Abstract
Caloric restriction (CR) has been shown to increase the life span and health span of a broad range of species. However, CR effects on in vivo brain functions are far from explored. In this study, we used multimetric neuroimaging methods to characterize the CR-induced changes of brain metabolic and vascular functions in aging rats. We found that old rats (24 months of age) with CR diet had reduced glucose uptake and lactate concentration, but increased ketone bodies level, compared with the age-matched and young (5 months of age) controls. The shifted metabolism was associated with preserved vascular function: old CR rats also had maintained cerebral blood flow relative to the age-matched controls. When investigating the metabolites in mitochondrial tricarboxylic acid cycle, we found that citrate and α-ketoglutarate were preserved in the old CR rats. We suggest that CR is neuroprotective; ketone bodies, cerebral blood flow, and α-ketoglutarate may play important roles in preserving brain physiology in aging.
Keywords: Aging, Brain metabolism, Cerebral blood flow, Neuroimaging, Ketone bodies, α-ketoglutarate, Mammalian target of rapamycin
1. Introduction
Brain energy demands are among the highest of all organs. As a result, the cerebral metabolic rates of glucose (CMRGlc) and cerebral blood flow (CBF) are quite high at baseline. A widely accepted cause of the functional losses that accompany aging is decreased brain metabolic and vascular functions (Bentourkia et al., 2000; Wallace, 2005). In support of this viewpoint, a host of neuro-imaging studies show that CMRGlc and CBF decline with age (Bentourkia et al., 2000; Lin and Rothman, 2014; Wallace, 2005) and decline still more rapidly and profoundly in Alzheimer's disease (AD) (Cunnane et al., 2011; Hoyer, 1991; Nagata et al., 1997). The metabolic and hemodynamic reductions precede brain structural alteration (gray matter and white matter atrophy) and cognitive impairment (Bookheimer et al., 2000; Cunnane et al., 2011; Reiman et al., 2001). Therefore, preserving brain metabolism (i.e., glucose oxidative capacity) and hemodynamics are critical for optimizing health span (Stranahan and Mattson, 2012).
Caloric restriction (CR) is the most studied antiaging manipulation and has been shown to increase the life span of a broad range of species (Choi et al., 2011; Colman et al., 2009; Rahat et al., 2011). In the nervous system, CR has been shown to reduce oxidative stress, enhance neurotrophin levels, restore neuronal structure (Stranahan et al., 2009), and enhance cognitive function (Fontan-Lozano et al., 2008; Mattson, 2010; Valdez et al., 2010; Witte et al., 2009). In a recent study, we found that Fischer 344 Brown-Norway F1 (F344BNF1) rats under chronic CR had preserved mitochondrial function and neuronal activity (Lin et al., 2014). Specifically, compared with young rats, old rats with CR diet had similar fluxes of neuronal tricarboxylic acid (TCA) cycle and gluta-mate (Glu)-glutamine (Gln) neurotransmitter cycling (Lin et al., 2014). As TCA cycle flux is associated with metabolism (e.g., CMRGlc) and neuronal activity is associated with vascular integrity (e.g., CBF) (Fox and Raichle, 1986; Lin et al., 2010), this indicates that CR may also be able to impede the age-related decline of brain metabolic and vascular functions.
In this study, our goal was to identify CR effects on cerebral metabolism and blood flow in the same animal model. Specifically, we used in vivo neuroimaging to measure CMRGlc and CBF. We also used mass spectroscopy to determine the brain metabolites and identify the metabolic pathway associated with the changes under CR. We hypothesized that CR can preserve metabolic and vascular physiology in aging brain.
2. Material and methods
2.1. Animal
Experiments were conducted using male F344BNF1 rats because this particular strain has demonstrated extended longevity under CR (Turturro et al., 1999). Young control (5 months, N = 6), old control, and old calorie-restricted rats (24 months, N = 6 for each group) were obtained from the National Institute on Aging Caloric Restricted Colony. The sample size was determined with power analysis to perform the comparison at a 0.05 level of significance, with a 90% chance of detecting a true difference of all the measurements between the 3 groups.
At National Institute on Aging, all rats were fed ad libitum (National Institutes of Health [NIH]-31 diet) until 14 weeks of age. The CR regimen was initiated by incremental caloric reduction of 10% per week over 4 weeks, reaching full 40% CR by week 16. The vitamin-fortified NIH-31 (NIH-31 fortified) diet fed to CR rats provided 60% of the calories and additional vitamins supplement consumed by ad libitum rats. After arriving at our facilities, rats were housed individually (1 rat per cage) in a specific pathogen-free facility and were fed the same diet 1 hour before the onset of the dark cycle. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio according to NIH guidelines.
2.2. Animal preparation for functional neuroimaging
Rats were anesthetized with 4.0% isoflurane for induction and then maintained in a 1.2% isoflurane and air mixture using a face mask. Heart rate (90–130 bpm.), respiration rate, and rectal temperature (37 ± 0.5 °C) were continuously monitored. A water bath with circulating water at 45–50 °C was used to maintain the body temperature. Heart rate and blood oxygen saturation level were recorded using a MouseOx system (STARR Life Science, Oakmont, PA, USA) and maintained within normal physiological ranges.
2.3. Cerebral metabolic rate of glucose (CMRGlc) measurements
CMRGlc was measured using fluorodeoxyglucose (18FDG) positron emission tomography (PET) methods (Focus 220 microPET, Siemens, Nashville, TN, USA). A quantity of 0.5 mCi of 18FDG dissolved in 1 mL of physiologic saline solution was injected through the tail vein. Forty minutes were allowed for 18FDG uptake before scanning. Animals were then moved to the scanner bed and placed in the prone position. Emission data were acquired for 20 minutes in a 3-dimensional (3D) list mode with intrinsic resolution of 1.5 mm. For image reconstruction, 3D PET data was rebinned into multiple frames of 1-second duration using a Fourier algorithm. After rebinning the data, a 3D image was reconstructed for each frame using a 2D-filtered back projection algorithm.
Decay and dead time corrections were applied to the reconstruction process. Cerebral metabolic rate of glucose was determined using the mean standardized uptake value (SUV) equation: SUV (A × W)/Ainj, where A is the activity of the region of interest (i.e., brain region in the study), W is the body weight of the rat, and Ainj is the injection dose of the 18FDG, as described in a previous study (Lin et al., 2012b).
2.4. Cerebral blood flow (CBF) measurements
We used magnetic resonance imaging (MRI) to measure CBF. Quantitative CBF (with units of mL/g per minute) was obtained with MRI-based continuous arterial spin labeling (CASL) techniques on a horizontal 7 T/30 cm magnet (Bruker, Billerica, MA, USA), as described previously (Lin et al., 2012b). A circular surface coil was placed on top of the head and a circular labeling coil was placed at the heart position for CASL. The 2 coils were positioned parallel to each other and were actively decoupled. Paired images were acquired in an interleaved fashion with field of view = 12.8 × 12.8 mm2, matrix = 128 × 128, slice thickness = 1 mm, 10 slices, labeling duration = 2100 ms, repetition time = 3000 ms, and echo time = 20 ms. CASL image analysis employed codes written in MATLAB (Natick, MA, USA) and STIMULATE software (University of Minnesota, Minneapolis, MN, USA) to obtain CBF.
2.5. Brain metabolites measurements
We used high-performance liquid chromatography-electrospray ionization-mass spectrometry (HPLC-ESI-MS) to measure brain metabolites. Rats were sacrificed at the end of the MRI study. Brain tissues from the cortex and hippocampus were separated and frozen with liquid N2. Those brain tissues were then homogenized and extracted with icy cold 80% aqueous methanol and maintained on ice for 1 hour. The cell extracts were centrifuged at 13,800g for 10 minutes and the supernatants were transferred to glass auto-sampler vials for HPLC-ESI-MS analysis.
HPLC-ESI-MS analyses were conducted on a Thermo Fisher Q Exactive mass spectrometer with on-line separation by a Thermo Fisher/Dionex UltiMate 3000 HPLC. HPLC conditions were: column, Luna NH2, 3 μm, 2 × 150 mm (Phenomenex; Torrance, CA, USA); mobile phase A, 5% acetonitrile in water containing 20-mM ammonium acetate, and 20-mM ammonium hydroxide, pH 9.45; mobile phase B, acetonitrile; flow rate, 300 μL/min; gradient, 85% B to 1% B over 10 minutes and held at 1% B for 10 minutes. Pro-genesis CoMet Software (Nonlinear Dynamics) was used to process the raw data files. We quantified the concentration of metabolites by measuring the area under the curve and detected the metabolites that exhibited significant differences in abundance. Metabolite identification was performed through METLIN database searching using a 5-ppm mass tolerance, manual interpretation of the MS/MS fragment patterns, and agreement with the HPLC retention time of authentic standards.
2.6. Blood ketone bodies measurements
When the rats were sacrificed, blood sample was collected in a 2-mL BD tube coated with of Lithium Heparin (Vacutainer K2 EDTA) to avoid blood coagulation. Twenty five microliter of blood sample was used to measure blood ketone bodies level of each rat using an STAT-Site Analyzer-Ketone Photometer and STAT-Site β-hydroxybutyrate (BHB) test cards. (Standbio Laboratory, Boerne, TX, USA).
2.7. Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad, San Diego, CA, USA). Significance of differences among means of the 3 groups was evaluated using 1-way analysis of variance followed by Tukey's post hoc test. Evaluation of differences between 2 groups was done using Student t test. Values of p < 0.05 were considered significant.
3. Results
3.1. CR-reduced brain glucose metabolism
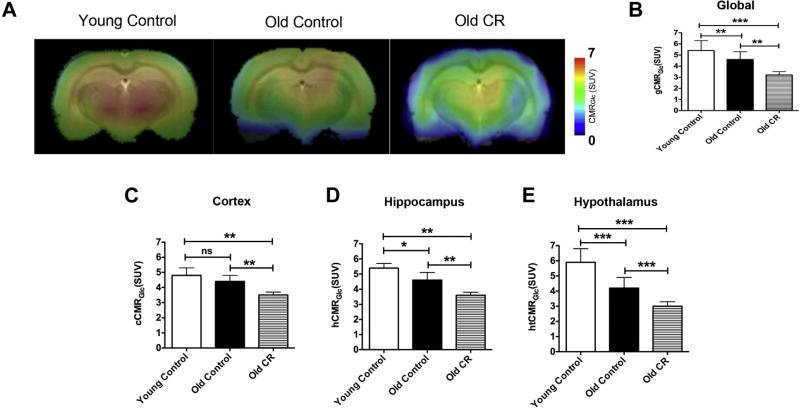
Fig. 1A shows a representative single slice of the CMRGlc maps overlaid on the corresponding anatomical image. Color bar shows the minimal and maximal CMRGlc in SUV in the linear scale. The maps show that old control rats overall had significantly lower CMRGlc compared with the young controls; the old CR had significantly lower global CMRGlc relative to both control groups. The quantitative CMRGlc values are shown in Fig. 1B. We further did regional analyses on the entire cortex, hippocampus, and hypothalamus. We chose cortex and hippo-campus because they are related to cognitive functions and the hypothalamus is related to glucose sensing. Similar to the pattern in global CMRGlc, old control rats showed lower regional CMRGlc than those in the young ones (though not significant in the cortex); old CR rats had significantly lower CMRGlc compared with the 2 control groups in cortex (Fig. 1C), hippo-campus (Fig. 1D), and hypothalamus (Fig. 1E). The results suggest that CMRGlc declines with age, and CR further reduces glucose utilization in aging.
Fig. 1.
Caloric restriction reduced cerebral metabolic rate of glucose (CMRGlc) (A) CMRGlc of representative control and CR rats; (B) Quantitative global CMRGlc (gCMRGlc); (C) Quantitative cortical CMRGlc (cCMRGlc); (D) Quantitative hippocampal CMRGlc (hCMRGlc); (E) Quantitative hypothalamic CMRGlc (htCMRGlc). N = 6 per experimental group. Data are presented as mean ± standard error of mean. *p < 0.05; **p < 0.01; ***p < 0.001. Abbreviations: CR, caloric restriction; SUV, standardized uptake value.
3.2. CR preserved CBF
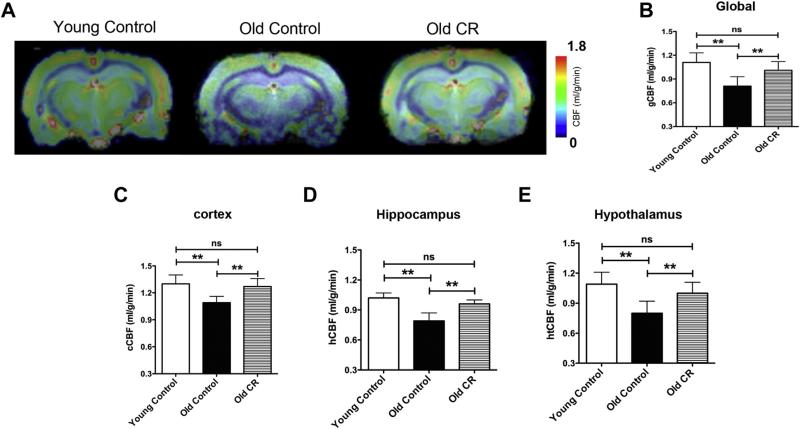
Fig. 2A shows a representative single slice of CBF maps overlaid on the corresponding anatomical image. Color bar shows the minimal and maximal CBF (in mL/g/min) in the linear scale. The maps show that old control rats overall had significantly lower CBF than that of the young controls. However, old CR rats had indistinguishable CBF compared with the young controls. Quantitation of global CBF can be seen in Fig. 2B. We also did regional analyses on cortex, hippocampus, and hypothalamus. Similar to global CBF, we found preserved CBF in old CR rats in cortex (Fig. 2C), hippocampus (Fig. 2D), and hypothalamus (Fig. 2E). The results indicate that CBF reduces with age, but CR is able to impede the decline.
Fig. 2.
Caloric restriction preserved cerebral blood flow (CBF). (A) CBF maps of representative control and CR rats obtained by ASL; (B) quantitative global CBF (gCBF); (C) quantitative cortical CBF (cCBF); (D) quantitative hippocampal CBF (hCBF); and (E) quantitative hypothalamic CBF (htCBF). N = 6 per experimental group. Data are presented as mean ± standard error of the mean.**p < 0.01. Abbreviation: CR, caloric restriction.
3.3. CR reduced lactate level and increased utilization of ketone bodies
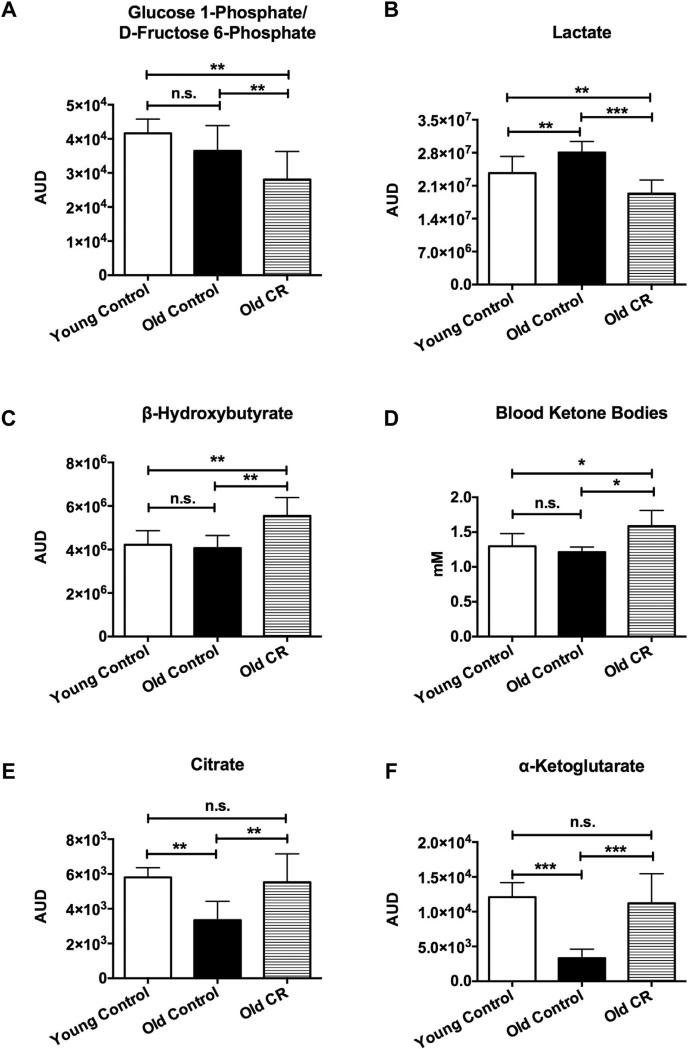
Using HPLC-ESI-MS methods, we observed significantly reduced glucose 1-phosphate/d-fructose 6-phosphate (Fig. 3A; p < 0.01) and lactate concentrations (Fig. 3B; p < 0.01) in cortex and hippocampus of the old CR rats compared with the age-matched controls. Glucose 1-phosphate is an intermediate metabolite between the glucose-glycogen recycling pathway and d-fructose 6-phosphate is an intermediate in the glycolytic pathway (from glucose to pyruvate); lactate is the end product of glycolysis. These results were consistent with our CMRGlc imaging findings that glucose utilization is lower in the old CR rats.
Fig. 3.
Caloric restriction reduced glycolysis, elevated ketone body metabolism, and preserved brain mitochondrial tricarboxylic acid (TCA) cycle metabolites. (A) Glucose 1-phosphate/d-fructose 6-phosphate, intermediates in the glycolytic metabolic pathway; (B) lactate, the end product of glycolysis; (C) brain ketone bodies, β-hydroxybutyrate (BHB); (D) blood ketone bodies; (E) citrate; and (F) α-ketoglutarate, 2 intermediates in the mitochondrial TCA cycle. N = 6 per experimental group. Data are presented as mean ± standard error of the mean. *p < 0.05; **p < 0.01; ***p < 0.001. Abbreviations: AUD, area under the curve; CR, caloric restriction.
As ketone bodies are the alternative fuel substrate in the brain when glucose utilization goes down (Akram, 2013), we examined concentration of ketone bodies, BHB. We found that BHB was significantly elevated in the CR group (Fig. 3C; 45% increase; p < 0.01). Similar findings were found in blood ketone bodies’ level, though the changes were not as high as in the brain (Fig. 3D; 25% increase; p < 0.05).
3.4. CR preserved mitochondrial metabolites
We further looked into metabolites related to TCA cycle in the cortex and hippocampus. We found that the level of citrate in the old CR animals was significantly higher (p < 0.01; Fig. 3E) compared with old control animals but was not significantly different compared with young controls. We also found significantly higher α-ketoglutarate (α-KG) in the old CR group relative to the old controls (p < 0.001; Fig. 3F), but indistinguishable from the young controls.
4. Discussion
In this study, we demonstrated that CR increases the level of ke-tone bodies and preserves CBF in aging F344BNF1 rats. We also observed that CR reduces glycolysis in the brain, including decreased glucose uptake, glucose 1-phosphate/d-fructose 6-phosphate level, and lactate concentration. These are consistent with previous findings that CR reduces the numbers of glucose transporter (e.g., GLUT1) and monocarboxylate transporter (e.g., MCT1) in aged rats (Roy et al., 2013). Despite the decrease in CMRGlc, we previously showed that the mitochondrial TCA cycle flux is preserved in CR old rats (Lin et al., 2014). This could be due to compensatory increases in the metabolism of ketone bodies (Hasselbalch et al., 1996; Roy et al., 2013). Preserved metabolic and vascular functions were observed both globally and regionally including in the cortex, hippocampus, and hypothalamus. Because the cortex and hippocampus are highly associated with cognitive functions (e.g., memory, learning, and perceptual attention), our findings are in good agreement with literature that CR slowed cognitive decline in aging F344BNF1 rats (Markowska and Savonenko, 2002). Similar findings were also reported in humans (Witte et al., 2009). Because the hypothalamus has glucose-sensing neurons, reduced CMRGlc could increase the electrical activity of hypothalamus orexin neurons (which are inhibited by glucose), but suppress the excitability of melanin-concentrating hormone neurons. The consequent increased release of orexin and decreased release of melanin-concentrating hormone stimulate wakefulness and activity (Burdakov et al., 2005). In line with this, CR-treated aging rats had higher physical activity than the age-matched controls (Carter et al., 2009). Collectively, this suggests that CR can preserve brain physiological, cognitive, and physical function in aging.
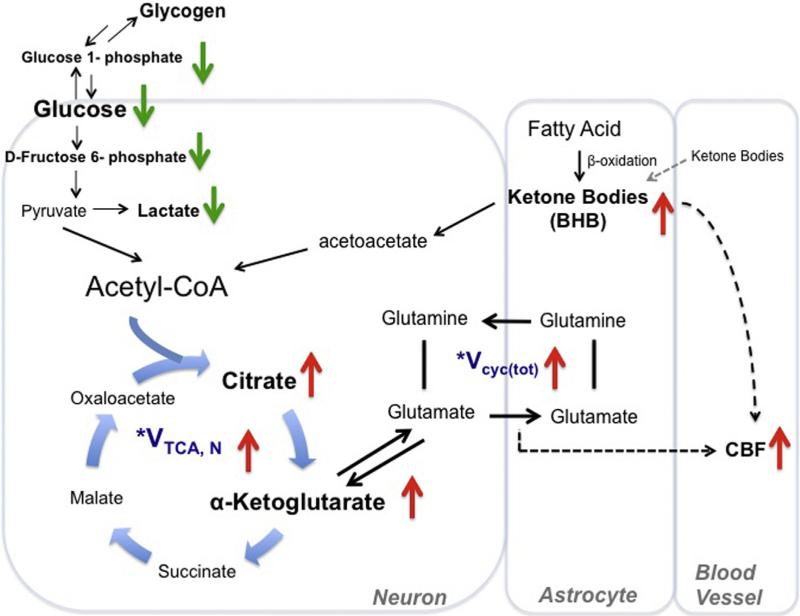
Based on our observations from the current and the previous study (Lin et al., 2014), we summarize the pathways in Fig. 4: CR reduces CMRGlc, but enhances ketone bodies, both globally and regionally. Ketone bodies may come from blood stream (generated by liver) and the fatty acid oxidation in astrocytes (Guzman and Blazquez, 2001; Maalouf et al., 2009). We found that ketone bodies were much higher in the brain than in the blood in the old CR rats, suggesting that astrocytes, not blood, contributed to increased ketone bodies. Ketone bodies are then converted to acetoacetate, further metabolized to acetyl-CoA, and enter into the mitochondrial TCA cycle in neurons, thus preserving mitochondrial bioenergetics (Lanza et al., 2012; Lin et al., 2014; Lopez-Lluch et al., 2006; Maalouf et al., 2009). As a result, we found preserved citrate and α-KG levels induced by CR. α-KG is transaminated to form Glu and rapidly exchange with Glu (Hertz, 2013). Glu-Gln neurotransmitter cycle communicates between neurons and astrocytes to sustain neuronal activity, including maintaining the neurotransmitter trafficking and eliciting blood-flow response to energy substrate delivery (e.g., oxygen, glucose, and ketone bodies) (Kida et al., 2001). Our finding is consistent with the preserved Glu-Gln neurotransmission seen in CR-treated aging rats (Lin et al., 2014). Because neuronal transmission and levels of ketone bodies are highly associated with CBF (Fox and Raichle,1986; Fox et al., 1988; Hasselbalch et al., 1996; Lin et al., 2010; Linde et al., 2006) and we observed preserved CBF, we suggest that CR can preserve brain metabolism, neuronal activity, and CBF and thus slow cognitive decline in aging.
Fig. 4.
Proposed metabolic and hemodynamic changes induced by caloric restriction (CR). This figure shows the comparison between old control and CR rats. CR downregulated glucose metabolic pathway (with reduced glucose uptake, glycolysis, lactate, and glucose-glycogen recycling) but upregulated ketogenic pathway. The ketone bodies may come from astrocytic fatty acid oxidation or blood stream. Ketone bodies are converted to acetoacetate and then further metabolized to acetyl-CoA. The altered metabolic pathway resulted in enhanced TCA cycle flux and glutamate-glutamine recycling (Vcyc(tot)) between neurons and astrocytes. Elevated CBF may be due to enhanced neuronal activity and increased ketone bodies levels. *This was shown in our previous study (Lin et al., 2014). Abbreviations: BHB, β-hydroxybutyrate; CBF, cerebral blood flow; TCA, tricarboxylic acid.
The shift from glucose metabolism to ketone bodies’ utilization under CR is consistent with findings that ketone bodies are an alternative fuel for brain cells when glucose availability is insufficient; ketosis proportionally spares glucose utilization in the brain (Zhang et al., 2013). Ketone bodies can support most, if not all, of basal (housekeeping) neuronal oxidative metabolism. In a recent study, Chowdhury et al. reported that acetyl-CoA oxidation from ketone bodies accounted for approximately 62% of neuronal TCA cycle flux, while the remaining 38% was contributed by glucose, in awake rats under hyperketonemia (Chowdhury et al., 2014). Because both brain glucose utilization and neuronal activity decline during aging, the shifted metabolism from glucose to ketone bodies under CR may be able to sustain brain energy supply and functions in aging brain.
The shifted metabolism may also play an important role in preserving memory in aging since glucose plays a critical role in forming and enhancing memory (Dash et al., 2006). As mentioned above, glucose consumption declines with age and in an accelerated manner in AD. Without additional or alternative fuel substrates, it is not surprising that the ability for memory processing goes down with age and more significantly in AD. Emerging evidence shows that lactate could be an additional energy source when extracellular glucose levels are not sufficient to maintain optimal cognitive functions (Suzuki et al., 2011). During intense cognitive functions, for example, astrocytic glycogenolysis is activated to provide lactate, which is transported to neurons, converted to pyruvate, and enters the TCA cycle (Brown et al., 2004; Pellerin and Magistretti, 1994). Using intrahippocampal infusion of lactate, Newman et al. recently showed that rats had enhanced spatial working memory (Newman et al., 2011). In this study, we showed that both brain glucose and lactate levels were lower in the CR rats, and the brain used ketone bodies as an alternative fuel substrate instead. Similar to lactate, ketone is generated via astrocytes (through fatty acid oxidation pathway), indicating that astrocytes play an important role in supplying energy substrates to neurons when glucose level is insufficient. Moreover, since the glucose level has been chronically lower in the brain, CR rats may have not relied on glucose as the main energy source and thus been protected from the age-related glucose metabolism impairment (including reduced glucose uptake, increased glucose intolerance, and increased insulin resistance). The preserved memory functions found in aged CR rats may be associated with the shifted metabolism from glucose to ke-tone bodies.
In addition to sustaining neuronal activity and memory, metabolism of ketone bodies has neuroprotective effects and therapeutic potential in a variety of different common and rare disease states (Akram, 2013; Veech, 2004). The most well known is the treatment efficacy for refractory epilepsy (Kinsman et al., 1992). Epilepsy is caused by hyper-synchronous firing of neurons. Ketone bodies are able to maintain neurotransmission balance in epilepsy by either increasing inhibitory neurotransmitters (e.g., γ-aminobutyric acid) or inhibiting vascular glutamate transporters (McNally and Hartman, 2012). Others have shown that a ketogenic diet has therapeutic effects on diseases of insulin resistance (Veech, 2013), diseases resulting from free radical damage, and hypoxia (Maalouf et al., 2007; Sullivan et al., 2004). In line with this, ketogenic diets have demonstrated improved structural and functional outcome in traumatic brain injury models, mild traumatic brain injury/concussion models, and spinal cord injury in preclinical studies employing both pre- and post-injury implementation (Prins and Matsumoto, 2014). In animal models with neurodegenerative disorders, increased ketone bodies metabolism (by administrating a ketone ester diet) exhibit anxiolytic and cognition-sparing properties, and lessen beta-amyloid and tau pathologies in mouse modeling human AD (Kashiwaya et al., 2013). This is consistent with the literature in that CR can reduce beta-amyloid and tau deposition, and restore memory in animals modeling human AD (Mouton et al., 2009).
Increased ketone bodies may also contribute to increased CBF. Two studies show that an acute increase in the concentration of ketone bodies by infusion of BHB increased the CBF without affecting the overall cerebral metabolic activity, suggesting that ketone bodies have directeffecton the cerebral endotheliumto increase CBF, independent of metabolic interactions (Hasselbalch et al., 1996; Linde et al., 2006). Mammalian target of rapamycin (mTOR) signaling may be involved in the process. Ketogenesis is associated with downregulated mTOR activity (Sengupta et al., 2010) and upregulated adenosine monophoshate–activated protein kinase (Blazquez et al., 1999). Both of these changes can activate endothelial nitric oxide synthase signaling and consequently increase CBF (Shafique et al., 2013). This is consistent with our previous findings that mTOR inhibition with rapamycin activates endothelial nitric oxide synthase and restores vascular functions (CBF and vascular density) in mice modeling human AD (Lin et al., 2013; Richardson et al., 2014). Thisisalso consistent withthe literature in that CR preserves vascular functions and vascular density in aging (Lynch et al., 1999; Ungvari et al., 2010).
Preserved levels of α-KG may also contribute to sustained brain viability. As mentioned above, α-KG involves regulating Glu-Gln neurotransmission cycling. In addition, a recent study showed that α-KG may mimic CR and play a key role in extending life span. Consistent with our observations in rats, Chin et al. reported that Caenorhabditis elegans had increased endogenous α-KG levels on starvation (Chin et al., 2014). They also found that the beneficial effects of α-KG depend on the target of rapamycin inactivation. Collectively, they suggested that α-KG is a key metabolite that mediates longevity by dietary restriction.
The goal of this study was to identify the chronic CR effects in normal aging brain physiology. In the future, it will be important to investigate the acute effects on young animals and to identify the age-dependent CR effects on brain functions as well as the correlation between the brain metabolism and peripheral energy metabolism. One limitation of our study is that we used a long-lived rodent model to investigate CReffects.Recent studies have shown that the life span response to a single level of CR (e.g., 40% CR) varies widely in mice with different genetic backgrounds (Liao et al., 2010). In some cases, CR shortened the life span in inbred mice. The main findings in the studies were that CR life extension correlated inversely with fat reduction–strains with the least reduction in fat were more likely to show life extension, and those with the greatest reduction were more likely to have shortened life span (Liao et al., 2011). As fatty acids in astrocytes are needed for ketone body metabolism, those with shorter life spans may not be able to upregulate ketone body utilization under CR. Reduction in CMRGlc without elevated ketone bodies may lead to shorter life span. Brain metabolic and vascular functions were not examined in these studies. Therefore, it will be important in the future to determine if CR also has adverse effects on brain functions during aging in rodent strains where deleterious effects on life span are observed.
Investigations of CR effects on brain functions are translatable. Previous studies show that CR can improve memory in humans (Witte et al., 2009). Because the PETand MRI imaging used in the study are readily used in humans, it would be important in future studies to identify CR-induced changes in metabolic and vascular functions in human brain aging using these multimetric imaging methods (Lin et al., 2012a; 2014; Uh et al., 2011).
In conclusion, we found that CR protects brain physiology in aging F344BNF1 rats. Upregulating ketone bodies metabolism, sustaining CBF and α-KG (all which involve in TOR pathway) may play a crucial role in preserving neuronal health span under CR. These results provide a rationale for CR-induced sustenance of brain health with extended life span. Understanding nutritional effects on brain function may have profound implications in human aging and other age-related neurodegenerative disorders.
Acknowledgements
The authors thank Drs. Brian Gold and Michael P. Murphy of the University of Kentucky and Dr. Arlan Richardson of University of Oklahoma Health Science Center for their valuable comments. The authors also thank Ms. Paula Thomason of the University of Kentucky for editing the article. Mass spectrometry analyses were conducted in the Metabolomics Core Facility of the Mass Spec-trometry Laboratory at the University of Texas Health Science Center at San Antonio. This research was supported by NIH grant K01AG040164 and American Federation for Aging Research Grant #A12474 to Ai-Ling Lin.
Footnotes
Disclosure statement
The authors declare no conflict of interest.
References
- Akram M. A focused review of the role of ketone bodies in health and disease. J. Med. Food. 2013;16:965–967. doi: 10.1089/jmf.2012.2592. [DOI] [PubMed] [Google Scholar]
- Bentourkia M, Bol A, Ivanoiu A, Labar D, Sibomana M, Coppens A, Michel C, Cosnard G, De Volder AG. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: effect of aging. J. Neurol. Sci. 2000;181:19–28. doi: 10.1016/s0022-510x(00)00396-8. [DOI] [PubMed] [Google Scholar]
- Blazquez C, Woods A, de Ceballos ML, Carling D, Guzman M. The AMP-activated protein kinase is involved in the regulation of ketone body production by astrocytes. J. Neurochem. 1999;73:1674–1682. doi: 10.1046/j.1471-4159.1999.731674.x. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. N. Engl. J. Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Baltan Tekkok S, Ransom BR. Energy transfer from astrocytes to axons: the role of CNS glycogen. Neurochem. Int. 2004;45:529–536. doi: 10.1016/j.neuint.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philosophical Trans. R. Soc. Lond. B Biol. Sci. 2005;360:2227–2235. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Leeuwenburgh C, Daniels M, Foster TC. Influence of calorie restriction on measures of age-related cognitive decline: role of increased physical activity. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:850–859. doi: 10.1093/gerona/glp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, Diep S, Lomenick B, Meli VS, Monsalve GC, Hu E, Whelan SA, Wang JX, Jung G, Solis GM, Fazlollahi F, Kaweeteerawat C, Quach A, Nili M, Krall AS, Godwin HA, Chang HR, Faull KF, Guo F, Jiang M, Trauger SA, Saghatelian A, Braas D, Christofk HR, Clarke CF, Teitell MA, Petrascheck M, Reue K, Jung ME, Frand AR, Huang J. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510:397–401. doi: 10.1038/nature13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Choi KM, Lee CK. Caloric restriction improves efficiency and capacity of the mitochondrial electron transport chain in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2011;409:308–314. doi: 10.1016/j.bbrc.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Chowdhury GM, Jiang L, Rothman DL, Behar KL. The contribution of ketone bodies to basal and activity-dependent neuronal oxidation in vivo. J. Cereb. Blood Flow Metab. 2014;34:1233–1242. doi: 10.1038/jcbfm.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, Pifferi F, Bocti C, Paquet N, Begdouri H, Bentourkia M, Turcotte E, Allard M, Barberger-Gateau P, Fulop T, Rapoport SI. Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition. 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moore AN. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. J. Neurosci. 2006;26:8048–8056. doi: 10.1523/JNEUROSCI.0671-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontan-Lozano A, Lopez-Lluch G, Delgado-Garcia JM, Navas P, Carrion AM. Molecular bases of caloric restriction regulation of neuronal synaptic plasticity. Mol. Neurobiol. 2008;38:167–177. doi: 10.1007/s12035-008-8040-1. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. U. S. A. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Guzman M, Blazquez C. Is there an astrocyte-neuron ketone body shuttle? Trends Endocrinol. Metab. 2001.;12:169–173. doi: 10.1016/s1043-2760(00)00370-2. [DOI] [PubMed] [Google Scholar]
- Hasselbalch SG, Madsen PL, Hageman LP, Olsen KS, Justesen N, Holm S, Paulson OB. Changes in cerebral blood flow and carbohydrate metabolism during acute hyperketonemia. Am. J. Physiol. 1996;270:E746–E751. doi: 10.1152/ajpendo.1996.270.5.E746. [DOI] [PubMed] [Google Scholar]
- Hertz L. The glutamate-glutamine (GABA) Cycle: Importance of late postnatal development and potential reciprocal interactions between biosynthesis and degradation. Front. Endocrinol. 2013;4(5 Pt 1):59. doi: 10.3389/fendo.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer S. Abnormalities of glucose metabolism in Alzheimer's disease. Ann. N. Y. Acad. Sci. 1991;640:53–58. doi: 10.1111/j.1749-6632.1991.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, Okun E, Clarke K, Mattson MP, Veech RL. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer's disease. Neurobiol. Aging. 2013;34:1530–1539. doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida I, Hyder F, Behar KL. Inhibition of voltage-dependent sodium channels suppresses the functional magnetic resonance imaging response to forepaw somatosensory activation in the rodent. J. Cereb. Blood Flow Metab. 2001;21:585–591. doi: 10.1097/00004647-200105000-00013. [DOI] [PubMed] [Google Scholar]
- Kinsman SL, Vining EP, Quaskey SA, Mellits D, Freeman JM. Efficacy of the ketogenic diet for intractable seizure disorders: review of 58 cases. Epilepsia. 1992;33:1132–1136. doi: 10.1111/j.1528-1157.1992.tb01770.x. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Zabielski P, Klaus KA, Morse DM, Heppelmann CJ, Bergen HR, 3rd, Dasari S, Walrand S, Short KR, Johnson ML, Robinson MM, Schimke JM, Jakaitis DR, Asmann YW, Sun Z, Nair KS. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab. 2012;16:777–788. doi: 10.1016/j.cmet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Gelfond JA, Diaz V, Nelson JF. Fat maintenance is a predictor of the murine lifespan response to dietary restriction. Aging Cell. 2011;10:629–639. doi: 10.1111/j.1474-9726.2011.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Coman D, Jiang L, Rothman DL, Hyder F. Caloric restriction impedes age-related decline of mitochondrial function and neuronal activity. J. Cereb. Blood Flow Metab. 2014;34:1440–1443. doi: 10.1038/jcbfm.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Fox PT, Hardies J, Duong TQ, Gao JH. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc. Natl. Acad. Sci. U. S. A. 2010;107:8446–8451. doi: 10.1073/pnas.0909711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Laird AR, Fox PT, Gao JH. Multimodal MRI neuroimaging bio-markers for cognitive normal adults, amnestic mild cognitive impairment, and Alzheimer's disease, Neurol. Res. Int. 2012a;2012:907409. doi: 10.1155/2012/907409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Poteet E, Du F, Gourav RC, Liu R, Wen Y, Bresnen A, Huang S, Fox PT, Yang SH, Duong TQ. Methylene blue as a cerebral metabolic and hemodynamic enhancer. PLoS One. 2012b;7:e46585. doi: 10.1371/journal.pone.0046585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Rothman DL. What have novel imaging techniques revealed about metabolism in the aging brain? Future Neurol. 2014;9:341–354. doi: 10.2217/fnl.14.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Zheng W, Halloran JJ, Burbank RR, Hussong SA, Hart MJ, Javors M, Shih YY, Muir E, Solano Fonseca R, Strong R, Richardson AG, Lechleiter JD, Fox PT, Galvan V. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer's disease. J. Cereb. Blood Flow Metab. 2013;33:1412–1421. doi: 10.1038/jcbfm.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde R, Hasselbalch SG, Topp S, Paulson OB, Madsen PL. Global cerebral blood flow and metabolism during acute hyperketonemia in the awake and anesthetized rat. J. Cereb. Blood Flow Metab. 2006;26:170–180. doi: 10.1038/sj.jcbfm.9600177. [DOI] [PubMed] [Google Scholar]
- Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CD, Cooney PT, Bennett SA, Thornton PL, Khan AS, Ingram RL, Sonntag WE. Effects of moderate caloric restriction on cortical microvascular density and local cerebral blood flow in aged rats. Neurobiol. Aging. 1999;20:191–200. doi: 10.1016/s0197-4580(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res. Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko A. Retardation of cognitive aging by life-long diet restriction: implications for genetic variance. Neurobiol. Aging. 2002;23:75–86. doi: 10.1016/s0197-4580(01)00249-4. [DOI] [PubMed] [Google Scholar]
- Mattson MP. The impact of dietary energy intake on cognitive aging. Front. Aging Neurosci. 2010;2:5. doi: 10.3389/neuro.24.005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally MA, Hartman AL. Ketone bodies in epilepsy. J. Neurochem. 2012;121:28–35. doi: 10.1111/j.1471-4159.2012.07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR, Chachich ME, Quigley C, Spangler E, Ingram DK. Caloric restriction attenuates amyloid deposition in middle-aged dtg APP/PS1 mice. Neurosci. Lett. 2009;464:184–187. doi: 10.1016/j.neulet.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Buchan RJ, Yokoyama E, Kondoh Y, Sato M, Terashi H, Satoh Y, Watahiki Y, Senova M, Hirata Y, Hatazawa J. Misery perfusion with preserved vascular reactivity in Alzheimer's disease. Ann. N. Y. Acad. Sci. 1997;826:272–281. doi: 10.1111/j.1749-6632.1997.tb48479.x. [DOI] [PubMed] [Google Scholar]
- Newman LA, Korol DL, Gold PE. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One. 2011;6:e28427. doi: 10.1371/journal.pone.0028427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. U. S. A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins M, Matsumoto J. The collective therapeutic potential of cerebral ke-tone metabolism in traumatic brain injury. J. Lipid Res. 2014;55:2450–2457. doi: 10.1194/jlr.R046706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahat O, Maoz N, Cohen HY. Multiple pathways regulating the calorie restriction response in yeast. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:163–169. doi: 10.1093/gerona/glq165. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Galvan V, Lin AL, Oddo S. How longevity research can lead to therapies for Alzheimer's disease: the rapamycin story. Exp. Gerontol. 2014 doi: 10.1016/j.exger.2014.12.002. pii: S0531-5565(14)00349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Hennebelle M, St-Pierre V, Courchesne-Loyer A, Fortier M, Bouzier-Sore AK, Gallis JL, Beauvieux MC, Cunnane SC. Long-term calorie restriction has minimal impact on brain metabolite and fatty acid profiles in aged rats on a Western-style diet. Neurochem. Int. 2013;63:450–457. doi: 10.1016/j.neuint.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- Shafique E, Choy WC, Liu Y, Feng J, Cordeiro B, Lyra A, Arafah M, Yassin-Kassab A, Zanetti AV, Clements RT, Bianchi C, Benjamin LE, Sellke FW, Abid MR. Oxidative stress improves coronary endothelial function through activation of the pro-survival kinase AMPK. Aging. 2013;5:515–530. doi: 10.18632/aging.100569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippo-campal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Metabolic reserve as a determinant of cognitive aging. J. Alzheimers Dis. 2012;30(Suppl 2):S5–S13. doi: 10.3233/JAD-2011-110899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann. Neurol. 2004;55:576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging Program. J. Gerontol. A Biol. Sci. Med. Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Uh J, Lin AL, Lee K, Liu P, Fox P, Lu H. Validation of VASO cerebral blood volume measurement with positron emission tomography. Magn. Reson. Med. 2011;65:744–749. doi: 10.1002/mrm.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Kang H, Clemenson GD, Jr., Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Veech RL. Ketone esters increase brown fat in mice and overcome insulin resistance in other tissues in the rat. Ann. N. Y. Acad. Sci. 2013;1302:42–48. doi: 10.1111/nyas.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kuang Y, Xu K, Harris D, Lee Z, LaManna J, Puchowicz MA. Ketosis proportionately spares glucose utilization in brain. J. Cereb. Blood Flow Metab. 2013;33:1307–1311. doi: 10.1038/jcbfm.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]