Abstract
Background
Surgical morbidity and mortality (M&M) for patients with disseminated malignancy (DMa) is high, and some have questioned the role of surgery. Therefore, we sought to characterize temporal trends in M&M among DMa patients, hypothesizing that surgical intervention would remain prevalent.
Methods
We queried the American College of Surgeons National Surgical Quality Improvement Program from 2006 to 2010. Excluding patients undergoing a primary hepatic operation, we identified 21,755 patients with DMa. Parametric/non-parametric statistics and logistic regression were used to evaluate temporal trends and predictors of M&M.
Results
The prevalence of surgical intervention for DMa declined slightly over the time period, from 1.9% to 1.6% of all procedures (p<0.01). Among DMa patients, the most frequent operations performed were bowel resection, other gastrointestinal procedures, and multivisceral resections, these all showed small statistically significant decreases over time (p<0.01). The rate of emergency operations also decreased (p<0.01). In contrast, the rate of preoperative independent functional status rose, while the rate of preoperative weight loss and sepsis decreased (p<0.01). Rates of 30-day morbidity (33.7 vs 26.6%), serious morbidity (19.8 vs 14.2%), and mortality (10.4 vs 9.3%) all decreased over the study period (p<0.05). Multivariate analysis identified standard predictors (e.g. impaired functional status, pre-operative weight loss pre-operative sepsis, and hypoalbuminemia) of worse 30-day M&M.
Conclusion
30-day morbidity, serious morbidity, and mortality have decreased incrementally for patients with DMa undergoing surgical intervention, but surgical intervention remains prevalent. These data further highlight the importance of careful patient selection and goal-directed therapy in patients with incurable malignancy.
Keywords: Disseminated Malignancy, Morbidity and Mortality, Perioperative Outcomes
Introduction
Patients with disseminated malignancy commonly present with complex surgical needs, whether for symptom palliation or to treat an acute condition such as bowel obstruction [1-3]. However, providing surgical intervention to patients with incurable cancer is not without risk. Surgical intervention, even for purposes of symptomatic palliation and improving the patient's quality of life, comes with substantial morbidity and mortality. Multiple studies have shown rates of post-operative morbidity and mortality to be approximately 28 – 44% and 9 – 11%, respectively [2, 4-6].
Although recent studies have highlighted the importance of estimating the risk of morbidity and mortality and defining goals of care prior to surgical intervention in patients with disseminated malignancy [4, 7], few studies have addressed whether this heightened attention on this unique patient population has impacted the frequency and outcomes of surgical operations among patients with disseminated malignancy. The purpose of this study, therefore, was to evaluate temporal trends among patients with disseminated malignancy undergoing surgical intervention with respect to frequency of operations performed as well as nature of the operations performed. We also sought to evaluate the predictors of morbidity and mortality among this patient population to determine other time-dependent changes. We hypothesized that despite greater awareness of the role of non-operative palliative care for patients with terminal disease [8, 9], surgical intervention would remain prevalent over time and that morbidity and mortality would remain high for this patient population.
Methods
We queried the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) from 2006 to 2010 to identify all patients with disseminated malignancy undergoing surgical intervention (N= 25,172 before exclusion). ACS NSQIP defines disseminated malignancy as “patients who have cancer that: (1) Has spread to one site or more sites in addition to the primary site AND (2) in whom the presence of multiple metastases indicates the cancer is widespread, fulminant or near terminal” [American College of Surgeons, 10]. For statistical analysis of patient characteristics, morbidity, and mortality, we chose to exclude patients undergoing a primary hepatic operation (N = 3,417), as research has shown that this can be a potentially curative operation and our goal was to identify patients with incurable cancer [11-14]. Our final cohort was 21,755 patients.
We abstracted data on 5 demographic, 10 preoperative, 3 intraoperative, and 22 postoperative variables. Using NSQIP definitions [15], preoperative functional status was defined as impaired if the patient required some or total assistance from another person for activities of daily living, such as bathing, feeding, dressing, toileting, or mobility. Preoperative sepsis was defined as a positive culture from suspected infection with two or more of the following: fever, tachycardia, tachypnea, leukocytosis, leukopenia, or anion gap acidosis. Preoperative weight loss was defied as a greater than 10% decrease in body weight 6 months prior to surgery excluding patients who have intentional lost weight. An operation was deemed as emergent if the operation was performed no later than 12 hours after onset symptoms or admission AND the surgery was reported as emergent by the surgeon and anesthesiologist.
Current procedural terminology (CPT) codes were used to classify procedures as orthopedic, skin and soft tissue, thyroid/parathyroid, hepatic, gastric, biopsy/lymph node excision, bowel resection, cholecystectomy/appendectomy/lysis of adhesions, vascular, other abdominal procedures which included various gastrointestinal procedures, and other. Multivisceral resections were identified based on the classification of the primary procedure CPT codes in combination with additional procedure CPT codes.
Post-operative morbidity was defined as a diagnosis of one or more of the following events within 30 days of the principal operation: superficial or deep wound infection, organ space infection, wound dehiscence, pneumonia, reintubation due to onset of respiratory or cardiac failure or requiring prolonged intubation which was defined as intubated for > 48 hours post-operatively, pulmonary embolism, progressive renal insufficiency which was defined as creatinine rise >2 mg/dl from preoperative value, acute renal failure which was defined as renal dysfunction requiring dialysis post-operatively, urinary tract infection, stroke, coma for > 24 hours, peripheral nerve injury, cardiac arrest, myocardial infarction, graft/prosthesis/flap failure complications, deep vein thrombosis, reoperation, sepsis, and septic shock. Septic shock was defined as meeting the criteria of sepsis, as noted above, with documented organ and/or circulatory dysfunction. We defined post-operative serious morbidity as pulmonary embolism, respiratory or cardiac failure requiring reintubation, prolonged intubation, acute renal failure requiring dialysis, reoperation, stroke, coma, cardiac arrest or systemic shock within 30 days after the principal operation [4].
Pearson chi-squared analysis was performed to evaluate differences over time with respect to the number of operations performed, type of operations performed, patient functional status, DNR status, weight loss prior to surgery, and pre-operative sepsis. One way ANOVA was used to evaluate differences over time with regards to age, BMI, pre-operative creatinine, albumin, hematocrit, and white blood cell count. Multiple comparisons were performed using the Tukey procedure.
Logistic regression analysis was performed to identify independent predictors of 30-day morbidity and mortality. Gender, age, BMI, functional status, DNR status, pre-operative weight loss, sepsis, creatinine, albumin, white blood cell count, hematocrit, emergency operations and multivisceral resections were selected as potential predictors for analysis based on prior research [4]. Missing data were most common for BMI (2.3%), pre-operative creatinine (5.1%), albumin (21.2%), white blood cell count (3.8%), and hematocrit (3.8%). All other variables had less than 1% missing data consistent with prior research demonstrating the reliability and completeness of NSQIP data [15]. As missing data could not be assumed to be missing at random [16], multiple imputation, using SPSS software (IBM SPSS Statistics Version 22), was used to provide values to the missing predictor variables. Significance was set at P<0.05. Since all patient information was de-identified, this study was exempt from University of California Davis Institutional Review Board approval.
Results
Baseline Characteristics
From 2006 to 2010, we identified 1,300,956 patients in the NSQIP database who underwent surgical procedures. For the entire study period, 1.9% (n = 25,172) were identified with a diagnosis of disseminated malignancy. After excluding 3,417 patients who underwent a primary hepatic operation (13.6% of total disseminated malignancy patients), we identified 21,755 (1.7%) patients who met entry criteria.
The demographic data for these patients are depicted in Table 1. The median age was 63 years (range 16 to 90+ years), 51% (n = 11,078) were female, mean BMI was 27 (± 6.6), and the majority was Caucasian (n = 16,888, 77.6%). 3,644 (16.7%) had impaired functional status, 3,693 (17%) presented with preoperative sepsis, 3,070 (14.1%) had greater than 10% weight loss in the past 6 months, 4,299 (19.8%) underwent chemotherapy in the last 30 days prior to surgery, 1,660 (7.6%) underwent radiation therapy 90 days prior to surgery, and 644 (3.0%) had a DNR order.
Table 1. Baseline Pre-Operative Characteristics (N=21,755).
| Demographics | Mean (±SD) or N (%) |
|---|---|
| Age | 62 (±14) |
| Female | 11078 (50.9%) |
| Ethnicity | |
| Native American | 130 (0.6%) |
| Asian | 570 (2.6%) |
| Black | 2110 (9.7%) |
| Caucasian | 16888 (77.6%) |
| Unknown | 1793 (8.3%) |
| Hispanic Ethnicity | 763 (3.5%) |
| BMI | 27.1 (± 6.6) |
| DNR | 644 (3.0%) |
| Impaired Functional Status | 3644 (16.7%) |
| Pre-operative Sepsis | 3693 (17.0%) |
| >10% Loss of body weight | 3070 (14.1%) |
| Recent Therapy | |
| Chemotherapy <30 days | 4299 (19.8%) |
| Radiotherapy last 90 days | 1660 (7.6%) |
| Laboratory Values | |
| Pre-op Creatinine | 1.01(± 0.77) |
| Pre-op Albumin | 3.39 (± 0.82) |
| Pre-op Bilirubin | 0.83 (± 1.31) |
| Pre-op WBC | 8.5 (± 5.1) |
| Pre-op Hematocrit | 35.2 (± 5.8) |
| Pre-op Platelets | 269 (± 127) |
| Pre-op INR | 1.15 (± 0.34) |
Case Mix and Outcomes
As depicted in Table 2, the operations most frequently performed included bowel resections (28.5%), other gastrointestinal procedures (25.5%), and multivisceral resections (9.5%). The median length of hospital stay was 7 days (range 0 – 369). Overall morbidity was 29.4% (n = 6,405), serious morbidity was 16.5% (n = 3,595) and mortality was 10.4% (n = 2,266).
Table 2. Patient characteristics by Procedure Type.
| Procedure | N (%) | Age Mean (±SD) | Female N (%) | BMI Mean (±SD) | DNR N (%) | Independent Functional Status N (%) | Pre-op Sepsis N (%) | >10% Weight Loss N (%) | Emergency Operations N (%) |
|---|---|---|---|---|---|---|---|---|---|
| Bowel Resection | 6210 (28.5%) | 63 (13) | 3086 (49.8%) | 26.6 (6.3) | 178 (2.9%) | 5168 (83.2%) | 1277 (20.7%) | 1052 (16.9%) | 1315 (21.2%) |
| Other Gastrointestinal Procedures | 5548 (25.5%) | 61 (13) | 2581 (46.6%) | 26.8 (6.5) | 184 (3.3%) | 4675 (84.3%) | 920 (16.7%) | 965 (17.4%) | 807 (14.5%) |
| Multivisceral Resection | 3455 (9.5%) | 60 (13) | 1067 (51.8%) | 27.0 (6.2) | 29 (1.4%) | 1854 (89.8%) | 236 (11.5%) | 302 (14.6%) | 187 (9.1%) |
| Celiotomy/Lysis of Adhesions | 1501 (6.9%) | 61 (14) | 759 (50.5%) | 26.3 (6.9) | 60 (4.0%) | 1109 (73.6%) | 451 (30.1%) | 271 (18.0%) | 419 (27.8%) |
| Cholecystectomy/ Appendectomy | 805 (3.7%) | 60 (14) | 416 (51.8%) | 27.7 (6.6) | 19 (2.4%) | 722 (89.8%) | 192 (24.0%) | 81 (10.1%) | 172 (21.4%) |
| Gastrectomy | 248 (1.1%) | 66 (13) | 106 (42.7%) | 26.3 (6.3) | 10 (4.0%) | 199 (80.2%) | 54 (22.0%) | 55 (22.2%) | 46 (18.5%) |
| Skin and Soft Tissue | 1377 (6.3%) | 59 (14) | 1205 (87.8%) | 28.7 (7.1) | 12 (0.9%) | 1264 (91.8%) | 98 (7.2%) | 65 (4.7%) | 66 (4.8%) |
| Orthopedic | 1145 (5.3%) | 63 (15) | 547 (47.8%) | 27.4 (7.0) | 56 (4.9%) | 817 (71.4%) | 178 (15.7%) | 107 (9.3%) | 129 (11.3%) |
| Biopsy | 989 (4.5%) | 59 (14) | 471 (87.8%) | 28.2 (6.8) | 12 (1.2%) | 932 (94.2%) | 43 (4.4%) | 106 (10.7%) | 28 (2.8%) |
| Vascular | 675 (3.1%) | 69 (12) | 250 (37.1%) | 26.5 (6.1) | 31 (4.6%) | 480 (71.1%) | 152 (22.7%) | 67 (9.9%) | 223 (33.0%) |
| Thyroid/ Parathyroid | 280 (1.3%) | 59 (15) | 170 (60.7%) | 29.3 (7.1) | 1 (0.4%) | 265 (94.6%) | 4 (1.4%) | 10 (3.6%) | 3 (1.1%) |
| Other | 2798 (12.9%) | 62 (14) | 1405 (50.4%) | 27.4 (6.4) | 79 (2.8%) | 2303 (82.3%) | 309 (11.2%) | 282 (10.1%) | 218 (7.8%) |
For emergency operations, overall morbidity (47.6%) and mortality (28.0%) were significantly greater compared to non-emergent operations (26%, and 7.1%, respectively, p < 0.01). As shown in Table 2, emergent operations occurred most frequently for vascular operations (33.0%), celiotomies and lysis of adhesions (27.8%), cholecystectomies and appendectomies (21.4%) and bowel resections (21.2%). Overall morbidity was also significantly greater for patients undergoing a multivisceral resection, 44.1% vs. 27.9% (p < 0.01) than for patients undergoing other types of procedures.
Temporal Trends in Procedures for Disseminated Malignancy
Overall, despite an absolute increase in the number of operations reported in NISQP from 2006 to 2010 (118,560 cases in 2006 to 363,431 in 2010) with hepatic cases excluded, there was a small, but statistically significant, decrease in percentage of cases performed on patients with disseminated malignancy. In 2006, 1.9% (n = 2190) of all NSQIP operations performed involved patients with disseminated malignancy patients. In subsequent years, the frequency was 1.7% in 2007 (n = 3,642), 1.7% in 2008 (n = 4,465), 1.7% in 2009 (n = 5,549), and 1.6% (n = 5,909) in 2010 (p < 0.01).
We also observed a shift in case mix over time. As shown in Figure 2, the proportion of bowel resections declined from 31.0% in 2006, 32.7% in 2007, 29.9% in 2008, 26.7% in 2009, to 25.8%% in 2010 (p < 0.01). A similar trend was also noted for celiotomies/lysis of adhesions, from 8.4% in 2006, 8.2% in 2007, 6.4% in 2008, 7.1% in 2009, to 5.8% in 2010 (p < 0.01), and for appendectomies and cholecystectomies which declined from 6.6% in 2006, 4.0% in 2007, 4.1% in 2008, 2.8% in 2009, to 2.9% in 2010 (p < 0.01). There was also a small, but statistically significant, decline in multivisceral resections, from 11.1% in 2006, 9.8% in 2007, 9.8% in 2008, 8.5% in 2009, to 9.5% in 2010 (p < 0.01). Importantly, the rate of emergency cases among patients with disseminated cancer also declined significantly from 17.4% in 2006 to 15.0% in 2009 and 2010, respectively (p<0.01).
Figure 2. Rates of bowel resection, celiotomy/lysis of adhesions and cholecystectomy/appendectomy among patients with disseminated malignancy from 2006 to 2010.

Bar graph showing percentage of patients undergoing bowel resections, celiotomy/lysis of adhesions, and appendectomy/cholecystectomy performed on an annual basis. Pearson chi squared analysis showed statistically significant decline over the study period.
Temporal Trends in Patient Characteristics
Overall, although there was no significant difference in BMI, DNR status, creatinine, or hematocrit in our patient cohort over time (p >0.05), we did observe changes in other important patient characteristics. For example, in 2006, the mean age of patients with disseminated malignancy undergoing surgery was slightly younger than the mean age of patients in 2009 (61 ± 13 vs. 62 ± 13 years), p < 0.01. In addition, the mean serum albumin rose from 3.46 g/dL ± 0.82 in 2006 to 3.55 g/dL ± 0.79 in 2010 (p<0.01). In contrast, the mean WBC count on presentation decreased slightly from 8.7 ± 5.3 × 103/μL in 2006 to 8.2 ± 4.7 × 103/μL in 2010 (p < 0.01).
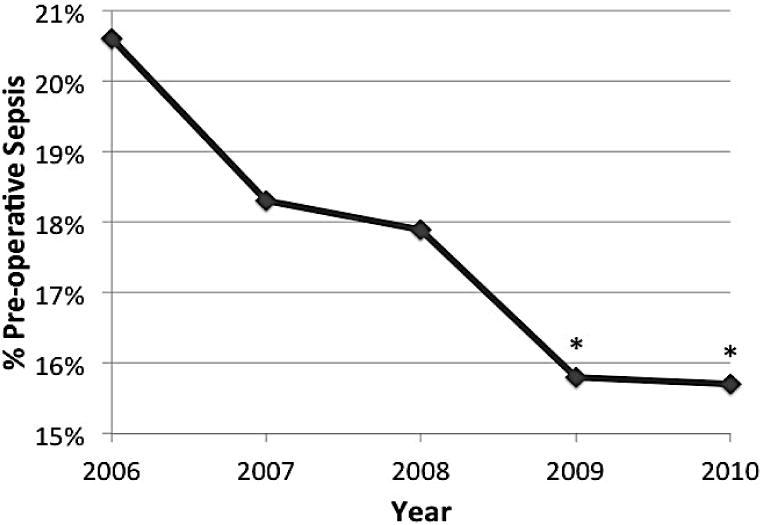
As shown in Figure 3, we also observed a significant increase in pre-operative independent functional status in our patient cohort. In 2006, 82.3% were classified as independent compared to 86.1% in 2010 (p < 0.01). Furthermore, there was also a statistically significant decline in the percentage of patients with weight loss greater than 10% of body weight in the 6 months prior to surgery. In 2006, this level of weight loss was present among 14.4% of patients, while in 2010, it dropped to 12.8% of patients (p < 0.01). A similar decline was observed in the incidence of pre-operative sepsis, dropping from 20.6% in 2006 to 15.7% in 2010 (p < 0.01).
Figure 3.


a. Independent function status among patients with disseminated malignancy who underwent a surgical operation from 2006 to 2010.
Line graph demonstrating percentage of patients with independent functional status undergoing operation. Pearson chi squared analysis showed a statistically significant increase over the study period. * p<0.01.
b. Preoperative weight loss of greater than 10% of patients' body weight in the 6 months prior to surgery among patients with disseminated malignancy who underwent a surgical operation from 2006 to 2010.
Line graph demonstrating percentage of patients with weight loss of greater than 10% of their body weight in the 6 months prior to the principal operation. Pearson chi squared analysis showed a statistically significant decrease over the study period. * p<0.01.
c. Preoperative diagnosis of sepsis among patients with disseminated malignancy who underwent a surgical operation from 2006 to 2010.
Line graph demonstrating percentage of patients with a diagnosis of pre-operative sepsis. Pearson chi squared analysis showed a statistically significant decrease over the study period. * p<0.01.
Morbidity and Mortality
As shown in Figure 4, morbidity significantly decreased over time, dropping from 33.7% in 2006 to 26.6% in 2010 (p < 0.01). When analyzing serious morbidity (i.e. respiratory or cardiac failure requiring reintubation, prolonged intubation, acute renal failure requiring dialysis, reoperation, pulmonary embolism, stroke, coma, cardiac arrest or systemic shock), there was also a significant decline over time, declining from 19.8% in 2006, 18.0% in 2007, 16.0% in 2008, 17.2% to 14.2% in 2010 (p < 0.01). Similarly, mortality showed a statistically significant, although modest decline, from 10.4% in 2006 to 9.3% in 2010 (p < 0.05).
Figure 4. Morbidity and mortality trends among patients with disseminated malignancy undergoing surgical intervention from 2006 to 2010.
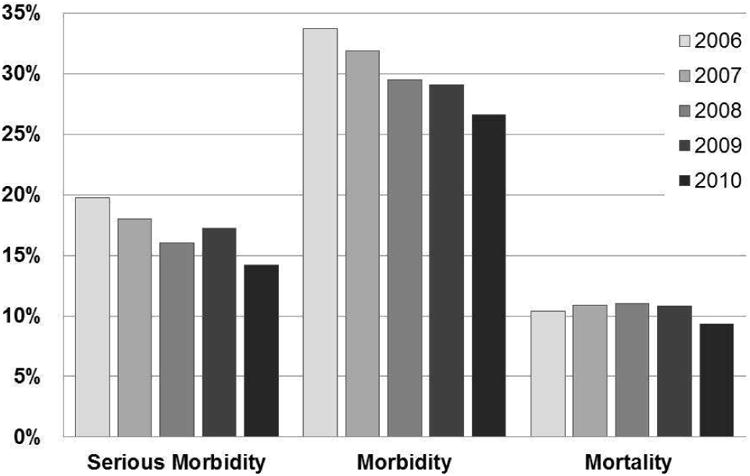
Bar graph showing percentage of patients experiencing post-surgical morbidity, serious morbidity and mortality. Pearson chi squared analysis demonstrated a statistically significant decline in all three of these outcomes over the study period, p < 0.05.
On multivariate logistic regression analysis, male gender, greater BMI, impaired functional status, pre-operative weight loss of >10% of body weight, pre-operative sepsis, emergency operations, multivisceral resections, lack of DNR status, greater WBC count and creatinine, decreased hematocrit, and decreased albumin predicted worse 30-day morbidity, p < 0.05. Predictors or 30-day mortality were similar, including male sex, greater BMI, impaired functional status, >10% of body weight pre-operative weight loss, preoperative sepsis, emergency operations, greater WBC count and creatinine and decreased hematocrit and albumin, p < 0.05. However, multivisceral resection was not a significant predictor of mortality (p > 0.05). In addition, the presence of DNR status, not absence, and increasing age predicted a higher risk of postoperative mortality (p < 0.05).
Discussion
As predicted, there were a substantial number of operations performed on patients with disseminated malignancy, although we did observe a slight percentage decline over the selected time period. This suggests that despite greater attention to the potential risks of surgical intervention in this high risk population, surgical procedures remain prevalent among patients with stage IV cancer patients. These findings are consistent with prior research.[4, 6, 17] For example, the incidence of malignant bowel obstruction has been estimated to be 5 – 51% in ovarian malignancies and 10 – 28% in gastrointestinal cancers, and rates of operations performed on stage IV cancer patients with bowel obstructions have consistently ranged from 57 – 67% [1, 3, 7, 18].
It is, however, unclear the exact motivation of such surgical intervention, whether the operations were performed with hopes of cure, extending life, or palliating symptoms. Prior to and during this time period, studies have supported the concept that the performance of palliative operations may provide effective symptom relief in patients with advanced malignancies [2, 5, 19]. These and other studies may have supported the decision of surgeons, patients and families to undergo surgical intervention [20]. Furthermore, surgeons as well as other clinicians have been observed to be overly optimistic when estimating life expectancy among patients with advanced malignancy, including in cases where surgical intervention is contemplated [5, 21]. This suggests that both surgeon optimism regarding patient life expectancy and hopes of providing symptom relief may help explain, in part, the persistent prevalence of surgical interventions among stage IV cancer patients.
As predicted, overall morbidity and mortality remained substantial in this cohort at 29.4% and 10.4% respectively. This is consistent with prior research from Miner et al (2004) and Tseng et al (2011), among others, which demonstrated overall 30-day morbidity rates of 28 – 29% and mortality of 9 – 11%, respectively. However, interestingly, we observed a trend over the time period of this study (which was statistically significant) for declining rates of serious and overall morbidity. In addition, during this time period, there was also a significant increase in patients with independent functional status and a significant decrease in the percentage of emergency operations and patients with pre-operative sepsis and pre-operative weight loss. These factors may help explain the decreasing trends in morbidity over time, as these were also found to be significant predictors of morbidity. These data suggest that despite the persistent use of surgical intervention in patients with disseminated malignancy, there does appear to be evidence for improved selection for patients undergoing operation over time, and this may help explain the decreased morbidity over time.
These data further emphasize the importance of patient selection in the decision-making process when considering surgical intervention for patients with incurable cancer. This decision-making process should include an assessment of the risks of the procedure based on patient and procedural characteristics as well as probability of symptom resolution, the effect of overall quality of life, pain control, cost-effectiveness, and individual patient goals [3, 22]. Important patient characteristics to consider include patient functional status, as we and others have observed this factor to be a significant predictor of the risks of morbidity and mortality [4, 18].
However, even with improved patient selection, we observed that 30-day mortality remained elevated and largely static over the 5-year period, except for a small, but statistically significant decline from 10.4% in 2006 to 9.3% in 2010. Therefore, surgeons need to remain cautious with their optimism regarding patient survival with surgical intervention, as there is a persistently high mortality risk among patients with stage IV cancer. Furthermore, these results represent outcomes at 30 days, and it is possible, and likely probable, that greater numbers of patients are experiencing additional surgical morbidity and mortality past the 30-day NSQIP reporting period.
Consistent with prior research, there was an increased risk of mortality with the presence of a do not resuscitate order [23, 24]. As Scarborough et al. reasoned, this may be due to DNR patients being less likely than non-DNR patients to receive aggressive therapy for major postoperative complications, so-called failure to pursue rescue [24, 25]. However, interestingly and of critical importance for the design of possible prospective intervention studies, very few disseminated malignancy patients in this cohort (3.0%) who underwent an operation during this time period had a DNR order. This may be due to multiple factors including the tendency for surgeons to have a higher threshold for surgical interventions on patients with a DNR order, leading to an under-representation of patients with a DNR order undergoing operation
Another important implication of this study (as has been shown previously by others) is that patients, families, and care providers (including surgeons) may delay discussions of goals of care and management priorities for end-of-life when caring for disseminated malignancy patients who present with acute surgical conditions [23, 26]. For example, Mack et al. observed that for patients who had died during their reporting period, the first end of life discussion occurred for patients at a median of 33 days before their death [26]. Delaying this end of life discussion has significant consequences for patients, as it is associated with delayed referrals to palliative care and hospice. Early palliative care referrals have been associated better quality of life among patients with advanced cancer [8, 9]. This highlights the importance of early end-of-life care discussions between patients and physicians, including surgeons, to facilitate improved quality of life and to ensure that patient goals of care are aligned with their providers' expectations and unwanted and/or maximally invasive procedures are avoided.
It is important to acknowledge limitations of our study. Most notably, we were unable to determine the patients' primary cancer diagnosis, the goals of the operation by the surgeon, whether the goal was to extend survival or palliate symptoms, and whether the operation was successful in palliating symptoms and mortality risk. Knowledge of the patients' primary cancer diagnoses is important, as not all stage 4 cancers are equal in prognosis, and some patients may achieve long-term survival with multimodality therapy [27, 28]. Furthermore, it remains to be shown whether surgical intervention is better or worse at enabling patients with disseminated malignancy to resume intensive multimodality therapy for their underlying cancer diagnosis. Another unresolved question is the effect of medical versus surgical management of these “surgical” conditions on the important outcomes of overall survival, quality of life, and effective palliation of symptoms. Further research with matched surgical and non-surgical comparator groups evaluating these important outcomes is needed.
In summary, our analysis of the temporal trends among patients with disseminated malignancy undergoing surgical intervention demonstrate continued utilization of surgical intervention over time, despite greater attention to the role of palliative care. In addition, we identified a significant decline in morbidity, potentially due to improved patient selection, but with a largely unchanged high mortality. These data highlight the importance of careful patient selection and an evaluation of the goals of therapy and end of life goals among this high risk patient population.
Figure 1. Percentage of NSQIP operations performed on patients with disseminated malignancy, excluding hepatic cases, from 2006 to 2010.

Bar graph showing percentage of patients with disseminated malignancy undergoing surgical intervention (excluding hepatic cases) among all NSQIP operations performed on an annual basis. Pearson chi-squared analysis showed a statistically significant decline in procedures over the study period.
Acknowledgments
This work was supported by funding from the National Institutes of Health (UC Davis Paul Calabresi K12 Career Development Award NIH 1K12CA138464-01A2).
Footnotes
Presented in part at the Academic Surgical Congress 10th Annual Meeting, February 2 – 4, 2015, Las Vegas, NV.
Author Disclosure Statement: The authors report no proprietary or commercial interest in any project mentioned or concept discussed in this article.
Author Contributions: SB Bateni – conception and design, analysis and interpretation of data, writing the manuscript
FJ Meyers – analysis and interpretation of data, critical revision of the article
RJ Bold – critical revision of the article
RJ Canter – conception and design, analysis and interpretation of data, writing the manuscript, critical revision of the manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feuer DJ, Broadley KE, Shepherd JH, Barton DP. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. The Cochrane database of systematic reviews. 2000;(4):CD002764. doi: 10.1002/14651858.CD002764. [DOI] [PubMed] [Google Scholar]
- 2.Miner TJ, Brennan MF, Jaques DP. A Prospective, Symptom Related, Outcomes Analysis of 1022 Palliative Procedures for Advanced Cancer. Annals of Surgery. 2004;240:719–727. doi: 10.1097/01.sla.0000141707.09312.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson TJP, Pinkerton C, Brasel KJ, Schwarze ML. Palliative Surgery for Malignant Bowel Obstruction from Carcinomatosis: A Systemic Review. JAMA Surgery. 2014;149(4):383–392. doi: 10.1001/jamasurg.2013.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tseng WH, Yang X, Wang H, Martinez SR, Chen SL, Meyers FJ, Bold RJ, Canter RJ. Nomogram to predict risk of 30-day morbidity and mortality for patients with disseminated malignancy undergoing surgical intervention. Annals of surgery. 2011;254(2):333–338. doi: 10.1097/SLA.0b013e31822513ed. [DOI] [PubMed] [Google Scholar]
- 5.McCahill LE, Smith DD, Borneman T, Juarez G, Cullinane C, Chu DZ, Ferrell BR, Wagman LD. A prospective evaluation of palliative outcomes for surgery of advanced malignancies. Annals of surgical oncology. 2003;10(6):654–663. doi: 10.1245/aso.2003.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Krouse RS, Nelson RA, Farrell BR, Grube B, Juarez G, Wagman LD, Chu DZ. Surgical palliation at a cancer center: incidence and outcomes. Archives of surgery. 2001;136(7):773–778. doi: 10.1001/archsurg.136.7.773. [DOI] [PubMed] [Google Scholar]
- 7.Henry JC, Pouly S, Sullivan R, Sharif S, Klemanski D, Abdel-Misih S, Arradaza N, Jarjoura D, Schmidt C, Bloomston M. A scoring system for the prognosis and treatment of malignant bowel obstruction. Surgery. 2012;152(4):747–756. doi: 10.1016/j.surg.2012.07.009. discussion 756-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui D, Kim SH, Roquemore J, Dev R, Chisholm G, Bruera E. Impact of timing and setting of palliative care referral on quality of end-of-life care in cancer patients. Cancer. 2014;120(11):1743–1749. doi: 10.1002/cncr.28628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, Moore M, Rydall A, Rodin G, Tannock I, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721–1730. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 10.Surgeons ACo. User Guide for the 2010 Participant Use Data File: American College of Surgeons National Surgical Quality Improvement Program. Chicago, IL: American College of Surgeons; 2011. [Google Scholar]
- 11.Harmon KE, Ryan JA, Jr, Biehl TR, Lee FT. Benefits and safety of hepatic resection for colorectal metastases. American journal of surgery. 1999;177(5):402–404. doi: 10.1016/s0002-9610(99)00070-7. [DOI] [PubMed] [Google Scholar]
- 12.Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, Curley SA, Zorzi D, Abdalla EK. Solitary colorectal liver metastasis: resection determines outcome. Archives of surgery. 2006;141(5):460–466. doi: 10.1001/archsurg.141.5.460. discussion 466-467. [DOI] [PubMed] [Google Scholar]
- 13.Adam R, Chiche L, Aloia T, Elias D, Salmon R, Rivoire M, Jaeck D, Saric J, Le Treut YP, Belghiti J, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Annals of surgery. 2006;244(4):524–535. doi: 10.1097/01.sla.0000239036.46827.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D'Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(29):4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 15.Shiloach M, Frencher SK, Jr, Steeger JE, Rowell KS, Bartzokis K, Tomeh MG, Richards KE, Ko CY, Hall BL. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. Journal of the American College of Surgeons. 2010;210(1):6–16. doi: 10.1016/j.jamcollsurg.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton BH, Ko CY, Richards K, Hall BL. Missing data in the American College of Surgeons National Surgical Quality Improvement Program are not missing at random: implications and potential impact on quality assessments. Journal of the American College of Surgeons. 2010;210(2):125–139 e122. doi: 10.1016/j.jamcollsurg.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Badgwell B, Krouse R, Cormier J, Guevara C, Klimberg VS, Ferrell B. Frequent and early death limits quality of life assessment in patients with advanced malignancies evaluated for palliative surgical intervention. Annals of surgical oncology. 2012;19(12):3651–3658. doi: 10.1245/s10434-012-2420-5. [DOI] [PubMed] [Google Scholar]
- 18.Francescutti V, Miller A, Satchidanand Y, Alvarez-Perez A, Dunn KB. Management of bowel obstruction in patients with stage IV cancer: predictors of outcome after surgery. Annals of surgical oncology. 2013;20(3):707–714. doi: 10.1245/s10434-012-2662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podnos YD, Juarez G, Pameijer C, Choi K, Ferrell BR, Wagman LD. Impact of surgical palliation on quality of life in patients with advanced malignancy: results of the decisions and outcomes in palliative surgery (DOPS) trial. Annals of surgical oncology. 2007;14(2):922–928. doi: 10.1245/s10434-006-9238-y. [DOI] [PubMed] [Google Scholar]
- 20.Badgwell B, Bruera E, Klimberg SV. Can patient reported outcomes help identify the optimal outcome in palliative surgery? Journal of surgical oncology. 2014;109(2):145–150. doi: 10.1002/jso.23466. [DOI] [PubMed] [Google Scholar]
- 21.Glare PA, Sinclair CT. Palliative medicine review: prognostication. Journal of palliative medicine. 2008;11(1):84–103. doi: 10.1089/jpm.2008.9992. [DOI] [PubMed] [Google Scholar]
- 22.Miner TJ, Cohen J, Charpentier K, McPhillips J, Marvell L, Cioffi WG. The palliative triangle: improved patient selection and outcomes associated with palliative operations. Archives of surgery. 2011;146(5):517–522. doi: 10.1001/archsurg.2011.92. [DOI] [PubMed] [Google Scholar]
- 23.Speicher PJ, Lagoo-Deenadayalan SA, Galanos AN, Pappas TN, Scarborough JE. Expectations and outcomes in geriatric patients with do-not-resuscitate orders undergoing emergency surgical management of bowel obstruction. JAMA surgery. 2013;148(1):23–28. doi: 10.1001/jamasurg.2013.677. [DOI] [PubMed] [Google Scholar]
- 24.Scarborough JE, Pappas TN, Bennett KM, Lagoo-Deenadayalan S. Failure-to-pursue rescue: explaining excess mortality in elderly emergency general surgical patients with preexisting “do-not-resuscitate” orders. Annals of surgery. 2012;256(3):453–461. doi: 10.1097/SLA.0b013e31826578fb. [DOI] [PubMed] [Google Scholar]
- 25.Olson TJ, Schwarze ML. Failure-to-Pursue Rescue: Truly a Failure? Annals of surgery. 2013 doi: 10.1097/SLA.0000000000000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mack JW, Cronin A, Taback N, Huskamp HA, Keating NL, Malin JL, Earle CC, Weeks JC. End-of-life care discussions among patients with advanced cancer: a cohort study. Annals of internal medicine. 2012;156(3):204–210. doi: 10.1059/0003-4819-156-3-201202070-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrogh M, Park A, Norton L, King TA. Changing indications for surgery in patients with stage IV breast cancer: a current perspective. Cancer. 2008;112(7):1445–1454. doi: 10.1002/cncr.23319. [DOI] [PubMed] [Google Scholar]
- 28.Livhits M, Li N, Yeh MW, Harari A. Surgery is associated with improved survival for adrenocortical cancer, even in metastatic disease. Surgery. 2014;156(6):1531–1541. doi: 10.1016/j.surg.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]


