional and distant metastatic recurrence in the context of HPV tumor status and salvage therapy.
Surgical salvage therapy in the oropharynx has traditionally been associated with increased morbidity such as gastrostomy tube dependence27 and need for a midline mandibulotomy. However, the landscape of recurrent OPSCC is changing with the rise of HPV-related disease1,2 as well as advancements in surgical approaches to the oropharynx. Transoral robotic surgery (TORS) has become a viable option for select recurrent OPSCC with decreased reported morbidity including shorter hospital stays, margin control, and decreased tracheostomies and gastrostomy tubes.28 Based on extent of recurrent disease, minimally invasive surgery for locoregional disease may not always be possible. It was not used in the majority of locoregional recurrences reviewed herein (n=4), thus improvement in overall survival associated with salvage surgery was likely not dependent on minimally invasive techniques.
Salvage surgery for distant metastatic disease extended median overall survival from 12.5 to 35 months. This reduction in risk of death remained significant after adjustment for HPV tumor status, age, time to recurrence and multiple sites of disease recurrence (aHR 0.19, p=0.018). Surgery for metastatic disease was primarily performed for lung metastases. As observed in this cohort, minimally invasive pulmonary procedures are also now widely available.29 An important consideration that is not captured by the present analysis is the quality of life after salvage surgery for metastatic disease.
Surgery for metastatic disease poses a departure from the traditional management of metastatic disease in head and neck cancer. Only chemotherapeutic treatment of distant metastases is supported by strong level 1 evidence within the current National Comprehensive Cancer Network (NCCN) guidelines.30 Although surgical salvage for distant metastases is an option for patients with good performance status and limited distant disease, evidence for non-chemotherapy treatments are based on lower level 2A evidence and data defining surgically appropriate distant metastases are lacking.30 In other anatomic sites such as colorectal cancer, NCCN guidelines have integrated site-specific recommendations for surgical resection of liver and pulmonary metastases based on an established body of evidence.31-35 Non-surgical approaches for localized treatment of metastatic disease (e.g. radiosurgery) are frequently used for distant metastases and also warrant further investigation. Our data in the context of current guidelines underscore the need to prospectively collect analogous surgical and non-surgical salvage data to elucidate the potential role of surgery and radiation therapy in the treatment of distant metastatic OPSCC to inform future guidelines.
Surgical salvage was associated with improved survival for both HPV-positive and HPV-negative patients, although this survival advantage was more prominent in HPV-negative patients. HPV-positive patients with recurrence may be more responsive to salvage chemotherapy treatments,14 thus attenuating the survival advantage of surgical salvage. Few studies have characterized the impact of HPV tumor status on recurrent OPSCC.36 Our data are consistent with recent observations that HPV-positive patients who develop recurrence retain an HPV-positive clinical phenotype including race and smoking history, and do no resemble HPV-negative patients.12 In concordance with recent literature, anatomic site distribution of recurrences did not differ by HPV tumor status.12
In this cohort, HPV-positive tumor status was associated with a longer time to recurrence. However, this data is consistent with previous reports that most failures, regardless of HPV status, occur within two years.12 Differences in time to recurrence may be explained by frequent post-treatment imaging in clinical protocols which could expedite diagnosis of HPV-positive recurrences. By contrast, less than half of recurrences (43%) in our cohort were diagnosed with imaging studies, most of which were HPV-positive (80.4%). Notably, longer time to recurrence was independently associated with a significant reduction in risk of death. This has not previously been reported in the context of HPV tumor status.27 Further studies will elucidate the role of HPV tumor status on timing of recurrence and its implications on the intensity of post-treatment surveillance in the clinical practice setting.
A limitation of this study is that HPV tumor status was classified based on tumor status of primary disease rather than recurrence, although a majority of HPV-positive patients (45 of 78, 57.7%) had available HPV tumor status of recurrence. However, studies have shown that in patients with prior HPV-positive OPSCC, recurrent disease is HPV-positive in 92-97% of cases, including pulmonary recurrences.18,19
The primary limitations of this study are biases inherent to retrospective analyses. In particular, there are potential selection biases that remain unaccounted for including patient and physician preferences, co-morbidities, performance status and extent of recurrent disease. A prospective study would be necessary to ascertain rationale for treatment choices and patient eligibility or ineligibility for treatment. Furthermore, complications and morbidity associated with these treatments could be reliably characterized with prospective data collection. Additional biases may include tertiary institutional referral patterns and non-uniform clinical practices both at the time of primary diagnosis and recurrence. Future prospective studies of surgical treatment of recurrent and distant metastatic OPSCC will elucidate these relevant clinical questions.
In conclusion, patients with recurrent OPSCC should be counseled on the potential survival advantages of surgical salvage, regardless of HPV tumor status or site of recurrence (locoregional or distant). HPV tumor status remains an important prognostic marker in the setting of recurrence. These data argue for inclusion of HPV tumor status and receipt of surgical salvage in design and analyses of clinical trials. The independent survival advantage of surgical salvage of distant metastatic disease highlights the need to prospectively collect data to inform future NCCN guidelines with higher levels of evidence than currently available.
Acknowledgments
This work was supported by P50DE019032, 2T32DC000027-26, and the Milton J Dance Jr. Head and Neck Center.
Footnotes
There are no financial disclosures from any authors.
References
- 1.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008 Feb 1;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011 Nov 10;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007 May 10;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 4.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008 Feb 20;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 5.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010 Jul 1;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized Phase III Trial of Concurrent Accelerated Radiation Plus Cisplatin With or Without Cetuximab for Stage III to IV Head and Neck Carcinoma: RTOG 0522. J Clin Oncol. 2014 Aug 25; doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfister DG, Fury MG. New chapter in our understanding of human papillomavirus-related head and neck cancer. J Clin Oncol. 2014 Oct 20;32(30):3349–3352. doi: 10.1200/JCO.2014.56.5754. [DOI] [PubMed] [Google Scholar]
- 8.Huang SH, Perez-Ordonez B, Liu FF, et al. Atypical clinical behavior of p16-confirmed HPV-related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2012 Jan 1;82(1):276–283. doi: 10.1016/j.ijrobp.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Huang SH, Perez-Ordonez B, Weinreb I, et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol. 2013 Jan;49(1):79–85. doi: 10.1016/j.oraloncology.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Ruzevick J, Olivi A, Westra WH. Metastatic squamous cell carcinoma to the brain: an unrecognized pattern of distant spread in patients with HPV-related head and neck cancer. J Neurooncol. 2013 May;112(3):449–454. doi: 10.1007/s11060-013-1075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller S, Khuri FR, Kono SA, Beitler JJ, Shin DM, Saba NF. HPV positive squamous cell carcinoma of the oropharynx. Are we observing an unusual pattern of metastases? Head Neck Pathol. 2012 Sep;6(3):336–344. doi: 10.1007/s12105-012-0355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014 Oct 20;32(30):3365–3373. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Argiris A, Li S, Ghebremichael M, et al. Prognostic significance of human papillomavirus in recurrent or metastatic head and neck cancer: an analysis of Eastern Cooperative Oncology Group trials. Ann Oncol. 2014 Jul;25(7):1410–1416. doi: 10.1093/annonc/mdu167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermorken JB, Psyrri A, Mesia R, et al. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: retrospective analysis of the phase III EXTREME trial. Ann Oncol. 2014 Apr;25(4):801–807. doi: 10.1093/annonc/mdt574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008 Sep 11;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 16.El-Mofty SK, Zhang MQ, Davila RM. Histologic identification of human papillomavirus (HPV)-related squamous cell carcinoma in cervical lymph nodes: a reliable predictor of the site of an occult head and neck primary carcinoma. Head Neck Pathol. 2008 Sep;2(3):163–168. doi: 10.1007/s12105-008-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003 Dec 15;9(17):6469–6475. [PubMed] [Google Scholar]
- 18.Bishop JA, Ogawa T, Chang X, et al. HPV analysis in distinguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2012 Jan;36(1):142–148. doi: 10.1097/PAS.0b013e3182395c7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vainshtein J, McHugh JB, Spector ME, et al. Human papillomavirus-related oropharyngeal cancer: HPV and p16 status in the recurrent versus parent tumor. Head Neck. 2015 Jan;37(1):8–11. doi: 10.1002/hed.23548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993 Dec 1;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin WJ., Jr Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope. 2000 Mar;110(3 Pt 2 Suppl 93):1–18. doi: 10.1097/00005537-200003001-00001. [DOI] [PubMed] [Google Scholar]
- 22.Zafereo ME, Hanasono MM, Rosenthal DI, et al. The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer. 2009 Dec 15;115(24):5723–5733. doi: 10.1002/cncr.24595. [DOI] [PubMed] [Google Scholar]
- 23.Ho AS, Kraus DH, Ganly I, Lee NY, Shah JP, Morris LG. Decision making in the management of recurrent head and neck cancer. Head Neck. 2014 Jan;36(1):144–151. doi: 10.1002/hed.23227. [DOI] [PubMed] [Google Scholar]
- 24.Kano S, Homma A, Hayashi R, et al. Salvage surgery for recurrent oropharyngeal cancer after chemoradiotherapy. Int J Clin Oncol. 2013 Oct;18(5):817–823. doi: 10.1007/s10147-012-0449-x. [DOI] [PubMed] [Google Scholar]
- 25.Nichols AC, Kneuertz PJ, Deschler DG, et al. Surgical salvage of the oropharynx after failure of organ-sparing therapy. Head Neck. 2011 Apr;33(4):516–524. doi: 10.1002/hed.21480. [DOI] [PubMed] [Google Scholar]
- 26.Matoscevic K, Graf N, Pezier TF, Huber GF. Success of salvage treatment: a critical appraisal of salvage rates for different subsites of HNSCC. Otolaryngol Head Neck Surg. 2014 Sep;151(3):454–461. doi: 10.1177/0194599814535183. [DOI] [PubMed] [Google Scholar]
- 27.Kostrzewa JP, Lancaster WP, Iseli TA, Desmond RA, Carroll WR, Rosenthal EL. Outcomes of salvage surgery with free flap reconstruction for recurrent oral and oropharyngeal cancer. Laryngoscope. 2010 Feb;120(2):267–272. doi: 10.1002/lary.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White H, Ford S, Bush B, et al. Salvage surgery for recurrent cancers of the oropharynx: comparing TORS with standard open surgical approaches. JAMA Otolaryngol Head Neck Surg. 2013 Aug 1;139(8):773–778. doi: 10.1001/jamaoto.2013.3866. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima J, Takamoto S, Tanaka M, Takeuchi E, Murakawa T, Fukami T. Thoracoscopic surgery and conventional open thoracotomy in metastatic lung cancer. Surg Endosc. 2001 Aug;15(8):849–853. doi: 10.1007/s004640090005. [DOI] [PubMed] [Google Scholar]
- 30.Head and Neck Cancer (Version 2.2014) [Accessed October 6, 2014];NCCN Clinical Practice Guidelines in Oncology. 2014 http://www.nccn.org/professionals/physician_gls/pdf/head-andneck.pdf.
- 31.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004 Jun;239(6):818–825. doi: 10.1097/01.sla.0000128305.90650.71. discussion 825-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997 Mar;15(3):938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto T, Tsubota N, Iwanaga K, Yuki T, Matsuoka H, Yoshimura M. Pulmonary resection for metastases from colorectal cancer. Chest. 2001 Apr;119(4):1069–1072. doi: 10.1378/chest.119.4.1069. [DOI] [PubMed] [Google Scholar]
- 34.Colon Cancer. (Version 2.2015) [Accessed October 7, 2014];NCCN Clinical Practice Guidelines in Oncology. 2015 http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- 35.Rectal Cancer. (Version 1.2015) [Accessed October 7, 2014];NCCN Clinical Practice Guidelines in Oncology. 2015 http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
- 36.Psyrri A, Rampias T, Vermorken JB. The current and future impact of human papillomavirus on treatment of squamous cell carcinoma of the head and neck. Ann Oncol. 2014 Nov;25(11):2101–2115. doi: 10.1093/annonc/mdu265. [DOI] [PubMed] [Google Scholar]
- 37.Trosman S, AK S, Koyfman SA, et al. Distant metastatic failure patterns in squamous cell cancer of the oropharynx (SCCOP) treated with chemoradiation: the impact of human papillomavirus (HPV) Int J Radiat Oncol Biol Phys. 2014;88(2):471. [Google Scholar]


