Abstract
In a recent study, we demonstrated that sleep-dependent consolidation of declarative memories is preserved in older adults. The present study examined whether this benefit of sleep for declarative learning in older adults reflects a passive role of sleep in protecting memories from decay or an active role in stabilizing them. Young and older adults learned a visuo-spatial task and recall was probed following sleep or wake. Although a reduction in performance was observed following sleep and wake, task-related interference prior to recall had a larger detriment on performance in the wake condition. This was true for young and high performing older adults only. Low performing older adults did not receive a benefit of sleep on the visuo-spatial task. Performance changes were associated with early night NREM sleep in young adults and with early night REM sleep in high performing older adults. These results demonstrate that performance benefits from sleep in older adults as a result of an active memory stabilization process; importantly, the extent of this benefit of sleep is closely linked to the level of initial acquisition of the episodic information in older adults.
Keywords: Sleep, Memory, Declarative, Consolidation, Aging, Interference
1. Introduction
The ancient Greek philosopher, Heraclitus, once said, “Even a soul submerged in sleep is hard at work and helps make something of the world” (Haxton, 2001). Indeed, the last two decades have produced a number of studies that provide evidence supporting this notion. For instance in young adults, recall of declarative memories is greater following sleep compared to equivalent intervals of wake (Rasch et al., 2007; Wilson et al., 2012).
Sleep’s benefit on memory may be passive or the result of an active memory process (Ellenbogen, Payne & Stickgold, 2006). By the passive account, newly encoded information is benefited by being undisturbed by interference from waking activities while asleep. The active account posits that memories are stabilized through continued processing such as hippocampal reactivation of memory traces. Support for an active role of sleep in memory consolidation in young adults is based on two lines of evidence. First, the amount of memory protection over sleep correlates with specific measures of sleep physiology and not merely total sleep time. In other words, if sleep’s role in memory was through passive protection from waking interference, more time spent asleep should yield greater memory benefits. Such is not the case. Rather, performance benefits are associated with early night sleep physiology (Plihal and Born, 1997), particularly slow-wave sleep (SWS) and the EEG spectral power of the slow waves, or slow-wave activity (SWA) associated with it (Peigneux et al., 2004; Marshall et al., 2006). In addition, the arrangement of non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep stages in a cyclical, organized fashion across sleep bouts has been implicated in memory processing (Giuditta, 1995; Diekelmann and Born, 2010; Spencer, 2013). For instance, Ficca and colleagues (2000) found greater post-sleep recall when NREM-REM sleep cycles were uninterrupted by wake, compared to when cycles were disrupted or disorganized. Moreover, using multichannel recordings in rodent hippocampus, Grosmark and colleagues (2012) demonstrated that the firing rates of hippocampal CA1 neurons increased during NREM sleep, while interleaving REM bouts served to not only decrease firing rates, but also increased synchrony of neuronal firing. Thus, it has become increasingly apparent that NREM and REM sleep exert sequential effects in the process of memory consolidation.
A second line of evidence for the active role of sleep in memory consolidation in young adults is that memories are less susceptible to interference following sleep, as would be expected if they were consolidated into long-term storage. Ellenbogen and colleagues (2006) used an interference paradigm to demonstrate this effect in young adults. Participants were trained on a word-pair learning task using A-B word pairs. After a period of 12 hours, consisting of either daytime wake or overnight sleep, participants were trained on interfering A-C word pairs. When memory for the original A-B word pairs was subsequently tested, performance of the group that slept in between sessions was significantly superior to performance of the group that stayed awake (see also Diekelmann et al., 2011; Alger et al., 2012). These results suggest that, in young adults, memories are actively stabilized over an interval of sleep, leaving them more resistant to interference.
However sleep, much like other physiological processes, undergoes radical changes with age (Neikrug & Ancoli-Israel, 2010). Of these, the most notable is the increase in nighttime awakenings and the consequent increase in sleep fragmentation (Bliwise, 1993). Some studies have also reported a reduction in slow-wave sleep (SWS) quantity, quality and distribution across sleep (Cajochen et al., 2006; Carrier et al., 2001; Lombardo et al., 1998), as well as a reduction in rapid-eye movement (REM) density (the number of REMs per minute; Darchia et al., 2003). Concurrent with these alterations in sleep is a general decline in episodic memory with age, particularly after the age of 60 yrs (Ronnlund et al., 2005). Young adults also demonstrate steeper learning curves compared to older adults with respect to declarative learning tasks (Vakil and Agmon-Ashkenazi, 1997; Davis et al., 2003). Consequently, one would expect a reduction in the processing of declarative memories over sleep with age. Indeed, studies of middle-age (Backhaus et al., 2007) and older adults (Cherdieu et al., 2013; Mander et al., 2013; Scullin, 2013) suggest reduced sleep-dependent consolidation with age. However, in contrast with these findings, and despite age-related changes in sleep, sleep has been shown to have a benefit on autobiographical memory (Aly and Moscovitch, 2010) and word-pair learning (Wilson et al., 2012). Notably, in these studies, declarative memory performance post-sleep was nonetheless reduced (not protected or enhanced) relative to pre-sleep performance, but this reduction in performance was to a lesser extent than that observed following wake. As such, the performance advantage following sleep may be the result of a passive protection of memories due to reduced interference from ongoing waking activities, as opposed to an active role of sleep in stabilizing the memories (see Mednick et al., 2011).
To address whether sleep plays an active role in memory processing, in the present study we compared post-wake and post-sleep stability of visuo-spatial memory using an Interference paradigm (see Diekelmann et al., 2011; Ellenbogen et al., 2006; Alger et al., 2012) in young and older adults. Stemming from previous work, we hypothesized that performance of young adults on a visuo-spatial memory task would benefit from a 12-hr interval of sleep, such that memories would be rendered more resistant to retroactive interference. Moreover, owing to the relationship between SWS-rich early night sleep and declarative memory consolidation (Ekstrand et al., 1977; Peigneux et al., 2004; Plihal & Born, 1999), we hypothesized the benefit of sleep on visuo-spatial memory would be driven by time spent in SWS early in the night, as well as the EEG spectral power of slow-wave activity (SWA) during this time. Additionally, stemming from the finding that changes in firing rates and in the synchrony of neuronal firing over NREM sleep may be attributed to interleaving REM sleep (Grosmark et al., 2012), we examined the role of the first NREM-REM-NREM triplet in the night in performance changes over sleep. We predicted that the sequential effects of NREM and REM sleep across this triplet would be crucial for active visuo-spatial memory consolidation. Finally, and of particular interest, was whether sleep similarly protected memories from interference in older adults. Based on previous studies demonstrating preserved over-sleep declarative memory consolidation in older adults (Aly and Moscovitch, 2010; Wilson et al., 2012), we hypothesized that active visuo-spatial memory consolidation would persist in older adults, but perhaps by means of altered sleep-dependent mechanisms owing to age-related changes in sleep physiology, namely a reduction in SWS and disruption of NREM-REM sleep cycles.
2. Methods
2.1 Participants
Participants were 128 healthy young adults (18-30 yrs) and 91 healthy older adults (50-79 yrs) who received payment or course credit for their time. Individuals were excluded if they had been diagnosed with a neurological disease, congestive heart failure, or a myocardial infarction, or had a history of stroke, head trauma, or heart surgery. Additionally, participants were excluded if they used sleep-affecting medications or if they habitually slept less than 5 hrs or more than 11 hrs per day. We confirmed that participants had unimpaired or corrected-to-normal vision (20/30 or less) using a standard vision chart. To assess cognitive function in older adults, we administered the Mini-Mental State Exam (MMSE; Rovner and Folstein, 1987) and the National Adult Reading Test (NART; Nelson, 1991). A minimum score of 27 out of 30 on the MMSE and 70% on the NART was required for inclusion.
Within each age group (Young and Older adults), participants were assigned to either a Wake or Sleep group based on joint availability for both the participant and experimenters. Further assignment of individuals in each Wake and Sleep group to “No Interference” and “Interference” conditions (see section 2.2. below for details), was made by alternating assignment to these conditions throughout the data collection phase.
2.2 Visuo-Spatial Task
The task was a visuo-spatial learning task similar to the game Memory (also known as Concentration; Fig. 1a) adapted from Kurdziel and colleagues (2013). There were four phases: preview, encoding, immediate recall, and delayed recall. In the preview phase, 20 images representing common nouns (e.g., “nurse,” “dog,” or “cherries”) arranged in a 5×4 matrix, were presented on a computer screen. The preview was presented for 30 s for young adults and 60 s for older adults. The extended preview in older adults was done in an effort to equate initial learning, since it has previously been demonstrated that older adults display more gradual learning curves as compared to young adults in declarative learning tasks (Vakil and Agmon-Ashkenazi, 1997; Davis et al., 2003). In the encoding phase, the images were virtually “flipped over” and a single image was displayed on the right side of the screen and participants were asked to click on the location within the matrix where the matching image was located. The item in the chosen location was subsequently presented for 1 s (i.e., feedback). After all items in the matrix had been tested, if accuracy was < 15%, the preview of the image matrix was presented again. The encoding phase continued until participants reached a criterion of 65% correct or until the full set of images had been probed 10 times (as in: Wilson et al., 2012; Donohue & Spencer, 2011; Plihal & Born, 1997). In the immediate recall phase, each image location was tested just once and no feedback was provided (to prevent further encoding).
Figure 1. Experimental design.
a) The visuo-spatial task. b) Experimental procedures for the Wake and Sleep groups.
In the Interference condition, an additional preview and encoding phase took place. Items in the matrix contained the same images used during the initial encoding phase but were in new locations. Interference encoding continued until accuracy was 65% or until all items had been probed 4 times (this was lower than session one in order to avoid substantial levels of interference, resulting in a floor effect with respect to memory for the original image locations). Following encoding of the new matrix, participants were shown a movie (“Planet Earth”) in order to prevent active rehearsal of the image locations and to equate activities across participants. In the No Interference condition, no additional encoding occurred; however, in order to equate time spent in the lab, they watched the movie (“Planet Earth”) for 30 minutes. Finally, the delayed recall phase was identical to the immediate recall phase.
2.3 Sleep Assessments
Participants were queried for average sleep time using the Pittsburgh Sleep Quality Index (PSQI), a questionnaire used to determine an individual’s sleep quality over the previous 30 days (Buysse et al., 1989). An abbreviated Waketime Diary was given to assess subjective sleep quantity and quality during the preceding night, while an abbreviated Sleeptime Diary was given to assess daytime activities including napping and caffeine intake. Participants also completed the Stanford Sleepiness Scale (SSS; Hoddes et al., 1973) to assess differences in subjective sleepiness across groups and conditions.
2.4 Procedures
Procedures were approved by the University of Massachusetts, Amherst Institutional Review Board. Written informed consent was obtained before the experiment commenced. Participants within each age group were assigned to either the Wake group or the Sleep group and to either the No Interference or the Interference condition, resulting in a total of 8 experimental groups (Table 1).
Table 1.
Sample descriptive statistics
| Descriptive | Young Adults
|
p-value | Older Adults
|
p-value | ||
|---|---|---|---|---|---|---|
| Wake | Sleep | Wake | Sleep | |||
| No Interference Condition | ||||||
| N | 20 | 38 | 11 | 25 | ||
| Mean Age (SD) | 21.52 (2.50) | 20.66 (2.37) | 0.182 | 65.54 (7.56) | 63.17 (7.44) | 0.363 |
| Sex | 11M, 9F | 19M, 19F | 0.973 | 4M, 7F | 8M, 17F | 0.878 |
| Handedness | 1L,19R | 38R | 0.402 | 11R | 25R | 0.922 |
| Interference Condition | ||||||
| N | 23 | 30 | 16 | 23 | ||
| Mean Age (SD) | 21.81(3.22) | 21.29(2.61) | 0.506 | 67.29(7.27) | 66.26(6.93) | 0.671 |
| Sex | 8M, 15F | 14M, 16F | 0.667 | 4M, 12F | 6M, 17F | 0.54 |
| Handedness | 23R | 2L,28R | 0.963 | 16R | 23R | 0.229 |
| Conditions Combined | ||||||
| PSQI | 3.91 (1.80) | 4.27 (1.91) | 0.327 | 4.15 (1.63) | 3.34 (1.74) | 0.053 |
| SSS1 | 2.86 (1.10) | 3.03 (1.28) | 0.474 | 2.03 (0.98) | 2.57 (1.16) | 0.046 |
| SSS2 | 2.67 (1.41) | 2.66 (1.46) | 0.964 | 2.67 (1.00) | 2.02 (1.05) | 0.012 |
| # Loops at Encoding Immediate | 2.42 (1.12) | 2.24 (1.5) | 0.387 | 4.43 (3.1) | 3.27 (2.63) | 0.372 |
| Recall Accuracy | 0.72 (0.12) | 0.70 (0.13) | 0.387 | 0.65 (0.12) | 0.65 (0.12) | 0.925 |
Handedness, Left-handed = L, Right-handed = R; Sex, Male = M, Female = F. Also shown here are mean questionnaire scores across experimental groups (higher questionnaire scores indicate poorer outcomes): Pittsburgh Sleep Quality Index (PSQI), Stanford Sleepiness Scale during session one (SSS1) and session two (SSS2), as well as differences in baseline performance. Values in parentheses represent standard deviations. p-values are provided for Wake vs. Sleep comparisons within each age group.
Participants in all groups took part in two sessions (Fig. 1b). Session one and two were separated by a 12-hr interval. Session one took place between 8-10 AM for those assigned to the Wake group, with session two occurring 12 hrs later, between 8-10 PM, following an interval spent fully awake. Participants were instructed not to nap or consume alcohol during this time. The Sleep group performed session one between 8-10 PM and session two 12 hrs later between 8-10 AM the following morning after an interval consisting of overnight sleep.
Following informed consent procedures, session one began with the preview phase, which was immediately followed by the encoding phase. Subsequently, during a 20 min interval, participants completed the PSQI, Sleeptime Diary (Sleep groups) or the Waketime Diary (Wake groups) and the SSS (SSS1). This interval was followed by the immediate recall phase of the visuo-spatial learning task. At the start of session two, 12 hrs later, the SSS (SSS2) and Waketime Diary (Sleep groups) or the Sleeptime Diary (Wake groups) were completed. Participants in the Interference conditions then encoded the additional matrix. After participants viewed a movie (“Planet Earth”) for 30 mins, the delayed recall phase occurred.
2.5 Polysomnography
For a subset of the participants that were assigned to the Sleep groups (Young adults, No Interference N=17, Interference N = 15; Older adults, No Interference N=14, Interference N = 11), polysomnography (PSG) was recorded in the overnight interval using the Aura PSG wireless/ambulatory system (Grass Technologies, Astro-Med Inc., West Warwick, RI). Two hours prior to the participant’s habitual bedtime, the PSG montage was applied in the participant’s residence. The montage included seven EEG leads (O1, O2, C3, C4, F1, F2, Cz), two EOG leads (one on the side of each eye), two chin EMG leads, two mastoid electrodes and one ground electrode on the forehead.
2.6 Data Analyses
PSG data was analyzed according to the revised American Academy of Sleep Medicine manual (Iber et al., 2007), and all records were scored for NREM-REM sleep cycles as per the criteria provided by Griessenberger and colleagues (2012). In short, a NREM-REM cycle was defined as a period of continuous NREM sleep, followed by REM sleep (without interruption by a wake bout > 2 mins) for a minimum duration of 30 mins.
EEG spectral power analyses were conducted using BrainVision Analyzer 2.0 software (Brain Products, Munich, Germany). Raw data was subjected to segmentation such that only the SWS bouts were selected. Segmented data was then filtered for frequencies between 0.3 and 35 Hz, followed by semi-automatic raw data inspection for large artifacts, such as arousals, motion artifacts and transient electrical interference that render epochs unscorable: artifacts were automatically detected by the software, but were subsequently confirmed or rejected by visual inspection. Inspected data was then segmented into 4 s bins and subjected to semi-automatic artifact rejection for the detection of more minute frequency and amplitude fluctuations that may have been missed during the raw data inspection. Spectral power density (μV2/Hz) in the delta frequency range (0.5 – 4 Hz) was calculated over the central and frontal electrodes using Fast-Fourier transform analysis with a 10% Hanning window with no overlap (Marshall et al., 2006).
To compare group differences in questionnaire measures and baseline performance on the task, Age Group (young vs. Older) by Interval Type (Wake vs. Sleep) ANOVAs were conducted. To compare sleep physiology measures, independent samples t-tests were used. For all t- tests, if the Levene’s test for homogeneity was found to be significant, the adjusted t-statistics and p- values are reported. In the case where significant differences between groups (Young vs. Older, Wake vs. Sleep) were detected in sleepiness in session one (SSS1) or session two (SSS2), the questionnaire scores were used as covariates in the behavioral comparisons between experimental groups.
In order to compare post-wake and post-sleep performance, we used accuracy at delayed recall as the dependent variable in a three-way Interval Type (Wake vs. Sleep) by Age Group (Young vs. Older) by Condition (No Interference vs. Interference) between-subjects ANCOVA with accuracy at immediate recall as a covariate (to control for baseline differences in performance). For this analysis, we used a Sidak-Bonferroni correction to correct for multiple comparisons. Based on the outcome of this three-way ANCOVA, we performed post-hoc analyses to further illuminate Wake and Sleep differences in performance across conditions and age groups: a one-way Wake vs. Sleep ANOVA within each condition (No Interference and Interference) in young adults, and likewise in older adults. To explore whether the level of interference impacted accuracy at delayed recall, we examined the relationship between accuracy of recall in the interference round and accuracy at delayed recall for young and older adults, using separate Pearson correlations for each age group.
To explore the relationship between sleep parameters and performance changes over sleep, Pearson correlations were computed between each sleep parameter and the change in accuracy from immediate recall to delayed recall, calculated as a Difference Score (Delayed Recall Accuracy – Immediate Recall Accuracy). All correlational analyses conducted within the older adult age group were controlled for age (age was added as a covariate), owing to progressive changes in sleep structure and physiology in this broad age range. The specific sleep parameters that we were interested in were SWS, REM sleep and NREM sleep (a combination of NREM Stage 2 and SWS). We explored the relationship between performance changes over sleep and total time spent in each of these stages across the night. In addition, we contrasted the relationship between performance changes and early vs. late night sleep (i.e. first half vs. second half of the night).
Finally, to test whether the interaction between NREM in the first sleep cycle (NREMSC1), REM in the first sleep cycle (REMSC1) and NREM in the second sleep cycle (NREMSC2), across the first NREM-REM-NREM triplet of the night impacted over-sleep changes in performance, a multiple regression analysis was conducted that included the interaction terms associated with these variables (for triplet analysis, see Grosmark et al., 2012). This methodological approach was consistent with previous studies (Mednick et al., 2003; McDevitt et al., 2015; Stickgold et al., 200). The following variables were centered around their average values and entered into the multiple regression: NREMSC1, REMSC1, NREMSC2, and the interaction terms (NREMSC1 × REMSC1, REMSC1 × NREMSC2, NREMSC1 × NREMSC2, and NREMSC1 × REMSC1× NREMSC2). The Difference Score was used as the dependent variable for this regression analysis.
3. Results
3.1 Group Characteristics
Table 1 provides descriptive statistics for all groups. Young adults were excluded for taking a nap in between sessions (n=8) and for having a PSQI score > 7 indicating significant sleep disturbances (n=9). We used Waketime Diary data to screen for individuals that slept < 5 hrs or > 11 hrs the previous night (for the Wake group); however, there were no such individuals in our sample. Average sleep duration prior to the experiment was 6.99 hrs (SD = 1.17). Therefore, final analyses are based on 111 young adult participants.
Older adults were excluded for taking a nap in between sessions (n=4) and for having a PSQI score > 7 (n=12). Notably, all older adult participants scored >27 out of a possible 30 on the MMSE and >70% on the NART, suggesting they were free from significant cognitive deficits. No individuals failed to meet the required sleep of < 5 hrs or > 11 hrs the previous night (for the Wake group). Average sleep duration prior to the experiment for the older adults was 6.85 hrs (SD = 1.26). Therefore, final analyses are based on 75 older adult participants. Due to a sampling bias, wherein a greater number of female older adults responded to our advertisements, we had fewer male older adult participants in our sample than female (Males, N = 22; Females, N = 53).
Table 1 also provides mean scores for young and older adults for each questionnaire measure. Note that PSQI and SSS scores were unavailable for one older adult leaving 74 older adults for these measures. With regard to habitual sleep quality as measured by the PSQI, the Age Group (Young vs. Older) by Interval Type (Wake vs. Sleep) ANOVA did not reveal a main effect of Age Group (F(1,185) = 1.495, p = 0.223) or Interval Type (F(1,185) = 0.649, p = 0.422). However, there was a significant Age Group × Interval Type interaction (F(1,185) = 4.352, p = 0.038). For sleepiness in session one (SSS1), we found a main effect of Age Group (F(1,185) = 12.512, p = 0.001), a main effect of Interval Type (trend-level; F(1,185) = 3.820, p = 0.052), but no Age Group × Interval Type interaction (F(1,185) = 1.040, p = 0.309). For sleepiness in session two (SSS2), we did not find a main effect of Age Group (F(1,185) = 2.602, p = 0.108), Interval Type (F(1,185) = 2.681, p = 0.103), nor an Age Group × Interval Type interaction (F(1,185) = 2.479, p = 0.117). We conducted post-hoc planned comparisons with each age group (Young and Older) in order to look at the effect of Interval Type (Wake vs. Sleep) on each measure, the results of which are displayed in Table 1.
Due to observed group differences with relation to sleepiness at the time of testing (specifically in session one), all behavioral comparisons between experimental groups (Young vs. Older, Wake vs. Sleep) included SSS1 and SSS2 scores as covariates; this was done in order to ensure that any observed differences in behavior between groups were a result of differential consolidation processes, rather than the level of alertness at the time of testing.
3.2 Performance on the Visuo-Spatial Task
3.2.1 Baseline Differences in Visuo-Spatial Learning
Table 1 provides mean values for young and older adults for baseline performance, specifically the number of exposures to the items during the encoding phase (maximum of 10) required to reach criterion (65%) as well as accuracy at immediate recall. Due to a computer error, we do not have data on the number of exposures to the items during the encoding phase for individuals assigned to the Interference condition, for whom the number of loops required to reach criterion in the Interference round alone was recorded. Therefore, comparisons involving this measure are from individuals in the No Interference condition alone.
In spite of extended time in the preview phase, the Age Group (Young vs. Older) by Interval Type (Wake vs. Sleep) ANOVA revealed a main effect of Age Group (F(1,76) = 8.189, p = 0.006) on the number of loops required to reach criterion. However, there was no main effect of Interval Type (F(1,76) = 1.822, p = 0.181), or Age Group × Interval Type interaction (F(1,76) = 1.462, p = 0.231). Likewise, with respect to accuracy at immediate recall, we found a significant main effect of Age Group (F(1,186) = 8.023, p = 0.005), but no main effect of Interval Type (F(1,186) = 0.246, p = 0.620), or Age Group × Interval Type interaction (F(1,186) = 0.404, p = 0.526).
3.2.2 Change in Performance over Wake and Sleep Intervals
The three-way Interval Type × Age Group × Condition ANCOVA revealed a significant main effect of Interval Type (F(1,171) = 14.878, p < 0.001), Age Group (F(1,171) = 5.549, p = 0.020) and Condition (F(1,171) = 216.115, p < 0.001) on delayed recall. However, neither the Interval Type × Age Group (F(1,171) = 0.525, p = 0.470), Age Group × Condition (F(1,171) = 0.576, p = 0.449) interactions, nor the three-way Interval Type × Age Group × Condition interaction (F(1,171) = 1.363, p = 0.245) were significant. We did find a significant Interval Type × Condition interaction (F(1,171) = 4.744, p = 0.031); however, the confidence intervals for this effect included 0, and thus was not considered a true significant effect. Table 2 provides the parameter estimates and confidence intervals for each effect.
Table 2.
Parameter estimates and confidence intervals for main effects and interactions.
| Variable | β | SE | p-value | 95% CI |
|---|---|---|---|---|
| Intercept | -0.033 | 0.059 | 0.574 | (-0.149, 0.083) |
| Immediate Recall | 0.715 | 0.071 | <0.001 | (0.575, 0.855) |
| SSS1 | -0.011 | 0.008 | 0.156 | (-0.026, 0.004) |
| SSS2 | -0.018 | 0.007 | 0.01 | (-0.032, 0.004) |
| Age Group (Young, Older) | 0.093 | 0.032 | 0.004 | (0.030, 0.156) |
| Interval Type (Wake, Sleep) | -0.075 | 0.037 | 0.044 | (-0.148, -0.002) |
| Condition (No Interference, Interference) | 0.259 | 0.033 | <0.001 | (0.193, 0.325) |
| Age Group × Interval Type | -0.69 | 0.049 | 0.165 | (-0.166, 0.029) |
| Age Group × Condition | -0.07 | 0.045 | 0.121 | (-0.158, 0.019) |
| Interval Type × Condition | 0.036 | 0.056 | 0.524 | (-0.075, 0.146) |
| Age Group × Interval Type × Condition | 0.084 | 0.072 | 0.245 | (0.058, 0.227) |
In order to further examine differences between the Wake and Sleep groups within each condition (No Interference/ Interference), we performed post-hoc ANOVAs in young adults with delayed recall as the dependent variable and immediate recall as a covariate. In the No Interference condition, we found no difference between the Wake and Sleep groups with respect to delayed recall (F(1,55) = 0.443, p = 0.508; Fig. 2a). However in the Interference condition, the Sleep group significantly outperformed the Wake group (F(1,47) = 14.776, p < 0.001; Fig. 2a). Likewise, for older adults, the post-hoc ANCOVAs (using SSS1 and SSS2 scores as a covariate due to Wake vs. Sleep differences in sleepiness) revealed no significant difference between groups in the No Interference condition (F(1,29) = 1.396, p = 0.447; Fig. 2b), while a benefit of sleep was observed in the Interference condition at trend-level (F(1,) = 3.446, p =0.072; Fig. 2b).
Figure 2. Effects of a wake vs. sleep on visuo-spatial memory consolidation.
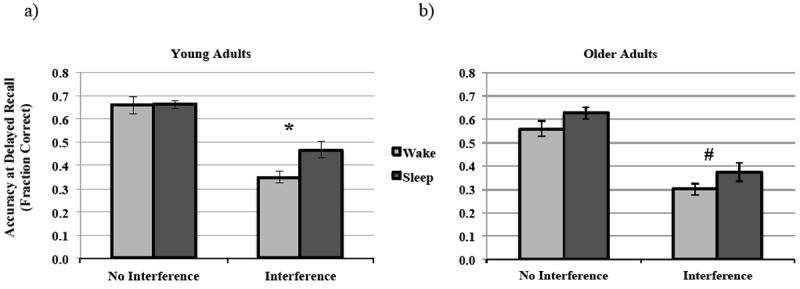
Comparison of accuracy at Delayed Recall in the No Interference and Interference conditions for a) Young adults, and b) Older adults. * p-value < 0.05.
In order to test whether this observed benefit of sleep in older adults in the Interference condition was reduced relative to young adults, we performed a post-hoc two-way Interval Type (Wake vs. Sleep) by Age Group (Young vs. Older) between-subjects ANCOVA (with immediate recall accuracy, SSS1 and SSS2 scores as covariates). Although we found a significant main effect of Interval Type (F(1,83) = 14.935, p < 0.001) and Age Group (F(1,83) = 4.961, p = 0.029) on delayed recall, we did not find a significant Interval Type × Age Group interaction (F(1,83) = 0.979, p = 0.325).
With regard to the Interference round, there was no difference between young and older adults with respect to the number of rounds required to reach criterion (t(58) = -1.072, p = 0.288). However, accuracy during the interference round (fraction correct) was significantly poorer for older adults (M = 0.64, SD = 0.129) compared to young adults (M = 0.71, SD = 0.11; t(84) = 2.538, p = 0.013). For young adults, accuracy in the Interference round was not correlated with performance at delayed recall (Pearson r = -0.107, p = 0.473); however, for older adults, greater accuracy during the interference round was associated with significantly better performance at delayed recall (Pearson r = 0.339, p = 0.035).
3.2.3 Over-Sleep Changes in Low vs. High Performance Older Adult Groups
To further examine whether the level of initial acquisition of the visuo-spatial material affected the extent of memory consolidation over sleep, we performed a median split analysis based on accuracy at immediate recall within the older adult group resulting in two groups: Low Performers (N=31) and High Performers (N=44). Immediate recall in high performing older adults was not significantly different from the young adults (t(132.565) = -1.673, p = 0.10). Although the Low Performers did not differ from the High Performers with respect to age (t(73) = 0.083, p = 0.934), they required greater task exposure to reach criterion at encoding (trend-level, t(20) = 1.934, p = 0.067) and they had lower accuracy at immediate recall (t(73) = -11.019, p < 0.001).
Here, too, we performed a one-way ANCOVA with factor Interval Type and immediate recall, SSS1 and SSS2 scores as covariates within each performance group. Unlike the Low Performers, for whom there was no observed difference between Wake and Sleep groups with respect to delayed recall accuracy (F(1,25) = 0.141, p = 0.710; Fig. 4a), the High Performers received a significant benefit of sleep on delayed recall (F(1,38) = 5.1.61, p = 0.029; Fig. 4a).
Figure 4. Encoding effects on post-sleep performance in older adults.
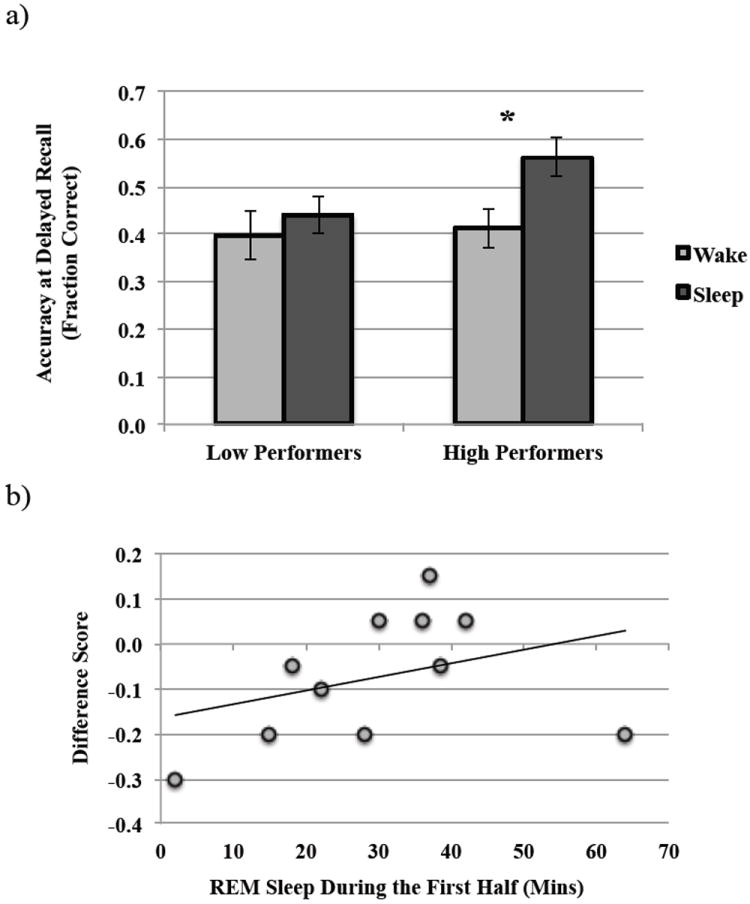
a) Comparison of post-sleep and post-wake performance (Delayed Recall Accuracy) in low and high performing older adults, and b) correlation between REM sleep during the first half of the night and change in performance over sleep (Difference Score) in High Performers. *p-value <0.05.
3.3 Group Differences in Sleep Architecture
Table 3 provides the means, standard deviations and p-values for comparisons of measures of sleep physiology across groups. There was no significant difference between the age groups with respect to total sleep time (TST; total time spent sleeping as opposed to in bed), or the distribution of sleep stages, namely time spent in SWS, NREM or REM sleep across the night. Consistent with prior studies (Buysse et al., 2005; Campbell & Dawson, 1992; O’Donnell et al., 2009), wake after sleep onset (WASO), or the amount of time spent awake after sleep onset and prior to awakening, was significantly greater in older adults than young adults. We did not find a difference between young and older adults in sleep efficiency (time spent in sleep relative to time in bed); however, this lack of difference was driven by three outliers (sleep efficiency was > 3 SD away from the mean), which when removed, revealed a significant age-related decline in sleep efficiency (YA, M = 92.85%, SD = 0.08; OA, M = 86.86%, SD = 0.09; t(52) = 2.649, p = 0.011). With respect to the organization of the sleep stages, no significant differences were observed between young and older adults for average number of cycles occurring throughout the night. However, the average cycle length was significantly greater for young adults than older adults. Additionally, young adults spent a greater amount of time in complete, uninterrupted sleep cycles (total cycle time, TCT; as defined above) than did older adults, and this remained so when looking at the percent time spent in sleep cycles relative to total sleep time (TCT/TST).
Table 3.
Sleep characteristics as recorded by polysomnography in the Sleep groups.
| Sleep Measure | Young Adults | Older Adults | p-value |
|---|---|---|---|
| Across the Night (mins) | |||
| TST | 407.60 (68.02) | 396.33 (55.43) | 0.521 |
| Sleep Latency | 13.33 (16) | 11.30 (10.58) | 0.601 |
| WASO | 17.59 (19.35) | 45.70 (33.21) | 0.001 |
| Sleep Efficiency (%) | 88.23 (15.54) | 87.21 (8.72) | 0.764 |
| Total SWS | 124.73 (45.53) | 115.48 (33.33) | 0.416 |
| Total NREM | 291.17 (58.50) | 270.65 (38.98) | 0.153 |
| Total REM | 71.08 (25.86) | 69.70 (24.89) | 0.845 |
| Sleep Cycle Characteristics | |||
| # Cycles | 3.46 (1.25) | 3.30 (1.13) | 0.664 |
| Average Cycle Length (mins) | 111.39 (44.28) | 88.55 (18.34) | 0.037 |
| TCT | 357.88 (90.92) | 303.98 (76.05) | 0.041 |
| TCT/TST (%) | 86.18 (15.71) | 73.80 (14.52) | 0.010 |
| First Half of the Night (mins) | |||
| Total SWS | 89.41 (36.45) | 77.78 (24.84) | 0.223 |
| Total NREM | 159.59 (38.33) | 144.28 (30.73) | 0.149 |
| Total REM | 15.45 (12.35) | 30.83 (15.30) | < 0.001 |
| Second Half of the Night (mins) | |||
| Total SWS | 34.09 (20.91) | 39.23 (25.18) | 0.445 |
| Total NREM | 131.64 (26.98) | 121.10 (21.61) | 0.155 |
| Total REM | 55.78 (21.83) | 42.28 (21.89) | 0.040 |
| Delta Power Density of SWS (μV2/Hz) | |||
| Across the Night | 176.11 (86.14) | 80.89 (46.57) | <0.001 |
| First Half of the Night | 204.77 (102.27) | 87.92 (56.75) | <0.001 |
Values in parentheses represent standard deviations. p-values in bold represent p< 0.05.
Young adults spent marginally more time in NREM sleep during the first half of the night compared to older adults (Table 3). There was no difference between young and older adults in terms of time spent in NREM sleep during the second half of the night. Additionally, there were no significant differences between young and older adults for time spent in SWS in the first half of the night or the second half of the night. However, delta power density during SWS in first half of the night was greater for young adults compared to older adults (Fig. 3).
Figure 3. Relationship between sleep physiology and memory performance in young adults.
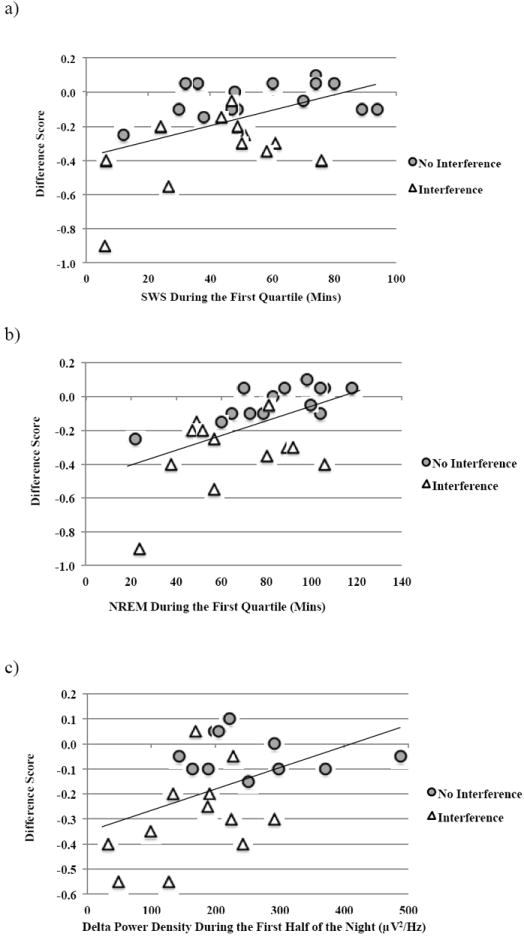
Correlations for young adults between post-sleep performance on the visuo-spatial task (accuracy at Delayed Recall) and a) SWS in the first quartile, b) NREM in the first quartile, and c) delta power density during SWS in the first half.
Older adults showed a more diffuse distribution of REM sleep across the night compared to young adults. Specifically, older adults spent a greater amount of time in REM sleep during the first half of the night (Table 3), but significantly less time in REM sleep during the second half of the night compared to young adults.
3.4 Relationship between Sleep Measures and Performance on the Visuo-Spatial Task
We assume an identical sleep process was at work in both No Interference and Interference conditions (as the intervention happened after sleep). Therefore, in the subsequent analyses, No Interference and Interference conditions were combined to increase statistical power.
3.4.1 The Role of NREM Sleep
For young adults, performance changes over sleep (Difference Score) were correlated with NREM sleep (N2 and SWS combined) across the night at trend-level (Pearson r = 0.328, p = 0.077), but were strongly correlated with NREM sleep during the first half of the night (Pearson r = 0.476, p= 0.01). With regard to SWS specifically, over-sleep changes in performance were not correlated with total time spent in SWS across the night (Pearson r = 0.032, p = 0.867), but were correlated with time spent in SWS during the first half of the night at trend-level (Pearson r = 0.436, p = 0.071). On closer examination, we found a robust correlation between the Difference Score and SWS during the first quartile of the night (Pearson r = 0.486, p = 0.009; Fig. 3a), as well as NREM sleep during the first quartile (Pearson r = 0.553, p = 0.002; Fig. 3b). Furthermore, delta power density during SWS across the night (Pearson r = 0.495, p = 0.019) as well as during the first half of the night (Pearson r = 0.449, p = 0.036; Fig. 3c) was significantly correlated with over-sleep performance changes.
For older adults, no relationship was found between over-sleep performance changes and time spent in SWS across the night (Pearson r = -0.015, p = 0.946), during the first half of the night (Pearson r = 0.331, p = 0.166) or during the first quartile of the night (Pearson r = -0.096, p = 0.695). Likewise, there was no relationship between the Difference Score and NREM sleep across the night (Pearson r = -0.188, p = 0.402), during the first half of the night (Pearson r = -0.217, p = 0.372), or during the first quartile of the night (Pearson r = -0.272, p = 0.260). In addition, there was no significant relationship between the Difference Score and delta power density during SWS across the night (Pearson r = 0.242, p = 0.333), or during the first half of the night (Pearson r = 0.227, p = 0.365). We did not find any correlations between performance changes over sleep and either NREM sleep or SWS for either low or high performing older adults.
3.4.2 The Role of REM Sleep and NREM/REM Interactions
REM sleep either during the first quartile (YA, Pearson r = 0.146, p = 0.487; OA, Pearson r = -0.143, p = 0.611), first half (YA, Pearson r = -0.001, p = 0.997; OA, Pearson r = -0.339, p = 0.217), second half (YA, Pearson r = 0.268, p = 0.194; OA, Pearson r = 0.049, p = 0.863), or across the night (YA, Pearson r = -0.019, p = 0.921; OA, Pearson r = -0.043, p = 0.849) did not confer any benefits to performance on the visuo-spatial task. Although low and high performing older adults did not differ with respect to any of the sleep parameters, for High Performers alone (for whom a sleep benefit was observed), greater time spent in REM sleep in the first half of the night was associated with performance benefits post-sleep (Pearson r = 0.761, p =0.011; Fig. 4b).
It has been previously demonstrated that memory consolidation is dependent on the integrity of the NREM/REM sleep cycles (Ficca et al., 2000) and perhaps on the sequential effects of NREM and REM sleep that each exert differential effects on neuronal excitability in the hippocampus (Grosmark et al., 2012). We did not find a significant correlation between percent time spent in sleep cycles relative to total sleep time (TCT/TST) and over-sleep performance changes (Difference Score) in young or older adults. However, in order to examine the effect of REM sleep when embedded in early NREM sequences on over-sleep performance changes on the visuo-spatial task (Difference Score), we conducted a multiple regression analysis, which revealed a 3-way interaction between NREMSC1, REMSC1 and NREMSC2 that trended towards significance in young adults (β = 2.801, t(19) = 2.039, p = 0.064) suggesting a sequential role of REM and NREM sleep. This analysis did not result in any significant interactions in older adults.
4. Discussion
In the current study, we introduced task-related interference to a visuo-spatial learning task in order to investigate post-sleep stability of declarative memories in young and older adults. We report an active benefit of sleep on visuo-spatial memory consolidation in both age groups, an effect that does not appear to be reduced in older adults relative to young adults.
4.1 Wake vs. Sleep Effects in the No Interference and Interference Conditions
In the No Interference Condition, we did not find a significant difference between the Wake and Sleep groups for either age group with respect to delayed recall accuracy. Previous studies using a similar visuo-spatial paradigm have used image matrices that contain a greater number of images (Rasch et al., 2007; Cherdieu et al., 2014). Therefore, our task consisting of 20 images may have been less challenging than those used in previous studies, resulting in equivalent performance levels across wake and sleep groups. Furthermore, without a control task to equate the level of cognitive demands relative to the Interference task, the No Interference condition was markedly less challenging than the Interference condition. This notion is supported by the fact that we see little forgetting overall in both the Wake and Sleep conditions, with the Adjusted Score clustering close to zero in both age groups. In addition to the relative ease of the No Interference task in the current study, previous studies employed alternative measures of memory accuracy: Rasch and colleagues (2007) used 15 pairs of cards and accuracy was measured as percent card pairs correctly remembered. Cherdieu and colleagues (2014) used accuracy during the learning phase (where feedback was provided and learning continued until a criterion of 75% was achieved) as the baseline measure of memory performance. Since performance changes were measured between the learning phase and the recall phase, the additional feedback provided in the learning phase was confounded with the measure of over-sleep performance changes. By probing immediate recall (without feedback) a more accurate measure was obtained but likely had less magnitude to detect change.
In the Interference condition, the memory system was challenged with competing information, thus revealing a clear benefit of sleep in both age groups. This result provides us with the first line of evidence that active consolidation of visuo-spatial memories occurs during a period of overnight sleep in older adults. Both wake and sleep intervals resulted in memory decay. However, the memory representations that persisted post-interval were more resistant to interference following sleep than wake, suggesting that the process of memory stabilization occurs maximally over an interval of sleep. Importantly, the two-way Age (Young vs. Old) by Interval Type (Wake vs. Sleep) ANCOVA did not reveal a significant Age × Interval Type interaction, indicating that this benefit of sleep was not reduced in older adults relative to young adults.
4.2 Young Adults: Mechanism of Sleep-Dependent Visuo-Spatial Memory Consolidation
In the young adult group, greater amount of time spent in NREM sleep during the first quartile of the night, specifically SWS, was associated with better performance on the task the following morning. These results are in line with previous literature that states that declarative memory consolidation is NREM-dependent, occurring maximally during the early part of the night that is dominated by SWS (Plihal and Born, 1997; Peigneux et al., 2004). Furthermore, Siapas and Wilson (1998) demonstrated that slow-wave activity across the neocortex is responsible for synchronizing the occurrence of hippocampal sharp-wave ripples and neocortical spindles, thereby facilitating the transfer of hippocampal-dependent memories to long-term neocortical stores. Likewise, we report a strong correlation between performance benefits on the visuo-spatial task and delta power density during SWS in young adults.
A number of recent studies suggest that alternating NREM and REM bouts contribute to the memory consolidation in a sequential fashion (Ficca et al., 2000; Chauvette et al., 2012; Griessenberger et al., 2012; Grosmark et al., 2012). Likewise, the results of the regression analyses in the current study revealed an important role of the sequential effects of NREM and REM bouts during the first NREM- REM-NREM triplet on visuo-spatial memory consolidation. Grosmark and colleagues (2012) previously demonstrated that REM episodes are responsible for a reduction in neuronal discharge rates (i.e. synaptic depotentiation). Previously associated with NREM sleep, the process of synaptic depotentiation is crucial for creating an ideal neural environment for additional learning post- sleep due to the increase in synaptic potentiation across wake (Tononi and Cirelli, 2003). In contrast, Grosmark and colleagues (2012) demonstrated an increase in neuronal discharge rates during NREM sleep, as a result of high-frequency hippocampal sharp-wave ripples. This is consistent with the mechanism of memory consolidation that is driven by long-term potentiation (LTP) and an increase in neuronal firing rates (Bliss and Collingrdige, 1993).
Collectively, the results suggest that the benefit of sleep on visuo-spatial memory in young adults is sensitive to the organization of sleep stages, perhaps reflecting three crucial steps in the process. First, during slow-wave activity soon after sleep onset (the first NREM bout), local upscaling of the neuronal circuits that were activated while encoding the image locations occurs (Chauvette et al., 2012). Retrieval expectancy in an experimental setup is sufficient to “tag” memories as important, thus prioritizing their consolidation over sleep (Wilhelm et al., 2011). Second, subsequent REM sleep results in global downscaling of synaptic strengths in the hippocampus, serving as a “filter,” wherein memories that are not of future relevance are depotentiated (Grosmark et al., 2012; Wilhelm et al., 2011). Third, SWA following REM-dependent global downscaling of synaptic strengths, further strengthens and stabilizes the memory traces associated with the image locations, ultimately resulting in the reorganization of the memories into neocortical stores from where they can be retrieved more efficiently the following morning.
4.4 Older Adults: Mechanism of Sleep-Dependent Visuo-Spatial Memory Consolidation
Sleep physiology was observed to be markedly different in older adults compared to young adults. Firstly, older adults appeared to have a broader distribution of REM sleep across the night, an observation that has previously been reported in aging populations (Lombardo, 1998) and has been attributed in part to changes in the circadian regulation of sleep (Pace-Schott and Spencer, 2011). Specifically, although young and older adults in this study did not differ with respect to total time spent in REM sleep across the night, older adults spent greater time in REM sleep early in the night and less time in REM sleep later in the night, as compared to their younger counterparts. We also report age-related changes in the electrophysiological properties of SWS: older adults had reduced delta power density during SWS, corroborating previous reports in aging populations (Cajochen et al., 2006; Carrier et al., 2001; Ohayon et al., 2004). Sleep architecture is particularly sensitive to body temperature, and to hormonal and neurochemical modulations (Porkka-Heiskanen, Zitting and Wigren, 2013), which are known to alter with age (Duffy et al., 2002; Monk, 2005). In line with this, older adults in the present study hade greater amount of WASO, reduced sleep efficiency, spent less time in uninterrupted sleep cycles relative to total sleep time and had shorter sleep cycles on average compared to young adults.
We sought evidence of active memory consolidation in older adults by means of behavioral manipulations and sleep physiology. First, through the use of task-related interference, we were able to demonstrate that sleep stabilized visuo-spatial memories and thus protected them from retroactive interference in older adults. Second, despite a reduction in slow wave activity (delta power density of the slow waves) relative to young adults, we observed that older adults benefited equally from a period of overnight sleep compared to young adults with respect to performance on the visuo-spatial task. A possible explanation for this is that the relationship between SWS and memory weakens with age, a finding that is corroborated by Scullin (2013) who demonstrated a negative relationship between SWS and episodic memory in healthy older adults.
Wilhem and colleagues (2012) previously demonstrated that pre-sleep performance levels predict the extent of memory consolidation occurring over sleep. Likewise, Tucker and colleagues (2011) suggested that in older adults, sleep-dependent memory consolidation might not occur in the event that encoding was inadequate. However, these observations were made with respect to procedural tasks. Therefore, in order to examine whether the contributions of NREM and REM sleep to memory consolidation were dependent on the level of initial acquisition of the visuo-spatial information, we dichotomized our older adult group into “Low Performers” and “High Performers.” With respect to performance on the visuo-spatial task, we observed a benefit of sleep relative to wake for the High Performers only. Furthermore, for high performing older adults, REM sleep during the first half of the night benefited memory performance, such that greater time spent in REM sleep resulted in less forgetting post-sleep. As mentioned previously, by means of its synaptic depotentiation effect (Grosmark et al., 2012), REM sleep may in fact play a beneficial role in declarative memory consolidation. Older adults may be more reliant on this property of REM and thus benefit from having larger bouts of REM early in the night. Furthermore, the decrease in SWS properties with a concurrent increase in REM sleep early in the night in older adults perhaps facilitates a greater dependence on REM-mediated declarative memory consolidation processes. In the Low Performers, an interval of sleep following learning did not render any additional benefits to performance on the visuo-spatial task as compared to an interval of wake.
These findings are consistent with previous studies, which demonstrate that pre-sleep performance on a particular memory task is predictive of the extent of over-sleep consolidation (Wilhelm et al., 2012). Presumably, in low performing older adults, the memory may be too weak to be subject to over-sleep processing (Baran, Wilson, & Spencer, 2010).
We note the limitations of our study. First, young adults reported having poorer subjective sleep quality and were significantly sleepier at the time of testing compared to older adults. As a result, although young adults demonstrated over-sleep memory consolidation, they might have had reduced efficiency in task performance (due to sleepiness), thus reducing our ability to detect age-related differences in sleep-dependent memory consolidation. However we find this to be unlikely given that sleepiness scores were used as a covariate in our analyses, thus controlling for differences in sleepiness between age groups, and age-related differences in task performance remained. Second, there was a disproportionately larger percentage of women in our older adult sample. Age-related changes in sleep architecture and EEG spectral characteristics occur earlier, and to a larger extent, in men (Ehlers & Kupfer, 1997; Bliwise, 2005). This may explain the relatively equivalent amount of SWS in our older adult and young adult samples, contrary to previous findings that demonstrate significant reductions in SWS with age (Lombardo et al., 1998; Cajochen et al., 2006; but see Cherdieu et al., 2014; Aly & Moscovitch, 2010). Finally, we combined the No Interference and Interference conditions for the purpose of the correlational analyses with sleep parameters. Although there are similar declarative memory consolidation processes at play in the No Interference and Interference conditions, the potential ceiling effect observed in the No Interference condition might have diminished our capacity to detect the relationship between sleep parameters and over-sleep performance changes.
4.5 Conclusions
We compared post-wake and post-sleep performance on a visuo-spatial learning task in healthy young and older adults specifically with the goal of dissociating the passive and active roles of sleep in declarative memory consolidation. Sleep was found to protect declarative memories from subsequent interference in both young and older adults, providing support for the active, strengthening effects of sleep on declarative memories. As a consequence of age-related changes in sleep architecture, the underlying sleep- dependent mechanisms in older adults do not appear to be dependent on NREM sleep early in the night as do in young adults, but rather with REM sleep early in the night. Furthermore, this compensatory mechanism for declarative memory consolidation is only present in those older adults that have superior encoding abilities, and thus a greater level of initial acquisition of the declarative information. Thus, future research should focus on understanding how age-related cognitive decline impacts the traditional hippocampal-dependent declarative memory system, and how this might affect sleep-dependent memory consolidation.
Highlights.
Visuo-spatial memories are stabilized over sleep and are thus more resistant to retroactive interference, compared to wake, in young and high performing older adults.
In young adults, the sleep benefit on visuo-spatial memory is correlated with SWS and SWA early in the night.
NREM and REM sleep early in the night sequentially contribute to memory consolidation in young adults.
In high performing older adults, the sleep benefit on visuo-spatial memory is associated with REM sleep early in the night.
Acknowledgments
The study was supported by NIH R01 AG040133.
Footnotes
Disclosure Statement
The authors do not have any actual or potential conflicts of interest. All procedures were approved by the Institutional Review Board (IRB) of the University of Massachusetts, Amherst.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alger SE, Lau H, Fishbein W. Slow wave sleep during a daytime nap is necessary for protection from subsequent interference and long-term retention. Neurobiology of Learning and Memory. 2012;98:188–196. doi: 10.1016/j.nlm.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Aly M, Moscovitch M. The effects of sleep on episodic memory in older and younger adults. Memory. 2010;18(3):327–334. doi: 10.1080/09658211003601548. [DOI] [PubMed] [Google Scholar]
- Bakhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K. Midlife decline in declarative memory consolidation is correlated with decline in slow wave sleep. Learning and Memory. 2007;14:336–341. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran B, Wilson J, Spencer RMC. REM-dependent repair of competitive memory suppression. Experimental Brain Research. 2010;23:471–477. doi: 10.1007/s00221-010-2242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16(1):40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Normal aging. Principles and Practice of Sleep Medicine. 2005;4:24–38. [Google Scholar]
- Buboltz WC, Brown F, Soper B. Sleep habits and patterns of college students: a preliminary study. Journal of American College Health. 2001;50(3):131–135. doi: 10.1080/07448480109596017. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Monk TH, Carrier J, Begley A. Circadian patterns of sleep, sleepiness, and performance in older and younger adults. Sleep. 2005;28(11):1365–1376. doi: 10.1093/sleep/28.11.1365. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Munch M, Knoblauch V, Blatter K, Wirz-Justice A. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiology International. 2006;23(1-2):461–474. doi: 10.1080/07420520500545813. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Dawson D. Aging young sleep: a test of the phase advance hypothesis of sleep disturbance in the elderly. Journal of Sleep Research. 1992;1(3):205–210. doi: 10.1111/j.1365-2869.1992.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20-60 years old) Psychophysiology. 2001;38(2):232–242. [PubMed] [Google Scholar]
- Chauvette S, Seigneur J, Timofeev I. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron. 2012;75(6):1105–1113. doi: 10.1016/j.neuron.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherdieu M, Reynaud E, Uhlrich J, Versace R, Mazza S. Does age worsen sleep- dependent memory consolidation? Journal of Sleep Research. 2014;23(1):53–60. doi: 10.1111/jsr.12100. [DOI] [PubMed] [Google Scholar]
- Darchia N, Campbell IG, Feinberg I. Rapid eye movement density is reduced in normal elderly. Sleep. 2003;26(8):973–977. doi: 10.1093/sleep/26.8.973. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nature Reviews Neuroscience. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Buchel C, Born J, Rasch B. Labile or stable: Opposing consequences for memory when reactivated during waking and sleep. Nature Neuroscience. 2011;14(3):381–386. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk DJ, Czeisler CA. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. American Journal of Physiology Endocrinology and Metabolism. 2002;282(2):E297–303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Kupfer DJ. Slow-wave sleep: do young adult men and women age differently? Journal of Sleep Research. 1997;6:211–215. doi: 10.1046/j.1365-2869.1997.00041.x. [DOI] [PubMed] [Google Scholar]
- Ekstrand BR, Barrett TR, West J, Maier WG. Neurobiology of Sleep and Memory. London: Academic Press; 1977. The effect of sleep on human long-term memory. [Google Scholar]
- Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: Sleep, declarative memory, and associative interference. Current Biology. 2006;16(13):1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Ficca G, Lombardo P, Rossi L, Salzarulo P. Morning recall of verbal material depends on prior sleep organization. Behavioural Brain Research. 2000;112(1-2):159–163. doi: 10.1016/s0166-4328(00)00177-7. [DOI] [PubMed] [Google Scholar]
- Giuditta A, Ambrosini MV, Montagnese P, Mandile P, Contugno M, Grassi ZG, Vescia S. The sequential hypothesis of the function of sleep. Behavioural Brain Research. 1995;69(1-2):157–166. doi: 10.1016/0166-4328(95)00012-i. [DOI] [PubMed] [Google Scholar]
- Griessenberger H, Hoedlmoser K, Heib DP, Lechinger J, Klimesch W, Schabus M. Consolidation of temporal order in episodic memories. Biological Psychology. 2012;91(1):150–155. doi: 10.1016/j.biopsycho.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosmark AD, Mizuseki K, Pastalkova E, Diba K, Buzsaki G. REM sleep reorganizes hippocampal excitability. Neuron. 2012;75(6):1001–1007. doi: 10.1016/j.neuron.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxton B. Fragments: The Collected Wisdom of Heraclitus. New York City, NY: Viking Adult; 2001. [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: A new approach. Psychophysiology. 1973;10(4):431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. 1. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Lombardo P, Formicola G, Gori S, Gneri C, Massetani R, Murri L, Salzarulo P, et al. Slow wave sleep (SWS) distribution across night sleep episode in the elderly. Aging. 1998;10(6):445–448. doi: 10.1007/BF03340157. [DOI] [PubMed] [Google Scholar]
- Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. Journal of Adolescent Health. 2010;46(2):124–132. doi: 10.1016/j.jadohealth.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin J, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nature Neuroscience. 2013;16:357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- McDevitt EA, Duggan KA, Mednick SC. REM sleep rescues learning from interference. Neurobiology of Learning and Memory. 2015 doi: 10.1016/j.nlm.2014.11.015. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC, Cai DJ, Shuman T, Anagnostraras S, Wixted JT. An opportunistic theory of cellular and systems consolidation. Trends in Neuroscience. 2011;34(1):504–514. doi: 10.1016/j.tins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nature Neuroscience. 2003;6(7):697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- Monk TH. Aging human circadian rhythms: Conventional wisdom may not always be right. Journal of Biological Rhythms. 2005;20(4):366–374. doi: 10.1177/0748730405277378. [DOI] [PubMed] [Google Scholar]
- Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult – a mini-review. Gerontology. 2010;56(2):181–189. doi: 10.1159/000236900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE, Willison J. National Adult Reading Test (NART) Nfer-Nelson; 1991. [Google Scholar]
- O’Donnell D, Silva EJ, Munch M, Ronda JM, Wang D, Duffy JF. Comparison of subjective and objective assessments of sleep in healthy older subjects without sleep complaints. Journal of Sleep Research. 2009;18(2):254–263. doi: 10.1111/j.1365-2869.2008.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM. Sleep and the elderly. Journal of Psychosomatic Research. 2004;56(5):463–464. doi: 10.1016/j.jpsychores.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RM. Age-related changes in the cognitive function of sleep. Progress in Brain Research. 2011;191:75–89. doi: 10.1016/B978-0-444-53752-2.00012-6. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Maquet P, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44(3):535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. Journal of Cognitive Neuroscience. 1997;9(4):534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Zitting KM, Wigren HK. Sleep, its regulation and possible mechanisms of sleep disturbances. ActaPhysiologica. 2013;208(4):311–328. doi: 10.1111/apha.12134. [DOI] [PubMed] [Google Scholar]
- Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315(5817):1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- Ronnlund M, Nyberg L, Backman L, Nilsson LG. Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychology and Aging. 2005;20(1):3–18. doi: 10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- Rovner BW, Folstein MF. Mini-mental state exam in clinical practice. Hospital practice. 1987;22(1A):99–110. [PubMed] [Google Scholar]
- Scullin K. Sleep, memory, and aging: The link between slow-wave sleep and episodic memory changes from younger to older adults. Psychology and Aging. 2013;28(1):105–114. doi: 10.1037/a0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21(5):1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Spencer RMC. Neurophysiological basis of sleep’s function on memory and cognition. ISRN Physiology. 2013 doi: 10.1155/2013/619319. Article ID 619319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: a multi-step process occurring during sleep. Journal of Cognitive Neuroscience. 2000;12(2):246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: A hypothesis. Brain Research Bulletin. 2003;62(2):143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Tucker M, McKinley S, Stickgold R. Sleep optimizes motor skills in older adults. Journal of the American Geriatric Society. 2011;59:603–609. doi: 10.1111/j.1532-5415.2011.03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Diekelmann S, Molzow I, Ayoub A, Mölle M, Born J. Sleep selectively enhances memory expected to be of future relevance. The Journal of Neuroscience. 2011;31(5):1563–1569. doi: 10.1523/JNEUROSCI.3575-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Metzkow-Meszaros M, Knapp S, Born J. Sleep-dependent consolidation of procedural motor memories in children and adults: The pre-sleep level of performance matters. Developmental Science. 2012;15(4):506–515. doi: 10.1111/j.1467-7687.2012.01146.x. [DOI] [PubMed] [Google Scholar]
- Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RM. Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiology of Aging. 2012;33(5):991–1000. doi: 10.1016/j.neurobiolaging.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


