Abstract
Introduction
Nicotine intake from electronic cigarette (e-cigarettes) increases with user’s experience. This suggests that smokers who switched from tobacco to electronic cigarettes compensate for nicotine over the time to get as much nicotine as they need. One of the mechanism by which smokers may compensate for nicotine is by modifying their puffing behavior. The aim of the study was to assess the changes in puffing behavior after switching from conventional to electronic cigarettes among regular smokers.
Materials and Methods
Twenty smokers (11 female, aged 31±10, CPD 16±8, FTND 4±3, and exhaled CO 16±17 (mean±SD)) who were naïve to e-cigarettes participated in this study. They were asked to substitute their regular tobacco cigarettes with first generation e-cigarettes (labelled 18 mg nicotine) for two weeks. Puffing topography (number of puffs, puff volume, intervals between puffs, and average puff flow rate) was measured at the initial use (baseline), as well as after one and two weeks of product use. We tested changes in puffing topography outcomes using repeated measures ANOVA.
Results
We found that after one week of using e-cigarettes, participants significantly increased the average time they puffed on e-cigarettes from 2.2±0.1 (mean±SEM) to 3.1±0.3 sec (p<0.05). The average puff flow rate decreased from 30.6±2.3 to 25.1±1.8 ml/sec after one week of e-cigarette use (p<0.05).
Conclusions
Our data show that smokers modify their puffing behavior after switching from tobacco to electronic cigarettes by taking longer and slower puffs. The potential reason for changing puffing behavior is to compensate for less efficient nicotine delivery from e-cigarettes.
Keywords: electronic cigarettes, e-cigarettes, puffing topography, nicotine, compensation, vapor
1. Introduction
Electronic cigarettes (e-cigarettes) are promoted as an alternative product to conventional tobacco cigarettes (Hajek et al, 2014a). The use of e-cigarettes has been increasing rapidly around the world, especially among tobacco cigarette smokers and young people (Carroll Chapman & Wu, 2014). E-cigarettes are battery-driven devices which vaporize nicotine solutions in a form of inhalable aerosol. These products contain tobacco-derived nicotine and flavorants such as tobacco, menthol, fruit, and coffee. Nicotine, flavorants and other additives are dissolved in propylene glycol, glycerin or a mixture of both substances. There is a consensus among researchers and public health advocates that e-cigarettes are less harmful to individual users and bystanders than tobacco cigarettes since e-cigarettes contain fewer hazardous chemicals than conventional cigarettes (Cahn & Siegel, 2011; Grana & Glantz, 2014; Goniewicz et al., 2014; Gualano et al. 2014).
Nicotine is the primary pharmacologically active substances in e-cigarettes. It is responsible for addictiveness of tobacco products and administration of nicotine from other sources than tobacco cigarettes has been shown to alleviate withdrawal symptoms among abstinent smokers (Benowitz, 2013). Studies have shown that vapor generated from e-cigarettes contain variable yields of nicotine (Goniewicz et al. 2013, Goniewicz, Hajek, & McRobbie, 2014). It has been shown that e-cigarettes can deliver nicotine effectively to blood while reducing exposure to combustion toxicants and carcinogens among their users (Farsalinos et al., 2014; Goniewicz, Hajek & McRobbie, 2014; Goniewicz et al., 2014; Vansickel & Eissenberg, 2013).
The nicotine delivery from e-cigarettes is likely to play a major role in the patterns of product use among smokers who are interested in substituting their regular cigarettes with safer alternatives. Some pharmacokinetic studies on e-cigarettes revealed that nicotine intake from the devices on initial use is lower compared to tobacco cigarettes (Eissenberg, 2010; Hajek et al., 2014b). However, studies on long-term e-cigarette users found that those users who have significant experience with these devices actually can achieve plasma nicotine levels similar to levels observed among smokers (Vansickel & Eissenberg, 2013; Farsalinos et al., 2014). Our group has also shown that nicotine uptake from e-cigarettes increases with practice (Hajek et al., 2014b). Using pharmacokinetic data from six smokers, we found that 4 weeks of practice generated a 24% increase in the peak plasma concentrations and a 79% increase in overall nicotine intake when compared to initial e-cigarette use. The studies cited above suggest that although e-cigarettes may deliver less nicotine than conventional cigarettes but that intake may improve with practice. At this time, it remains unclear how smokers increase nicotine intake over time. It has been suggested that there is a ‘learning period’ during which naïve users learn how to puff on e-cigarettes to get desired doses of nicotine (Hajek et al., 2014b). The aim of the study was to assess the changes in puffing behavior among adult smokers who were naïve to e-cigarettes and who switched from conventional to electronic cigarettes for two weeks.
2. Materials and Methods
2.1. Subjects
Cigarette smokers willing to switch to potentially reduced exposure product to reduce their smoking were recruited from the Silesia metropolitan area in Poland using advertisements in campus, local media, and by word of mouth. A total of 20 adult smokers (11 female) who were naïve to e-cigarettes were recruited for the study. All participants were provided written informed consent. The average age of participants was 31 years (SD=10, range 20–52). Participants smoked on average 16 tobacco cigarettes per day (SD=8, range 5–35), for on average 13 years (SD=7, range 5–35), the mean exhaled CO level was 16 ppm (SD=17, range 3–85), and the average FTND score was 4 points (SD=3, range 0–9).
2.2. Product
Participants were provided with the e-cigarette M201 type (Mild, Poland) with cartridges containing 11.0±1.5 mg of nicotine as determined in a previous study (labeled 18 mg) (Goniewicz et al, 2013). Participants were also provided with 20 cartridges for one week of use, one spare battery, and a charger. Verbal and written instruction on how to use, recharge, and store the product were given to all participants.
2.3. Study protocol
Participants were asked to substitute their regular tobacco cigarettes with e-cigarettes for two weeks and refrain from smoking any combustible tobacco products. They were required to attend three clinic visits in the mornings on the same days of the week: during Day #1 (baseline), during Day #7 (Week 1), and during Day #14 (Week 2) of the study. Participants were asked to refrain from using e-cigarettes at least 8 hours before each clinic visit. At each visit, subjects participated in an experimental puffing session during which they were asked to puff ad lib on e-cigarettes. They were allowed to take as many puffs as desired. Participants provided measures of puffing topography with CressMicro monitor with a connector for e-cigarettes (Borgwaldt Ltd, Germany). The topography monitor was calibrated before each puffing session using a piston-like smoking machine and a square-shaped puff profile. Measurement variables of puffing topography included number of puffs, puff volume, intervals between puffs, and puff flow rate. The study protocol was reviewed and approved by the IRB at the Medical University of Silesia, Poland.
2.4. Statistical analysis
To analyze the effect of experience with e-cigarette use on puffing behavior, we compared average puffing variables measured during initial e-cigarette use (baseline) with variables registered over two weeks (during the first and the second follow-up sessions). Statistical comparisons were performed using repeated measures ANOVA with Statistica 9.0 software (Statsoft, USA). Difference were considered significant if p-value was <0.05.
3. Theory
We hypothesized that smokers who switched from tobacco to electronic cigarettes compensate to get as much nicotine as they need. The compensation refers to adjustment of behaviors associated with product use in such a way that nicotine uptake remains the same. Compensatory behaviors have been studied among smokers who switched from cigarettes with high nicotine yields to low-nicotine (denicotinized) cigarettes (Hammond, Fong, Cummings, & Hyland, 2005; Kassel et al., 2007). These studies have shown that smokers compensate for nicotine by taking deeper puffs or puffing on cigarettes more frequently. Thus, we hypothesized that smokers who switch from tobacco cigarettes to e-cigarettes will also compensate for nicotine by modifying their puffing behavior.
4. Results
All subjects reported significant reduction in number of tobacco cigarettes smoked per day (on average from 16.2 to 0.6 CPD) and 8 subjects reported complete tobacco abstinence during the study. A substantial variability in puffing topography between smokers was found. Table 1 presents average puffing topography outcomes on initial use of e-cigarettes, as well as after one week and after two weeks of product use. Smokers took on average 19.3±2.5 (3–40) (mean±SEM (range)) puffs on initial e-cigarette use; 23.7±2.4 (8–40; p>0.05) puffs after one week; and 21.3±2.4 (6–40; p>0.05) puffs after two weeks of product use. The average interval between per puffs was 19.2±2.7 (5.4–52.8), 15.2±2.2 (0.9–45.3; p>0.05), and 22.1±4.9 (4.5–84.5; p>0.05) sec during the first, second, and third session, respectively. Smokers took an average of 64.0±4.8 (37.4–109.4) ml puffs when using e-cigarettes for the first time, 66.5±3.7 (37.2–94.3; p>0.05) and 63.3±5.2 (30.3–126.6; p>0.05) ml puffs after one and two weeks of experience with the products.
Table 1.
Puffing topography among 20 smokers who switched from tobacco to electronic cigarettes for two weeks.
| E-cigarette Puffing Topography (mean±SEM, n=20) | |||
|---|---|---|---|
| Initial use | After 1 week | After 2 weeks | |
| Puff Count (n) | 19.3±2.5 | 23.7±2.4 | 21.3±2.4 |
| Interval between Puffs (sec) | 19.2±2.7 | 15.2±2.2 | 22.1±4.9 |
| Puff Volume (ml) | 64.0±4.8 | 66.5±3.7 | 63.3±5.2 |
| Puff Duration (sec) | 2.2±0.1 | 3.1±0.3* | 2.9±0.2* |
| Puff Flow Rate (ml/sec) | 30.6±2.3 | 25.1±1.8* | 24.8±1.9* |
Note:
indicates significant change compared to initial use (repeated measures ANOVA; p<0.05)
Statistical analysis revealed significant changes in puff duration and puff flow rate during the study. Figure 1 show changes in puff duration (Figure 1A; left) and puff flow rate (Figure 1B; right) from initial e-cigarette use to one and two weeks of product use. The average puff duration increased from 2.2±0.1 (1.1–3.8) to 3.1±0.3 (1.2–6.0; p<0.05) sec between initial and first week of e-cigarette use. The average puff flow rate decreased from 30.6±2.3 (16.2–60.3) to 25.1±1.8 ml/sec (range; p<0.05) after one week of e-cigarette use. No additional significant changes were observed between the first and second week of e-cigarette use.
Figure 1.
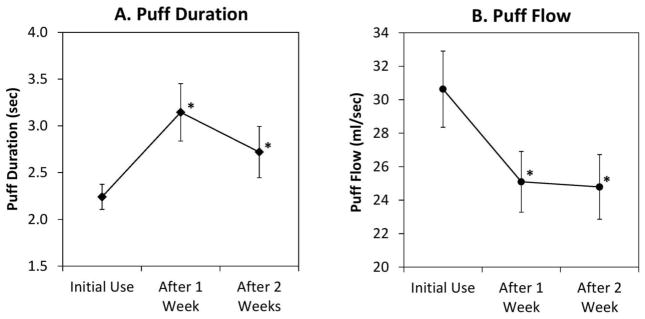
Changes in puff duration (A, left) and puff flow rate (B, right) over two weeks of electronic cigarette use among 20 naïve smokers (mean±SEM).
Note: * indicates significant change compared to initial use (repeated measures ANOVA; p<0.05)
5. Discussion
We presented novel data on how smokers change their puffing behavior after switching from tobacco to electronic cigarettes. Although puffing behavior was highly variable between smokers who participated in this study, we were able to detect significant changes in puff duration and puff flow rate over time. The study showed that after one week of e-cigarette use, smokers took longer puffs and puffed slower on the devices when compared to initial use of the product. A week of practice generated, on average, an almost 40% increase in the puff duration and an almost 20% decrease in the average puff flow rate compared to initial use.
Results of this study suggest that smokers who make a switch to e-cigarettes adapt to the new product in relatively short time. Significant changes in puffing behavior were only observed during the first week of product use and no changes were observed during the second week of the study. This suggests that smokers can adjust to new product in just few days, which has important implications for future clinical studies with e-cigarettes, including clinical trials to assess efficacy of e-cigarettes for smoking cessation. Participants in these trials should be instructed that there will be few days during which they will discover how to use e-cigarettes and adjust to the new product. In addition, puffing topography should not be measured among subjects who are naïve to e-cigarettes and who lack experience with the devices.
This study looked at the puffing topography among smokers who switched from tobacco to electronic cigarettes over two weeks. The average puff duration observed after one and two weeks of product use was slightly shorter than previously reported among experienced e-cigarette users. Farsalinos et al. (2014) video-recorded 45 experienced e-cigarette users and found that they puffed on their devices on average for 4.2 sec. Hua et al. (2014) analyzed YouTube videos showing e-cigarettes users and determined the average puff duration of 4.3 sec. Eissenberg et al. (2014) used a mouthpiece-based device in a similar way as described in our study and found that e-cigarette users took larger and longer puffs with lower flow rates relative to previously reported data for tobacco cigarette smokers. These findings are consistent with our observations.
In the current study we did not examine whether changes in puffing behavior were correlated with changes in nicotine intake. However, our previous research showed that smokers who were naïve to e-cigarettes increased overall nicotine intake by 79% over 4 weeks of practice when compared with using e-cigarette for the first time (Hajek et al., 2014b). Thus, it is likely that smokers in current study took longer and slower puffs in order to increase nicotine intake and inhale desired doses of the drug. However, described changes in puffing topography among smokers who switched to e-cigarettes are somehow different than changes observed in smokers who switched from high-yield to low-yield nicotine tobacco cigarettes. In the study by Hammond et al. (2005), adult smokers who switched to a low-yield cigarette increased their total smoke intake per cigarette by 40% by taking higher puff volumes while maintaining similar nicotine uptake. Kassel et al. (2007) studied changes in smoking topography in response to denicotinized nicotine cigarettes in adolescent smokers and found that adolescents who smoked denicotinized cigarettes took significantly more puffs per cigarette than did those who smoked a high-yield cigarette. Future studies are needed to evaluate whether e-cigarette users titrate doses of nicotine delivered from various devices by modifying puffing topography.
The current study was a pilot project and has several limitations. Firstly, the sample size in our study was relatively small. We were not able to control for potential confounders and assess whether demographic characteristics of participants influenced measured outcomes. Secondly, the study was conducted in a laboratory setting. We are not aware about published studies comparing e-cigarette puffing topography measured inside and outside a laboratory. Thirdly, the monitor used in this study was designed to measure puffing topography in smokers of conventional cigarettes and has not been validated for use in studies with e-cigarettes yet. We approached this limitation by calibrating the device using a smoking machine. Finally, we did not measure how participants had puffed on their regular tobacco cigarettes before they made a switch to e-cigarettes. Future studies should compare puffing behaviors of tobacco cigarette smokers and e-cigarette users.
HIGHLIGHTS.
Nicotine intake from e-cigarette has been shown to increase with experience.
We assessed puffing behavior after switching from tobacco to electronic cigarettes.
E-cigarette puffing behavior differed significantly between participants.
Smokers modified their puffing behaviors after switching to e-cigarettes.
The study showed that smokers took longer puffs and puffed slower after one week of e-cigarette use.
Acknowledgments
Role of Funding Sources
This study was conducted while the corresponding author was at Medical University of Silesia, Poland and was supported by the Ministry of Science and Higher Education of Poland under grant number N N404 025638. The study sponsor had no involvement in the study design, collection, analysis and interpretation of data, the writing of the manuscript or the decision to submit the manuscript for publication.
We thank Craig Steger and Noel Leigh for editorial help.
Footnotes
Contributions
MLG designed the study and wrote a protocol. MG collected the data. YHL conducted literature searches and provided summaries of previous studies. MLG and YHL analyzed the data and wrote the first draft of the manuscript. All contributors approved the final version of the manuscript.
Conflict of interest
MLG received research funding from Pfizer, manufacturer of stop smoking medications. The other authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cahn Z, Siegel M. Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? J Public Health Policy. 2011;32(1):16–31. doi: 10.1057/jphp.2010.41. [DOI] [PubMed] [Google Scholar]
- 3.Carroll Chapman SL, Wu LT. E-cigarette prevalence and correlates of use among adolescents versus adults: a review and comparison. J Psych Res. 2014;54:43–54. doi: 10.1016/j.psychires.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eissenberg T. Electronic nicotine delivery devices: ineffective nicotine delivery and craving suppression after acute administration. Tob Control. 2010;19(1):87–88. doi: 10.1136/tc.2009.033498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10(6):2500–2514. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. doi: 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction. 2014;109(3):500–507. doi: 10.1111/add.12410. [DOI] [PubMed] [Google Scholar]
- 8.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, 3rd, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15(1):158–166. doi: 10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- 10.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gualano MR, Passi S, Bert F, La Torre G, Scaioli G, Siliquini R. Electronic cigarettes: assessing the efficacy and the adverse effects through a systematic review of published studies. J Public Health (Oxf) 2014 doi: 10.1093/pubmed/fdu055. First published online: August 9, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Hajek P, Etter JF, Benowitz N, Eissenberg T, McRobbie H. Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction. 2014a;109(11):1801–1810. doi: 10.1111/add.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajek P, Goniewicz ML, Phillips A, Myers Smith K, West O, McRobbie H. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res. 2014b doi: 10.1093/ntr/ntu153. First published online: August 13, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond D, Fong GT, Cummings KM, Hyland A. Smoking topography, brand switching, and nicotine delivery: results from an in vivo study. Cancer Epidemiol Biomakers Prev. 2005;14(6):1370–1375. doi: 10.1158/1055-9965.EPI-04-0498. [DOI] [PubMed] [Google Scholar]
- 15.Hua M, Yip H, Talbot P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube Videos. Tob Control. 2013;22(2):103–106. doi: 10.1136/tobaccocontrol-2011-050226. [DOI] [PubMed] [Google Scholar]
- 16.Kassel JD, Greenstein JE, Evatt DP, Wardle MC, Yates MC, Veilleux JC, Eissenberg T. Smoking topography in response to denicotinized and high-yield nicotine cigarettes in adolescent smokers. J Adolesc Health. 2007;40(1):54–60. doi: 10.1016/j.jadohealth.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Norton KJ, June KM, O’Connor RJ. Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes. Tob Induc Dis. 2014;12(1):17. doi: 10.1186/1617-9625-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: The effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob Res. 2014 doi: 10.1093/ntr/ntu186. First published online: September 19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15(1):267–270. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]


