Abstract
Most people find it profoundly difficult to name familiar smells. This difficulty persists even though perceptual odor processing and visual object naming are unimpaired, implying deficient sensory-specific interactions with the language system. In this article, we synthesize recent behavioral and neuroimaging data to develop a biologically informed framework for olfactory lexical processing in the human brain. Our central premise is that the difficulty in naming common objects through olfactory (compared to visual) stimulation is the end result of cumulative effects occurring at three successive stages of the olfactory language pathway: object perception, lexical-semantic integration, and verbalization. Understanding the neurocognitive mechanisms by which the language network interacts with olfaction can yield unique insights into the elusive nature of olfactory naming.
Keywords: Olfaction, language, perception
Olfactory naming
Object naming and categorization enable the human brain to impose structure on the external world. However, objects come in many shapes, sizes, and sensory modalities, and how the senses differ in their capacity to interact with the language system may provide new insights in human cognition [1–3]. Although the ability to view a banana and conjure up the word “banana” comes quite easily and rapidly, the corresponding ability to smell a banana and conjure up the word “banana” can be extremely effortful. The comparison of olfactory naming to visual naming is of particular interest because many common odor objects (e.g., banana) are otherwise primarily identified through visual perceptual channels. The tenuous link between odors and names might have been noted already by Plato, who wrote that “the varieties of these smells have no name, but are indicated by two distinctive terms only, ‘pleasant’ and ‘painful’” [4]. Although the difficulty to name odors has been demonstrated empirically for decades, the underlying mechanisms remain elusive [5–7].
The key goal of this article is to provide a neurocognitive framework for olfactory language that incorporates recent psychophysical and neuroimaging research findings. Our approach to olfactory naming is inspired by recent models for understanding visual processing [8,9] and language pathways [10–12]. First, we present key empirical observations regarding olfactory perception and cognition. Second, we propose key neural mechanisms within a three-stage framework. Finally, we discuss how this framework might be used to address outstanding questions for future research.
Behavioral and perceptual insights into odor perception and naming
Below, four lines of study are reported that are of particular relevance in informing theoretical views of olfactory neural interactions with language.
Naming failure
Compared to naming visual objects, our ability to name the source of odors is exceptionally unimpressive. Pioneering studies showed that in healthy participants, only 20–50% of common odors (e.g., pine, chocolate) were successfully named, compared to nearly 100% of common pictures in an equivalent naming task [13,14]. Although naming failure could be the result of deficient olfactory perception, this generally appears not to be the case: common odors are easily discriminable from each other when presented pairwise, and critically, performance increases dramatically when odors are matched to labels in a multiple-choice testing format [15,16]. Although sensory impairments in olfaction would also lead to impaired odor naming and identification, the everyday phenomenon of olfactory naming failure is more likely based on poor lexical access and/or verbalization of odors. This concept is often described as a “weak link” between odors and words [5], though until recently its neural foundations had been poorly understood.
Configural perception
Poor olfactory naming might be partly explained by the tendency of the olfactory system to generate ‘configural’ object representations, thereby subsuming individual features that could otherwise improve lexical mapping. Most commonplace odors, such as spices or flowers, are chemically complex, yet there is little cognitive access to their constituent components [17]. In pioneering studies, participants were presented with up to five common odors as part of a mixture and asked to identify its components from a list. A majority of both novices and wine experts were incapable of identifying more than three components, and consistently underestimated the complexity of the mixture [18,19]. Olfactory configural perception critically relies on synthesis or blending of associative features; for example, repeated exposure to a binary odor mixture (e.g., lemon and mushroom) or an odor-taste pairing (e.g., lemon and sucrose) leads to a persistent blending of perceptual qualities, such that even pure lemon obtained a hint of “mushroom” or “sweetness” for those exposed to the mixtures [20,21]. By comparison, although the visual system enables configural processing, most notably in face perception, specific features (e.g., eyes, mouth, nose) remain fully accessible. Thus, in the olfactory system, the lack of access to distinctive features may impair mapping precision onto lexical-semantic space.
Early-stage object encoding
Configural odor ‘objects,’ as described above, are often viewed as key building blocks of olfactory cognition and emotion [22,23]. An alternative theory holds that odor naming is instead constrained by an initial, valence-based encoding format that resists verbal translation [24]. According to this theory, hedonic valence might be critical because it typically emerges as a primary dimension of olfactory perception [25,26], and because variations in odor valences correlate with activation patterns in the olfactory neuroepithelium and the brain [27,28]. Whether configural objects or valences constitute the initial perceptual odor encoding stage should be reflected by the speed for ‘object-based’ versus ‘valence-based’ decisions. A recent study suggested that when rapid behavioral responses were based on accessing the valence of a familiar odor, decision latencies were consistently longer (> 100 ms) and more variable compared to an object categorization task [29]. Similar results were obtained in a follow-up study where odors were subject to binary classifications based on their valence or object properties [30]. These findings suggest, at least for familiar smells, that odor objects manifest early in the processing stream [31].
Elusiveness of lexical descriptors
In-depth analysis of failed attempts to name odors shed additional light on how odor information interacts with lexical semantics. When familiar visual objects elude naming, a subjective “tip-of-the-tongue” state is elicited wherein partial lexical information (e.g., the first letter of the word) is often accessible [32]. When the naming of common odors was compared to pictures of well-known faces [33,34], naming failure was common in both sensory modalities but “tip-of-the-tongue” experiences were less common for odors. When subjects did have these experiences with odor naming, they were resolved less often and were poorer predictors of subsequent recognition. Importantly, partial lexical representations (e.g., what is its first letter?) were more accessible for pictures than odors, whereas broader semantic associations (e.g., is it edible?) were more common for odors than pictures [33]. After failed odor naming attempts, subsequent performance is rescued only by feedback regarding the veridical odor names [35]. These findings suggest that whereas visual naming failure results from an incomplete retrieval of an active lexical representation, odor naming failure results from an inefficient activation of the lexical representation.
A neuro-cognitive framework for odor naming
Anatomical comparisons between olfactory and visual object-naming pathways may aid our understanding of why odor naming is much poorer than visual naming. The language network is widely distributed across frontal, temporal, and parietal cortices [36,11,37]. Inferior and middle parts of the left temporal lobe support visual semantic processing of visual objects and written word stimuli [38–40]. Regions adjacent to the left Sylvian fissure are critical for visual object naming [41,42], and anteriorly, the inferior frontal gyrus (IFG) is particularly relevant for lexical-semantic selection and/or access for verbalization [43,37]. The superior temporal gyrus supports phonological processing in object naming and other verbal tasks [44,45]. Finally, the left temporal pole (TP) plays an important role in linking lexical representations to representations of unique and familiar sensory objects [46,47]. Here we review recent findings from human neuroimaging studies relevant for understanding odor naming, organized according to three main stages of processing: perception, lexical-semantic integration, and verbalization.
Odor perception
The first stage of our model begins with the arrival of odor inputs from the olfactory bulb into the piriform cortex (PC). Human imaging data increasingly point towards the PC being the principal associative brain area of the olfactory system [48]. For example, sequential presentation of two odors sharing the same perceptual quality (e.g., lemon and orange share a citrus quality) evoked fMRI ‘cross-adaptation’ in the posterior PC, suggesting that category-based perceptual information was processed in this area [49]. Multivariate fMRI pattern analyses have shown that distributed ensemble representations in the PC exhibit greater pattern overlap for odor stimuli belonging to the same perceptual category [50]. A recent study demonstrated that the posterior PC encodes value-based ensemble codes of a complex natural food-odor (peanut butter), but not its chemical components [51], consistent with the idea of configural processing in this area. Non-olfactory cues may also elicit odorspecific distributed patterns in the posterior PC in advance of odor input [52,53], suggesting that this area receives top-down projections to facilitate olfactory predictive coding and rapid processing [29].
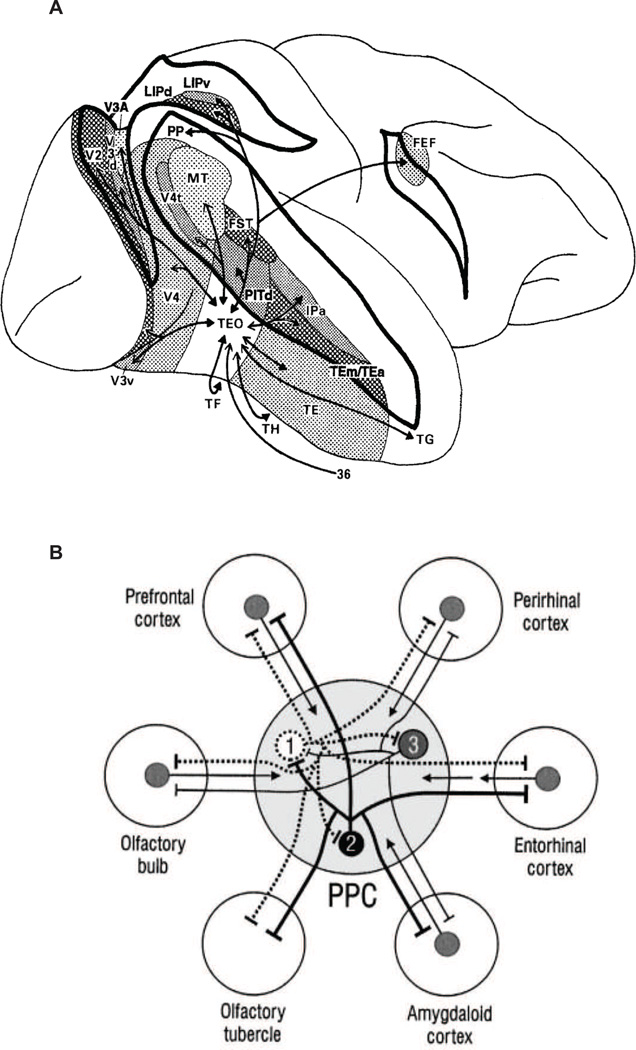
The role of the PC in establishing configural representations of odor object quality implies that the PC is the olfactory analog of visual associative areas in the ventral temporal cortex [48,54]. Of note, these olfactory and visual regions differ profoundly in their capacity for object analysis and their connectivity with the language network. For visual objects, a widely-distributed network of associated nodes along the ventral stream provide an excellent feature analysis [55–57] (Fig. 1a). These inputs converge onto global object-based representations in visual associative cortices (in monkeys, areas TE and TEO), which are densely and reciprocally interconnected with numerous other nodes in occipital, parietal, temporal, and frontal cortices [58,59]. This rich network of cortical connections enables parallel and iterative access to feature-selective detail, allowing for the assembly of richly endowed visual object configurations, and critically, offering multiple entry points into the lexical-semantic network.
Fig. 1.
Connectivity of visual and olfactory object-specific areas. (A) In the visual system, associative representations in macaque areas TEO and TE are densely and reciprocally integrated with neocortical areas in the temporal, parietal, and frontal lobes. Reprinted from [16] with permission from Wiley Periodicals Inc. In humans, such pathways offer rich opportunities for endowing visual object information with associative content to inform lexical-semantic processing and verbalization. (B) In the olfactory system, associative representations in rodent piriform cortex are densely and reciprocally integrated with limbic and paralimbic areas in the medial temporal lobe and basal forebrain. In humans, such pathways offer opportunities for endowing olfactory object information with limbic associative content to inform emotion, memory, and behavior, but lack robust connectivity with cortical language areas to optimize odor naming. Reprinted from [34] with permission from the Society for Neuroscience.
By comparison, in the olfactory system, the olfactory associative cortex (PC) is densely connected with limbic and paralimbic regions, but poorly connected with cortical areas that might otherwise embellish odor object representations with lexical-semantic content. Anatomical tracer studies in rodents show that individual PC neurons project to target regions in the basolateral amygdala, perirhinal cortex, entorhinal cortex, insula, orbital cortex, and olfactory tubercle [60] (Fig. 1b). The functional consequence is that while configural olfactory objects in the PC are endowed with emotion, value, memory, and experience, the ability to enrich these configurations with lexical-semantic content is relatively meager. This limitation poses an initial challenge to generating odor names.
Lexical-semantic integration
The second stage of our model provides a basis for integrating odor objects with lexical-semantic information. Critical elements in this process include the temporal pole (TP) and orbitofrontal cortex (OFC), which receive direct olfactory input from the PC.
In crossmodal priming tasks, odor cues (e.g., orange odor) or visual cues (e.g., picture of an orange) were followed by a matching or non-matching word (e.g., “orange” or “wood”). Results showed that semantic word processing was affected by its congruency with the preceding cue, and critically, depended on the sensory modality of the preceding cue [61]. Event-related potentials highlighted differential scalp distributions and timing of the N400 effect, an index of semantic integration, as a function of cue modality. fMRI response profiles revealed response decrement (cross-adaptation) in both OFC and dorsomedial TP when odor (but not visual) cues were followed by non-matching vs. matching word targets. Involvement of the OFC in integrating olfactory and lexical-semantic content likely represents just one aspect of its broader associative role in synthesizing converging information streams from different sensory modalities [62–69].
Evidence for the role of the TP in odor identification and naming has further emerged from studying patients with primary progressive aphasia (PPA), who exhibit progressive deficits in naming and identifying visual objects [70–72]. Patients with PPA had considerable difficulty naming common smells, but performance increased significantly and approached controlgroup levels when visual and verbal cues were available. The degree of left-hemisphere TP atrophy across patients correlated with deficits of odor matching and odor familiarity, reinforcing the idea that this brain region is critically involved in mediating the access of odor objects to lexical-semantic representations. Imaging data increasingly suggest that the TP serves as an associative gateway that supports identification and naming [73,46,74]. In particular, the dorsomedial TP is perhaps the most plausible anatomical candidate for bridging olfactory and left hemisphere language networks, with tracing experiments in monkeys revealing that the PC directly projects to the dorsomedial rim of the TP [75].
Importantly, odors are already integrated with lexical representations at the third synapse from receptor neuron input. This could put olfaction at a disadvantage compared to the visual system, where multiple subcortical and cortical sites create object representations prior to lexical-semantic integration by integrating features at different spatial scales [76]. It has been noted that within the visual object stream, short synaptic links that provide coarse object processing are complemented by more complex processing networks which are needed to establish detailed object features [77,78]. In the current framework, this distinction is invoked to explain differences between olfactory and visual source naming. Indeed, neural processing of lexical semantics might be initiated more rapidly when a word is preceded by an odor prime, compared to a visual prime, yet behavioral matching performance is ultimately less accurate for odor-word integration [61,79]. The reliance on early, coarse lexical integration of odors poses a second challenge to generating odor names.
Verbalization
The third stage of our model culminates in the verbalization of olfactorylexical representations. As noted above, odor-based naming failures are common despite intact odor discrimination and intact knowledge of lexical-semantic concepts. The brain region most consistently associated with object naming across sensory modalities is the inferior frontal gyrus (IFG) and adjacent regions [43]. Early evidence for the role of IFG in odor object naming came from a study showing that odor-evoked fMRI activity in the IFG was linearly correlated with the familiarity of the odor [80]. More recently, neural activation was compared for odors that were easy or hard to name in the context of a working memory task [81]. While unnameable odors activated the PC as a putative “working-memory buffer,” easily named odors activated the IFG [81], suggesting a primary role for the IFG in successful identification and verbalization of odors.
Evidence from neurological patients with PPA provides complementary insights on the causal role of the IFG [72]. Those patients with the highest degree of cortical thinning in the IFG were most impaired in odor naming, as well as in visually-cued odor identification [72]. In terms of effect-size, odor naming was more impaired than visual naming in these patients [72]. Converging findings from regional atrophy and functional neuroimaging studies thus underscore a key role of the IFG in identifying and verbalizing familiar odors.
Neural substrates of odor naming: an integrated perspective
We propose that the common failure to name and identify odors might be tied to inherent properties of the designated network for olfactory language. The olfactory processing hierarchy is characterized by direct cortical links from the olfactory bulb to PC, and from the PC to TP and OFC, leaving odor object information minimally elaborated before interacting with the lexical-semantic system. Although ‘direct access’ might be beneficial in very simple tasks, lexical integration and naming require a higher degree of cortical elaboration than the odor object recognition system typically provides. Olfactory representations might thus be conducted to the language network in an unconstrained, indeterminate format, causing an unspecific activation of latent object concepts.
Our framework for odor naming (Fig. 2) is broadly consistent with the idea that visual perceptual learning involves back-projections from high-level object representations onto sparse lower-level sensory inputs [8], and that top-down predictions refine perception of bottom-up inputs [9]. Here, we extend this notion to cross-modal visual influences on odor object matching and identification. In fact, access to odor names improves performance on a variety of olfactory tasks, including quality discrimination [82]. We hypothesize that as initial neocortical representations of odors are configural and relatively unembellished, access to visual cues or labels may thus exert strong “top-down” influences on olfactory representations.
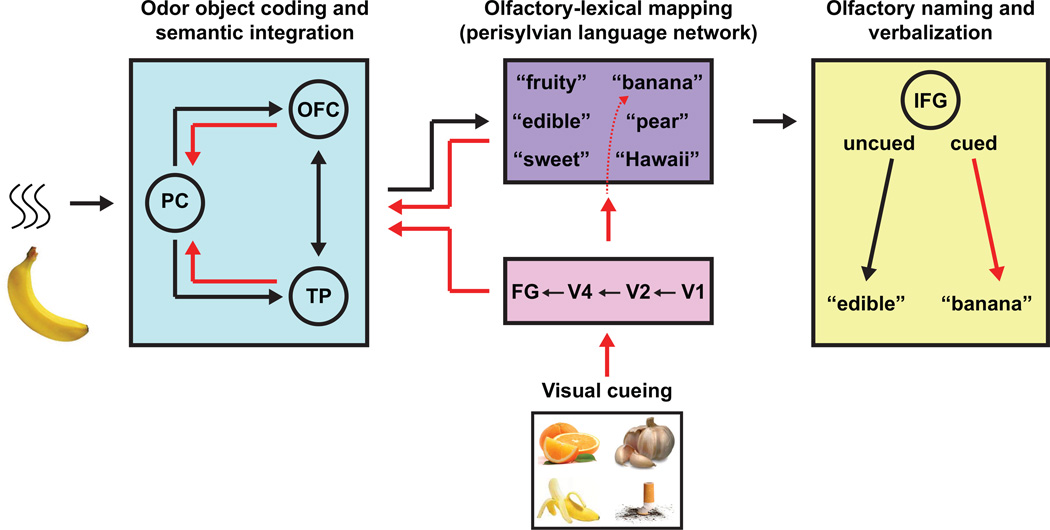
Fig. 2.
A neurocognitive model for olfactory naming and identification. In uncued naming, olfactory information (e.g., banana odor) is propagated along a feed-forward pathway from PC (black arrows) to OFC and TP (light blue box). Due to feature loss and mapping imprecisions at these initial levels of odor processing, a broad and unconstrained set of olfactory-lexical representations is activated in the perisylvian language network (purple box). This places greater challenges on IFG (yellow box) to retrieve and select from among the diverse set of lexical representations in order to generate an odor name. The example here shows verbalization of a superordinate categorical name (“edible”). In contrast, with access to visual cues, olfactory identification benefits from feed-back information (red arrows) along the ventral stream (pink box), helping to predict and specify olfactory representations at the levels of odor object coding in PC, semantic integration in OFC and TP, and lexical processing in the language network. Together the availability of predictive codes enables correct identification of the odor as “banana”.
In odor naming (when cues are absent), feed-forward projections from the PC present relatively coarse odor object information directly to the language network via the OFC and TP (Fig. 2, black arrows). The resulting unconstrained mapping onto lexical-semantic concepts manifests downstream as an exaggerated reliance on the IFG for odor verbalization. As a result, the correct name is occasionally retrieved, though more often a generic categorical word, an incorrect word, or an arbitrary autobiographical association is retrieved. By comparison, in cued odor identification tasks, access to visual cues or labels provides an entry point for developing lexical-semantic expectations about which odor is most likely to be encountered (Fig. 2, red arrows). Predictive feedback from visual associative areas effectively constrains activation of relevant lexical concepts in the language network, and also helps refine olfactory object representations (in the PC) and their integration with semantic systems (in the TP and OFC). These feedback effects of visual cueing utilize the intrinsic associative depth of visual object representations to engage the language network, with the ultimate result of accurate verbal retrieval and identification.
Although the radically different capabilities to name olfactory and visual objects may be rooted in neuroanatomy, odor naming and perception are not immune to effects of experience [83,84]. Professional perfumers show enhanced thickness of the orbitofrontal cortex and piriform gyrus [85], and more experienced perfumers exhibit reduced fMRI activation in the piriform cortex upon recalling odors [86]. Experts and novices thus engage partly similar networks for olfactory perception and naming, but semantic-integrative resources might be expanded in experts through neuroplastic changes [87]. As it has been argued that the specific inability to name odor objects is due to cultural, rather than neurobiological, constraints [83], an important question for future research is whether olfactory experts may approach olfactory source naming performance levels comparable to those typically observed in visual naming.
Concluding remarks
This framework brings together recent neuroimaging findings on the cortical basis of olfactory-language interactions. It also reconciles previous behavioral findings of olfactory naming deficits with other characteristic perceptual features, including the lack of olfactory feature analysis and poor translation of odor objects to lexical representations. Thus, the concepts highlighted here provide an integrative foundation for future research to specify the roles of key nodes in the olfactory naming network, as well as emerging research on how cultural and experiential variability shapes individual differences (Box 1–2). Our framework also formally conceptualizes olfactory naming failure as a cumulative deterioration of signal quality over multiple processing stages in the cascade from odor input to verbal output. In this manner, the anatomical and functional organization of the olfactory system presents persistent challenges to its ability to map odors to names.
Box 1: Cultural differences and similarities in olfactory language.
Although most published research support a view that odors resist verbal description and uncued naming, it is unclear whether these results constitute evidence of a universal limitation on olfactory naming (compared to visual naming). Only a few experimental investigations have been conducted on olfactory perception and naming outside of industrialized societies, for example, in nomadic hunter-gatherers in the Malay Peninsula [83,88]. In contrast to English and related languages, where odors are named by providing source-based descriptors (e.g., “this smell is [coming from a] banana”), the Jahai and Maniq languages include over a dozen abstract olfactory terms that may be used to describe and categorize odors independently of their source. Of note, the actual abstract terms used to describe classes of odors – such as edible, roasted, fragrant, stink, musty, stinging, urine – do resemble the kinds of odor categorical descriptors often used by English speakers, though their usage in these communities is strikingly flexible and facile.
In one study, odor terms used by speakers of Maniq were subjected to a multidimensional scaling procedure. Results showed that two distinct perceptual dimensions, roughly corresponding to pleasantness and dangerousness, best captured the odor descriptions [88]. This two-dimensional structure is broadly similar to results obtained in speakers of English [89,26]. Native speakers of Jahai and English were asked to name 12 odors from the Brief Smell Identification Test [90]. Results revealed that for the Jahai speakers, odor naming was as accurate as color naming, and abstract terms were used to describe both odors and colors. In contrast, speakers of English provided source-based (e.g., “banana”) and evaluative (e.g., “pleasant”) words to describe odors, but used abstract words exclusively to describe colors. Critically, odor naming accuracy in both Jahai and English was well below color naming accuracy in English [83]. Although these results highlight cultural differences, as well as possible similarities in olfaction, they are compatible with the notion that olfactory object naming is limited, or at least subserves a different role, compared to visual object naming. More extensive cross-cultural research is needed to address whether naming is more fully integrated with visual compared to olfactory content.
Box 2: Outstanding questions.
-
-
Do olfactory inputs (as opposed to visual inputs) activate a larger and less constrained set of lexical-semantic concepts in the language network? Is the TP an obligatory bridge between olfactory percepts and the lexical-semantic system? The emergence of multivariate techniques [91] to analyze distributed patterns of fMRI information, and the use of non-invasive brain stimulation protocols [92] to induce virtual brain lesions, may be able to bring new insights to these questions.
-
-
How does the brain compare predictive and afferent inputs to emit an olfactory prediction error signal? Such mechanisms are critical in odor perception [93], and could be important for helping the olfactory system converge toward odor object recognition by iteratively comparing an olfactory input (e.g. rose) to predictions (vanilla? strawberry?), emitting prediction errors until the error signal is minimized, and a match to rose odor is made. Though recent evidence [53,72,94–96] suggests that the mediodorsal thalamus is well-positioned to mediate comparisons between afferent and predictive streams of information in human olfaction, further work is necessary to fill in this critical assumption of our framework presented here.
-
-
Are there similarities in the naming of odors compared to other non-visual sensory systems? We speculate that our framework could generalize to include touch and sound, given that naming of somatosensory objects and (non-verbal) auditory objects also generally relies on supporting visual cues, and may face some of the same mapping imprecisions and limited associative network processing. This would be an important area of investigation, to develop a more generalized model of naming that is applicable across all senses.
Higlights.
Naming common odors is astonishingly difficult, but mechanisms remain elusive
We review recent neuroimaging studies that provide mechanistic insight
Results suggest an odor-language neural system with three main processing stages
Poor odor naming is explained by a loss of signal fidelity across these stages
Acknowledgments
We thank Dr. Marsel Mesulam, Dr. Rob Hurley and Dr. Fredrik Jönsson for their input on the ideas outlined here, and Caitlin Hawley for proofreading the manuscript (www.cbhink.com). Dr. Olofsson’s work is supported by grants from the Swedish Research Council (421-2012-806) The Swedish Foundation for Humanities and Social Sciences (M14-0375:1) and a Pro Futura Scientia VII fellowship. Dr. Gottfried’s work is supported by grants from the National Institute on Deafness and Other Communication Disorders (1R01DC010014, 1R01DC013243, 1R01DC014426).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jonas K. Olofsson, Department of Psychology, Stockholm University, SE-10691 Stockholm, Sweden, and the Swedish Collegium for Advanced Study, SE-75238 Uppsala, Sweden.
Jay A. Gottfried, Department of Neurology, Northwestern University Feinberg School of Medicine, IL 60611, Chicago, USA.
References
- 1.Barsalou LW. Grounded cognition. Ann. Rev. Psychol. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- 2.Clark A. Language, embodiment, and the cognitive niche. Trends Cogn. Sci. 2006;10:370–374. doi: 10.1016/j.tics.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Levinson SC, Majid A. Differential ineffability and the senses. Mind Lang. 2014;29:407–427. [Google Scholar]
- 4.Plato, translator. Plato in Twelve Volumes. Vol. 9. Harvard University Press; London: William Heinemann Ltd.; 1925. Timaeus, section 67a. [Google Scholar]
- 5.Herz RS, Engen T. Odor memory: Review and analysis. Psychon. Bull. Rev. 1996;3:300–313. doi: 10.3758/BF03210754. [DOI] [PubMed] [Google Scholar]
- 6.Jönsson FU, Stevenson RJ. Odor knowledge, odor naming, and the "tip-of-the-nose" experience. In: Schwartz BL, Brown AS, editors. Tip-of-the-Tounge States and Related Phenomena. Cambridge University Press; 2014. [Google Scholar]
- 7.Lorig TS. On the similarity of odor and language perception. Neurosci. Biobehav. Rev. 1999;23:391–398. doi: 10.1016/s0149-7634(98)00041-4. [DOI] [PubMed] [Google Scholar]
- 8.Ahissar M, Hochstein S. The reverse hierarchy theory of visual perceptual learning. Trends Cogn. Sci. 2004;8:457–464. doi: 10.1016/j.tics.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Summerfield C, de Lange FP. Expectation in perceptual decision making: neural and computational mechanisms. Nat. Rev. Neurosci. 2014;15:745–756. doi: 10.1038/nrn3838. [DOI] [PubMed] [Google Scholar]
- 10.Catani M, et al. Perisylvian language networks of the human brain. Ann. Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- 11.Dick AS, Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135:3529–3550. doi: 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- 12.Friederici AD, Gierhan SM. The language network. Curr. Op. Neurobiol. 2013;23:250–254. doi: 10.1016/j.conb.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Cain WS. Know with the nose - keys to odor identification. Science. 1979;203:467–470. doi: 10.1126/science.760202. [DOI] [PubMed] [Google Scholar]
- 14.Engen T, Ross BM. Long-term memory of odors with and without verbal descriptors. J. Exp. Psychol. 1973;100:221–227. doi: 10.1037/h0035492. [DOI] [PubMed] [Google Scholar]
- 15.Cain WS, Krause RJ. Olfactory testing: rules for odor identification. Neurol. Res. 1979;1:1–9. doi: 10.1080/01616412.1979.11739536. [DOI] [PubMed] [Google Scholar]
- 16.de Wijk RA, Cain WS. Odor quality: discrimination versus free and cued identification. Perc. Psychophys. 1994;56:12–18. doi: 10.3758/bf03211686. [DOI] [PubMed] [Google Scholar]
- 17.Thomas-Danguin T, et al. The perception of odor objects in everyday life: a review on the processing of odor mixtures. Front. Psychol. 2014;5 doi: 10.3389/fpsyg.2014.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laing DG, Francis GW. The capacity of humans to identify odors in mixtures. Physiol. Beh. 1989;46:809–814. doi: 10.1016/0031-9384(89)90041-3. [DOI] [PubMed] [Google Scholar]
- 19.Livermore A, Laing DG. Influence of training and experience on the perception of multicomponent odor mixtures. J. Exp. Psychol. Hum. Perc. Perf. 1996;22:267–277. doi: 10.1037//0096-1523.22.2.267. [DOI] [PubMed] [Google Scholar]
- 20.Stevenson RJ, et al. Resistance to extinction of conditioned odor perceptions: evaluative conditioning is not unique. J. Exp. Psychol. Learn. Mem. Cogn. 2000;26:423–440. doi: 10.1037//0278-7393.26.2.423. [DOI] [PubMed] [Google Scholar]
- 21.Stevenson RJ, et al. Confusing tastes and smells: how odours can influence the perception of sweet and sour tastes. Chem. Sens. 1999;24:627–635. doi: 10.1093/chemse/24.6.627. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson RJ, Boakes RA. A mnemonic theory of odor perception. Psychol. Rev. 2003;110:340–364. doi: 10.1037/0033-295x.110.2.340. [DOI] [PubMed] [Google Scholar]
- 23.Wilson DA, Stevenson RJ. The fundamental role of memory in olfactory perception. Trends Neurosci. 2003;26:243–247. doi: 10.1016/S0166-2236(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 24.Yeshurun Y, Sobel N. An odor is not worth a thousand words: From multidimensional odors to unidimensional odor objects. Ann. Rev. Psychol. 2010;61:219–241. doi: 10.1146/annurev.psych.60.110707.163639. [DOI] [PubMed] [Google Scholar]
- 25.Schiffman SS. Physicochemical correlates of olfactory quality. Science. 1974;185:112–117. doi: 10.1126/science.185.4146.112. [DOI] [PubMed] [Google Scholar]
- 26.Zarzo M. Psychologic dimensions in the perception of everyday odors: Pleasantness and edibility. J. Sens. Stud. 2008;23:354–376. [Google Scholar]
- 27.Haddad R, et al. Global features of neural activity in the olfactory system form a parallel code that predicts olfactory behavior and perception. J. Neurosci. 2010;30:9017–9026. doi: 10.1523/JNEUROSCI.0398-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapid H, et al. Neural activity at the human olfactory epithelium reflects olfactory perception. Nat. Neurosci. 2011;14:1455–1461. doi: 10.1038/nn.2926. [DOI] [PubMed] [Google Scholar]
- 29.Olofsson JK, et al. A time-based account of the perception of odor objects and valences. Psychol. Sci. 2012;23:1224–1232. doi: 10.1177/0956797612441951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olofsson JK, et al. High and low roads to odor valence? A choice response-time study. J. Exp. Psychol. Hum. Perc. Perf. 2013;39:1205–1211. doi: 10.1037/a0033682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olofsson JK. Time to smell: a cascade model of human olfactory perception based on response-time (RT) measurement. Front. Psychol. 2014;5:33. doi: 10.3389/fpsyg.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawless H, Engen T. Associations to odors: interference, mnemonics, and verbal labeling. J. Exp. Psychol. Hum. Learn. Mem. 1977;3:52–59. [PubMed] [Google Scholar]
- 33.Jonsson FU, Olsson MJ. Olfactory metacognition. Chem. Sens. 2003;28:651–658. doi: 10.1093/chemse/bjg058. [DOI] [PubMed] [Google Scholar]
- 34.Jonsson FU, et al. A metamemory perspective on odor naming and identification. Chem. Sens. 2005;30:353–365. doi: 10.1093/chemse/bji030. [DOI] [PubMed] [Google Scholar]
- 35.Cain WS, et al. Odor identification: perceptual and semantic dimensions. Chem. Sens. 1998;23:309–326. doi: 10.1093/chemse/23.3.309. [DOI] [PubMed] [Google Scholar]
- 36.Binder JR, et al. Where is the semantic system? A critical review and metaanalysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vigneau M, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Chao LL, et al. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat. Neurosci. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- 39.Gorno-Tempini ML, et al. The neural systems sustaining face and propername processing. Brain. 1998;121:2103–2118. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- 40.Vandenberghe R, et al. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- 41.DeLeon J, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130:1408–1422. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- 42.Gorno-Tempini ML, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann. Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogalski E, et al. Anatomy of language impairments in primary progressive aphasia. J. Neurosci. 2011;31:3344–3350. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Moore CJ, Price CJ. Three distinct ventral occipitotemporal regions for reading and object naming. NeuroImage. 1999;10:181–192. doi: 10.1006/nimg.1999.0450. [DOI] [PubMed] [Google Scholar]
- 46.Mesulam MM, et al. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain. 2013;136:601–618. doi: 10.1093/brain/aws336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patterson K, et al. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- 48.Gottfried JA. Central mechanisms of odour object perception. Nat. Rev. Neurosci. 2010;11:628–641. doi: 10.1038/nrn2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gottfried JA, et al. Dissociable codes of odor quality and odorant structure in human piriform cortex. Neuron. 2006;49:467–479. doi: 10.1016/j.neuron.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Howard JD, et al. Odor quality coding and categorization in human posterior piriform cortex. Nat. Neurosci. 2009;12:932–938. doi: 10.1038/nn.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howard JD, Gottfried JA. Configural and elemental coding of natural odor mixture components in the human brain. Neuron. 2014;84:857–869. doi: 10.1016/j.neuron.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez J, et al. Reading cinnamon activates olfactory brain regions. NeuroImage. 2006;32:906–912. doi: 10.1016/j.neuroimage.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 53.Zelano C, et al. Olfactory predictive codes and stimulus templates in piriform cortex. Neuron. 2011;72:178–187. doi: 10.1016/j.neuron.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haberly LB. Parallel-distributed processing in olfactory cortex: New insights from morphological and physiological analysis of neuronal circuitry. Chem. Sens. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- 55.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Ann. Rev. Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka K. Inferotemporal cortex and object vision. Ann. Rev. Neurosci. 1996;19:109–139. doi: 10.1146/annurev.ne.19.030196.000545. [DOI] [PubMed] [Google Scholar]
- 57.Vanessen DC, et al. Information-processing in the primate visual-system - an integrated systems perspective. Science. 1992;255:419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- 58.Distler C, et al. Cortical Connections of Inferior Temporal Area Teo in Macaque Monkeys. J. Comp. Neurol. 1993;334:125–150. doi: 10.1002/cne.903340111. [DOI] [PubMed] [Google Scholar]
- 59.Saygin ZM, et al. Anatomical connectivity patterns predict face selectivity in the fusiform gyrus. Nat. Neurosci. 2012;15:321–327. doi: 10.1038/nn.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson DMG, et al. New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that "primary" olfactory cortex functions like "association" cortex in other sensory systems. J. Neurosci. 2000;20:6974–6982. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olofsson JK, et al. A designated odor-language integration system in the human brain. J. Neurosci. 2014;34:14864–14873. doi: 10.1523/JNEUROSCI.2247-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bensafi M, et al. The effect of verbal context on olfactory neural responses. Hum. Brain Mapp. 2014;35:810–818. doi: 10.1002/hbm.22215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gottfried JA, Dolan RJ. The nose smells what the eye sees: crossmodal visual facilitation of human olfactory perception. Neuron. 2003;39:375–386. doi: 10.1016/s0896-6273(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 64.Gottfried JA, et al. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 65.Jones-Gotman M, Zatorre RJ. Olfactory identification deficits in patients with focal cerebral excision. Neuropsychologia. 1988;26:387–400. doi: 10.1016/0028-3932(88)90093-0. [DOI] [PubMed] [Google Scholar]
- 66.Potter H, Butters N. An assessment of olfactory deficits in patients with damage to prefrontal cortex. Neuropsychologia. 1980;18:621–628. doi: 10.1016/0028-3932(80)90101-3. [DOI] [PubMed] [Google Scholar]
- 67.Royet JP, Koenig O, Gregoire MC, Cinotti L, Lavenne F, Le Bars D, Froment JC. Functional anatomy of perceptual and semantic processing for odors. J. Neurosci. 1999;11:94–109. doi: 10.1162/089892999563166. [DOI] [PubMed] [Google Scholar]
- 68.Small DM, et al. Flavor processing: more than the sum of its parts. Neuroreport. 1997;8:3913–3917. doi: 10.1097/00001756-199712220-00014. [DOI] [PubMed] [Google Scholar]
- 69.Zatorre RJ, Jones-Gotman M. Human olfactory discrimination after unilateral frontal or temporal lobectomy. Brain. 1991;114:71–84. [PubMed] [Google Scholar]
- 70.Gorno-Tempini ML, et al. Classification of primary progressive aphasia and its variants. Neurol. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mesulam MM. Primary progressive aphasia. Ann. Neurol. 2001;49:425–432. [PubMed] [Google Scholar]
- 72.Olofsson JK, et al. A cortical pathway to olfactory naming: evidence from primary progressive aphasia. Brain. 2013;136:1245–1259. doi: 10.1093/brain/awt019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan D, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease. Ann. Neurol. 2001;49:433–442. [PubMed] [Google Scholar]
- 74.Rossell SL, et al. The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia. 2003;41:550–564. doi: 10.1016/s0028-3932(02)00181-1. [DOI] [PubMed] [Google Scholar]
- 75.Moran MA, et al. Neural Inputs into the Temporopolar Cortex of the Rhesus-Monkey. J. Comp. Neurol. 1987;256:88–103. doi: 10.1002/cne.902560108. [DOI] [PubMed] [Google Scholar]
- 76.Pelli DG, Tillman KA. The uncrowded window of object recognition. Nat. Neurosci. 2008;11:1129–1135. doi: 10.1038/nn.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lamme VAF, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- 78.Pessoa L, Adolphs R. Emotion processing and the amygdala: from a 'low road' to 'many roads' of evaluating biological significance. Nat. Rev. Neurosci. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smeets MA, Dijksterhuis GB. Smelly primes – when olfactory primes do or do not work. Front. Psychol. 2014;5:96. doi: 10.3389/fpsyg.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Savic I, Berglund H. Passive perception of odors and semantic circuits. Hum. Brain Mapp. 2004;21:271–278. doi: 10.1002/hbm.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zelano C, et al. A specialized odor memory buffer in primary olfactory cortex. PloS one. 2009;4:e4965. doi: 10.1371/journal.pone.0004965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rabin MD. Experience facilitates olfactory quality discrimination. Perc. Psychophys. 1988;44:532–540. doi: 10.3758/bf03207487. [DOI] [PubMed] [Google Scholar]
- 83.Majid A, Burenhult N. Odors are expressible in language, as long as you speak the right language. Cognition. 2014;130:266–270. doi: 10.1016/j.cognition.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 84.Royet JP, et al. The impact of expertise in olfaction. Front. Psychol. 2013;4:928. doi: 10.3389/fpsyg.2013.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delon-Martin C, et al. Perfumers' expertise induces structural reorganization in olfactory brain regions. NeuroImage. 2013;68:55–62. doi: 10.1016/j.neuroimage.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 86.Plailly J, et al. Experience induces functional reorganization in brain regions involved in odor imagery in perfumers. Hum. Brain Mapp. 2012;33:224–234. doi: 10.1002/hbm.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zatorre RJ, et al. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wnuk E, Majid A. Revisiting the limits of language: the odor lexicon of Maniq. Cognition. 2014;131:125–138. doi: 10.1016/j.cognition.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 89.Khan RM, et al. Predicting odor pleasantness from odorant structure: pleasantness as a reflection of the physical world. J. Neurosci. 2007;27:10015–10023. doi: 10.1523/JNEUROSCI.1158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doty RL, et al. Development of the 12-item cross-cultural smell identification test (CC-SIT) Laryngosc. 1996;106:353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 91.Kriegeskorte N, Bandettini P. Analyzing for information, not activation, to exploit high-resolution fMRI. NeuroImage. 2007;38:649–662. doi: 10.1016/j.neuroimage.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miniussi C, et al. Modelling non-invasive brain stimulation in cognitive neuroscience. Neurosci. Biobehav. Rev. 2013;37:1702–1712. doi: 10.1016/j.neubiorev.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 93.Koster EP, et al. A "Misfit" Theory of Spontaneous Conscious Odor Perception (MITSCOP): reflections on the role and function of odor memory in everyday life. Front. Psychol. 2014;5:64. doi: 10.3389/fpsyg.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Plailly J, et al. Attention to odor modulates thalamocortical connectivity in the human brain. J. Neurosci. 2008;28:5257–5267. doi: 10.1523/JNEUROSCI.5607-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sela L, et al. Spared and Impaired Olfactory Abilities after Thalamic Lesions. J. Neurosci. 2009;29:12059–12069. doi: 10.1523/JNEUROSCI.2114-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tham WW, et al. The impact of mediodorsal thalamic lesions on olfactory attention and flavor perception. Brain Cogn. 2011;77:71–79. doi: 10.1016/j.bandc.2011.05.008. [DOI] [PubMed] [Google Scholar]